Abstract
Objective
To assess the relation between plasma concentrations of clozapine and its 2 main metabolites, desmethyl clozapine and clozapine N-oxide, and clinical change in a sample of inpatients with schizophrenia who were resistant to conventional neuroleptics.
Method
Thirty-seven patients (27 men and 10 women, mean age 34.8 yr) with treatment-resistant schizophrenia were treated with clozapine for 18 weeks; dosage was adjusted according to clinical response, and plasma concentrations of clozapine and of its metabolites were measured weekly by high-performance liquid chromatography. Clinical status was also assessed weekly with the Positive and Negative Syndrome Scale (PANSS). Patients were considered “responsive” if they showed at least a 20% improvement over their baseline PANSS ratings.
Results
The mean endpoint clozapine dosage was 486.5 mg/day. There was a significant correlation between the daily dosage of clozapine and the plasma levels of clozapine and of its metabolites (p < 0.05). There was no correlation between the clozapine plasma level and the percent improvement on the PANSS. Clozapine plasma levels were not significantly different between those who responded to clozapine (n = 19) and those who did not (n = 18) and were not significantly different between patients who smoked (n = 28) and those who did not (n = 9). Receiver operating characteristic (ROC) curve analysis determined the plasma level threshold (above which a better clinical response was obtained) to be 550 ng/mL. Using the total of plasma levels of clozapine and its metabolites did not lead to a better sensitivity and specificity.
Conclusions
Our calculated plasma clozapine threshold was higher than that reported by others, but this may be related to the severity of symptoms of our patient sample. Monitoring plasma rates remains a useful tool, together with clinical evaluation, to establish the clozapine dosage for an optimum benefit–risk ratio.
Medical subject headings: antipsychotic agents; clozapine; dose-response relationship, drug; drug monitoring; schizophrenia
Abstract
Objectif
Évaluer le lien entre les concentrations plasmatiques de clozapine et de ses deux principaux métabolites (desméthyl clozapine et clozapine N-oxyde) et les changements cliniques chez un échantillon de patients hospitalisés atteints de schizophrénie résistant aux neuroleptiques classiques.
Méthode
37 patients (27 hommes et 10 femmes; âge moyen de 34,8 ans) atteints de schizophrénie réfractaire ont été traités à la clozapine pendant 18 semaines. On a rajusté la posologie en fonction de la réaction clinique et mesuré les concentrations plasmatiques de clozapine et de ses métabolites, la desméthyl clozapine et la clozapine N-oxyde, une fois par semaine par la chromatographie liquide à haut rendement. On a aussi évalué l'état clinique des sujets une fois par semaine en fonction de l'échelle des syndromes positifs et négatifs (PANSS). On a supposé que les patients « réagissaient » s'ils montraient une amélioration d'au moins 20 % par rapport à leurs résultats PANSS de référence.
Résultats
La posologie de virage moyenne de la clozapine s'est établie à 486,5 mg/jour. Il y avait un lien significatif entre la posologie quotidienne de clozapine et les concentrations plasmatiques de ses métabolites (p < 0,05). Il n'y avait pas de lien entre la concentration plasmatique de clozapine et le pourcentage d'amélioration selon l'échelle PANSS. Les concentrations plasmatiques de clozapine ne présentaient pas de différences significatives entre les sujets ayant réagi à la clozapine (n = 19) et ceux n'y ayant pas réagi (n = 18) et ne présentaient pas non plus de différence significative entre les patients fumeurs (n = 28) et non fumeurs (n = 9). L'analyse de la courbe des caractéristiques opérantes de l'opérateur (COO) a permis de déterminer que le seuil de concentration plasmatique (au-dessus duquel on obtient une meilleure réponse clinique) s'établit à 550 ng/mL. L'utilisation du total des concentrations plasmatiques de clozapine et de ses métabolites n'a pas produit de meilleurs taux de sensibilité et de spécificité.
Conclusions
Le seuil de concentration plasmatique de clozapine que nous avons calculé était plus élevé que celui dont d'autres chercheurs ont fait état, mais l'écart peut être relié à la gravité des symptômes chez nos patients. Le contrôle des concentrations plasmatiques est un moyen utile, conjugué à l'évaluation clinique, d'établir la posologie de clozapine nécessaire afin d'obtenir un ratio avantage-risque optimal.
Introduction
The effectiveness of neuroleptics to treat acute-phase schizophrenia and prevent relapses is well established, but optimal dosage is essentially determined in an empirical manner and may lead to therapeutic failure or unforeseeable major side effects. With conventional neuroleptics, the titration of the dose to arrive at the threshold for minimum side effects leads to setting the minimum effective dose that is likely to improve symptoms without significant adverse effects. This strategy for optimizing treatment is not used with atypical neuroleptics, which, by definition, are less liable to induce extrapyramidal symptoms. Several studies have been carried out to assess the correlation between neuroleptic dosage, plasma level and clinical response, with the aim of optimizing treatment in patients with schizophrenia.
Clozapine was the first neuroleptic described as atypical. It is metabolized in the liver through the action of cytochrome P-450; 2 main metabolites, desmethyl clozapine (or norclozapine) and clozapine N-oxide, may also show pharmacologic activity, and it has been suggested that factors such as cigarette smoking may affect the metabolism of the molecule.1,2 This may partially explain the wide interindividual variations of clozapine plasma concentrations, which may vary by a factor of 45 for a given dosage.3 These pharmacokinetic characteristics highlight the benefits of monitoring plasma levels to optimize the use of clozapine and avoid serious adverse effects.3
Early studies in the 1970s did not indicate a correlation between clozapine plasma levels and antipsychotic activity.4,5 However, Perry et al6 subsequently defined a plasma concentration threshold of 350 ng/mL for optimum clinical response, and other studies have determined similar threshold levels.1,3,7,8,9 Some suggest that a lower plasma level (i.e., 250 ng/mL) might be sufficient to obtain clinical response with less risk of inducing adverse side effects.10
Few studies have been conducted over long enough periods to enable an accurate and complete assessment of clinical response. Many studies have been conducted over 4 to 12 weeks, but 12 weeks seems to be the minimum period to assess clinical effectiveness of treatment in patients with schizophrenia with chronic evolution.
The patients in this study had chronic resistant schizophrenia and were highly symptomatic when they were prescribed clozapine. The aim of this study was to assess the correlation between plasma levels and the antipsychotic action of clozapine over an 18-week period and to identify a threshold plasma level for clinical improvement. We measured plasma levels of the 2 main metabolites (i.e., desmethyl clozapine and clozapine N-oxide) because we thought inclusion in the threshold calculation might improve the sensitivity or specificity. The influence of smoking on plasma levels and tolerance to clozapine were also evaluated.
Methods
This study formed part of an open study to assess the clinical action of clozapine in patients with treatment-resistant schizophrenia.11 The patients, inpatients in the Adult Psychiatry Department at Hospital Sainte-Marguerite, Marseilles, France, were over 18 years of age, displayed schizophrenia according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (as assessed by P.M.L. and J.F.) and were considered to be resistant to neuroleptics. Resistance criteria were as defined by Kane et al;12 that is, they had received a neuroleptic treatment with 2 chemically dissimilar classes, with a chlorpromazine equivalent of 1000 mg/day, over at least 3 separate 6-week periods in the previous 5 years, with no significant clinical improvement. All patients gave written consent to take part in the study.
Patients were excluded if there was a diagnosis other than schizophrenia in axis I of the DSM-IV (schizoaffective conditions in particular), a history of toxic substance abuse, severe somatic pathology (neurologic conditions in particular) or ongoing treatments with normothymic medication, antidepressants or electroconvulsive therapy.
Study design
Before the study, patients had been taking conventional neuroleptics and, in some cases, hypnotics or tranquilizers. Transition from one treatment to clozapine was done by progressively decreasing the neuroleptic treatment during the first week, at the end of which, the patients were administered clozapine only.
In this 18-week open study, dosage of clozapine was adjusted according to the clinical response of the patient. It was prescribed in incremental steps, increasing by 50 mg every 2 days (with a few variations depending on clinical conditions) and up to the dosage of 400 mg/day on the 16th day. Daily dosage was then adjusted according to the clinical response of the patient. Clozapine was given once a day, at 8 pm, or twice a day, at 6 pm and 8 pm (depending on tolerance) to avoid using hypnotics. The only additional medication prescribed was lorazepam as required (up to 10 mg/d) for anxiety or insomnia.
A hematologic analysis was performed once a week.
Clinical assessment
An expert psychiatrist (C.L.) assessed the patients once a week using the Positive and Negative Syndrome Scale (PANSS)13 in its validated French version.14,15 A baseline score was established before the patients began taking clozapine. For analysis, we used the scores obtained at weeks 4, 8, 12 and 18. For patients who did not complete the study, we used results of the last PANSS evaluation (i.e., endpoint).
Extrapyramidal symptoms were evaluated weekly using the Extrapyramidal Symptoms Rating Scale (ESRS).16
“Clinical response” was defined as a minimum 20% improvement of the PANSS global score at endpoint. The patient sample could therefore be divided into 2 groups — responders and nonresponders — to assess the relation between clinical response and plasma levels.
Plasma measurement
Blood samples were taken at 8 am, 12 hours after the last intake of clozapine. Sampling periodicity was established in parallel with the compulsory hematologic follow-up — weekly for the first 18 weeks and then monthly. Plasma levels at weeks 4, 8, 12 and 18 were used in the statistical analyses. For patients who did not complete the study, results of the last test that had been done were used. As soon as blood samples were collected, they were centrifuged at 2000 g for 15 minutes at 4°C. Plasma was then separated and stored at –20°C until it was analyzed.
Clozapine, clozapine N-oxide and desmethyl clozapine concentrations were assessed using a high-performance liquid chromatography (HPLC) method developed by Disdier et al.17
Precision and accuracy were established through multiple analyses (n = 6) of blank serum samples spiked with increasing concentrations of standard solutions of each of the compounds. For the intraday analysis, 10, 50, 100, 500 and 1000 ng/mL concentrations of each of the products were tested. For the interday analysis, 100, 500 and 1000 ng/mL concentrations of each of the compounds were tested. Precision (given by the coefficient of variation) and accuracy (given by the bias) were satisfactory in all instances. For reproducibility, coefficients of variation varied from 1.1% to 6.2% for clozapine, 2.9%–7.3% for desmethyl clozapine and 4.6%–8.0% for clozapine N-oxide, and the bias remained below 5%. For clozapine and its metabolites, the lower limit of detection was 10 ng/mL.
Statistical analysis
Patients who responded to clozapine and those who did not were compared on demographic and clinical characteristics and plasma concentrations (of clozapine, desmethyl clozapine, clozapine N-oxide and their totals) using the Student's t-test. The PANSS baseline, final scores and subscores of the responders and nonresponders were compared using the Mann-Whitney U test. Two-tailed t-tests were performed to compare the ESRS symptom ratings at baseline and at week 18 of clozapine treatment.
A Spearman rank test was used to assess the correlation between daily clozapine dosage and plasma levels, and Pearson correlation coefficients were used to assess the correlation between clinical improvement (i.e., on PANSS) and plasma level.
Categorical analysis was carried out by means of receiver operating characteristic (ROC) curves to determine the dosage threshold for optimum sensitivity and specificity6,9 Once this threshold was set, the proportions of responders and nonresponders on either side of the threshold value were compared using a χ<2 test.
Results
Twenty-seven men and 10 women inpatients with schizophrenia were recruited (Table 1). These patients met the inclusion criteria and had been admitted consecutively in 1 of the 3 units of the Adult Psychiatry Department at the Hôpital Sainte-Marguerite. According to DSM-IV criteria, diagnoses were 295.10 (n = 6), 295.30 (n = 17) and 295.90 (n = 14).
Table 1

Eleven patients (30%) did not complete the full 18 weeks of the treatment. One patient withdrew consent, 7 dropped out because they found clozapine ineffective and 3 because of side effects. One of the patients dropped out at 6 weeks, 3 at 8 weeks, 1 at 10 weeks, 4 at 12 weeks and 2 at 14 weeks. There were no significant differences between the patients who completed the study and those who did not on sociodemographic characteristics or PANSS baseline scores.
Clozapine dosage and plasma levels
The mean dosage of clozapine at endpoint was 486.5 mg/day (standard deviation [SD] 137.3, range 200–800 mg/day) for mean plasma levels of 543.8 ng/mL (SD 228.9, range 155–1240 ng/mL) for clozapine, 333.2 ng/mL (SD 148.3, range 128–637 ng/mL) for desmethyl clozapine and 326.8 ng/mL SD 129.8, range 80–548 ng/mL) for clozapine N-oxide.
There was a significant correlation between daily dosage of clozapine and clozapine plasma level (Spearman correlation coefficient 0.37, p = 0.026); significant correlations were also found for both metabolites (p < 0.05).
Clinical response and plasma levels
There was a significant overall improvement (from baseline to endpoint) on global PANSS scores and on positive, negative and general psychopathology subscores (p < 0.001 for each).
There were no significant correlations between the clozapine plasma level at endpoint and improvement on any of the PANSS scores. Similarly, there was no significant correlation between the sum of the plasma levels for clozapine and its 2 metabolites and improvement on the PANSS global score.
We also assessed correlations between clozapine plasma level at endpoint and the percent improvement on 5 PANSS factors we defined in a previous study (i.e., positive, negative, hostility, cognitive, depressive),18 but there were no significant correlations detected on these either.
Smoking
Twenty-eight (75.7%) of the 37 patients smoked. The mean clozapine plasma level for smokers was 526 (SD 218.0) ng/mL and for nonsmokers was 599.4 (SD 271.9) ng/mL. There was no significant difference between the groups on clozapine plasma level, the totalled clozapine and metabolite levels at endpoint, or clozapine plasma adjusted for daily dosage and weight at endpoint.
Responders, nonresponders and plasma levels
By the end of the study, 19 (51.4%) patients had shown a decrease of 20% or more on the total PANSS score. In this “responders” group, 1 patient dropped out at 6 weeks, 1 at 10 weeks, 1 at 12 weeks and 1 at 14 weeks. In the nonresponders group, 3 dropped out at 8 weeks, 3 at 12 weeks and 1 at 14 weeks.
Responsive and nonresponsive patients were not significantly different in terms of age, sex, baseline weight, baseline clozapine dose or clozapine dosage at the end of 18 weeks (or at the end of the observation period) (Table 2). The intensity of their baseline symptoms, as measured by the PANSS total score and positive, negative and general psychopathology subscores, did not differ between groups, and there were no differences on plasma levels at 18 weeks (or at the end of the observation period).
Table 2
An ROC test with 50 ng/mL increments of clozapine is shown in Fig. 1. For clozapine, a plasma threshold of 550 ng/mL was associated with sufficient sensitivity (63.9%) and relatively good specificity (51.4%). Results of a χ2-test with Pearson correction to compare responsive and nonresponsive patients using the 550 ng/mL threshold were not significant.
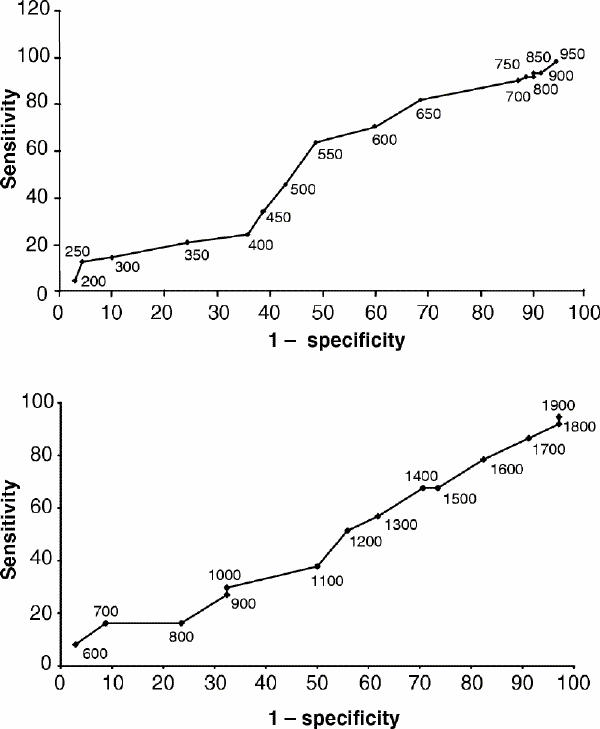
Fig. 1: Receiver operating characteristic (ROC) curves for the relation between responder status and plasma level of clozapine (upper panel) and the sum of clozapine, desmethyl clozapine and clozapine N-oxide (lower panel).
We also performed an ROC with 100 ng/mL increments for the sum of the clozapine plasma level and its 2 metabolites (Fig. 1). For a 1200 ng/mL plasma threshold, sensitivity is 51.4% and specificity is 44.1%.
Tolerance
Only 3 patients dropped out the study because of adverse effects — 2 because of significant sedation and 1 because of hypersalivation. We did not observe any cases of agranulocytosis.
Subjects showed significant decreases on extrapyramidal symptoms as measured with scores on the ESRS (p < 0.001 for dyskinesia, acuteness of parkinsonism, dystonia and parkinsonism level). Decreases were observed in as many responsive patients as they were in the nonresponsive patients.
Discussion
Tolerance to clozapine and its potential toxic effects highlight the importance of studying ways to optimize its use, and the study of plasma levels is one of the means used to determine optimum dosage.3
The aim of our study was to assess the strength of the correlation between clozapine plasma level and clinical response in patients with chronic resistant schizophrenia. In clinical practice, the notion of plasma threshold is particularly useful for adjusting the therapy for inpatients whose response is often partial.
This study was conducted over an 18-week period, and was thus longer than many of the previously published studies (Table 3). The change in patients with chronic development often occurs relatively slowly, especially when negative symptoms are present. Some authors have highlighted a continuing clinical improvement in patients treated with clozapine after several months.20
Table 3

For clinical evaluation, we used the French validated version of the PANSS,14 whose psychometric properties are superior to the Brief Psychiatric Rating Scale (BPRS) used in most correlation studies.13
Plasma levels were assessed with a validated HPLC procedure,17 and we measured the 2 main clozapine metabolites to determine their importance in plasma-level monitoring. Totals of clozapine, desmethyl clozapine and clozapine N-oxide enabled us to understand, more specifically, this molecule's pharmacologic activity.
The patients in this study had chronic schizophrenia which evolved over a long period, and they remained symptomatic despite the antipsychotic therapies administered to them. This is representative of patients in France who are prescribed clozapine. It is noteworthy that the baseline symptoms of our sample were more severe (mean score on BPRS 61.97) than in most similar studies.6,9,21 As was highlighted by Olesen et al,22 more intense symptoms are often associated with higher daily doses of clozapine. Our mean clozapine dosage of 486.5 mg/day is in the higher range of the dosages reported in the published research.
The response rate in our sample was relatively high (51.4%) compared with other studies.9 However, the definition of a 20% improvement in clinical response, without an absolute total score to be reached, in a sample that was initially very symptomatic yielded a group of responsive patients who still had high PANSS scores in the 18th week (i.e., mean PANSS score of responsive patients was 73.1 [SD 12.7]). Despite the persistence of symptoms, the improvement has positive consequences in terms of autonomy and potential hospital discharge. The pretreatment patient status and our definition of “responsive” may explain some of the discrepancies between our findings and those of other studies.9
We found a significant correlation between clozapine dosage and the clozapine plasma level. The mean clozapine plasma level in our study (543.8 ng/mL) is higher than that reported in other studies. Our ratio between mean plasma concentration and mean dose (expressed as [ng/mL]/[mg/day]) is 1.12, close to the 1.25 [ng/mL]/[mg/day] reported by Spina et al.9 The variability in this ratio (from 0.5510 to 1.2822 in published research) may be related to metabolic differences associated with genetic, developmental and environmental factors.
By defining clinical response as an improvement of at least 20% of the baseline BPRS score, several studies have identified a threshold clozapine plasma concentration for therapeutic response, making it possible to predict such a response in a specific and sensitive manner. Perry et al6 reported a threshold clozapine plasma concentration for therapeutic response of 350 ng/mL after 4 weeks of constant-dosage treatment. Other studies have reported thresholds of 420 ng/mL,1 400 ng/mL,7 350 ng/mL,8 370 ng/mL3 and 350 ng/mL.9
Our clozapine plasma threshold was higher, at 550 ng/mL. If we take into account the concentration/dose ratio, the dose used to arrive at such a threshold is 491.1 mg/day.
The higher threshold may be due to several factors:
· The dosing schedules and time between medication intake and sampling may have introduced an important variation in the observed plasma levels — even with the same daily doses.10 Patients were given clozapine in 1 or 2 administrations, either at 8 pm or at 6 pm and 8 pm, and blood was taken at 8 am the next day. This interval between the last clozapine intake and sampling may have favoured higher plasma levels at the time of blood sampling.
· Our mean clozapine dosage was high because of the severity of the symptoms at baseline. The plasma level/dosage ratios suggest nonlinear kinetics may be at play at higher doses.10 This would have to be confirmed in future studies, however.
· Dosage procedures used in our study may differ from those used in other studies.
· The definition of a clinical response (i.e., “responder” may differ across studies).
We did not find any correlation between clozapine plasma level and changes in the PANSS positive, negative or general psychopathology subscores or to the response on various PANSS factors, which were previously validated.18 This contrasts with the results of Spina et al,9 who reported a correlation between clozapine plasma levels and BPRS reduction. However, the use of BPRS subfactors to evaluate positive and negative symptoms remains imprecise, the clinical parameters being less specific than those explored with the PANSS.
Clozapine N-oxide likely has limited pharmacologic activity,23 and desmethyl clozapine seems to be a relatively weak metabolite. The measurement of clozapine plasma levels as well as its 2 metabolites and the use of their total did not help improve the predictive value of the clozapine plasma level on its own for clinical improvement.
Threshold determination enables one to define a plasma level at which most patients improve,9,10 but some patients may respond at levels much lower than the pre-established threshold. In our sample, the plasma level of 2 responsive patients was below 250 ng/mL, and 8 patients responded at levels below the threshold defined by us. However, obtaining a plasma level above threshold must constitute only one of the elements in the benefit–risk calculation, which is essential when deciding on a clozapine treatment that may involve important adverse effects.
Clozapine significantly improved extrapyramidal symptoms, one of the major advantages of the atypical antipsychotics. Despite of the high doses used, only 3 patients in our study suffered adverse effects that led them to discontinue treatment. We did not observe any epileptic seizures or evidence of confusion. Our patients were not taking any other medications during the study (except for lorazepam). In other similar studies,21,22 patients were also taking additional treatments (e.g., neuroleptics and benzodiazepines), a practice which likely increases the risk of adverse effects and confusion.24
Conclusion
Eighteen weeks is sufficiently long to observe clinical changes in a population of patients with highly symptomatic chronic schizophrenia. Over half of the patients in this study showed a significant clinical response. A plasma threshold of 550 ng/mL was associated with a clinical response with satisfactory sensitivity and specificity. Although this threshold is higher than that reported in other studies to date, tolerance to the treatment remained good, with few observed adverse effects and significant improvement in extrapyramidal symptoms.
In a patient population showing significant residual symptoms that may adversely affect the possibility of autonomy, it is essential to ensure that patients be given every opportunity to improve with clozapine treatment.
Despite of the limitations of the methods used (i.e., open study, flexible clozapine dosages), our study confirms the importance of clozapine plasma level monitoring to optimize the effectiveness of therapy and improve the benefit–risk calculation when refining a treatment plan.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Pierre-Michel Llorca, Centre Médico-Psychologique B, Centre Hospitalier Universitaire, BP 69, 63003 Clermont-Ferrand Cedex 1, France; fax 33 47 375-2126; pmllorca@chu-clermontferrand.fr
Submitted Jan. 29, 2001 Revised May 15, 2001 Accepted May 30, 2001
References
- 1.Hasegawa M, Gutierrez-Esteinou R, Way L, Meltzer HY. Relationship between clinical efficacy and clozapine concentrations in plasma in schizophrenia: effect of smoking. J Clin Psychopharmacol 1993;13:383-90. [PubMed]
- 2.Lane H, Chang Y, Chang W, Lin S, Tseng Y, Jann M. Effects of gender and age on plasma levels of clozapine and its metabolites analyzed by critical statistics. J Clin Psychiatry 1999;60:36-40. [DOI] [PubMed]
- 3.Freeman DJ, Oyewumi LK. Will routine therapeutic drug monitoring have a place in clozapine therapy? Clin Pharmacokinet 1997;32:93-100. [DOI] [PubMed]
- 4.Ackenheil VM, Brau H, Burkhart A, Franke A, Pacha W. Antipsychotic efficacy in relation to plasma levels of clozapine. Arneimittelforschung 1976;26:1156-8. [PubMed]
- 5.Thorup M, Fog R. Clozapine treatment of schizophrenic patients: plasma concentration and coagulation factor. Acta Psychiatr Scand 1977;66:123-6. [DOI] [PubMed]
- 6.Perry P, Miller D, Arndt S, Cadoret R. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am J Psychiatry 1991;148:231-5. [DOI] [PubMed]
- 7.Potkin SG, Bera R, Gulasekaran B, Costa J, Hayes S, Jin Y, et al. Plasma clozapine concentrations predict clinical response in treatment-resistant schizophrenia. J Clin Psychiatry 1994;55:133-6. [PubMed]
- 8.Kronig M, Munne R, Szymanski S, Safferman A, Pollack S, Cooper T, et al. Plasma clozapine levels and clinical response for treatment-refractory schizophrenic patients. Am J Psychiatry 1995;152:179-82. [DOI] [PubMed]
- 9.Spina E, Avenoso A, Facciola G, Scordo M, Ancione M, Madia A, et al. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventionnal neuroleptics. Psychopharmacology 2000;148:83-9. [DOI] [PubMed]
- 10.Vander Zwaag C, McGee M, McEvoy J, Freudenreich O, Wilson W, Cooper T. Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry 1996;153:1579-84. [DOI] [PubMed]
- 11.Llorca P-M, Lançon C, Farisse J, Scotto J-C. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry 2000;24:274-83. [DOI] [PubMed]
- 12.Kane J, Honigfeld G, Singer J, Meltzer HY. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45:789-96. [DOI] [PubMed]
- 13.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261-76. [DOI] [PubMed]
- 14.Lançon C, Reine G, Llorca P-M, Auquier P. Validity and reliability of the french langage version of the positive and negative syndrome scale (PANSS). Acta Psychiatr Scand 1999;100 (3): 237-43. [DOI] [PubMed]
- 15.Lepine JP. Positive and Negative Syndrome Scale. In: Guelfi JD, editor. L'évaluation clinique et standardisée en psychiatrie. Vol. 2. Castres: Editions médicales Pierre Fabre; 1996. p. 585-90.
- 16.Chouinard G, Ross-Chouinard A, Annable L, Jones BD. The extrapyramidal symptom rating scale. Can J Neurol Sci 1980;7:233.
- 17.Disdier B, Llorca P-M, Lançon C, Farisse J, Bun H, Cornet M-C. Concentrations plasmatiques de la clozapine et ses métabolites chez les patients schizophrènes résistants. J Pharm Clin 1999; 18 (3): 244-250
- 18.Lançon C, Aghababian C, Llorca P-M, Auquier P. Factorial structure of the Positive and Negative Symptom Scale (PANSS): A forced five-dimensional factor analysis. Acta Psychiatr Scand 1998;98:369-76. [DOI] [PubMed]
- 19.Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry 1992;49(5):345-53. [DOI] [PubMed]
- 20.Meltzer HY, Burnett S, Bastani B, Ramirez LF. Effect of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Commun Psychiatry 1990;41:892-7. [DOI] [PubMed]
- 21.Centorrino F, Baldessarini RJ, Kando JC, Frankenburg FR, Volpicelli SA, Flood JG. Clozapine and metabolites: concentrations in serum and clinical findings during treatment of chronically psychotic patients. J Clin Psychopharmacol 1994;14:119-25. [PubMed]
- 22.Olesen OV, Thomsen K, Jensen PN, Wulff CH, Rasmussen NA, Refshammer C, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross-sectionnal study. Psychopharmacology 1995;117:371-8. [DOI] [PubMed]
- 23.Ackenheil M. Clozapine:pharmacokinetic investigations and biochemical effects in man. Psychopharmacology 1989;99:32-7. [DOI] [PubMed]
- 24.Faisal I, Lindenmayer JP, Taintor Z, Cancro R. Clozapine–benzodiazepine interactions. J Clin Psychiatry 1997;58:547-8. [DOI] [PubMed]



