Abstract
Purpose. The goals of this study were to investigate whether β-defensins are differentially expressed in the conjunctival epithelium of patients with moderate dry eye when compared with normal subjects and whether proinflammatory cytokines or bacteria can modulate the expression of human β-defensins (hBDs)-1, -2, and -3 by conjunctival epithelial cells.
Methods. RNA extracted from conjunctival impression cytology specimens of eight normal subjects and nine patients with moderate dry eye was used in RT-PCR to detect mRNA for hBDs-1, -2, and -3. Two conjunctival epithelial cell lines and primary cultured conjunctival epithelial cells were treated with proinflammatory cytokines or heat-killed Pseudomonas aeruginosa. RT-PCR and immunoblot analysis were used to detect mRNA for hBD-1, -2, and -3 and protein secretion of hBD-2, respectively.
Results. hBD-2 message was detected in RNA samples of eight of nine patients with dry eye, but not in any of the normal subjects’ samples, whereas hBD-1 and -3 were detected in all subjects tested. RT-PCR revealed an upregulation of hBD-2 but no difference in expression of hBD-1 and -3 in cultured conjunctival cells after a 24-hour treatment with 10 ng/mL interleukin (IL)-1β, IL-1β and tumor necrosis factor-α (10 ng/mL) or heat-killed Pseudomonas aeruginosa (1 million colony-forming units; n = 3). hBD-2 expression was upregulated from 4 hours of treatment with IL-1β (at 10 ng/mL; (n = 2–3) and at a concentration of 0.1 ng/mL IL-1β (24-hour treatment; n = 2–3). Immunoblots demonstrated protein secretion results corresponding to the RT-PCR data.
Conclusions. hBD-2 was expressed only in the conjunctival epithelium of patients with moderate dry eye. Because cytokines such as IL-1β and TNF-α induced the expression of hBD-2 by conjunctival epithelial cells and because increased proinflammatory cytokine activity is a feature of dry eye disease, it can be speculated that the hBD-2 upregulation observed in subjects with moderate dry eye is mediated by proinflammatory cytokine activity.
Dry eye arises either from decreased tear secretion or increased tear evaporation.1 Stern et al.2 have proposed that subclinical inflammation is a feature of all forms of dry eye. Recent evidence clearly demonstrates increased proinflammatory cytokine activity and inflammatory cell-surface markers at the ocular surface epithelia of subjects with Sjögren’s or non-Sjögren’s dry eye.3,4 Increased levels of the proinflammatory cytokines interleukin (IL)-1α and -1β have been shown in subjects with Sjögren’s syndrome or ocular rosacea.4,5 The ocular surface in dry eye disease is compromised and therefore at risk for microbial infections.6,7 Although the concentration of antibacterial proteins such as lactoferrin are lower in the tear film of subjects with dry eye, there is no increase in the number of colonies of common conjunctival bacterial flora such as Staphylococcus aureus.8,9 Seal et al.8 proposed that subjects with dry eye may have an alternative mechanism to protect the ocular surface from infection. One possibility is that naturally occurring antimicrobial peptides, such as human β-defensins (hBDs), provide alternate means of defense for the compromised ocular surface in dry eye, thus reducing the risk of corneal and conjunctival infections.
Defensins are antimicrobial peptides that are involved in innate host defense.10,11 Two families of defensins, α and β, have been identified in humans. α-Defensins are produced by neutrophils and the Paneth cells of the intestine. β-defensins are secreted by epithelial cells.11 Six hBDs—hBD-1, -2, -3, -4, and recently, -5 and -6 — have been identified.12–15 Defensins are active in vitro against several different bacteria, fungi and enveloped viruses and are thought to perform their microbicidal functions by forming pores in microbial cell membranes.16,17 Apart from their antimicrobial effects, defensins appear to influence a variety of cellular activities such as proliferation,18 cytokine production,19,20 chemotaxis,21,22 and stimulation of mast cell histamine release.23 In most tissues, hBD-1 is constitutively expressed, whereas hBD-2 and -3 are inducible by proinflammatory cytokines and bacterial by-products.24 hBD-4 expression in human airway epithelia was upregulated by bacterial infection and activation of protein kinase C,25 but was unchanged by proinflammatory cytokines that induce the expression of hBD-2 and -3. The effect of inflammatory cytokines or bacteria on the expression of the two most recently identified β-defensins, hBD-5 and -6, is unknown.
It has been shown that hBD-1 is constitutively expressed in the corneal and conjunctival epithelia.26–28 hBD-2 expression is variable in the conjunctiva of human subjects.26 The expression of hBD-2 in the cornea is inducible by proinflammatory cytokines such as IL-1α and -1β, tumor necrosis factor-α (TNF-α), Gram-positive and -negative bacteria, and bacterial by-products such as lipopolysaccharide.29,30 hBD-3, -4, -5, and -6 have not been studied in conjunctival tissue.
Because strong evidence exists for increased proinflammatory cytokine activity on the ocular surface in dry eye, we hypothesized that a differential expression of β-defensins (hBD-1, -2, and -3) would be observed in subjects with moderate dry eye when compared with normal subjects.2–4 Based on an earlier study30 and results from our laboratory,29 we speculated that proinflammatory cytokines and heat-killed Pseudomonas aeruginosa (PA) would differentially regulate β-defensin expression by human conjunctival epithelial cells. Some of these results have been presented in preliminary form (Narayanan S, et al. IOVS 2001;42:ARVO Abstract 2624).
Methods
Human Subjects
All procedures involving human subjects were approved by the University of Houston institutional review board and were in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects. All study subjects had been examined at the University Eye Institute (University of Houston) and had a normal ocular surface or dry eye disease. Eight normal subjects (three men, five women; age range, 20–43 years) and nine subjects with dry eye (one man, eight women; age range, 23–40 years) took part in this study. There was no significant difference (one-way ANOVA P = 0.47) between the mean ages of the normal subjects (28.37 ± 7.1 years [SD]) and subjects with dry eye (26.22 ± 4.9 years). None of the subjects were current contact lens wearers. All subjects with dry eye used tear supplements, whereas the normal subjects did not. Serologic testing was not done to rule-out Sjögren’s syndrome. However, no subject reported a history of connective tissue disorders or xerostomia, which would be associated with this autoimmune disease.
Human Subjects: Subjective Assessment
A scoring-system–based dry eye questionnaire (Narayanan S, unpublished data, 2001) was used to aid the initial classification of subjects as normal or having moderate dry eye. A total questionnaire score below 17 was considered to be normal, a score above 32 was considered positive for dry eye, and scores between 17 and 32 were considered to indicate the possible presence of dry eye.
Human Subjects: Objective Assessment
This involved examination of general ocular surface health with a biomicroscope, grading of bulbar and limbal injection (Cornea and Contact Lens Research Unit [CCLRU] grading scale; School of Optometry, University of New South Wales, Sydney, NSW, Australia), and vital staining of the corneal and conjunctival epithelia using fluorescein and lissamine green31–33; measuring tear secretion by the phenol red thread test34; measuring tear osmolality using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT); and estimating tear stability by measuring the fluorescein tear break-up time (Dry Eye Test; Akorn, Chicago, IL).35 One-way analysis of variance (ANOVA) was used to test for differences between the two groups in the tear osmolality and phenol red thread test data, whereas grades of staining and injection were analyzed with nonparametric tests.
Conjunctival Impression Cytology Sample Collection
A single drop of 0.5% proparacaine hydrochloride (Akorn) was instilled in the eye. A 3 × 8-mm preautoclaved polyether sulfone membrane (Supor; Pall Gellman Sciences, East Hills, NY), was then placed on the temporal bulbar conjunctiva for 5 to 10 seconds. The membrane was gently removed and placed directly in 100 μL ice-cold TRIzol (Invitrogen, Carlsbad, CA). Samples were stored at −80°C until RT-PCR analysis.
Reverse Transcription and Duplex PCR
A modified phenol-chloroform extraction procedure36 was used for total RNA extraction from the impression cytology specimens. RNA was precipitated with isopropanol and washed in ethanol before reconstitution in nuclease-free water. A portion of total RNA (250 ng) was used to generate 20 μL of cDNA with oligo dT primers, using a commercially available first-strand synthesis system (Superscript; Invitrogen).
A constitutively expressed gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to ensure equal amounts of starting cDNA in each reaction. Duplex PCR (Fast-Taq kit; Roche Diagnostics Inc., Indianapolis, IN) was performed for GAPDH+hBD-1 and for GAPDH+hBD-2. The primer sequences used and expected product sizes were as follows: GAPDH30 forward 5′-GTGAAGGTCGGAGTCAACGGATTT-3′, reverse 5′-CACAGTCTTCTGGGTGGCAGTGAT-3′, 555 bp; hBD-127 forward 5′-CCCAGTTCCTGAAATCCTGA-3′, reverse 5′-CAGGTGCCTTGAATTTTGGT-3′, 215 bp; hBD-230 forward 5′-CCAGCCATCAGCCATGAGGGT-3′, reverse 5′-GGAGCCCTTTCTGAATCCGCA-3′, 257 bp; hBD-314 forward 5′-AGCCTAGCAGCTATGAGGATC-3′, reverse 5′-CTTCGGCAGCATTTTCGGCCA-3′, 206 bp. PCR amplification was performed for 35 cycles of denaturation at 94°C (50 seconds), annealing 60°C (1 minute) and primer extension at 72°C (1 minute). Ethidium bromide–stained 1.3% agarose gels were used to analyze the PCR products. An Alpha Imager (Alpha Innotech, San Leandro, CA) gel-documentation system was used to obtain digital images and analyze them semiquantitatively. One-step RT-PCR (described later) was used to detect hBD-3 mRNA in the conjunctival impression cytology samples. Control experiments in which either nucleic acid or reverse transcriptase was omitted were also performed, and in all cases no product was obtained (data not shown).
Cell Culture
All cell culture reagents were obtained from Invitrogen, unless otherwise stated. Chang conjunctival cells (Wong-Kilbourne derivative) were obtained from American Type Culture Collection (CCL 20.2; Manassas, VA) and grown in M199 medium with 10% calf serum.37 Normal human conjunctival (IOBA-NHC) epithelial cells (Diebold Y, et al. IOVS 2002;43:ARVO E-Abstract 3170) were cultured in DMEM-F12 (1:1 vol/vol), containing 10% fetal bovine serum, 2 ng/mL mouse epidermal growth factor (EGF; Sigma-Aldrich, St. Louis, MO), 1 μg/mL bovine insulin (Sigma-Aldrich), 0.1 μg/mL cholera toxin (Sigma-Aldrich), 5 μg/mL hydrocortisone (Sigma-Aldrich), 2.5 μg/mL amphotericin B, and a penicillin streptomycin mixture (5000 U/mL and 5000 μg/mL, respectively). Human conjunctival tissue from three donors (54, 37, and 39 years of age) was obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). Primary conjunctival epithelial cells were cultured as described by Gamache et al.38 Briefly, conjunctival tissue was incubated overnight at 4°C in a 1:1 (vol/vol) solution of cell culture medium (EpiLife; Cascade Biologics, Portland, OR) and dispase (20 U/mL). Epithelial cells were then scraped free and seeded in the culture medium into 25 cm2 flasks coated with a coating mix to enhance attachment (FNC; AthenaES, Baltimore, MD). The cells grew to confluence by 1 week and were then passaged in trypsin-EDTA. The epithelial nature of the primary cultured cells was studied by immunolabeling for epithelial-specific cytokeratin. All cells labeled positively for the antibody (data not shown). Primary-cultured cells of passages 1 to 3 were used for the experiments.
Preparation of Heat-Killed
Pseudomonas aeruginosa
A single isolated PA colony was used to inoculate 5 mL of nutrient broth (NB) overnight at 37°C. Fifty microliters of this bacterial suspension was used to inoculate 50 mL of fresh NB, which was incubated for 2.5 hours with vigorous shaking at 37°C to achieve midlog-phase growth. Twenty-five milliliters of the warm PA culture was centrifuged at 3100g for 10 minutes, and the bacterial cell pellet was resuspended in cold PB (10 mM phosphate buffer [8.2 mM Na2HPO4, 1.8 mM KH2PO4, pH = 7.4]) at 107 cfu/mL. Bacteria were killed by heating 100-μL aliquots at 65°C for 30 minutes. To establish the efficacy of this procedure, a sample of heat-killed or live PA were grown on individual agar plates at 37°C for 24 hours. The heat-killed plates did not demonstrate any evidence of bacterial growth, whereas plates with live bacteria showed complete coverage by colonies (data not shown). The heat-killed bacteria were stored at −80°C until needed.
Cytokine and PA Treatment of Conjunctival Cells
Chang cells and NHC cells were serum-starved overnight, whereas primary cultured cells were placed in extract-free cell culture medium (EpiLife; Cascade Biologics) overnight before each experiment. The cells were treated for 24 hours with IL-1β (10 ng/mL), TNF-α (10 ng/mL), a combination of IL-1β and TNF-α (both at 10 ng/mL), IFN-γ (40 ng/mL), or heat-killed PA (106 cfu). Experiments were also performed in which the cells were treated with various concentrations (0.1–100 ng/mL) of IL-1β for 24 hours or 10 ng/mL IL-1β for 2 to 24 hours. At the end of the treatment period, cells were collected and stored at −80°C until RNA extraction. Cell culture supernatants were also collected, centrifuged to remove any cells, and stored at −80°C until immunoblot analysis was performed.
One-Step RT-PCR
Total RNA was extracted from the cells using mini kits (RNeasy; Qiagen, Valencia, CA). One-step RT-PCR (Superscript I; Invitrogen) was performed with 250 ng of total RNA and 25 pmol of gene-specific primers (described earlier), to detect mRNA for GAPDH and hBD-1, 2, and -3. Reverse transcription was performed at 50°C for 1 hour to generate the cDNA, followed by 5 minutes at 94°C to denature the enzyme. PCR was performed for 40 cycles, and products were analyzed as described earlier.
Immunoblot Analysis
One hundred microliters of culture supernatant was applied by gravity to a nitrocellulose membrane using a dot-blot apparatus. Nonspecific binding sites were blocked by incubating in Tris-buffered saline (TBS) containing 5% nonfat powdered milk for 30 minutes at room temperature. The membrane was then incubated overnight with rabbit anti-human hBD-2 diluted 1:2000 in TBS containing 5% nonfat powdered milk, 5% goat serum, 0.05% Tween 20, and 0.02% sodium azide. After the incubation, the membrane was washed (3 × 10 minutes, TBS) and incubated for 1 hour at room temperature with a horseradish peroxidase–conjugated goat anti-rabbit antibody (Jackson Laboratories, West Grove, PA), diluted 1:10,000 in TBS containing 5% nonfat powdered milk. Immunoreactivity was visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ). The membrane was scanned using a desktop scanner to document the results.
Results
Subjective and Objective Examination of Study Participants
A significant difference (P = 0.002; one-way ANOVA) was found between the mean dry eye questionnaire score of the normal subjects (11.33 ± 6.3 points [SD]), when compared with the subjects with moderate dry eye (43.33 ± 19.5 points). Biomicroscopic examination did not reveal meibomian gland dysfunction in any subject. Bulbar conjunctival injection was significantly higher (P = 0.015; Kruskal-Wallis test) in the subjects with dry eye (grade 0.50 ± 0.53 [SD]) compared with the normal subjects (mean grade, 0). Mean tear osmolality was significantly lower (P = 0.02; one-way ANOVA) in the normal subjects (292.67 ± 13.93 mOsm/kg [SD]) when compared with the subjects with dry eye (308.66 ± 16.02 mOsm/kg). The mean tear break-up time was significantly (P = 0.037), shorter in the subjects with dry eye (6.67 ± 3.21 seconds [SD]) than in the normal subjects (12.12 ± 8.08 seconds). The other clinical tests performed did not demonstrate statistically significant differences between the two groups.
hBD Expression in the Conjunctival Epithelium
As shown in the examples in Figures 1A and 1C, hBD-1 and -3 mRNA expression in the conjunctiva, as determined by RT-PCR, was observed in all subjects tested. However, as illustrated in Figure 1B, hBD-2 mRNA was expressed only in the conjunctival epithelium of the subjects with dry eye. Figure 2 shows the mRNA expression of the three β-defensins in cultured conjunctival epithelial cells. hBD-1 and -3 mRNA were constitutively expressed by all the cells (n = 3 passages for cell lines, n = 3 donors for primary cultured cells). A weak baseline expression of hBD-2 mRNA was observed in the primary cultured conjunctival epithelial cells from all three donors, whereas the two cell lines did not express hBD-2 at baseline.
Figure 1.
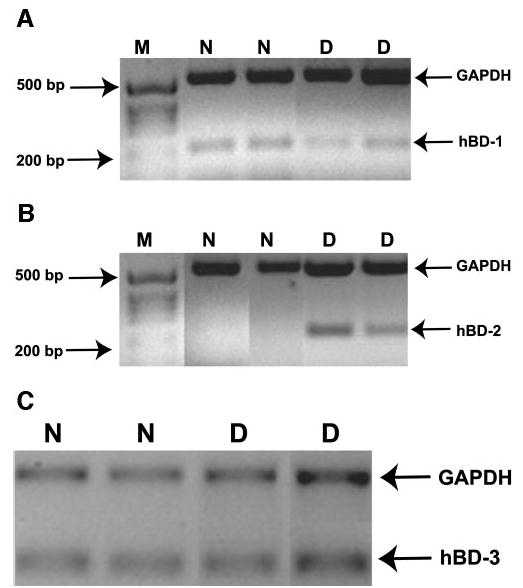
hBD expression in human subjects. Duplex RT-PCR was performed for GAPDH+hBD-1 (A) or GAPDH+hBD-2 (B) on RNA extracted from conjunctival impression cytology specimens. One-step RT-PCR was performed to detect GAPDH and hBD-3 mRNA (C). M, size marker; N, normal subject; D, subject with dry eye. Data are from two representative subjects in each group.
Figure 2.
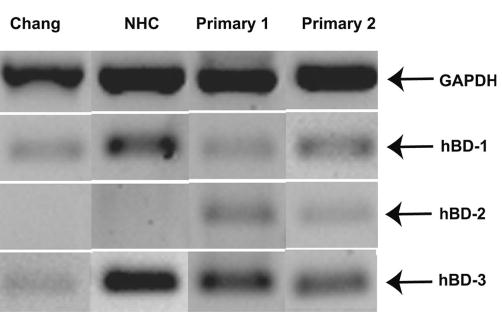
hBD expression by human conjunctival epithelial cells in culture. RT-PCR was performed on samples collected from Chang and NHC cells and primary cultured conjunctival cells that were treated with serum-free growth media alone. Products of the constitutively expressed GAPDH gene are shown as well as the 3-hBD. The first two lanes are representative results (one of three experiments) from the cell lines and the last two lanes represent results from primary cultured cells from two of three donors tested.
Effects of Cytokine or PA Treatment on hBD Expression by Conjunctival Epithelial Cells in Culture
Primary cultured human conjunctival epithelial cells were treated with proinflammatory cytokines or heat-killed PA to test differential expression of hBD (n = 3). As shown in Figure 3A, hBD-2 mRNA upregulation was observed in primary cultured cells after treatment with IL-1β, TNF-α, IL-1β+TNF-α, IFN-γ, or heat-killed PA. Semiquantitative analysis relative to GAPDH revealed a 2 to 2.7 fold-increase of hBD-2 expression with the various treatments, compared with the media-treated controls. Though the combination of IL-1β and TNF-α resulted in an hBD-2 band of higher intensity (2.7-fold increase) when compared with treatment with IL-1β alone (2.5-fold increase), the difference was not significant. There was no difference in the expression of hBD-1 or -3 with cytokine or bacteria treatment. The results from the cell lines were the same, with the exception that treatment with either TNF-α or IFN-γ alone was unable to induce hBD-2 expression (Fig. 3B; n = 3).
Figure 3.
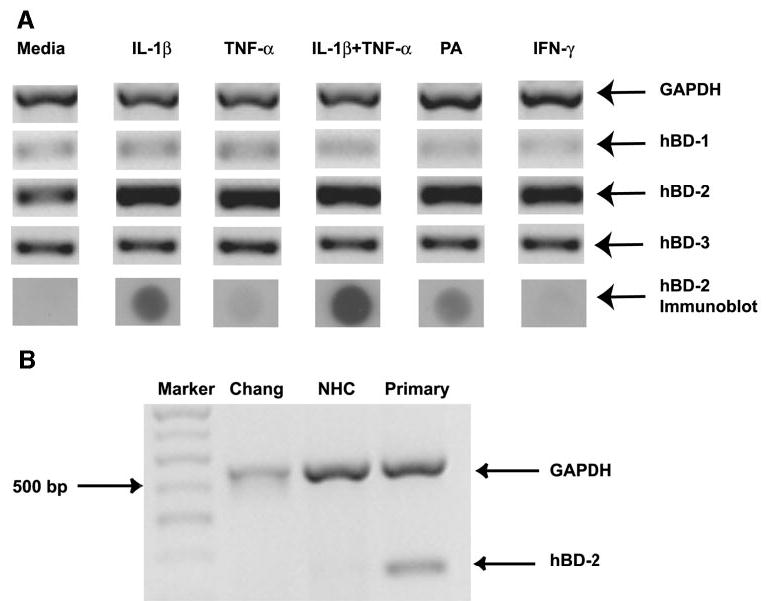
hBD mRNA and protein expression by primary cultured conjunctival epithelial cells treated with cytokines or bacteria. Primary conjunctival cells (n = 3) were treated with cytokines or PA for 24 hours. (A) RT-PCR results for GAPDH and hBD-1, -2, and 3, and immunoblot analysis to detect hBD-2 protein secretion. (B) Comparison of the effect of TNF-α on hBD-2 mRNA expression by different human conjunctival cells in culture. M, size marker.
Effect of Various Concentrations and Treatment Duration of IL-1β on hBD-2 Expression by Conjunctival Epithelial Cells
We investigated the effect of various concentrations of IL-1β (n = 2 cell lines; n = 1 primary cells) and incubation time (n = 2 cell lines; n = 1 primary cells) on hBD-2 expression. It is evident from Figure 4A, that hBD-2 expression by primary cultured cells increased with the addition of 0.1 ng/mL of IL-1β and peaked at 10 ng/mL. Semiquantitative analysis relative to GAPDH levels, showed a 1.2-fold hBD-2 mRNA upregulation with 0.1 ng/mL IL-1β treatment and a peak of 1.6-fold upregulation with the addition of 10 ng/mL IL-1β. hBD-2 protein secretion was also upregulated (Fig. 4B) in the same manner. As illustrated in Figure 5A, hBD-2 mRNA expression was increased as early as 2 hours of treatment with 10 ng/mL IL-1β and plateaus at 12 hours. Semiquantitative analysis relative to GAPDH levels revealed a 1.3-fold hBD-2 mRNA upregulation at 2 hours and a peak of 1.7-fold upregulation at the 12-hour time point. Immunoblot analysis (Fig. 5B) revealed that hBD-2 protein secretion was upregulated after 8 hours of treatment with 10 ng/mL IL-1β.
Figure 4.
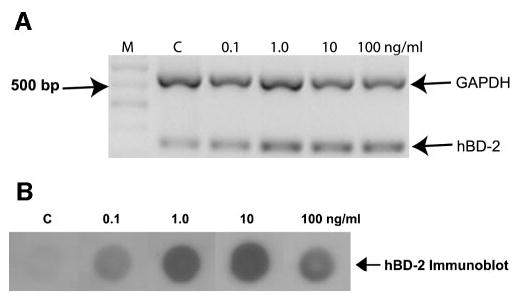
The effect of various concentrations of IL-1β on hBD-2 expression in primary cultured conjunctival epithelial cells. (A) RT-PCR products for GAPDH and hBD-2 from primary conjunctival cells (n = 1) treated 24 hours with various concentrations of the proinflammatory cytokine IL-1β. M, size marker; C, media-treated control cells. (B) The changes in hBD-2 protein secretion by primary cultured conjunctival cells in this experiment. Similar results were obtained with the cell lines (n = 2).
Figure 5.
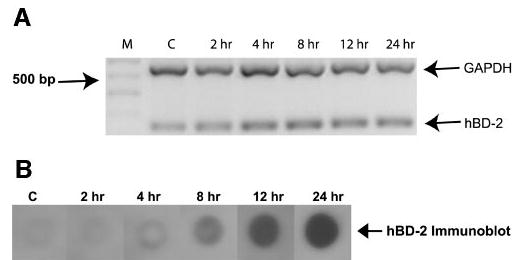
The effect of various periods of incubation with IL-1β on hBD-2 expression by primary conjunctival epithelial cells. (A) RT-PCR products for GAPDH and hBD-2 from primary conjunctival cells (n = 1) treated with IL-1β for various lengths of incubation at a concentration of 10 ng/mL. M, size marker; C, media-treated controls. (B) The changes in hBD-2 protein secretion in this experiment. Similar results were obtained with the cell lines (n = 2).
Discussion
We have shown that hBD-2 mRNA is expressed in the conjunctival epithelium of subjects with moderate dry eye but not in normal subjects. In contrast, hBD-1 and -3 were constitutively expressed in both groups. We also observed that the expression of hBD-2 mRNA and protein can be upregulated in conjunctival cells in culture by proinflammatory cytokines and heat-killed PA.
To establish the presence of dry eye, we assessed tear osmolality, tear stability, tear volume, ocular surface health and performed a symptom assessment using a dry eye questionnaire. The subjects with moderate dry eye had significantly higher tear osmolality and shorter tear break-up time, attributes known to be changed in dry eye.1 Our subjects did not undergo serologic testing for Sjögren’s syndrome. However, as none reported a history of connective tissue disorders or xerostomia, conditions that provide a firm basis to suspect Sjögren’s syndrome,39 we believe that our subjects with dry eye had aqueous-deficient non-Sjögren’s dry eye.
We used RT-PCR to study the expression of hBD-1, -2, and -3 in the conjunctival epithelium of the two groups of subjects. hBD-1 was found to be constitutively expressed in both groups; however, we observed hBD-2 expression only in the subjects with moderate dry eye. Hattenbach et al.26 previously found variable expression of hBD-2, though they did not specify the ocular surface status of their subjects. hBD-3, which had not previously been studied was constitutively expressed.
We cannot completely eliminate the possibility that goblet cells populating the conjunctival epithelium may also express defensins. However, based on the evidence that only 4% to 10% of the conjunctival epithelium comprises goblet cells40,41 and subjects with dry eye have decreased goblet cell densities,42,43 we believe that our results are reflective of the epithelial cell population. Dry eye syndromes have been linked to gender showing greater prevalence in postmenopausal women.1,44–46 The two subject groups in this study consisted of mainly women. Because the mean ages of the normal (28.37 years) and dry eye (26.22 years) subjects were much below menopausal ages, and tear physiology is not affected by hormonal changes in this age group,47 our impression cytology results are unlikely to have been influenced by hormonal factors.
We used RT-PCR and immunoblot analysis to study the expression of hBD by cultured human conjunctival epithelial cells. Our results show that hBD-1 and -3 were constitutively expressed, whereas hBD-2 was inducible. hBD-1 has been shown to be constitutively expressed in corneal and other ocular cells,27,28,30,48 thus agreeing with our data. In our study, hBD-2 expression was induced by various cytokines as well as by PA. Similar findings have been reported for corneal epithelial and other cells.49–54 Treatment with TNF-α or IFN-γ upregulated hBD-2 mRNA but not protein secretion in the primary cultured cells. The reason for this is unclear, but one possibility is that they were unable to activate the hBD-2 secretory pathway. These cytokines were also unable to induce hBD-2 mRNA or protein expression in the cell lines tested. Evidence from gene array studies (Narayanan S, unpublished observation, 2002) and indirect observations that IFN-γ can alter Chang cell gene expression37,55 suggests that the NHC and Chang cell lines possess TNF-α and IFN-γ receptors. However, we are unsure why these cytokines were unable to upregulate hBD-2 mRNA. One reason may be differences in postreceptor intracellular pathways between primary cultured cells and the cell lines. Several studies have demonstrated that hBD-3 expression can be induced by cytokines and bacteria.14,56 However, we did not observe this in conjunctival epithelial cells, a finding in keeping with an earlier observation of ours in corneal epithelial cells.29
Our results indicated that IL-1β was able to induce hBD-2 mRNA expression rapidly (by 2 hours) and was effective at concentrations as low as 0.1 ng/mL. As expected, secretion of hBD-2 was delayed, only becoming detectable after 8 hours of treatment with 10 ng/mL IL-1β. These results show that IL-1β is a potent inducer of hBD-2 in conjunctival epithelial cells. Notably, the level of IL-1β in the tear film of subjects with dry eye is 80 to 180 pg/mL, whereas that of normal subjects is 30 pg/mL.5 Therefore, tear film levels of IL-1β in subjects with dry eye approximate to the lowest concentration of this cytokine, which induced hBD-2 expression in conjunctival epithelial cells in culture.
We have shown that hBD-1 and -3 were constitutively expressed in the conjunctival epithelium of subjects with normal ocular surface as well as dry eye, whereas hBD-2 was only expressed in the dry eye group. Based on our results that proinflammatory cytokines upregulate hBD-2 expression in cultured conjunctival cells and the evidence from other studies for an inflammatory basis for dry eye disease,2 we hypothesize that hBD-2 expression is induced by increased activity of proinflammatory cytokines in our subjects with dry eye. Because the three β-defensins do not have identical spectra of antimicrobial activity,13,14,25,56 it is possible that baseline protection is provided by hBD-1 and -3, whereas hBD-2 widens the spectrum and provides additional defense especially for subjects with dry eye where the ocular surface is compromised. The higher tear osmolalities observed in subjects with dry eye may affect the activity of secreted defensins because some are known to be salt-sensitive.13 However, physiological conditions at the ocular surface of subjects with dry eye do not preclude significant antimicrobial effects. The decreased tear quantity in subjects with dry eye may effectively increase the concentration of antimicrobial agents. Also, synergistic interactions between antimicrobial peptides may counteract the effect of high salt concentration.
Although hBDs are primarily antimicrobial in nature, they can influence cellular activities such as proliferation,18 cytokine production,19,20 and histamine release by mast cells.23 Defensins also have chemotactic effects on mast cells, T-cells, dendritic cells, and monocytes and promote dendritic cell maturation.21,22,57 At least some of these functions appear to be mediated by receptors such as toll-like receptor-4,58 and CC-chemokine receptor-6.59 These other activities have led to the suggestion that defensins are a link between innate and adaptive immunity.24 It is possible that expression of hBD-2 plays a chemotactic role in mediating the increase in T-cell subpopulations observed in subjects with non-Sjögren’s dry eye.60 Further, hBD-2 stimulated histamine release by conjunctival mast cells may elicit signs and symptoms of ocular irritation. Therefore, while an increased expression of hBD-2 may be beneficial in terms of antimicrobial protection, it may also contribute to ocular surface damage observed in subjects with dry eye.
Acknowledgments
The authors thank Yolanda Diebold and Margarita Calonge (IOBA, Universidad de Valladolid, Valladolid, Spain) for the generous gift of IOBA-NHC cells; Tomas Ganz (University of California Los Angeles) for the kind gift of rabbit-anti-human hBD-2; Jan Bergmanson, Julie Jackson, and Norman Leach for assistance in examining human subjects; and Ling-Chi Huang for preparing the bacterial cultures.
Footnotes
Disclosure: S. Narayanan, None; W.L. Miller, None; A.M. Mc-Dermott, None
Supported by grants from University of Houston, College of Optometry, Vision Research Support Grant (SN), Texas Higher Education Coordinating Board Advanced Research Program Grant (AMM), and National Eye Institute of the National Institutes of Health Grant EY13175 (AMM).
References
- 1.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 2.Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Pflugfelder SC. Differential diagnosis of dry eye conditions. Adv Dent Res. 1996;10:9–12. doi: 10.1177/08959374960100011801. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 6.Hemady R, Chu W, Foster CS. Keratoconjunctivitis sicca and corneal ulcers. Cornea. 1990;9:170–173. [PubMed] [Google Scholar]
- 7.Lemp MA. Is the dry eye contact lens wearer at risk? Yes. Cornea. 1990;9(Suppl 1):S48–S50. doi: 10.1097/00003226-199010001-00020. discussion S54. [DOI] [PubMed] [Google Scholar]
- 8.Seal DV, McGill JI, Mackie IA, et al. Bacteriology and tear protein profiles of the dry eye. Br J Ophthalmol. 1986;70:122–125. doi: 10.1136/bjo.70.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danjo Y, Lee M, Horimoto K, Hamano T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol (Copenh) 1994;72:433–437. doi: 10.1111/j.1755-3768.1994.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T, Lehrer RI. Antimicrobial peptides of vertebrates. Curr Opin Immunol. 1998;10:41–44. doi: 10.1016/s0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 12.Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 13.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 14.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Nagase T, Makita R, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–2523. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 16.Diamond G, Bevins CL. beta-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 17.White SH, Wimley WC, Selsted ME. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CJ, Foster BA, Mannis MJ, Selsted ME, Reid TW. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–413. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 19.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, et al. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 20.Chaly YV, Paleolog EM, Kolesnikova TS, et al. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 21.Yang D, Chertov O, Bykovskaia SN, et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 22.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 23.Befus AD, Mowat C, Gilchrist M, et al. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–953. [PubMed] [Google Scholar]
- 24.Raj PA, Dentino AR. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett. 2002;206:9–18. doi: 10.1111/j.1574-6968.2002.tb10979.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia JR, Krause A, Schulz S, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–1821. [PubMed] [Google Scholar]
- 26.Hattenbach LO, Gumbel H, Kippenberger S. Identification of beta-defensins in human conjunctiva. Antimicrob Agents Chemother. 1998;42:3332. doi: 10.1128/aac.42.12.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83:737–741. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann OJ, Hussain IR, Watt PJ. Investigation of beta defensin gene expression in the ocular anterior segment by semiquantitative RT-PCR. Br J Ophthalmol. 2000;84:523–526. doi: 10.1136/bjo.84.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott A, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNamara NA, Van R, Tuchin OS, Fleiszig SM. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 31.Norn MS. Lissamine green: vital staining of cornea and conjunctiva. Acta Ophthalmol (Copenh) 1973;51:483–491. doi: 10.1111/j.1755-3768.1973.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 32.Eliason JA, Maurice DM. Staining of the conjunctiva and conjunctival tear film. Br J Ophthalmol. 1990;74:519–522. doi: 10.1136/bjo.74.9.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terry RL, Schnider CM, Holden BA, et al. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci. 1993;70:234–243. doi: 10.1097/00006324-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Hamano H, Hori M, Hamano T, et al. A new method for measuring tears. CLAO J. 1983;9:281–289. [PubMed] [Google Scholar]
- 35.Korb DR, Greiner JV, Herman J. Comparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the Dry Eye Test (DET) method. Cornea. 2001;20:811–815. doi: 10.1097/00003226-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.De Saint Jean M, Brignole F, Feldmann G, Goguel A, Baudouin C. Interferon-gamma induces apoptosis and expression of inflammation-related proteins in Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:2199–212. [PubMed] [Google Scholar]
- 38.Gamache DA, Dimitrijevich SD, Weimer LK, et al. Secretion of proinflammatory cytokines by human conjunctival epithelial cells. Ocul Immunol Inflamm. 1997;5:117–128. doi: 10.3109/09273949709085060. [DOI] [PubMed] [Google Scholar]
- 39.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjogren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren’s Syndrome. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman HE, Barron BA, McDonald MB. The Cornea. 2nd ed. Boston: Butterworth-Heinemann; 1998;xxi,1109.
- 41.Yeo AC, Carkeet A, Carney LG, Yap MK. Relationship between goblet cell density and tear function tests. Ophthalmic Physiol Opt. 2003;23:87–94. doi: 10.1046/j.1475-1313.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JA, Perrigin JA. Relationship of impression cytology and tear ferning to reports of dry eye. J Am Optom Assoc. 1999;70:187–192. [PubMed] [Google Scholar]
- 43.Nelson JD. Impression cytology. Cornea. 1988;7:71–81. [PubMed] [Google Scholar]
- 44.Sullivan DA, Krenzer KL, Sullivan BD, et al. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–1265. [PubMed] [Google Scholar]
- 45.Sullivan DA, Wickham LA, Rocha EM, et al. Androgens and dry eye in Sjogren’s syndrome. Ann N Y Acad Sci. 1999;876:312–324. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 46.Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119. doi: 10.1097/00003226-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson A, Pearce EI, Simmons PA, Blades K. Effect of oral contraceptives on tear physiology. Ophthalmic Physiol Opt. 2001;21:9–16. [PubMed] [Google Scholar]
- 48.McDermott AM, Redfern RL, Zhang B. Human beta-defensin 2 is upregulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 49.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathews M, Jia HP, Guthmiller JM, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes RJ, McElveen JE, Dua HS, Tighe PJ, Liversidge J. Expression of human beta-defensins in intraocular tissues. Invest Ophthalmol Vis Sci. 2000;41:3026–3031. [PubMed] [Google Scholar]
- 52.O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 53.O’Neil DA, Cole SP, Martin-Porter E, et al. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect Immun. 2000;68:5412–5415. doi: 10.1128/iai.68.9.5412-5415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krisanaprakornkit S, Kimball JR, Weinberg A, et al. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Saint Jean M, Debbasch C, Rahmani M, et al. Fas- and interferon gamma-induced apoptosis in Chang conjunctival cells: further investigations. Invest Ophthalmol Vis Sci. 2000;41:2531–2543. [PubMed] [Google Scholar]
- 56.Garcia JR, Jaumann F, Schulz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity: its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257–264. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 57.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 58.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 59.Hoover DM, Boulegue C, Yang D, et al. The structure of human macrophage inflammatory protein-3alpha/CCL20: linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem. 2002;277:37647–7654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 60.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]


