Abstract
Purpose. Human β-defensin-2 (hBD-2) and cathelicidin LL-37 are salt-sensitive cationic antimicrobial peptides expressed by ocular surface epithelia. The goal of this study was to investigate the effect of preservative-free artificial tears on hBD-2 and LL-37 antimicrobial activity against Pseudomonas aeruginosa.
Methods. P. aeruginosa was incubated with hBD-2 or LL-37 in the absence or presence (70% vol/vol) of different preservative-free artificial tears—Visine Tears (300 mOsm/kg), Tears Naturale Free (261 mOsm/kg), TheraTears (185 mOsm/kg), and Refresh Plus (325 mOsm/kg)—for 2 hours at 37°C. In some experiments, P. aeruginosa was incubated with hBD-2 or LL-37 and Visine Tears or Tears Naturale Free with or without carboxymethylcellulose (0.5% vol/vol final concentration). Plates were inoculated with samples of each reaction mixture and then incubated for 24 hours at 37°C.
Results. Visine Tears and Tears Naturale Free had little or no effect on the antimicrobial activity of 100 μg/mL hBD-2 or LL-37. In the presence of Refresh Plus and TheraTears, the activity of 100 μg/mL hBD-2 or LL-37 was reduced by 90% to 100%. Carboxymethylcellulose, at a concentration comparable to that present in Refresh Plus, reduced the effectiveness of hBD-2 or LL-37 by 40% to 90% in the presence of Tears Naturale Free and Visine Tears.
Conclusion. Human β-defensin-2 and cathelicidin LL-37 inhibit the growth of P. aeruginosa in vitro, but this activity is markedly reduced in the presence of Refresh Plus and TheraTears. These results suggest that carboxymethylcellulose-containing artificial tears may reduce the activity of the endogenously produced antimicrobial peptides.
Keywords: Carboxymethylcellulose, Cationic antimicrobial peptides, Human β-defensin-2, Human cathelicidin LL-37, Preservative-free artificial tears, Pseudomonas aeruginosa
Secretion of small cationic antimicrobial peptides by epithelia is an important component of the innate immune response.1 These peptides, which include β-defensins and cathelicidins, insert themselves into microbial cell membranes and, by pore formation or electrostatic disruption, cause release of intracellular contents leading to death of the organism.2 By regulating mammalian cell functions, such as chemotaxis and proliferation, defensins and cathelicidins also provide a link between the innate and adaptive immune systems and regulate wound-healing processes.3,4
The human corneal epithelium expresses three β-defensins. Human β-defensin (hBD)-1 and hBD-3 are constitutively expressed, whereas hBD-2 expression is induced by proinflammatory cytokines and bacterial products and after injury.5–7 The authors have recently identified that the corneal epithelium also expresses LL-37, an antimicrobial peptide of the cathelicidin family, which is up-regulated after injury.8 This pattern of expression suggests that hBD-1 and hBD-3 provide baseline defense against infection, whereas after injury, additional antimicrobial protection may be afforded by hBD-2 and LL-37. It also raises the possibility that hBD-2 and LL-37 may have important roles in modulating corneal epithelial cell behavior during wound healing. Not only are de-fensins and LL-37 important endogenously expressed molecules, but they have great potential as pharmaceutical agents that would simultaneously provide antimicrobial protection and stimulate corneal epithelial healing.
Preservative-free artificial tears have become one of the most widely used modalities for the relief of discomfort from dryness of the eyes and for conservative treatment of ocular surface diseases, such as meibomian gland dysfunction.9 The goal of this study was to determine whether commonly used preservative-free artificial tear solutions affect the antibacterial activity of hBD-2 and LL-37 against a common ocular pathogen, Pseudomonas aeruginosa.
MATERIALS AND METHODS
Preparation of Pseudomonas aeruginosa
Pseudomonas aeruginosa (ATCC 27853 and two clinical isolates from corneal scrapings of patients with bacterial keratitis) was tested in this study. ATCC 27853 is known to invade an intact cornea and produce severe ocular infection in experimentally infected animal models of bacterial keratitis.10,11 Most of the current studies were carried out by using ATCC 27853, and selected experiments were repeated with the two clinically isolated P. aeruginosa strains.
One single isolated P. aeruginosa colony was used to inoculate 5 mL of nutrient broth overnight at 37°C. Fifty microliters of this bacterial suspension was used to inoculate 50 mL of fresh nutrient broth, which was then incubated for 2.5 hours with vigorous shaking at 37°C to achieve mid log phase growth. Twenty-five milliliters of the warm P. aeruginosa culture was centrifuged at 3,100g for 10 minutes, and the bacterial cell pellet was resuspended in cold phosphate buffer (8.2 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). Optical density of the suspension was adjusted to 0.2 at 620 nm (approximately 107 colony-forming units [CFU]/mL) by adding an appropriate volume of phosphate buffer.
Effect of Preservative-Free Artificial Tears or Sodium Chloride on the Antimicrobial Activity of hBD-2 and LL-37
Human β-defensin-2 (Peninsula, San Carlos, CA) and LL-37 (American Peptide Company, Sunnyvale, CA) were dissolved in 0.01% acetic acid at a concentration of 1 mg/mL and stored at −20°C. The antimicrobial assay procedure was adapted from that described by Tomita et al.12 Reaction mixtures (final volume of 50 μL) containing 10 μL 107 CFU/mL P. aeruginosa, 5 μL hBD-2 or LL-37 diluted in phosphate buffer (each at a final concentration of 100 μg/mL) were incubated in the absence and presence of 70% vol/vol preservative-free artificial tears. The peptide concentration was chosen based on studies in which the authors found 100 μg/mL to be 100% effective against P. aeruginosa (data not shown) and published reports of 90% lethal dose and minimum inhibitory concentration values.13,14 Four different preservative-free artificial tears were tested: Visine Tears (Pfizer, Inc., New York, NY), Tears Naturale Free (Alcon, Fort Worth, TX), TheraTears (Advanced Vision Research, Woburn, MA), and Refresh Plus (Allergan, Irvine, CA). Osmolality of the artificial tears was measured using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT). The osmolality and the ingredients of the four preservative-free artificial tears tested are summarized in Table 1. Because of other constituents of the reaction mixture, 70% vol/vol was the maximum artificial tear concentration obtainable in these experiments. Reaction mixtures were incubated at 37°C for 2 hours with vigorous shaking. In each experiment, reaction mixtures containing 5 μL 0.01% acetic acid, the vehicle for diluting hBD-2 or LL-37, acted as a control. At the end of the incubation, each reaction mixture was serially diluted with nutrient broth, and then samples at the same dilution factor were used to inoculate nutrient broth agar plates. Samples were spread evenly over the surface of the plates by using sterile glass spreaders. After incubation at 37°C for 24 hours, the agar plates were placed on a light board, and a digital image was captured using an Alpha Imager documentation system (Alpha Innotec, San Leandro, CA).
TABLE 1.
Active Ingredients and Osmolality of the Preservative-Free Artificial Tear Solutions
| Refresh Plus | Tears Naturale Free | Visine Tears | TheraTears | |
|---|---|---|---|---|
| Osmolality (mOsm/kg) | 325 | 261 | 300 | 185 |
| Dextran 70 (0.1%) | X | |||
| Hydroxypropylmethylcellulose (0.2–0.3%) | X | X | ||
| Carboxymethylcellulose CMC (0.5%) | X | X | ||
| Polyethylene glycol 400 1% | X | |||
| Glycerin 0.2% | X |
Inactive ingredients for Refresh Plus: CaCl2, MgCl2, KCl, NaCl, purified water, sodium lactate. May also contain HCl and NaOH to adjust pH.
Inactive ingredients for Tears Naturale Free: KCl, purified water, sodium borate, NaCl. May also contain HCl and NaOH to adjust pH.
Inactive ingredients for Visine Tears: ascorbic acid, dextrose, glycine, MgCl2, KCl, purified water, sodium citrate, sodium lactate.
Inactive ingredients for TheraTears: borate buffer, CaCl2, MgCl2, KCl, NaCl, purified water, sodium bicarbonate, sodium phosphate.
In some experiments, sodium chloride was used in place of artifical tears so that the effect of a solution with osmolality matched to that of Refresh Plus (325 mOsm/kg, the highest osmolality among the four artificial tears tested) could be determined. In other experiments, antimicrobial assays were performed with hBD-2 or LL-37 (100 μg/mL) in the absence or presence of Visine Tears or Tears Naturale Free, with or without added carboxymethylcellulose (CMC) (0.5% vol/vol final concentration). CMC is the cellulose polymer present in TheraTears (0.25% vol/vol CMC) and Refresh Plus (0.5% vol/vol of CMC).
RESULTS
Effect of Artificial Tears on hBD-2 and LL-37 Antimicrobial Activity Against Pseudomonas aeruginosa
When tested in the presence of Refresh Plus and TheraTears, the antibacterial activity of 100 μg/mL hBD-2 and LL-37 against P. aeruginosa (ATCC 27853) was eliminated completely (n = 5). In contrast, Visine Tears and Tears Naturale Free had no effect on the antimicrobial activity of hBD-2 or LL-37 (n = 5). Figure 1 is representative data from one of the five experiments. The same results were obtained when experiments were repeated twice with each of the two other P. aeruginosa strains.
FIG. 1.
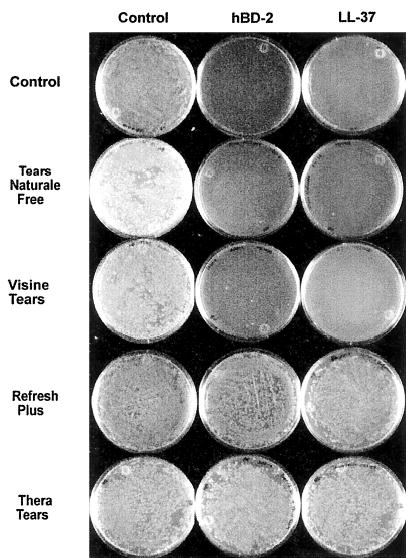
Effect of preservative-free artificial tears on hBD-2 and LL-37 antimicrobial activity. Pseudomonas aeruginosa (107 CFU/mL ATCC 27853) was incubated with 0.01% acetic acid (control), with hBD-2 and LL-37 (100 μg/mL), or with hBD-2 and LL-37 (100 μg/mL) in the presence of four preservative-free artificial tears at 37°C for 2 hours. When tested in the presence of Refresh Plus and TheraTears, the antibacterial activity of 100 μg/mL hBD-2 and LL-37 was markedly reduced (n = 5). Tears Naturale Free or Visine Tears did not reduce the activity of hBD-2 or LL-37 (n = 5).
Sodium Chloride Solution (322 mOsm/kg) Does Not Affect hBD-2 and LL-37 Antimicrobial Activity Against Pseudomonas aeruginosa
Previously, it has been shown that the antimicrobial activity of some cationic peptides, including hBD-2 and LL-37, is attenuated in the presence of high salt content.15–18 These effects depend on the concentration of peptide being used, with the higher concentrations being little affected.19 Therefore, at the peptide concentration used in the current study, one would not expect to find that antimicrobial activity is compromised by salt present in the artificial tear solutions. To confirm this hypothesis, the authors tested the antimicrobial activity of the peptides in the presence of a solution of sodium chloride at 322 mOsm/kg. This osmolality was approximately equivalent to that of Refresh Plus (325 mOsm/kg), which had the highest osmolality of the four artificial tears tested. That the antimicrobial activity of the peptides was not reduced under these conditions indicates that salt in the artificial tear solutions was not responsible for loss of peptide activity (Fig. 2).
FIG. 2.
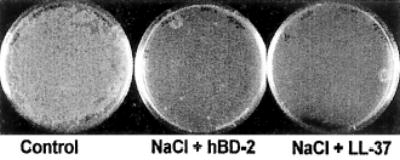
Effect of sodium chloride (NaCl) solutions on hBD-2 and LL-37 antimicrobial activity. Pseudomonas aeruginosa (107 CFU/mL ATCC 27853) was incubated with 0.01% acetic acid, with hBD-2 and LL-37 (100 μg/mL), or with hBD-2 and LL-37 (100 μg/mL) in the presence of sodium chloride solution (322 mOsm/kg) at 37°C for 2 hours (n = 3).
Carboxymethylcellulose Reduces hBD-2 and LL-37 Antimicrobial Activity Against Pseudomonas aeruginosa
Refresh Plus and TheraTears contain CMC, an anionic polymer, whereas Tears Naturale Free and Visine Tears contain hydroxypropylmethylcellulose (HPMC), a nonionic polymer (Table 1). To determine whether the CMC in Refresh Plus and TheraTears was responsible for the loss of hBD-2 and LL-37 antimicrobial activity, antimicrobial assays were performed in which CMC was added to Tears Naturale Free and Visine Tears to give a final concentration of 0.5% vol/vol CMC. As shown in Figure 3, in the presence of added CMC, Tears Naturale Free partially (40%–50%) and Visine Tears almost completely (80%–90%) impaired hBD-2 and LL-37 antibacterial activity against P. aeruginosa (ATCC 27853).
FIG. 3.
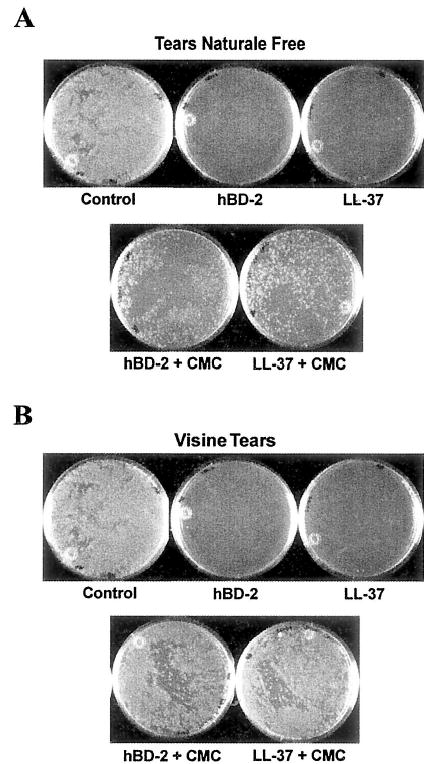
Effect of carboxymethylcellulose (CMC) on hBD-2 and LL-37 antimicrobial activity against Pseudomonas aeruginosa. P. aeruginosa (107 CFU/mL ATCC 27853) was incubated with 0.01% acetic acid (control), with hBD-2 and LL-37 (100 μg/mL), or with hBD-2 and LL-37 (100 μg/mL) in the presence of Tears Naturale Free or Visine Tears containing 0.5% CMC at 37°C for 2 hours. With the addition of CMC, Tears Naturale Free (A) and Visine Tears (B) partially or completely impaired the activity of hBD-2 and LL-37 (n = 2).
DISCUSSION
Preservative-free artificial tears are often recommended instead of preserved artificial tears for patients as a palliative therapy for ocular surface disorders because of potential adverse reactions to commonly used ophthalmic preservatives.20 Because cationic antimicrobial peptides, such as hBD-2 and LL-37, are important components of the innate immune defense produced by ocular surface epithelia in response to infection and inflammation,5,6,8 the effect of preservative artificial tears on hBD-2 and LL-37 antibacterial activity was studied.
The current study showed that the ability of hBD-2 and LL-37 to inhibit the growth of P. aeruginosa in vitro was markedly reduced in the presence of Refresh Plus and TheraTears, but not Tears Naturale Free or Visine Tears. Comparable findings were obtained using a laboratory and two clinical strains of P. aeruginosa. It has been shown that the antimicrobial activity of some defensins, including hBD-2 and LL-37, is attenuated in the presence of high salt concentrations.15–18 However, this response depends on the concentration of the peptide being used, so by using a relative high concentration of hBD-2 and LL-37 (100 μg/mL), the authors eliminated any effect of salt present in the artificial tears. The exact concentration of hBD-2 and LL-37 at the ocular surface in vivo has yet to be determined, although Oren et al.21 recently showed that physiologic concentrations of these peptides are approximately 10 μg/mL in other epithelial tissues. Although the current study used a supraphysiologic concentration of the peptides, the authors would expect to see that artificial tears have the same effect on physiologic levels.
The current data suggest that the differential effects of the preservative-free artificial tears are the result of the difference in the cellulose polymers present in the solutions. CMC, an active ingredient in Refresh Plus and TheraTears, is an anionic compound, and through ionic interaction, it would be capable of binding to hBD-2 and LL-37, which are both positively charged peptides. HPMC, an active ingredient in Tears Naturale Free or Visine Tears, is a nonionic compound, which would not be expected to interact with the peptides. Based on the differences in the chemical properties between CMC and HPMC, the loss of hBD-2 and LL-37 activity in the presence of Refresh Plus is likely the result of the peptides being bound to CMC. It is noteworthy that similar findings were reported in a recent study that showed that binding of CMC to polyhexamethylene biguanide, a cationic disinfectant, was accompanied by reduced antibacterial activity of polyhexamethylene biguanide.22
When comparing the outcome of added CMC, the authors observed that reduction of hBD-2 and LL-37 antimicrobial activity was greater with Visine Tears plus CMC than with Tears Naturale Free plus CMC. This may be attributed to differences in the composition between the two preservative-free artificial tears. Variation in the ionic makeup of Tears Naturale Free and Visine Tears may differentially influence the putative binding of CMC to the peptides, leading to a change in the availability of hBD-2 and LL-37 and, hence, variability in their antimicrobial effectiveness.
An obvious conclusion from the current study is that CMC-containing artificial tears may reduce the activity of endogenously produced cationic antimicrobial peptides. Furthermore, because hBD-2 and LL-37 have been shown to modulate cell migration and proliferation and, as a consequence, have been implicated as regulatory factors for wound healing,23–25 it is possible that CMC-containing tear solutions may also impair the ability of these peptides to stimulate corneal wound healing. However, extrapolation of the in vitro data to the in vivo situation should be done with extreme caution. Indeed, the ocular surface is equipped with several other antimicrobial substances26 and numerous factors believed capable of regulating wound healing.27 Thus, even if the activity of cationic antimicrobial peptides is compromised by the use of CMC-containing solutions, redundancy at the ocular surface in most cases will ensure that adequate antimicrobial protection and responses to injury still exist.
Defensins and LL-37, in addition to being antimicrobial, are known to be chemotactic for various immune cells.28–32 They have also been implicated as mediators of inflammation simulating the release of cytokines and histamine from innate and adaptive immune cells.33–36 Furthermore, several studies have shown high concentrations of antimicrobial peptides, such as defensins, to be cytotoxic to various mammalian cell types.37 Therefore, if present in excess, these peptides may be detrimental to the ocular surface for a variety of reasons, such as uncontrolled inflammation or cellular toxicity. Notably, CMC-based solutions have been shown to improve objective measurements in patients with dry eye38 and preserve ocular surface health after laser in situ keratomileusis.39,40 Therefore, in keeping with the concept that CMC has cytoprotective properties,22 an alternative interpretation of the results of the current study is that neutralization of hBD-2 and LL-37 by CMC at the ocular surface may actually be advantageous in preventing unwanted effects that may result from having an excess of these peptides.
Acknowledgments
The authors thank Dr. Bradley Mitchell of Baylor College of Medicine for providing the clinical P. aeruginosa isolates.
Footnotes
The authors have no financial interest in any of the commercial products used in this study.
Ms. Jean was supported by NEI Summer Research Program T35 EY007088. This work was funded in part by NIH grant EY13175 to Dr. McDermott.
References
- 1.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van ’t Hof W, Veerman EC, Helmerhorst EJ, et al. Antimicrobial peptides: Properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 3.Diamond G, Bevins CL. β-Defensins: Endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 4.Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 6.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: Multiple signalling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott AM, Redfern RL, Zhang B. Human β-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Proske RJ, McDermott AM. Expression of the peptide antibiotic LL-37/hCAP18 (cathelicidin) by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:ARVO E-abstract 1335. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero JM, Biser SA, Perry HD, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30:14–19. doi: 10.1097/01.ICL.0000095229.01957.89. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan RJ, Engel LS, Hobden JA, et al. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest Ophthalmol Vis Sci. 1996;37:534–543. [PubMed] [Google Scholar]
- 11.Hobden JA, Masinick SA, Barrett RP, et al. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci. 1995;36:1107–1114. [PubMed] [Google Scholar]
- 12.Tomita T, Hitomi S, Nagase T, et al. Effect of ions on antibacterial activity of human beta defensin 2. Microbiol Immunol. 2000;44:749–754. doi: 10.1111/j.1348-0421.2000.tb02559.x. [DOI] [PubMed] [Google Scholar]
- 13.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 14.Turner J, Cho Y, Dinh NN, et al. Activities of LL-37, a cathelicidin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bals R, Wang X, Wu Z, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travis SM, Anderson NN, Forsyth WR, et al. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68:2748–2755. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka D, Miyasaki KT, Lehrer RI. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: A cathelicidin found in human leukocytes and epithelium. Oral Microbiol Immunol. 2000;15:226–231. doi: 10.1034/j.1399-302x.2000.150403.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka I, Hirota S, Yomogida S, et al. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 20.Enzenauer RW, Kao A, Williams T, et al. Relative costs of various preserved artificial tear solutions for the treatment of dry eye conditions. Eye Contact Lens. 2003;29:238–240. doi: 10.1097/01.icl.0000090882.94633.1C. [DOI] [PubMed] [Google Scholar]
- 21.Oren A, Ganz T, Liu L, et al. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 22.Vehige JG, Simmons PA, Anger C, et al. Cytoprotective properties of carboxymethyl cellulose (CMC) when used prior to wearing contact lenses treated with cationic disinfecting agents. Eye Contact Lens. 2003;29:177–180. doi: 10.1097/01.ICL.0000074106.82322.17. [DOI] [PubMed] [Google Scholar]
- 23.Redfern RL, Proske RJ, McDermott AM. Effect of defensins on corneal cell migration and cytokine production. Invest Ophthalmol Vis Sci. 2004;45:E-abstract 4866. [Google Scholar]
- 24.Huang L, Proske RJ, McDermott AM. Functional roles of the epithelial-derived antimicrobial peptide LL-37 at the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:E-abstract 4940. [Google Scholar]
- 25.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin antimicrobial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 26.Sack RA, Nunes I, Beaton A, et al. Host-defense mechanism of the ocular surfaces. Biosci Rep. 2001;21:463–480. doi: 10.1023/a:1017943826684. [DOI] [PubMed] [Google Scholar]
- 27.Sotozono C. Growth factors and cytokines in corneal wound healing. In: Nishida N, ed. Corneal Healing Response to Injuries and Refractive Surgeries. The Hague: Kugler Publications, 1998, pp 29–40.
- 28.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 30.Territo MC, Ganz T, Selsted ME, et al. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niyonsaba F, Iwabuchi K, Someya A, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, et al. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 34.Chaly YV, Paleolog EM, Kolesnikova TS, et al. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 35.Befus AD, Mowat C, Gilchrist M, et al. Neutrophil defensins induce histamine secretion from mast cells: Mechanisms of action. J Immunol. 1999;163:947–953. [PubMed] [Google Scholar]
- 36.Niyonsaba F, Someya A, Hirata M, et al. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: Antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 38.Grene RB, Lankston P, Mordaunt J, et al. Unpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivitis sicca. Cornea. 1992;11:294–301. doi: 10.1097/00003226-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Albietz JM, Lenton LM, McLennan SG, et al. A comparison of the effect of Refresh Plus and Bion Tears on dry eye symptoms and ocular surface health in myopic LASIK patients. CLAO J. 2002;28:96–100. [PubMed] [Google Scholar]
- 40.Ahee JA, Kaufman SC, Samuel MA, et al. Decreased incidence of epithelial defects during laser in situ keratomileusis using intraoperative nonpreserved carboxymethylcellulose sodium 0.5% solution. J Cataract Refract Surg. 2002;28:1651–1654. doi: 10.1016/s0886-3350(01)01348-7. [DOI] [PubMed] [Google Scholar]


