Abstract
PECAM-1/CD31 is a cell adhesion and signaling molecule that is enriched at the endothelial cell junctions. Alternative splicing generates multiple PECAM-1 splice variants, which differ in their cytoplasmic domains. It has been suggested that the extracellular ligand-binding property, homophilic versus heterophilic, of these isoforms is controlled by their cytoplasmic tails. To determine whether the cytoplasmic domains also regulate the cell surface distribution of PECAM-1 splice variants, we examined the distribution of CD31-EGFPs (PECAM-1 isoforms tagged with the enhanced green fluorescent protein) in living Chinese hamster ovary cells and in PECAM-1-deficient endothelial cells. Our results indicate that the extracellular, rather than the cytoplasmic domain, directs PECAM-1 to the cell-cell borders. Furthermore, coculturing PECAM-1 expressing and deficient cells along with transfection of CD31-EGFP cDNAs into PECAM-1 deficient cells reveal that this PECAM-1 localization is mediated by homophilic interactions. Although the integrin αvβ3 has been shown to interact with PECAM-1, this trans-heterophilic interaction was not detected at the borders of endothelial cells. However, based on cocapping experiments performed on proT cells, we provide evidence that the integrin αvβ3 associates with PECAM-1 on the same cell surface as in a cis manner.
INTRODUCTION
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a 130 kDa transmembrane glycoprotein that belongs to the immunoglobulin gene superfamily (IgGSF) (Albelda et al., 1990; Newman et al., 1990). It is expressed on the surface of platelets, endothelial cells (ECs), monocytes, neutrophils, and specific T-cell subsets. In particular, PECAM-1 concentrates at the junctions of ECs (Albelda et al., 1991; Newman and Albelda, 1992). Various studies have shown a role for PECAM-1 in endothelial cell-cell adhesion (DeLisser et al., 1994b), leukocyte-EC interactions (Bogen et al., 1992), transendothelial migration (Berman and Muller, 1995; Muller, 1995), angiogenesis (Horak et al., 1992; DeLisser et al., 1997), and the development of the early cardiovascular system (Baldwin et al., 1994).
PECAM-1 is composed of six extracellular Ig-homology domains, a short transmembrane region, and a cytoplasmic tail of variable length due to the alternative splicing of exons 12–16 (Newman et al., 1990; Albelda et al., 1991; Xie and Muller, 1993; Kirschbaum et al., 1994). Analysis of PECAM-1 expression in the developing mouse embryo identified six murine PECAM-1 (muPECAM-1) isoforms designated full length (FL), Δ12, Δ14, Δ15, Δ12&15, Δ14&15, and Δ12,14&15 (Baldwin et al., 1994). The functional significance of these multiple splice variants is still unknown, but the different cytoplasmic domains partly regulate the ligand binding properties of the PECAM-1 extracellular domain, possibly by interacting with different intracellular molecules (DeLisser et al., 1994a; Yan et al., 1995; Famiglietti et al., 1997). Binding to the amino-terminal Src homology 2 domains (SH-2) of SHP-1 and SHP-2 (Jackson et al., 1997; Hua et al., 1998) requires the phosphorylation of the two highly conserved phosphatase-binding motifs, VQpY663TEV and TVpY686SEV, in PECAM-1. This interaction is abrogated in PECAM-1 splice variants that lack exon 14. Intracellular molecules that interact with Δ14 PECAM-1 isoforms have not been yet identified. Therefore, the cytoplasmic-encoding exon 14 apparently regulates binding of PECAM-1 to intracellular ligands. Moreover, exon 14 modulates the extracellular adhesive property of PECAM-1. Whereas the FL muPECAM-1 and exon 14 containing isoforms mediate calcium- and heparin-dependent heterophilic aggregation in transfected cells, PECAM-1 isoforms lacking exon 14 mediate calcium- and heparin-independent homophilic cell aggregation (Yan et al., 1995; Sun et al., 1996a). Furthermore, either loss of the Tyr-686 from exon 14 or its phosphorylation results in a change in PECAM-1 ligand specificity from heterophilic to homophilic binding (Famiglietti et al., 1997). PECAM-1 homophilic binding requires the extracellular Ig-homology domains 1 and 2 plus the proper spacing formed by the six Ig-homology domains (Fawcett et al., 1995; Sun et al., 1996b). The region required for PECAM-1 heterophilic binding has not been determined, although several heterophilic ligands have been identified, including CD38 (Horenstein et al., 1998), an unidentified molecule on T lymphocytes (Prager et al., 1996), and the integrin αvβ3 (Piali et al., 1995; Buckley et al., 1996).
Integrins are cell-surface receptors formed from two noncovalently associated subunits, α and β. They bind to a variety of extracellular matrix molecules (ECM), cell surface proteins, and intracellular molecules. Integrin αvβ3 ligands include ECM vitronectin, fibrinogen, von Willebrand factor, thrombospondin, osteopontin, fibronectin, and laminin (Horton, 1997). Besides binding to PECAM-1, it also interacts in trans with the neural cell adhesion molecules L1 (Montgomery et al., 1996) and ADAM-15/metargidin (Nath et al., 1999). Furthermore, it associates laterally (in cis) with several cell-surface proteins, including the integrin-associated protein (IAP) (Brown et al., 1990), insulin receptor (IR) β-subunit (Schneller et al., 1997), the phosphorylated insulin receptor substrate 1 (IRS-1) (Vuori and Ruoslahti, 1994), platelet-derived growth factor (PDGF) β-receptor (Schneller et al., 1997), and the urokinase-type plasminogen activator receptor CD87 (Xue et al., 1997).
In this study, we addressed the question of whether the PECAM-1 alternatively spliced cytoplasmic domains regulate the distribution of PECAM-1 isoforms on the surface of ECs. In particular, we determined whether specific PECAM-1 isoforms are differentially directed to the cell junctions and to the apical cell surface. To accomplish this, functional proteins comprising the different PECAM-1 splice variants fused to the enhanced green fluorescent protein (EGFP) were expressed in CHO cells and ECs. Furthermore, by using endothelioma cells obtained from PECAM-1 deficient mice, we determined whether this localization is mediated by homophilic or heterophilic interactions. In addition, we examined the interaction of PECAM-1 and integrin αvβ3 at the EC junctions and on proT cells.
MATERIALS AND METHODS
Cells
CHO cells were purchased from the American Type Tissue Culture Collection (Rockville, MD). Mouse thymic and mouse brain endothelioma cells (tEnd.1 and bEnd.5, respectively) were obtained from Dr. Werner Risau (Max Planck Institute, Bad Nauheim, Germany) and have been previously described (Bussolino et al., 1991; Reiss et al., 1998). The mouse proT-cell line FTF1.26 has been previously described (Imhof et al., 1991).
To establish the PECAM-1 deficient mouse brain endothelioma cell line (bEnd.PECAM-1.2neo), cerebral capillaries were isolatd from 4- to 10-day-old PECAM-1 deficient mice (Duncan et al., 1999), following a previously described procedure (Risau et al., 1990). The capillaries were cultured overnight in DMEM medium supplemented with 1% L-glutamine, 1% nonessential amino acids, 1% sodium-pyruvate, 10.000 U/ml penicillin-streptomycin (all PAA Laboratories, Colbe, Germany), 10−5M β-mercaptoethanol,and 1% (vol/vol) bovine retinal extract. These primary endothelial cells were infected with a recombinant retrovirus transducing the polyoma virus middle T-oncogene (Kiefer et al., 1994; Wagner and Risau, 1994), as previously described (Reiss et al., 1998). The bEnd.PECAM-1.2neo cell line retained their endothelial morphology and showed contact inhibition upon confluency.
CHO cells were cultured in Ham's F12 medium, whereas tEnd.1, bEnd.5, FTF1.26, and bEnd.PECAM-1.2neo cells were cultured in DMEM medium (Life Technologies, Paisley, Scotland), each supplemented with 10% FCS (PAA Laboratories, Linz, Austria), 2 mM L-glutamine, 1% nonessential amino acids, 1% sodium-pyruvate, 100 i.u./ml penicillin, and 100 μg/ml streptomycin (all Life Technologies). In addition, interleukin-2 was added to the culture media of FTF1.26 cells. For selection of CD31-EGFP transfected cells, G418 (1.5 mg/ml Geneticin, Life Technologies) was added to the culture media.
Antibodies
mAb GC51 is a rat IgG2b isotype that recognizes the first Ig-homology domain of muPECAM-1. In brief, splenocytes of Fisher rats immunized with a recombinant soluble form of muPECAM-1 (Piali et al., 1995) were fused with the SP2/0 myeloma. Hybridoma supernatants were then screened for PECAM-1-specific antibodies by ELISA on recombinant soluble PECAM-1 and by flow cytometry analysis using PECAM-1 transfected J558L cells (Piali et al., 1995). mAb GC51 was purified by affinity chromatography using Sepharose protein G (Amersham Pharmacia, Uppsala, Sweden).
The mAbs MK1.9 (anti-VCAM-1) and H202.106.74 (anti-JAM) have been previously described (Miyake et al., 1991; Malergue et al., 1998).
The following antibodies were also used: anti-GFP (Clontech Laboratories Inc., Basel, Switzerland, Cat.8363–2), antirat Ig (Southern Biotechnology Associates, Inc., Birmingham, AL, Cat.3010–01), Texas Red dye-conjugated AffiniPure Goat Anti-Rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., La Roche, Switzerland, Cat.111–075-144), anti-PECAM-1 (Santa Cruz Biotechnology, Basel, Switzerland, M20 clone), anti-PTP1D/SHP-2 (Transduction Laboratiories, Basel, Switzerland, Cat. P54420), anti-ICAM-2 (PharMingen, Basel, Switzerland, 3C4 (mIC2/4) clone), anti-CD51/integrin αv (PharMingen, H9.2B8 clone), anti-CD61/integrin β3 (PharMingen, 2C9 clone), Goat F(ab′)2 Anti-Hamster IgG (H+L)-RPE (PharMingen, Cat. 6062–09), Goat Anti-Rat IgG (H+L)-RPE (PharMingen, Cat.3050–09), Texas Red dye-conjugated AffiniPure Goat Anti-Rat IgG (Jackson ImmunoResearch Laboratories, Inc., Cat.112–075-143), biotin-conjugated anti-CD18/integrin β2 (PharMingen, Cat.01662D), biotin-conjugated anti-CD25/IL2R alpha chain (PharMingen, Cat.01091D), biotin-conjugated anti-CD29/integrin β1 (PharMingen, Cat.22632D), biotin-conjugated anti-CD61/integrin β3 (PharMingen, Cat.01862D), biotin-conjugated anti-MHC/H-2Dk (PharMingen, Cat.06152D), Oregon Green-488 conjugated goat-anti rat IgG (Molecular Probes, Leiden, The Netherlands, Cat.O-6382), and neutralite avidin texas red conjugate (Southern Biotechnology Associates Inc., Cat.7200–07).
Preparation of cDNA Constructs
The EGFP cDNA, excised from the pEGFP-1 vector (Clontech Laboratories Inc., Palo Alto, CA) at the 5′ HindIII and 3′ XbaI sites, was subcloned into pcDNA3 (Invitrogen, San Diego, CA) to produce the pcDNA3-EGFP vector.
MuPECAM-1 cDNAs were amplified from mouse placenta cDNA by PCR. The sequences of the primer pair used to generate the FL PECAM-1 were: 5′-ATTAAAGCT TCCACCATGCTCCTGGCTCTGGGACTCA-3′ (PFL forward primer) and 5′-TATTAG GGCCCTTAAGTTCCATTAAGGGAGCCTT-3′ (PFL reverse primer), with the HindIII and ApaI sequences in italics, respectively. The PCR product was subcloned into pcDNA3 at the HindIII and ApaI sites to produce the pcDNA3-FL-CD31 vector. The proper FL PECAM-1 DNA sequence was verified by sequencing. For PCR amplification of the remaining PECAM-1 cytoplasmic splice variant cDNAs, the forward primer 5′-GGTGGA TGAAGTTGTGATTTCC-3′ (annealed to exon 8, the sixth Ig-homology domain region, which contains an internal NheI site) was used with the PFL reverse primer. These PCR products of different lengths were subcloned into pcDNA3-FL CD31 at the NheI and ApaI sites to produce pcDNA3 vectors that carry the different PECAM-1 isoforms. The proper PECAM-1 cytoplasmic tail DNA sequences was verified by sequencing.
Each of the PECAM-1 splice variant cDNAs was amplified by PCR using the PFL forward primer and the reverse primer 5′-ATAATATCGATAGTTCCATTAAGGGAG CCTT-3′ (for isoforms that contain exon 15) or 5′-ATAATATCGATAGGGAGCCTT CCGTTCT-3′ (for isoforms that lack exon 15, this results in a change in their open reading frame), with the ClaI sequences in italics. These PCR products were cloned into pcDNA3-EGFP at the HindIII and ClaI sites to produce the seven pcDNA-CD31-EGFP vectors, in which the EGFP is fused to the C-terminal end of PECAM-1 and separated by an eight-amino acid linker (IDGPPVAT). To avoid repeated sequencing of the PECAM-1 extracellular domains, the HindIII/NheI fragment of the seven pcDNA-CD31-EGFP vectors was replaced by a corresponding sequenced fragment. The region between the 5′NheI and 3′ClaI sites (the PECAM-1 transmembrane and cytoplasmic domains) of each pcDNA-CD31-EGFP vectors was sequenced.
Murine L-selectin cDNA was amplified from mouse proT-cell (FTF1.26) cDNA. The PFL forward primer (annealed to the N-terminal end of PECAM-1) and the CD31/L-selectin linker primer 5′-TCCTTGCCCCATGGAAGAAAACCGGTAACCCCCTCTTCA TTCCTGTA-3′ (annealed to the C-terminal end of PECAM-1 and the N-terminal end of L-selectin), with the AgeI site in italics, were used in the initial PCR amplification. The PCR product was then used as a template for further amplification using the PFL primer and the LS reverse primer 5′-GAAAGGATGGATGATCCATACATCGATAATTA-3′, with the ClaI site in italics. The PCR product was then cloned into pcDNA3-CD31-EGFP at the HindIII and ClaI sites to obtain a construct that consists of the PECAM-1 extracellular domains, the L-selectin transmembrane and cytoplasmic domains, and the EGFP (LS-CD31-EGFP). The amino acid sequence at the transition of PECAM-1/L-selectin is PWKK/TG(AgeI site)/NPLF, and the transition between L-Selectin/EGFP is MDDPY/ID (ClaI site)/GPPVA.
cDNA Transfection
For the expression of CD31-EGFP fusion proteins, tEnd.1 cells were stably transfected with the pcDNA3-CD31-EGFP vectors by electroporation. In brief, 5 × 106 cells were electroporated at 280mV and 960 μF, in 500 μl PBS with 20 μg plasmids, using 0.2 cm gap cuvettes and a gene pulser (Bio-Rad Laboratories, Richmond, CA). Culture media was changed the following day, and G418 was added for selection. After 48 h, CD31-EGFP-expressing tEnd.1 cells were subcloned into 96-well plates using a FACStar Plus cell sorter (Becton-Dickinson, Mountain View, CA). Mutiple sortings were performed to obtain stable FL CD31-EGFP tEnd.1 cells and Δ14 CD31-EGFP tEnd.1 cells.
CD31-EGFP cDNAs were transiently transfected into CHO cells using TransIT polyamine (LT-1) in RPMI serum free medium, following the manufactures protocol (Pan Vera Corporation, Madison, WI). In brief, 1–3 μg of PECAM-1 cDNA and 60% confluent cells plated on 35-mm culture dishes were used in each transfection.
CD31-EGFP proteins were transiently expressed in bEnd.PECAM-1.2neo cells following procedures as described for tEnd.1 or CHO cells. A total of 20–32 μg plasmids and 1–5 × 106 cells were used, and electroporation was performed at 240mV.
Immunoprecipitation and Western Blot
Untransfected and FL/Δ14 CD31-EGFP transfected tEnd.1 cells were either untreated or treated with 100 μM pervanadate for 20 min at 37°C. Cells were then washed with cold PBS, lysed in 1 ml ice cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1 tablet BM complete protease inhibitor cocktail (Boehringer Mannheim, Germany)/25 ml) for 20 min on ice, and lysates were precleared with protein G sepharose (Amersham Pharmacia Biotech AB, Uppsala, Sweden) for 1 h at 4°C. CD31-EGFP and endogenous PECAM-1 were immunoprecipitated sequentially by adding protein G sepharose coupled with goat anti-EGFP (bound via rabbit antigoat Ig) then with rat anti-PECAM-1 (GC51, bound via rabbit antirat Ig) for 1 h at 4°C. Sepharose was then washed four times (twice with lysis buffer and twice in PBS) and boiled in SDS-PAGE sample buffer, and eluted proteins (1/4 sample used) were resolved on a 6% SDS-gel. After transfer to nitrocellulose (BDH Laboratory Supplies, England, Cat. 43610 5C) using a semidry blotter (Bio-Rad Laboratories, Richmond, CA), samples were probed with anti-EGFP (1:5000), anti-PECAM-1 (M21 clone, 1:5000) and anti-SHP-2 (1:5000) then developed with ECL (Amersham Pharmacia Biotech AB, Uppsala, Sweden) according to the manufacturer's instructions.
Fluorescence-activated Cell Sorting Analysis
bEnd.5 and bEnd.PECAM-1.2neo cells were either untreated or treated with 150 U human TNF-α overnight (18 h), removed by trypsin/EDTA (Life Technologies, Paisley, Scotland), washed with PBS, and resuspended in 2% BSA/PBS (Sigma, Steinheim, Germany, Cat.A-3294). Cells were then incubated with anti-PECAM-1 (GC51 supernatant), anti-ICAM-2 (1:200), anti-VCAM-1 (SUP), anti-αv (1:200), or anti-β3 (1:200) antibody on ice for 1 h. After two washes with PBS, cells were incubated with the appropriate isotype matched phycoerythrin (PE)-conjugated antibody (1:100) on ice for 1 h. Cells were then washed twice with PBS followed by flow cytometry using a FACScan (Becton-Dickinson, Mountain View, CA). The flow cytometer was calibrated using single PE stained cells. Results of individual EC lines are expressed as a plot of frequency versus log fluorescence.
Immunofluorescent Staining
Equal numbers (5000 or 10,000/well) of bEnd.PECAM-1.2neo and tEnd.1 (CD31-EGFP tEnd.1 and bEnd.5 cells, also) cells were plated on human fibronectin (10 μg/ml huFN, Collaborative Biomedical Products, Bedford, MA)-coated 8-well chamber glass slides (Lab-Tek, Nunc Inc., Naperville, IL) and cultured for 2 days until cells reached confuency. Next, cells were fixed in acetone/methanol (-20°C 1:1 solution) for 5 min, washed once in 2% BSA/PBS, blocked for 15 min in 2% BSA/PBS, air dried (all steps at room temperature [RT]), and stored at −20°C until use. Fixed cells were incubated with anti-PECAM-1 (GC51 SUP), anti-JAM (SUP), or anti-ICAM-2 (1:5000) for 1 h at RT. After 3 washes in 2% BSA/0.1% Tween/PBS, cells were incubated with a Texas Red-conjugated goat anti-rat antibody (1:200) for 1 h at RT. After 3 more washes, HAM's F12 media was added to the wells for fluorescence microscopy analysis.
Fluorescence and Time Lapse Microscopy
For time-lapse imaging, CD31-EGFP stably transfected tEnd.1 cells were detached with trypsin/EDTA, resuspended in Ham's F12 medium, and plated on 10 μg/ml huFN-coated 1-well chamber glass slide. Living cells were observed under an inverted fluorescent microscope (Zeiss-Axiovert 100, Zurich, Switzerland), using a PlanNeofluar X63 Fluar oil immersion objective (Zeiss, Zurich, Switzerland) and a FITC filter set (450–490, FT 510, LP 520) in an incubation chamber with the temperature and CO2 set at 37°C and 10%, respectively. Pictures were acquired with a Hamamatsu C4742–95-10 digital CCD camera (Hamamatsu Photonics, Japan) controlled by the Openlab software (Improvision, Coventry, England). Images of other cells were captured using the same equipment and the following objectives: the PlanNeofluar X32 Fluar objective for the CD31-EGFP transfected CHO cells, the LDX40 Fluar oil immersion objective for the bEnd.PECAM-1.2neo and bEnd.5 untransfected or transfected cells, and the PlanNeofluar X63 Fluar oil immersion objective for the cocultured tEnd.1 and bEnd.PECAM-1.2neo cells.
PECAM-1 Cap Formations and Confocal Microscopy
Teflon slides (Polyscience Inc., Geneva, Switzerland, Cat.18357) were rinsed with 70% ethanol, air dried, and coated with 25 μl per field of poly-L-lysine (Sigma, Steinheim, Germany, Cat.P8920, 1:10 in PBS) for 10 min at RT. Slides were then rinsed with water, air dried, and plated with 105 FTF1.26 cells per field for 15min at 37°C. Unbound cells were removed by two washes in ice cold PBS. This was followed by blocking in 1%BSA/5% normal mouse serum/PBS for 30 min at RT. Next, cells were incubated with the mAb GC51 (10 μg/ml) for 1 h on ice. After three washes in ice cold PBS, cells were incubated with the Oregon Green conjugated goat-anti-rat IgG antibody (1:100) for 1 h on ice and unbound antibodies were removed by three washes in ice cold PBS. Capping of PECAM-1 (clustering of the protein due to antibody cross-linking) was allowed for 30 min at 37°C. After the slides were cooled down on ice, 5% normal mouse serum/PBS plus biotin-conjugated anti-integrin β1 chain (1:100), anti-integrin β2 chain (1:50), anti-integrin β3 chain (1:50), or anti-MHC molecule (1:25) were added for 1 h incubation at 4°C. After three washes in ice, cold 5% normal mouse serum/PBS, cells were incubated with 5% normal mouse serum/PBS plus a neutralite avidin Texas Red conjugated antibody (1:100) for 1 h at 4°C. After three more washes, cells were fixed in 4% paraformaldehyde for 5 min on ice, washed once in PBS, and mounted in moviol (Hoechst, 35 4–88) containing 1,4-Diazabicyclo(2,2,2)octane (DAPCO) (Fluka, Buchs, Switzerland, Cat.33480) and analyzed by confocal fluorescence microscopy. Note that the anti-rat IgG (used for cocapping) does not interact with the hamster IgG (anti-integrin β3) based on flow cytometry experiments (our unpublished results).
RESULTS
Full-length and Δ14 PECAM-1 Splice Variants Preferentially Localize to the Endothelial Cellular Junctions
To study the dynamics of individual muPECAM-1 splice variants, specifically their junctional versus apical localization in physiologically relevant cells, EGFP was fused to the seven naturally expressed muPECAM-1 isoforms at the carboxyl termini (Figure 1). The cDNAs of two representative PECAM-1 splice variants, the FL CD31-EGFP as the heterophilic interactor and the Δ14 CD31-EGFP as the homophilic binder, were stably transfected into tEnd.1 cells to verify their proper biochemical properties before monitoring their cell surface distribution by fluorescence microscopy.
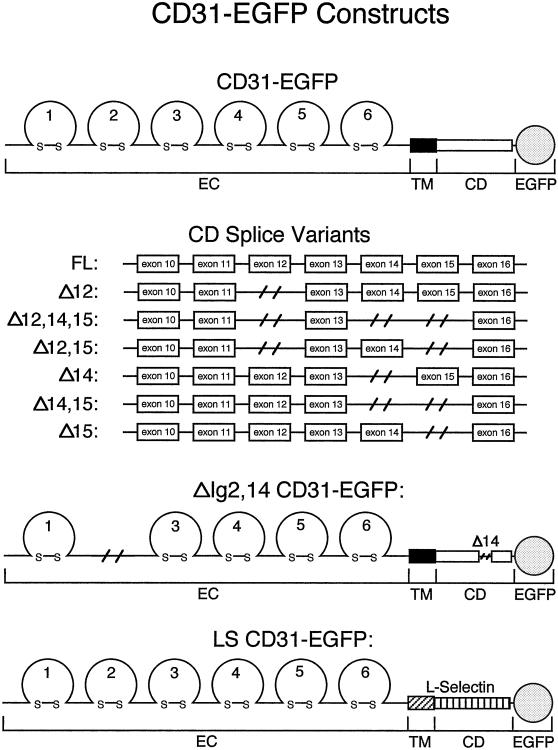
Figure 1.
Construction of CD31-EGFP fusion proteins. A schematic representation of the seven naturally expressed murine PECAM-1 cytoplasmic splice variants and the two artificial murine PECAM-1 constructs fused at the C-termini with the enhanced green fluorescent protein (EGFP). “EC”, “TM”, and “CD” indicate the PECAM-1 extracellular, transmembrane, and cytoplasmic domains respectively. FL, the full length PECAM-1 isoform; Δ, the exon(s), which is(are) spliced out from the PECAM-1 cytoplasmic domains. ΔIg2,14, a PECAM-1 construct that lacks the extracellular Ig-homology 2 region and exon 14. LS, a PECAM-1 chimeric molecule in which the PECAM-1 transmembrane and cytoplasmic domains are replaced by the analogous cell surface glycoprotein L-Selectin domains.
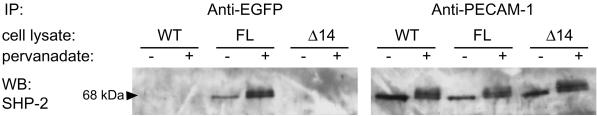
To verify that the binding of CD31-EGFP cytoplasmic tails to the known interactor PTP SHP-2 was not altered by the EGFP tag, CD31-EGFPs and endogenous PECAM-1 were sequentially immunoprecipitated from transfected and untransfected tEnd.1 cells (Figure 2). The 68 kDa PTP SHP-2 was coimmunoprecipitated (1) from the untransfected cells by anti-PECAM-1 but not anti-EGFP, (2) from the FL CD31-EGFP transfected cells by anti-EGFP and anti-PECAM-1, and (3) from the Δ14 CD31-EGFP transfected cells by anti-PECAM-1 but not anti-EGFP. Note the increased binding of SHP-2 to PECAM-1 in the presence of the PTP inhibitor pervanadate. This confirmed the specific interaction of SHP-2 to endogenous FL PECAM-1 and FL CD31-EGFP but not to endogenous Δ14 PECAM-1 and Δ14 CD31-EGFP. Furthermore, it shows that the presence of CD31-EGFPs does not affect the cytoplasmic binding properties of endogenous PECAM-1 to SHP-2. Based on these results, it can be assumed that the binding properties of the FL CD31-EGFP and Δ14 CD31-EGFP cytoplasmic domains are identical to that of endogenous PECAM-1. In addition, fluorescence-activated cell sorting analysis shows that similar levels of FL and Δ14 CD31-EGFP were expressed at the cell surface, ∼ 33% and 25% of the endogenous PECAM-1 level, respectively (our unpublished results).
Figure 2.
Biochemical analysis of FL and Δ14 CD31-EGFPs in transfected endothelioma cells. Untreated (-) and pervanadate treated (+) FL CD31-EGFP and Δ14 CD31-EGFP transfected tEnd.1 cells (denoted by “FL” and “Δ 14” respectively; “WT” indicates wild-type tEnd.1 cells) were lysed in lysis buffer. Sequential immunoprecipitations (IPs) were performed using anti-EGFP followed by anti-PECAM-1. Following SDS-PAGE and western blotting, blots were probed with anti-PTP SHP-2. The 68 kDa PTP SHP-2 coimmunoprecipitated with FL CD31-EGFP (by anti-EGFP in FL cells) and endogenous PECAM-1 (by anti-PECAM-1 IPs in all three cell lines) but not with Δ14 CD31-EGFP (by anti-EGFP in Δ14 cells).
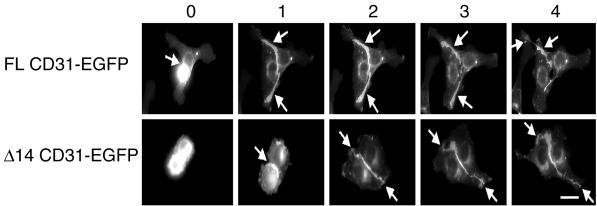
Next, we examined whether SHP-2 binding affected the cell surface localization of PECAM-1 splice variants, and in particular whether specific PECAM-1 isoforms were directed to the endothelial cellular junctions by interacting with SHP-2. The distribution of FL CD31-EGFP and Δ14 CD31-EGFP in the transfected tEnd.1 cells was observed during the formation of cell-cell contacts by using fluorescence time-lapse microscopy. Initially, both CD31-EGFP fusion proteins were distributed uniformly over the cell surface of isolated cells (our unpublished results). Upon formation of cell-cell contacts (within 1 h), the CD31-EGFP fluorescence rapidly concentrated at the intercellular junctions (Figure 3, arrows). PECAM-1 binding at the cell junctions did not prevent the contacting cells from moving apart (FL CD31-EGFP after 3 h and 4 h; our unpublished results) confirming that PECAM-1 is not involved in maintaining cell contacts. These data show that both PECAM-1 splice variants, independent of their ability to interact with SHP-2, preferentially localized to the endothelial cellular junctions. This implies that SHP-2 was not involved in the intercellular concentration of PECAM-1.
Figure 3.
Dynamics of FL and Δ14 CD31-EGFPs during endothelial monolayer formation. FL CD31-EGFP and Δ14 CD31-EGFP stably transfected tEnd.1 cells were plated on fibronectin-coated glass slides, and single images were collected every 2 min for 5–10 h during the cell monolayer formation. Elapsed time is indicated on top of each column in hrs (0, 1, 2, 3, and 4 respectively). The arrows indicate the points of cell-cell contact. Fluorescence of the FL CD31-EGFP and Δ14 CD31-EGFP splice variants are observed preferentially at the intercellular junctions. The 0-h designation is arbitrarily set as the first time point shown. Bar, 15 μm.
Localization of PECAM-1 Splice Variants to the Intercellular Junctions Is Mediated by Their Extracellular Domains
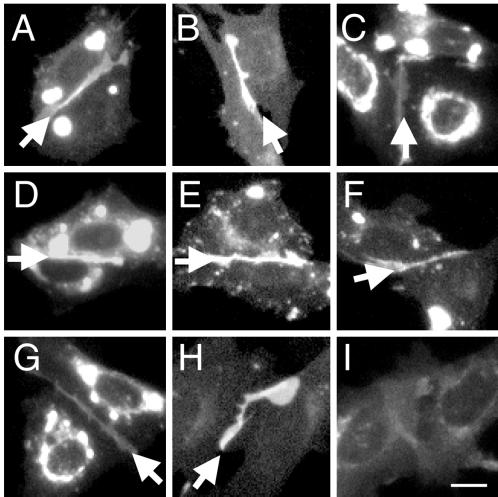
It is suggested that the different cytoplasmic tails, more specifically the presence or absence of exon 14, regulates the extracellular binding property of the PECAM-1 isoforms. To determine whether the PECAM-1 cytoplasmic domains also regulated the cell surface distribution (apical versus junctional) of PECAM-1 splice variants, the seven muPECAM-1 isoforms were transiently expressed in CHO cells. Their cell surface distribution was predominantly localized to the CHO intercellular junctions (Figure 4A-G, arrows), in a manner identical to the FL CD31-EGFP and Δ14 CD31-EGFP distribution in tEnd.1 cells.
Figure 4.
Surface distribution of CD31-EGFPs in CHO cells. The surface distribution of the nine CD31-EGFP constructs (Figure 1) were visualized by fluorescence microscopy on transiently transfected CHO cells. A: FL, B: Δ12, C: Δ12,14,15, D: Δ12,15, E: Δ14, F: Δ14,15, G: Δ15, H: LS and I: ΔIg2,14. All naturally expressed PECAM-1 isoforms and the LS CD31-EGFP preferentially localized to the cell-cell borders (arrows). In contrast, ΔIg2,14 was diffusely expressed over the whole cell surface. Images were not collected and manipulated under identical settings. Bar, 15 μm.
Our data showed that the distribution of PECAM-1 isoforms was not differentially regulated by their cytoplasmic domains. To eliminate the possibility that sequences common in all cytoplasmic isoforms (endcoded by exons 10, 11, and 13) were responsible for their junctional localization, a construct, in which the PECAM-1 cytoplasmic and transmembrane domains were replaced by the analogous cell surface glycoprotein L-selectin domains (LS CD31-EGFP, Figure 1), was transfected into CHO cells (Figure 4.). The cell surface distribution of L-selectin is not regulated by its cytoplasmic domain. As with the seven PECAM-1 splice variants, the LS CD31-EGFP accumulated at the cell-cell borders (Figure 4H). To confirm that the extracellular domain directs PECAM-1 isoforms to the cellular junctions, a construct that lacks the PECAM-1 extracellular Ig-homology domain 2 (ΔIg2,14 CD31-EGFP, Figure 1), a region required for homophilic interaction, was transiently expressed in CHO cells. In contrast to all the other CD31-EGFP constructs, the ΔIg2,14 CD31-EGFP remained diffusely distributed around the cells (Figure 4I). The lack of ΔIg2,14 CD31-EGFP at the intercellular junctions was not due to its inability to reach the cell surface since PECAM-1 was detected on the cell surface by immunofluorescence staining (the anti-PECAM-1 used recognizes the first Ig domain, our unpublished results).
Note that the images in Figure 4 were not collected and manipulated under identical settings, therefore, the fluoresence intensity cannot be compared between the different panels. The different levels of staining at the intercellular junctions correspond to the different levels of cell surface CD31-EGFP expression. In some cases when there was overexpression of the CD31-EGFP proteins, very bright spots were observed inside the cells. Nevertheless, identical results were obtained from low and high CD31-EGFP expressing cells (our unpublished results).
These data show that it is the interaction of the extracellular domain rather then the cytoplasmic domain of PECAM-1 that directs and maintains the protein to the endothelial cellular junctions. Moreover, the cytoplasmic domain is not necessary nor sufficient to localize PECAM-1 to the cell-cell borders.
PECAM-1 Splice Variants Mediate Homotypic Binding at the Intercellular Junctions of Endothelial Cells
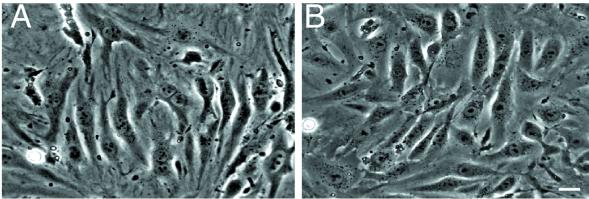
PECAM-1 splice variants containing exon 14 mediate homophilic and heterophilc binding, whereas Δ14 isoforms mediate homophilic binding only. To determine which type of interaction is responsible for the accumulation of PECAM-1 splice variants at the endothelial intercellular junctions, a PECAM-1 deficient brain endothelioma cell line (bEnd.PECAM-1.2neo) was prepared from PECAM-1 knocked-out mice. The morphology of these cells was similar to PECAM-1 expressing brain endothelioma cells (bEnd.5). Phase contrast images showed that both cell types formed a cell monolayer and appeared spindle-like (Figure 5). Besides the slower growth rate of the bEnd.PECAM-1.2neo cells, there were no further observable differences between these two cell lines.
Figure 5.
Morphology of bEnd. PECAM-1.2neo and bEnd.5 cells. Phase constrast images of confluent bEnd. PECAM-1.2neo (A) and bEnd.5 (B) cells. Both cell lines form an endothelial cell monolayer with no observable cellular morphological differences. Bar, 20 μm.
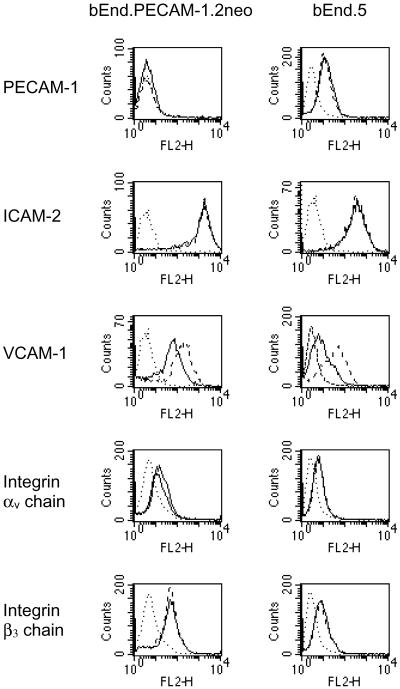
To determine the absence of PECAM-1 on bEnd.PECAM-1.2neo cells and whether this altered the expression of other cell surface proteins, flow cytometry analysis was performed on bEnd.PECAM-1.2neo and bEnd.5 cells under nonstimulated and stimulated conditions (Figure 6, not all protein profiles are shown). The expression of EC protein markers Endoglin and Meca 32, the constitutively expressed proteins ICAM-2, integrin chains αv and β3, and the proteins up-regulated by inflammatory agents VCAM-1, ICAM-1, E-selectin, and P-selectin were examined. As expected, PECAM-1 was not detected on bEnd.PECAM-1.2neo cells. This was confirmed by immunofluorescence assays (Figure 7C). ICAM-2, αv, and β3 were detected on both cell lines in the absence or presence of tumor necrosis factor α (TNF-α). Furthermore, incubation of the two cell lines with TNF-α or lipopolysaccharide (LPS) led to increased expression of VCAM-1, ICAM-1, E-selectin, and P-selectin. Although the expression level of these proteins appear to be different, the significant difference between the bEnd.PECAM-1.2neo and bEnd.5 cells is in their expression of PECAM-1.
Figure 6.
Expression of cell surface molecules on bEnd. PECAM-1.2neo and bEnd.5 cells. Surface expression of PECAM-1, ICAM-2, VCAM-1, integrin chains αv and β3 on nonstimulated (solid lines) and TNF-α stimulated (dashed lines) bEnd. PECAM-1.2neo and bEnd.5 cells were assessed by fluorescence-activated cell sorting. Dotted lines indicate the isotype matched negative control. The significant difference between the two cell lines is in their PECAM-1 expression.
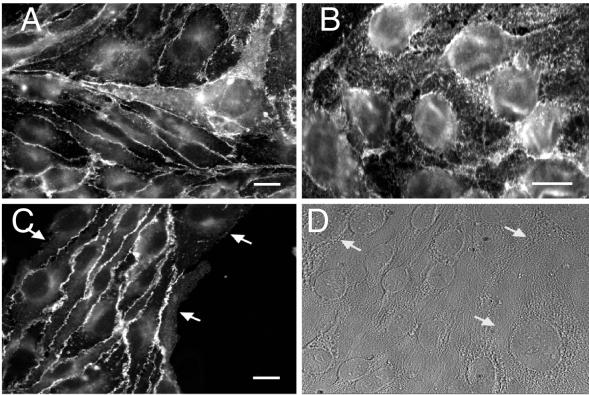
Figure 7.
Localization of cell adhesion molecules on cocultured tEnd.1 and bEnd. PECAM-1.2neo cells. Immunofluorescence staining of the cell adhesion molecules JAM (A), ICAM-2 (B), and PECAM-1 (C) on cocultured tEnd.1 and bEnd. PECAM-1.2neo cells. (D) represents a light image of (C) showing the prescence of bEnd. PECAM-1.2neo cells contacting tEnd.1 cells. The JAM and ICAM-2 controls showed the expected junctional and diffusive expression respectively. PECAM-1 localized to junctions formed by PECAM-1 expressing cells (C, left side) but not to junctions formed by PECAM-1 expressing and deficient cells (C, arrows). Bars, 15 μm.
The bEnd-CD31-KO cells thus provided us with a PECAM-1 deficient EC line to investigate the possible functional differences among the PECAM-1 splice variants. This approach is in contrast to the majority of PECAM-1 binding studies, which have been performed with non-EC lines that do not reflect the normal cellular environment of PECAM-1.
The bEnd.PECAM-1.2neo cells were then cocultured with tEnd.1, bEnd.5, or CD31-EGFP-transfected tEnd.1 cells (all cell lines produced the same results; data shown for tEnd.1 cell line) to compare the distribution of PECAM-1 with that of the cell junctional adhesion molecule (JAM) and the intercellular adhesion molecule-2 (ICAM-2). If PECAM-1 interacts heterophilically, then it should accumulate at the junctions of all contacting cells, whether or not they express PECAM-1. In contrast, if PECAM-1 interacts homophilically, then it should not localize to the cell-cell borders formed by PECAM-1 expressing and PECAM-1 deficient cells. Figure 7C shows that PECAM-1 concentrated only at the borders formed by cells that expressed PECAM-1. As expected in the controls, JAM preferentially localized to regions of all cell-cell contacts (Figure 7A, representative of all the stained cells) whereas ICAM-2 distributed over the whole cell surface (Figure 7B, representative of all the stained cells). The tEnd.1 cells were distinguished from the bEnd.PECAM-1.2neo cells by their smaller size. These data suggest that all PECAM-1 splice variants expressed in tEnd.1 cells mediate homotypic binding at the cell-cell junctions. Furthermore, it suggests that there are no heterophilic ligands for PECAM-1 at the EC junctions.
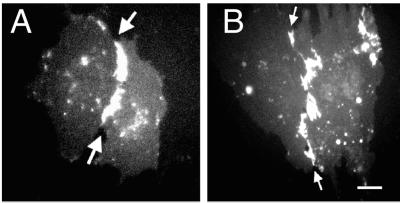
To further support that all PECAM-1 splice variants mediate homotypic binding at the intercellular junctions of ECs, an exon 14 containing (FL CD31-EGFP) and an exon 14 noncontaining (Δ14 CD31-EGFP) PECAM-1 splice variant were transiently expressed in bEnd.PECAM-1.2neo cells. Identical to the coculturing experiments, both PECAM-1 isoforms preferentially localized to the intercellular junctions of PECAM-1-expressing cells only (Figure 8, arrows; transfected cells are surrounded by nontransfected cells). These experiments further supported that the binding property of the PECAM-1 extracellular domain (homophilic binding) is responsible for directing PECAM-1 to the endothelial cell-cell borders.
Figure 8.
Surface distribution of FL and Δ14 CD31-EGFPs on bEnd. PECAM-1.2neo cells. Fluorescence microscopy showing the surface distribution of FL CD31-EGFP (A) and Δ14 CD31-EGFP (B) on transiently transfected bEnd. PECAM-1.2neo cells. Both PECAM-1 splice variants preferentially localized to the endothelial cell junctions of transfected cells (arrows), but not to borders formed by PECAM-1 expressing and deficient cells (confluent cell monolayer). Bar, 15 μm.
Integrin αvβ3 Associates with PECAM-1 in cis
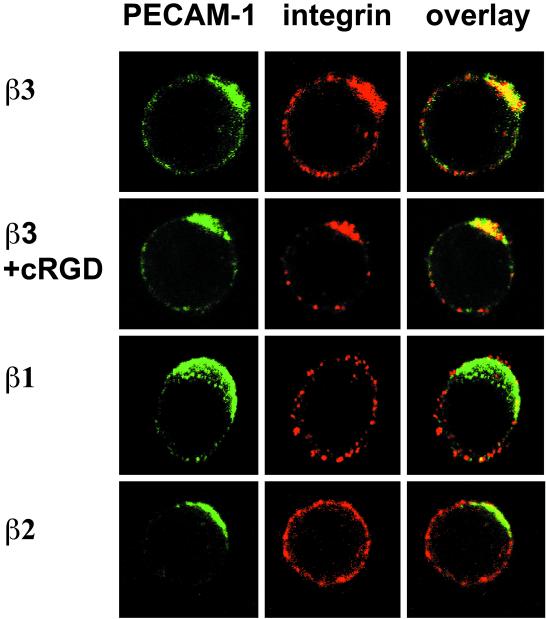
The results presented here show that PECAM-1 splice variants do not bind to integrin αvβ3 at the endothelial cell-cell junctions although these proteins can interact with each other and are expressed on ECs (Figure 6). We therefore investigated whether the two proteins interacted by a different mode. Since integrin αvβ3 interacts in cis with several cell surface proteins, we examined whether it also associated laterally on the same cell with PECAM-1 by performing cocapping experiments. First, specific antibodies were used to cross-link PECAM-1 (clustering of PECAM-1 into caps) on pro T-cells (FTF1.26). Successful formation of PECAM-1 caps were visualized as green fluorescence spots on the cell surface (Figure 9, PECAM-1). Next, other proteins were detected in red fluorescence. In principle, a protein which interacts with PECAM-1 will redistribute with the PECAM-1 cap and this colocalization of green and red fluorescence will result in a yellow fluorescence PECAM-1 cap. As speculated, the yellow cap produced from the cocapping experiments showed that integrin αvβ3 colocalized with PECAM-1 (Figure 9, overlay), suggesting that they associated in cis. PECAM-1 caps were formed on 23 out of 199 cells, and 22 of these (96%) showed colocalization of αvβ3 with PECAM-1. Furthermore, the addition of cyclic RGD peptides (a common binding motif for integrins) did not disrupt the colocalization of PECAM-1 and αvβ3, showing that their interaction did not involve the RGD motif. In this experiment, PECAM-1 caps formed on 8 out of 125 cells and all of these showed colocalization of the two proteins. In control experiments, colocalization of PECAM-1 with the β1 subunit was observed in 2/16 (13%) cells but never with the β2 subunit 0/9 (0%), major histocompatibitiy complex I (MHC I) molecule or the interleukin 2 receptor (IL2R) α chain (our unpublished results). Our result therefore supports the notion that PECAM-1 interacts with intergrin αvβ3 in a cis manner, and that this association is independent of the αvβ3 ligand ocupancy state.
Figure 9.
Distribution of integrin αvβ3 and PECAM-1 on the surface of FTF1.26 cells. PECAM-1 cap formation and its effect on the distribution of integrin β chains on the surface of proT-cells (FTF1.26) were analyzed by confocal fluorescencse microscopy. Cell surface PECAM-1 caps (green) were induced by specific antibody cross-linking followed by detection of integrin chains β1, β2, and β3 (red). Interacting proteins colocalize together and their overlapping fluorochromes results in a yellow cap. “cRGD” indicates the addition of the cyclic RGD peptide, a ligand binding motif for integrins, during the PECAM-1 cross-linking step. Only integrin chain β3, in the absence or presence of cRGD, colocalized with PECAM-1 (yellow caps in overlay). The integrin chains β1 and β2 did not colocalize with PECAM-1 (green caps in overlay).
DISCUSSION
The aim of this study was to determine the effect of alternative splicing on the surface distribution and ligand binding mode of PECAM-1 in ECs. In addition, we examined the heterophilic interaction between integrin αvβ3 and PECAM-1 at endothelial cell-cell junctions and on proT cells. Our major findings are as follows. First, the cytoplasmic domain of PECAM-1 is not involved in directing PECAM-1 splice variants to the intercellular junctions. This was demonstrated by expressing individual EGFP-tagged PECAM-1 splice variants in CHO cells and endothelioma cells. Second, we show that the accumulation of all PECAM-1 isoforms at the endothelial cell-cell junctions is mediated by trans-homophilic binding. This is shown by examining the localization of PECAM-1 in cocultured PECAM-1 expressing and deficient endothelioma cells as well as by the distribution of CD31-EGFP in transfected and nontransfected endothelioma cells. Third, we provide evidence that PECAM-1 associates with integrin αvβ3 in a cis manner based on cocapping experiments using proT cells.
PECAM-1 was identified in a search for novel EC adhesion molecules expressed on human ECs. Immunofluorescence assays showed that it preferentially localized to regions of endothelial cell-cell contacts (Muller et al., 1989; Newman et al., 1990). Later studies demonstrated that multiple PECAM-1 splice variants were present in ECs (Baldwin et al., 1994; Sheibani et al., 1997; Sheibani et al., 1999). The question of whether these isoforms distributed differentially (apical versus junctional), possibly by binding to specific intracellular interactors to mediate different functions, was not addressed. Yan et al. investigated the functional consequences of the alternatively spliced muPECAM-1 cDNAs. L-cells were transfected with cDNA for each isoform, and their ability to promote cell aggregation was compared. In this assay, FL muPECAM-1 and all three isoforms containing exon 14 mediated calcium- and heparin-dependent heterophilic aggregation. In contrast, the three muPECAM-1 variants lacking exon 14 mediated calcium- and heparin-independent homophilic aggregation (Yan et al., 1995). However, it remains unclear whether all PECAM-1 isoforms concentrate at the endothelial cell-cell borders and whether the ones localized at the junctions engage in homophilic or heterophilc binding.
To address the aforementioned questions, we first tagged the seven naturally expressed PECAM-1 splice variants with EGFP (Figure 1). This allowed us to examine the distribution of individual PECAM-1 isoform in living cultured cells, excluding experimental artifacts caused by immunohistochemical procedures. Our data show that all PECAM-1 isoforms and the LS CD31-EGFP concentrated at the intercellular borders of living CHO cells (Figure 4). This demonstrated that the cytoplasmic domain does not direct PECAM-1 to the intercellular junctions and suggested that the extracellular domain might be fulfilling this function.
The trans-homophilic binding of PECAM-1 is mediated by direct interaction of the Ig-homology domains 1 and 2 (Sun et al., 1996a). Furthermore, it requires the proper spacing provided by the six Ig-homology domains (Newton et al., 1997). In support of these results, a naturally spliced PECAM-1 isoform lacking Ig-homology domain 2 (ΔIg2 CD31) looses the ability to interact homophilically, resulting in its diffuse cell surface distribution (Litwin et al., 1997). In agreement with these published data, the ΔIg2,14 CD31-EGFP construct used in our study shows diffuse distribution around the transfected CHO cells. In contrast, all the other CD31-EGFP constructs containing the FL PECAM-1 extracellular domain accumulated at the cellular junctions (Figure 1 and Figure 4). These results clearly support the critical role of the extracellular domain and exclude the participation of the cytoplasmic domain in directing PECAM-1 to the cell-cell borders. This contradicts what DeLisser et al. (DeLisser et al., 1994a) observed when they transfected different PECAM-1 constructs into Cos-7 and 3T3 cells. While PECAM-1 with the full length and partially truncated cytoplasmic domains localized to the cell-cell borders, PECAM-1 lacking the entire cytoplasmic domain did not move to the intercellular junctions. This suggested to them that the cytoplasmic domain is required for PECAM-1 localization to cellular junctions. This discrepency may be due to differences in the experimental set up. In our experiments, we visualized the distribution of natural PECAM-1 splice variants in living CHO cells. In constrast, DeLisser et al. detected truncated PECAM-1 on fixed transfected fibroblasts. In addition, our data were confirmed in endothelial cells that mimick the physiological cellular environment of PECAM-1. The subcellular distribution of two representative PECAM-1 splice variants, the FL CD31-EGFP as the heterophilic interactor, and the Δ14 CD31-EGFP as the homophilic binder were visualized in the presence of multiple endogenous PECAM-1 isoforms in living ECs during the formation of cell-cell contact (Figure 3). Identical to the results obtained in CHO cells, both PECAM-1 splice variants, independent of exon 14, localized to endothelial cell-cell junctions. Taken together, we believe that our results are more likely to reflect the in vivo situation.
The coculture experiments with tEnd.1 (multiple PECAM-1 splice variants are expressed) and bEnd.PECAM-1.2neo cells (endothelioma cells obtained from PECAM-1 deficient mice) show that PECAM-1 accumulated at cell-cell borders of PECAM-1 expressing cells, but not at intercellular junctions between PECAM-1 expressing and deficient cells (Figure 7). This shows that the junctional localization of PECAM-1 isoforms is mediated by trans-homophilic binding. The Figure 7 data was confirmed by transfecting FL CD31-EGFP and Δ14 CD31-EGFP cDNAs into PECAM-1 deficient endothelioma cells. Both proteins localized exclusively to cellular borders of transfected cells, but not to junctions of transfected and nontransfected cells (Figure 8). Therefore, the localization of PECAM-1 splice variants to intercellular junctions is based on a trans-homophilic binding that is not dependent on the cytoplasmic tail. Nevertheless, we cannot rule out that in the absence of PECAM-1 expression, heterophilic ligands for it is also not expressed.
Famiglietti et al. identified a tyrosine residue, encoded by exon 14 (Tyr-686), that appeared to regulate the heterophilic versus homophilic binding mode of the PECAM-1 ectodomain in L-cells. This tyrosine residue, in combination with another tyrosine encoded by exon 13 (Tyr-663), served as a docking site for SH-2 domain containing phosphatases and kinases (Famiglietti et al., 1997). In addition, this study indicated that the PECAM-1 heterophilic binding mode requires a nonphosphorylated Tyr-686, whereas the homophilic binding mode required the absence or phosphorylation of this tyrosine residue. Because the phosphorylation state of Tyr-663 and Tyr-686 regulates PECAM-1 binding to SHP-2, we determined the binding mode of the FL CD31-EGFP construct indirectly by examining its interaction with SHP-2 from the transfected tEnd.1 cells, in the absence or presence of pervanadate, an inhibitor of PTPs (Figure 2). Our data showed that SHP-2 was coimmunoprecipitated with FL CD31-EGFP in both cases, with increased SHP-2/PECAM-1 binding in the presence of pervanadate. This indicated that a fraction of FL CD31-EGFP is phosphorylated under normal culture conditions and that phosphorylation of FL-CD31-EGFP can be increased. Taken together, this would suggest that FL CD31-EGFP is found in the homophilic and the heterophilic binding conformations (Famiglietti et al., 1997). While the homophilic binding mode for FL-CD31-EGFP was observed in our experimental system, the heterophilc binding mode was not detected (Figure 8). One possible explanation is that ECs do not express a PECAM-1 heterophilic ligand or require some undefined activation before their expression. Alternatively, PECAM-1 expressed on ECs may only interact heterophilcally with binders present on other cells possibly during transmigration.
The specific heterophilic interaction between integrin αvβ3 and PECAM-1 has been described by using soluble recombinant forms of murine and human PECAM-1 (Piali et al., 1995; Buckley et al., 1996). However, this interaction is not detected at the cellular level (Sun et al., 1996b). Furthermore, our data clearly demonstrated that, although integrin αvβ3 is present on ECs (Figure 6), PECAM-1 must be expressed on the opposing cells for its junctional localization. This indicates that αvβ3 is not a trans-cellular ligand for PECAM-1. Based on these contradicting results and on the fact that integrin αvβ3 interacts with several cell surface molecules in cis, we investigated, by performing cocapping studies, whether αvβ3 also associated with PECAM-1 laterally on the same cell. Because of technical difficulties, endothelioma cells were not suitable for these experiments. First, they adhered to the ECM by using part of their integrin receptors, thereby rendering these integrins immobile. Second, as shown here, PECAM-1 accumulated at the cell-cell junctions, thereby limiting its lateral movement in the plasma membrane. We therefore used the nonadherent proT cell line FTF1.26, which expresses high levels of integrin αvβ3 and PECAM-1. Cocapping experiments on these cells showed that integrin αvβ3 colocalizes with the PECAM-1 caps. This association was independent of the αvβ3 ligand occupancy, since the addition of cyclic RGD did not disrupt the colocalization.
Our data indicate that PECAM-1 and integrin αvβ3 associate with each other on the same cell surface (cis interaction). However, because FTF1.26 cells express multiple PECAM-1 splice variants (our unpublished observation), our result does not attribute the PECAM-1/integrin αvβ3 interaction to any specific isoform. Furthermore, it is uncertain whether these two proteins associate in cis on other cell types that express them. Nevertheless, we speculate that one functional consequence of FL PECAM-1/integrin αvβ3 cis-binding is the recruitment of SH-2 binding kinases and phosphatases to control the αvβ3 cytoplasmic tail association with the cytoskeleton. This can thereby regulate integrin αvβ3 mediated cell adhesion, spreading, and migration. Our hypothesis is supported by the finding that passage of neutrophils through the basement membrane during transendothelial migration is delayed in PECAM-1 deficient mice (Duncan et al., 1999). According to our cis interaction data, this may be due to the absence of a signal that is normally provided by PECAM-1 to regulate integrin αvβ3 activity. In further support of our hypothesis, the ITIM and ITAM motifs present in the cytoplasmic domain of PECAM-1 have been proposed to have important regulatory functions (Famiglietti et al., 1997; Newman, 1999). Moreover, it has been shown that engagement of PECAM-1 on endothelial cells can prevent apoptosis under serum starvation (Bird et al., 1999).
In summary, our results show that all PECAM-1 splice variants concentrate at the EC junctions, where they exclusively engage in trans-homophilic binding. This interaction is dependent on their extracellular domains and not on their cytoplasmic domains. We also provide evidence that the interaction between PECAM-1 and integrin αvβ3 occurs on the same cell surface in a cis manner. Future studies could focus on how the cis-interaction between PECAM-1 and integrin αvβ3 regulate cell adhesion and migration.
ACKNOWLEDGMENTS
We thank Claude Magnin for expert technical assistance in the generation and expression of the cDNA constructs; Dr. Tak W. Mak for providing the PECAM-1 deficient mice; and Dr. Ian Bird for critically reviewing the manuscript.
Abbreviations used:
- bEnd.PECAM-1.2.neo
PECAM-1 deficient brain endothelioma cell line
- EC
endothelial cell
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- FL
full length
- mu
murine
- PECAM-1/CD31
platelet endothelial cell adhesion molecule-1
- PTP
protein tyrosine phosphatase
- RT
room temperature
- SH-2
src homology 2 domain
Footnotes
This work was supported by the Yamanouchi Research Institute (Ph.D Studentship awarded to C.W.Y.W), the “Schweizerische Krebsliga” grant (No. KFS 412-1-1997), and the Swiss National Science Foundation grant (No. 31-49241.96).
REFERENCES
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, Buck CA. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM- 1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112:1989–1997. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- Bogen SA, Baldwin HS, Watkins SC, Albelda SM, Abbas AK. Association of murine CD31 with transmigrating lymphocytes following antigenic stimulation. Am J Pathol. 1992;141:843–854. [PMC free article] [PubMed] [Google Scholar]
- Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM- 1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Bussolino F, De Rossi M, Sica A, Colotta F, Wang JM, Bocchietto E, Padura IM, Bosia A, DeJana E, Mantovani A. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J Immunol. 1991;147:2122–2129. [PubMed] [Google Scholar]
- DeLisser HM, Chilkotowsky J, Yan HC, Daise ML, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol. 1994a;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994b;15:490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller W A, Senaldi G, Zukowski M M, Mak T W. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1- dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Famiglietti J, Sun J, DeLisser HM, Albelda SM. Tyrosine residue in exon 14 of the cytoplasmic domain of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) regulates ligand binding specificity. J Cell Biol. 1997;138:1425–1435. doi: 10.1083/jcb.138.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Buckley C, Holness CL, Bird IN, Spragg JH, Saunders J, Harris A, Simmons DL. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J Cell Biol. 1995;128:1229–1241. doi: 10.1083/jcb.128.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992;340:1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- Horenstein AL, Stockinger H, Imhof BA, Malavasi F. CD38 binding to human myeloid cells is mediated by mouse and human CD31. Biochem J. 1998;330:1129–1135. doi: 10.1042/bj3301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MA. The alpha v beta 3 integrin “vitronectin receptor.”Int. J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- Hua CT, Gamble JR, Vadas MA, Jackson DE. Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J Biol Chem. 1998;273:28332–28340. doi: 10.1074/jbc.273.43.28332. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Ruiz P, Hesse B, Palacios R, Dunon D. EA-1, a novel adhesion molecule involved in the homing of progenitor T lymphocytes to the thymus. J Cell Biol. 1991;114:1069–1078. doi: 10.1083/jcb.114.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Courtneidge SA, Wagner EF. Oncogenic properties of the middle T antigens of polyomaviruses. Adv Cancer Res. 1994;64:125–157. doi: 10.1016/s0065-230x(08)60837-4. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- Litwin M, Clark K, Noack L, Furze J, Berndt M, Albelda S, Vadas M, Gamble J. Novel cytokine-independent induction of endothelial adhesion molecules regulated by platelet/endothelial cell adhesion molecule (CD31) J Cell Biol. 1997;139:219–228. doi: 10.1083/jcb.139.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malergue F, Galland F, Martin F, Mansuelle P, Aurrand-Lions M, Naquet P. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol Immunol. 1998;35:1111–1119. doi: 10.1016/s0161-5890(98)00102-3. [DOI] [PubMed] [Google Scholar]
- Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AM, Becker JC, Siu CH, Lemmon VP, Cheresh DA, Pancook JD, Zhao X, Reisfeld RA. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin alpha v beta 3. J Cell Biol. 1996;132:475–485. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath D, Slocombe PM, Stephens PE, Warn A, Hutchinson GR, Yamada KM, Docherty AJ, Murphy G. Interaction of metargidin (ADAM-15) with alphavbeta3 and alpha5beta1 integrins on different hemopoietic cells. J Cell Sci. 1999;112:579–587. doi: 10.1242/jcs.112.4.579. [DOI] [PubMed] [Google Scholar]
- Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Albelda SM. Cellular and molecular aspects of PECAM-1. Nouv Rev Fr Hematol. 1992;34:S9–13. [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GCd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stockinger H. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med. 1996;184:41–50. doi: 10.1084/jem.184.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur J Immunol. 1998;28:3086–3099. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Risau W, Engelhardt B, Wekerle H. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990;110:1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. Embo J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996a;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996b;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Risau W. Oncogenes in the study of endothelial cell growth and differentiation. Semin Cancer Biol. 1994;5:137–145. [PubMed] [Google Scholar]
- Xie Y, Muller WA. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:5569–5573. doi: 10.1073/pnas.90.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Mizukami I, Todd RF, 3rd, Petty HR. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57:1682–1689. [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]