Abstract
Primary cultures of rat and mouse sensory neurons were used to study the entry of herpes simplex virus type 1 (HSV-1). Soluble, truncated nectin-1 but not HveA prevented viral entry. Antibodies against nectin-1 also blocked infection of rat neurons. These results indicate that nectin-1 is the primary receptor for HSV-1 infection of sensory neurons.
Herpes simplex virus type 1 (HSV-1) has a broad host range, and recent studies have identified a number of cell surface proteins that serve as receptors for viral entry (reviewed in reference 18). It appears that the abundance of the different receptors varies with the cell type, and this variation might influence the course of HSV-1 infection (4). The envelope of HSV-1 contains several glycoproteins, and studies with mutant virus have shown that the glycoproteins gB, gD, and gH are required for infection of rat neurons (1). An essential event in virus cell interaction is the binding of viral glycoprotein D (gD) to a cellular receptor. Glycoprotein D interacts with at least three structurally unrelated receptors: HveA (12, 13, 21), nectin-1 (4, 7), and 3-O-sulfated heparan sulfate (16). HveA, also known as HVEM, is a member of the tumor necrosis factor receptor superfamily. HveA mRNA is expressed in lymphoid cells and fibroblasts but only weakly in human brain tissue (8, 12). Nectin-1, also called HveC, is a member of the immunoglobulin superfamily. Nectin-1 is found at cellular junctions and is involved in cell-cell adhesion (14, 19) and in synapse formation (11). High levels of nectin-1 mRNA are expressed in the human central nervous system (2), in neuronal cell lines (4), and in mouse sensory, sympathetic, and parasympathetic neurons (5). Nectin-1 protein is found in abundance in rat sensory neurons but not in rat motor neurons (9). Wilcox and Johnson developed a model of HSV-1 latency in primary sensory neurons (22, 23). This model reproduces many of the characteristic features of a natural human HSV-1 latent infection, including restricted viral gene expression (3) and reactivation, to produce infectious virus (17, 23). Using this model and functional assays to measure virus entry, evidence was obtained that HSV-1 entry into rodent sensory neurons is mediated by nectin-1.
Effects of soluble HveA or nectin-1 receptors on HSV-1 entry.
Neuronal cultures were prepared from dorsal root ganglia of embryonic day 15 rats or mice as previously described (22, 23). Dulbecco's Eagle's medium-F12 (supplemented with 10% newborn bovine serum, 100 ng of 2.5-S mouse nerve growth factor/ml, and 20 μM 5-fluoro-2′-deoxyuridine to inhibit growth of nonneuronal cells) was used to establish neuronal cultures (neuronal maintenance medium). Rats and mice were treated according to institutional guidelines for animal use. Primary rat fibroblasts and HeLa cells were cultured in Dulbecco's Eagle's medium with 5% fetal bovine serum. For infection, a recombinant HSV-1 (17+ strain) expressing green fluorescent protein (GFP) fused to the C terminus of the immediate-early gene product ICP4 was used. This virus (HSVEGFP4) behaves essentially like wild-type strain 17+ virus (unpublished data). The 17+ virus strain can enter cells via HveA or nectin-1 (10, 12).
The blocking of HSV-1 entry into neurons or fibroblasts was assayed by incubating HSVEGFP4 with soluble HveA or nectin-1 before adding the virus to cells and quantifying the numbers of fluorescent cells present after incubation. Rat or mouse sensory neurons were plated in 24-well plates at approximately 5 × 103 cells per well. Soluble, truncated forms of HveA [HveA(200t)] or nectin-1 [HveC(346t)] were preincubated with the virus for 2 h at 4°C. A virus multiplicity of infection (MOI) of 100 PFU per neuron was used for each culture, and the soluble receptor concentration in the preincubation was 3.0 pg/PFU for nectin-1 and 0.7 pg/PFU for HveA. After preincubation, the virus and soluble receptor were added to neurons and the virus was allowed to adsorb for 1 h at 35°C. The inoculum was then removed and replaced with maintenance medium supplemented with 100 μM acyclovir to limit the infection. At 24 h after infection, neurons were identified by their morphology and GFP-positive neurons were visualized by epifluorescence. Each treatment group contained four cultures, and the fluorescent cells in four or five fields for each culture were counted. Data are expressed as the mean percentage of GFP-positive neurons per culture. Experiments using fibroblasts were performed as described above for neuronal cultures, except that fibroblasts were plated at 2 × 104 cells per well, an MOI of 10 PFU/cell was used, and at 8 h postinfection, cells were fixed in 4% paraformaldehyde prior to analysis of GFP expression.
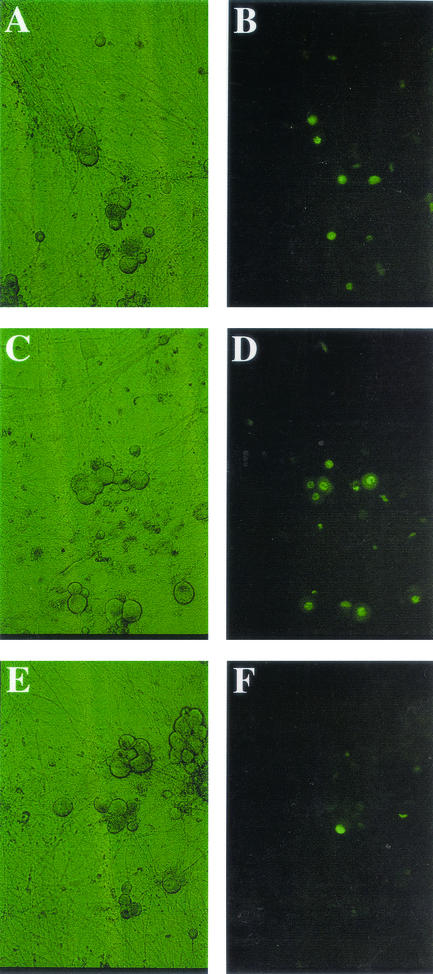
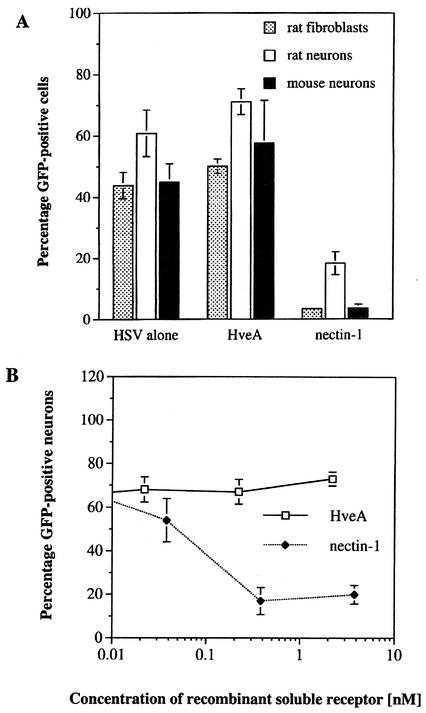
Previous studies have demonstrated that soluble forms of HveA and nectin-1 can block HSV-1 entry into cell lines (6, 12). Using similar experimental conditions, we tested the ability of these soluble receptors to inhibit HSV-1 infections of primary sensory neurons and fibroblasts. The effects of nectin-1 and HveA on rat neurons are shown in Fig. 1. Quantitative data for rat and mouse neurons and fibroblasts are shown in Fig. 2A. Rat neurons infected with HSV-1 alone resulted in 61% GFP-positive cells. Incubation of virus with HveA resulted in 71% GFP-positive cells, a value slightly higher than that for the control infection. The addition of nectin-1 to virus reduced the proportion of GFP-positive cells to 17% of that of the controls. Similar results were obtained with primary rat fibroblasts and mouse neuron cultures. A second recombinant virus that expresses GFP fused to ICP27 gave similar results (data not shown). To test the possibility that the amount of HveA or nectin-1 used in the previous experiments was not optimal, the virus was treated with additional concentrations of soluble receptors. Recombinant nectin-1 showed a dose-dependent decrease in HSV-1 entry into rat neurons, while incubations with a range of HveA concentrations did not inhibit viral entry (Fig. 2B).
FIG. 1.
Effects of soluble recombinant HveA or nectin-1 on viral entry into primary rat neurons. The panels shown depict representative neuronal cultures photographed using phase contrast (A, C, and E) or epifluorescence (B, D, and F) 24 h after infection. (A and B) HSVEGFP4 alone; (C and D) HSVEGFP4 with soluble HveA [HveA(200t)]; (E and F) HSVEGFP4 preincubated with soluble nectin-1 [HveC(346t)]. Images are shown at ×600 magnification.
FIG. 2.
Effect of soluble recombinant HveA or nectin-1 on viral entry into primary rat neurons, mouse neurons, and primary rat fibroblasts. (A) Virus was incubated with soluble receptor before being added to cells. An MOI of 100 PFU per cell was used for rat and mouse sensory neurons. An MOI of 10 PFU per cell was used for rat fibroblasts. Each bar represents the average percentage of GFP-positive cells per culture. Error bars indicate standard error of the mean (n = 4). (B) HSVEGFP4 virus was incubated with the indicated concentrations of recombinant HveA(200t) (open squares) or nectin-1(346t) (closed diamonds) prior to infection of rat sensory neurons. Each point represents the average percentage of GFP-positive cells per culture.
Effects of antibodies against HveA or nectin-1 on HSV-1 entry.
Blocking of infection by antibodies against soluble receptors was assayed using the experimental procedure described above, except that cell cultures were treated with antiserum for 1 h at 35°C prior to adding virus. Antisera were raised in rabbits against nectin-1 (R166) or HveA (R140) (20). A monoclonal antibody against nectin-1 (CK41) was also tested (6). Cells were incubated in medium containing 10% preimmune serum, 10% anti-HveA, 10% anti-nectin-1, or 1% monoclonal antibody CK41. The virus was added to serum-treated cells for 1 h to allow adsorption, and then the inoculum was replaced with medium supplemented with 100 μM acyclovir. Following incubation for 24 h at 35°C (neuronal cultures) or 8 h at 37°C (fibroblasts), the cultures were examined by fluorescent microscopy for expression of GFP.
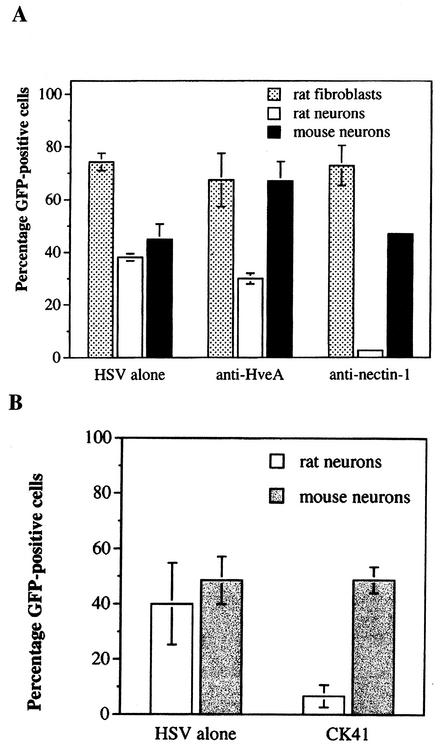
The results of the polyclonal antibody blocking experiments are shown in Fig. 3A. Anti-nectin-1 antiserum (R166) reduced the number of GFP-positive rat sensory neurons by more than 90%, while anti-HveA antiserum (R140) did not block infection. The ability of R166 anti-nectin-1 antiserum to interfere with HSV-1 entry was restricted to rat sensory neurons. Treatment with anti-nectin-1 antiserum did not affect HSV-1 entry into primary rat fibroblasts or mouse sensory neurons. That R166 anti-nectin-1 serum failed to block entry into mouse sensory neurons was unexpected, since R166 reacts with mouse nectin-1 by immunohistochemistry and flow cytometry (15). Monoclonal CK41 antibody against human nectin-1 blocks entry of HSV-1 into several types of cells (6). This antibody reduced virus entry into rat sensory neurons by 60% compared to virus entry into untreated neurons, but as was found with nectin-1 polyclonal antiserum, the CK41 monoclonal antibody did not block virus entry into mouse sensory neurons (Fig. 3B). Treatment of virus with anti-gD serum completely blocked infection (data not shown).
FIG. 3.
The effects of pretreatment of cells with antibodies to HveA or nectin-1 on viral entry. (A) Cells were left untreated (HSV alone) or were pretreated with rabbit antisera against HveA (R140) or nectin-1 (R166) prior to infection with HSV-1. (B) Rat or mouse neurons were left untreated (HSV alone) or were pretreated with monoclonal anti-nectin-1 antibodies (CK41) prior to infection with HSVEGFP4.
Western blot analyses of HveA and nectin-1 on primary sensory neurons and fibroblasts.
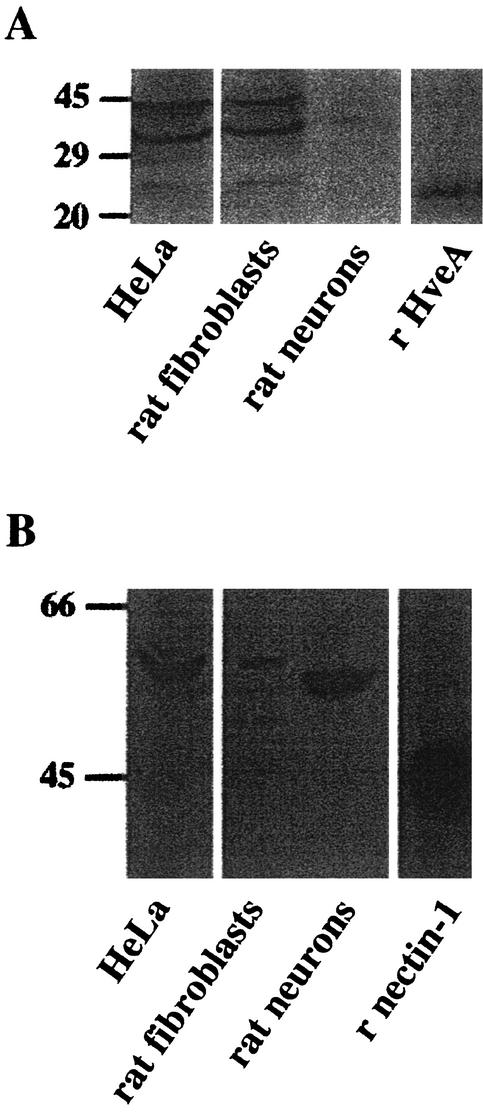
The inability of soluble HveA and HveA antiserum to reduce HSV-1 entry into neurons might be attributable to the absence of HveA from these cells or the presence of this receptor in an inactive form. To distinguish between these possibilities, Western blot analysis was carried out on protein from primary rat neuronal cultures, rat fibroblasts, and (as a control) HeLa cells. Cells were harvested in radioimmunoprecipitation assay buffer containing protease inhibitors, and proteins were resolved on sodium dodecyl sulfate-10% polyacrylamide gels and transferred to nitrocellulose for immunodetection. A chemiluminescent substrate for peroxidase (NEN Life Science, Boston, Mass.) was used to visualize protein bands. HveA was detected in primary rat fibroblasts and HeLa cells at the predicted molecular mass of about 35 kDa (Fig. 4A). A faint band was detected in the rat neuronal cultures. Nectin-1 was detected in HeLa cells and primary rat fibroblasts at approximately 63 kDa (Fig. 4B). In rat sensory neuronal cultures, an intense band was detected with anti-nectin-1 antibody. This band was smaller than that observed with HeLa and fibroblast cell extract and might represent the product of an alternatively spliced nectin-1 transcript (2). These results demonstrate that nectin-1 was abundant in rat neurons but HveA was present at low levels.
FIG. 4.
Detection of HveA and nectin-1 proteins by immunoblotting. For immunoblot analysis, 30 μg of protein from whole-cell lysates and 2 ng of soluble recombinant HveA(200t) or 10 ng of nectin-1(346t) were separated by electrophoresis and transferred to membranes for immunodetection. HveA was detected using rabbit anti-HveA serum (R140) (A), and nectin-1 was detected using rabbit anti-nectin-1 serum (R166) (B).
During infection of its natural host, HSV-1 encounters different types of cells. Viral entry into these cells is critical for replication of the virus in cells of the epithelium and for progression from the initial sites of infection to neurons and cells of the immune system. The viral receptors on these cells greatly influence which cells are productively infected, which cells establish latent infections, and the nature of the immune response. Therefore, to study the pathogenesis of HSV-1 infections, it is important to identify the viral receptors that function on target cells. The experiments described in this report addressed the nature of cellular receptors on sensory neurons, the cells involved in HSV-1 latency. The results show that nectin-1 is the primary, if not only, functional receptor on rodent sensory neurons in cultures. Consistent with functional assays, analysis of Western blots showed abundant nectin-1 on rat neurons but low levels of HveA. Fibroblasts expressed both HveA and nectin-1. While the data with rat neurons were consistent, two points arose with mouse neurons and rat fibroblasts. Anti-nectin-1 polyclonal and monoclonal antibodies failed to inhibit viral entry into mouse neurons. One possible explanation for this result is that the antibodies used were raised against human nectin-1 and have low affinity for the mouse homologue. The second point is that while the Western analyses of rat fibroblast extracts showed the presence of both HveA and nectin-1, soluble nectin-1 but not HveA inhibited infection. These data suggest that when HveA and nectin-1 are both on a cell, they are not equivalent in facilitating viral entry. The binding of soluble nectin-1 to gD in the virus restricted gD binding to cellular HveA. In contrast, soluble HveA-gD interactions did not interfere with cellular nectin-1 binding to the virus. The studies described in this report were carried out with cell-free HSV-1, and it should be informative to use infected cells to test whether soluble receptors and anti-receptor antibodies can disrupt the spread of virus from one cell type to another.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS-29046 to C. Wilcox and NS-30606 and NS-36731 to R. J. Eisenberg and G. H. Cohen), the National Institute of Allergy and Infectious Diseases (AI-18289 to R. J. Eisenberg and G. H. Cohen), and the College Research Council (C.L.W.).
We thank Michael Manchak for his editorial help.
REFERENCES
- 1.Babic, N., G. Rodger, J. Arthur, and A. C. Minson. 1999. A study of primary neuronal infection by mutants of herpes simplex virus type 1 lacking dispensable and non-dispensable glycoproteins. J. Gen. Virol. 80:2403-2409. [DOI] [PubMed] [Google Scholar]
- 2.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doerig, C., L. I. Pizer, and C. L. Wilcox. 1991. Detection of the latency-associated transcript on neuronal cultures during the latent infection with herpes simplex virus type 1. Virology 183:423-426. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 5.Haarr, L., D. Shukla, E. Rodahl, M. C. Dal Canto, and P. G. Spear. 2001. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology 287:301-309. [DOI] [PubMed] [Google Scholar]
- 6.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon, B. S., K. B. Tan, J. Ni, K. O. Oh, Z. H. Lee, K. K. Kim, Y. J. Kim, S. Wang, R. Gentz, G. L. Yu, J. Harrop, S. D. Lyn, C. Silverman, T. G. Porter, A. Truneh, and P. R. Young. 1997. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 272:14272-14276. [DOI] [PubMed] [Google Scholar]
- 9.Mata, M., M. Zhang, X. Hu, and D. J. Fink. 2001. HveC (nectin-1) is expressed at high levels in sensory neurons, but not in motor neurons, of the rat peripheral nervous system. J. Neurovirol. 7:476-480. [DOI] [PubMed] [Google Scholar]
- 10.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi, A., H. Nakanishi, K. Kimura, K. Matsubara, K. Ozaki-Kuroda, T. Katata, T. Honda, Y. Kiyohara, K. Heo, M. Higashi, T. Tsutsumi, S. Sonoda, C. Ide, and Y. Takai. 2002. Nectin: an adhesion molecule involved in formation of synapses. J. Cell Biol. 156:555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 13.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y.-F. Peng, K. Yamanishi, and Y. Takai. 2001. Requirement of interaction of nectin-1α/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J. Virol. 75:4734-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla, D., M. C. Dal Canto, C. L. Rowe, and P. G. Spear. 2000. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J. Virol. 74:11773-11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 17.Smith, R. L., L. I. Pizer, E. M. Johnson, Jr., and C. L. Wilcox. 1992. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology 188:311-318. [DOI] [PubMed] [Google Scholar]
- 18.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitbeck, J. C., S. A. Connolly, S. H. Willis, W. Hou, C. Krummenacher, M. Ponce De Leon, H. Lou, I. Baribaud, R. J. Eisenberg, and G. H. Cohen. 2001. Localization of the gD-binding region of the human herpes simplex virus receptor, HveA. J. Virol. 75:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox, C. L., and E. M. Johnson, Jr. 1988. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J. Virol. 62:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox, C. L., and E. M. Johnson, Jr. 1987. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J. Virol. 61:2311-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]