Abstract
Objective
To determine if there are significant differences in the temporal organization of rapid eye movement (REM) sleep microarchitecture between healthy controls and outpatients with major depressive disorder (MDD).
Methods
Forty age-matched subjects, 20 men and 20 women, half with MDD, were selected from an archive of sleep electroencephalography (EEG) data collected under identical conditions. Each participant spent 2 consecutive nights in the Sleep Study Unit of the University of Texas Southwestern Medical Center at Dallas, the first of which served as adaptation. The average amplitude in each of 5 conventional EEG frequency bands was computed for each REM period across the second night. Data were then coded for group and sex.
Results
Aside from REM latency, none of the key sleep macroarchitectural variables differentiated MDD patients from controls. REM latency was longest in men with MDD. Sleep microarchitecture, however, did show a number of between-group differences. In general, slower frequencies declined across REM periods, with a significant REM period effect for delta, theta and alpha amplitude. Group х sex interactions were also obtained for theta and alpha. Beta activity showed a unique temporal profile in each group, supported by a significant REM period !#!khcy; group х sex interaction. In addition, the temporal change in theta amplitude across REM periods was most striking in women with MDD.
Conclusions
This study suggests that, like during non-REM sleep, EEG amplitude shows a systematic temporal change over successive REM sleep periods and also shows elements that are both disease- and sex-dependent.
Medical subject headings: alpha rhythm; beta rhythm; delta rhythm; depressive disorder; electroencephalography; sex factors; sleep stages; sleep, REM; theta rhythm
Abstract
Objectif
Déterminer s'il y a des différences significatives dans l'organisation temporelle de la micro-architecture du sommeil paradoxal entre des témoins en bonne santé et des patients en service externe atteints de trouble dépressif majeur (TDM).
Méthodes
Quarante sujets jumelés selon l'âge, soit 20 hommes et 20 femmes, dont la moitié étaient atteints de TDM, ont été choisis dans une archive de données électroencéphalographiques (EEG) sur le sommeil recueillies dans des conditions identiques. Chaque participant a passé deux nuits consécutives à l'Unité d'étude du sommeil du Centre médical de l'Université du sud-ouest du Texas à Dallas, dont la première a servi de période d'adaptation. On a calculé l'amplitude moyenne de chacune des cinq bandes classiques de fréquence EEG pour chaque période de sommeil paradoxal au cours de la deuxième nuit. On a ensuite codé les données selon le groupe et le sexe.
Résultats
Outre la latence du sommeil paradoxal, aucune des variables macro-architecturales clés du sommeil n'a permis de distinguer les patients atteints de TDM des sujets témoins. La latence du sommeil paradoxal a été la plus longue chez les hommes atteints de TDM. La micro-architecture du sommeil a toutefois révélé des différences entre les groupes. En général, les fréquences plus lentes diminuaient au cours des périodes de sommeil paradoxal et l'amplitude des ondes delta, theta et alpha avait un effet important sur la période du sommeil paradoxal. On a aussi tiré des ondes theta et alpha des interactions groupe-sexe. L'activité beta a révélé un profil temporel singulier dans chaque groupe, appuyé par un lien groupe-sexe important pendant la période de sommeil paradoxal. En outre, le changement temporel de l'amplitude des ondes theta pendant les périodes de sommeil paradoxal a été le plus frappant chez les femmes atteintes de TDM.
Conclusions
Cette étude indique que, comme pendant le sommeil non paradoxal, l'amplitude EEG montre un changement temporel systématique pendant des périodes successives de sommeil paradoxal et montre aussi des éléments qui sont liés à la fois à la maladie et au sexe.
Introduction
There is ample evidence that the timing or distribution of rapid eye movement (REM) sleep and non-REM sleep stages are disrupted in those with major depressive disorders (MDD).1,2,3 Moreover, the microarchitecture of sleep based on quantitative electroencephalographic (EEG) analysis shows a number of abnormalities in patients with MDD. Elevated fast-frequency, decreased slow-wave activity and dysregulation of ultradian sleep EEG rhythms have all been reported as characteristic of those with MDD.4,5,6,7,8,9,10 Recent work also indicates that it is primarily men with MDD who show an aberrant time course of slow-wave activity in non-REM sleep, whereas women with MDD are more likely to show ultradian rhythm abnormalities.5
Most quantitative sleep EEG studies in MDD, however, have focused on non-REM sleep. To date, it is not clear whether these sex differences are also evident in REM sleep. Since women are at twice the lifetime risk of men for developing depression and since that risk occurs mainly during the reproductive years, it is reasonable to assume that there are biological factors that contribute to risk of depression.11 Sleep EEG studies provide some of the strongest support for the biological basis of depression and certainly present an ideal opportunity to investigate why women are at greater risk. Unfortunately, only a handful of sleep studies have assessed potential sex differences in depression or in healthy controls.12
The purpose of this preliminary report was to investigate potential sex differences in the temporal organization of REM sleep in depressed individuals and in healthy controls. It was expected that sex differences in the group with MDD would be larger than those observed in healthy adults.
Methods
The subjects were divided into groups on the basis of clinical diagnosis and sex. All participants were right-handed. The normal control (NC) group consisted of 10 men, 25–40 years of age (mean 30.8 [standard deviation (SD) 6.0] yr) and 10 women, 22–40 years of age (mean 30.4 [SD 5.0] yr). The MDD group consisted of 10 men, 22–40 years of age (mean 30.1 [SD 5.3] yr) and 10 women, 22–39 years of age (mean 30.6 [SD 5.4] yr). Outpatients who met criteria for nonpsychotic major depression (single or recurrent) but were otherwise physically healthy comprised the MDD group. Diagnoses were based on the Structured Clinical Interview for DSM-III-R (SCID)13 or DSM-IV.14 Patients were mild-to-moderately ill, with an average score of 21.4 (SD 3.9) on the 17-item Hamilton rating scale for depression (minimum 17). They were also medication free for a minimum of 2 weeks before the study (4 weeks for monoamine oxidase inhibitors, 6 weeks for fluoxetine). Other current Axis I disorders, current general medical conditions or substance abuse 12 months before baseline excluded subjects. All control subjects were medically fit and had no personal family history of Axis I disorders or substance abuse based on the SCID-NP (non-patient version). Independent sleep disorders such as narcolepsy, sleep apnea and bruxism were ruled out by medical history or polysomnogram. Shift work was also exclusionary.
Procedure
Each participant spent 2 consecutive nights in the University of Texas Southwestern Medical Center Sleep Study Unit, the first of which served as laboratory adaptation. All participants maintained regular bed- and rise-times for 5 days before the study, and this was verified by home diary. This habitual sleep schedule was also followed in the laboratory. Any subject with more than a half-hour of deviation in sleep schedule during the 5 days was excluded from the study. Subjects entered the laboratory for electrode application approximately 1.5 hours before the scheduled bedtime.
The electrode montage included left (C3) and right (C4) central EEG, left and right electro-oculograms (EOG) recorded from the upper and lower canthi, and a bipolar, chin–cheek electromyogram (EMG). The first night also served as an additional screening for independent sleep disorders (e.g., apnea, bruxism and periodic limb movements) and included leg leads, chest and abdomen respiration bands and a nasal–oral thermistor in the electrode montage. EEG electrodes were referenced to the earlobes connected to a 10-kΩ resistor to minimize nonhomogeneous current flow and potential artifactual hemispheric asymmetries,15 as is standard in our laboratory. EEG was transduced by GRASS P511 A/C amplifiers set at a sensitivity of 5 (50 μV, 0.5-s calibration), corresponding to a gain of 50 000. The half-amp low- and high-bandpass filters were set at 0.3 Hz and 30 Hz, respectively. A 60-Hz notch filter attenuated electrical noise. Signals were digitized online at 250 Hz (62.5 Hz for EOG and EMG) through a 16-bit MICROSTAR analogue-to-digital polygraph system, which was designed and validated in-house.
Signal processing
Period amplitude analysis (PAA) was used to quantify EEG activity and has been described in detail elsewhere.3,15,16,17 Briefly, PAA evaluates wave incidence and amplitude 250 times/s and accumulates over 30 s in each of delta (0.5 Hz to below 4 Hz), theta (4 Hz to below 8 Hz), alpha (8 Hz to below 12 Hz), sigma (12 Hz to below 16 Hz) and beta (16 Hz to below 32 Hz) frequency bands. For the purpose of this report, EEG amplitude (in μV2) in each frequency band was averaged across C3 and C4 electrodes.
Sleep records were scored from C3 electrodes in 30-s epochs, according to standard criteria,18 by research personnel trained at better than 90% agreement on an epoch-by-epoch basis. Scorers were blind to the clinical status and sex of study participants. Note that both computer and human evaluation of sleep EEG were based on identical 30-s epochs. All records were inspected visually, and epochs containing movement, breathing or muscle artifact or recording difficulties were excluded from the analysis. An average of 7.8 (SD 2.1) epochs were excluded, resulting in the loss of less than 5 minutes of EEG data over the 7- to 8-hour recording period, in any individual record.
Data analysis
The REM periods were defined by 3 rules. First, a minimum 3-minute duration was required to begin a REM period. At least 10 consecutive epochs of non-REM or awake were required to terminate a single REM period. This criterion was chosen to be comparable with non-REM period definitions in previous work. Second, subsequent REM periods had to be at least 60 epochs (i.e., 30 min) after the preceding REM period, in accordance with the rules of Rechtschaffen and Kales.18 Finally, the last REM period had to end either in accordance with the rules above or terminated by the morning awakening. After each REM period was defined, amplitude and incidence measures were summed and averaged for each subject. Note that because intervening wake, movement and non-REM epochs do occur within each REM period, all amplitude measures were based only on the REM epochs (i.e., net REM) within each REM period. Thus, the definition dictated that a minimum of 75 000 samples (250 Hz х 30 s х 5 min) would be available in which amplitude could be evaluated in each REM period. For statistical purposes, only the first 3 REM periods were included for analysis because only some of the participants had 4 or more complete REM periods for the night.
Data were then coded for group (MDD or NC) and sex. Repeated-measures analyses of variance (ANOVAS) were computed, treating REM period as a 3-level repeated measure and group and sex as between-group variables. Between-group interactions were tested first, followed by between-group main effects if no interaction was evident. Least-squares multiple comparisons tested differences between individual means at an experiment-wise p < 0.05, to protect against possible type I errors.
Results
Although overall sleep microarchitecture was more disturbed in the MDD group, none of the group main effects reached statistical significance (p > 0.05). Group х sex interactions were obtained for REM latency (p < 0.04), and multiple comparisons confirmed longer REM latency in men with MDD than in women with MDD and NC men (p < 0.02). As seen in Table 1, women with MDD had more slow-wave (stage 3 and 4) sleep, but this difference only approached significance (p < 0.08). Further, the latencies and durations of individual REM periods did not differ between groups. As a result, no further analyses were conducted on these measures.
Table 1
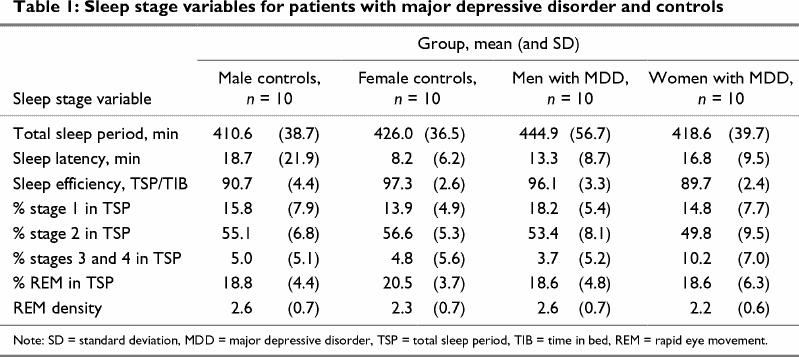
Sleep microarchitecture, however, did show a number of between-group differences. The means and SDs for sigma, alpha and delta amplitude measures are shown in Table 2. Between-group differences in theta and beta amplitude are illustrated in Fig. 1 and Fig. 2, respectively. A significant REM period repeated-measures effect was evident for delta amplitude (p < 0.01). Significant REM period and group х sex effects were also obtained for theta amplitude (p < 0.0003 and p < 0.02, respectively) and alpha amplitude (p < 0.002 and p < 0.005, respectively). Beta amplitude showed a group х sex х REM period interaction (p < 0.04).
Table 2
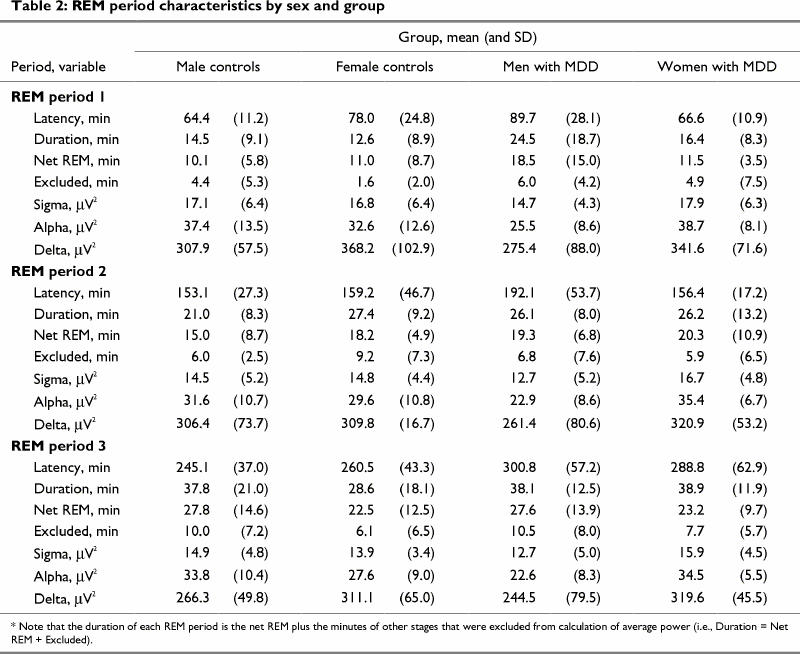
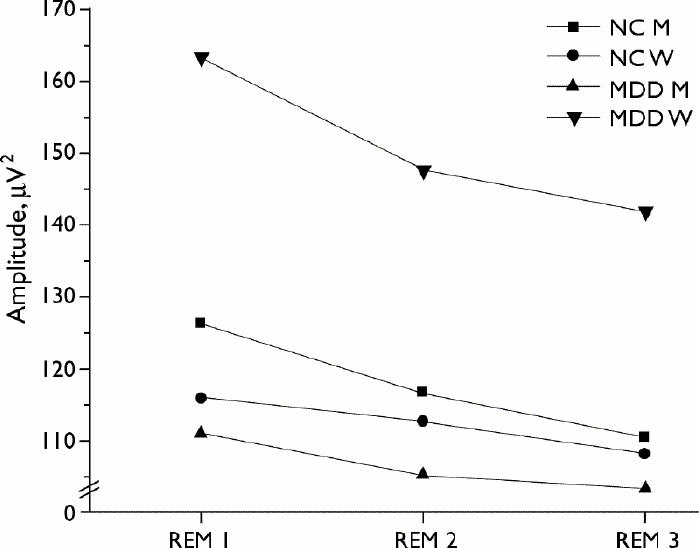
Fig. 1: Average theta (4.0–8.0 Hz) amplitude in the first 3 REM periods by sex and group (n = 10 per group). See Table 2 (net REM min) for average sample length per REM period.
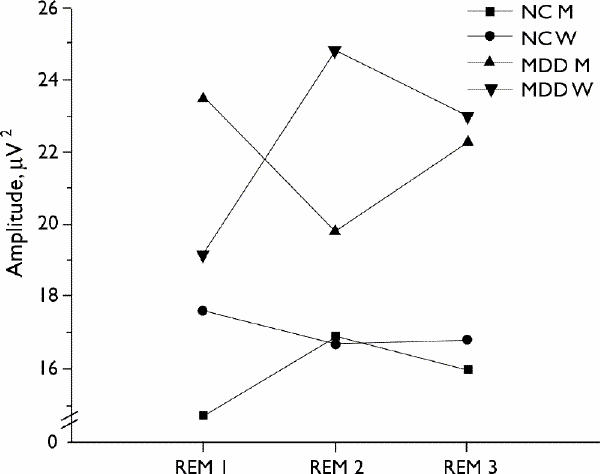
Fig. 2: Average beta (16–32 Hz) amplitude in the first 3 REM periods by sex and group (n = 10 per group).
In general, EEG amplitude in slower frequencies showed a decline across successive REM periods. Women in both MDD and NC groups had higher overall delta amplitude than their male counterparts, confirmed by multiple comparisons (p < 0.05). This effect was restricted to the first REM period. Moreover, the repeated-measure effect (i.e., the decline in delta over REM period) was larger than the sex difference. With regard to alpha amplitude, men with MDD had lower alpha amplitude than MDD women in all 3 REM periods (p < 0.05). Multiple comparisons also confirmed higher theta amplitude in the women with MDD compared with all other groups for each REM period (p < 0.01). Women with MDD also showed a stronger decline over the REM sleep periods.
By contrast, each group showed distinct and different temporal changes in fast-frequency activity. Beta amplitude was higher in MDD groups than in the corresponding NC groups, as expected from previous work. However, MDD men had higher beta amplitude than MDD women in the first REM period, an effect that reversed by the second REM period, with no sex difference in the third REM period. Moreover, sex differences in the NC group were in the opposite direction. None of the between-group multiple comparisons were significant, indicating that is the pattern of change across REM periods (i.e., the repeated measure) that differentiated the groups and produced the significant group х sex interaction (Fig. 2).
Discussion
The results of this study suggest that, like non-REM sleep, EEG amplitude shows a systematic temporal change over successive REM sleep periods, particularly for delta amplitude. Theta amplitude also declined over REM periods, but the effects were most pronounced in women with MDD. The trends, however, appear less systematic in faster frequency bands with a unique temporal profile in each group. Temporal changes in beta and theta amplitude also showed stronger sex differences in the MDD group than in the NC group. These findings are in general agreement with our previous studies identifying robust group х sex interactions in non-REM sleep EEG5,19 and provide further evidence that the pathophysiology of depression differs in men and women.
The strong results reported here are remarkably concordant with the results and conclusions of Antonijevic et al.20,21 In fact, both sleep macro- and microarchitecture data from the 2 research groups are quite similar. In addition, Antonijevic et al21 have suggested a sexual dimorphism in neuroendocrine function that is disease-dependent. It may very well contribute to the sex differences in sleep and depression between men and women. Further support for this notion has come from Rubin and colleagues,22,23 who have demonstrated sexual dimorphism in cholinergic neurotransmitter function and hypothalamic-pituitary-adrenal axis activity in depression. Moreover, recent reviews have highlighted a number of sex differences in sleep and mood regulation that are related to gonadal hormone regulation.11,12 These biological factors may contribute to the sex difference observed here and in other studies.
Our results also suggest a dynamic temporal organization of EEG activity in REM sleep like that demonstrated in non-REM sleep in numerous studies of healthy individuals.24,25,26,27,28,29 A caveat may be necessary, however, since the temporal organization is influenced by group and sex.6
There were some surprising findings in this study. First, enhanced theta amplitude might be expected to occur in longer duration, more phasic REM periods.17 Thus, higher theta amplitude would be expected in the latter REM periods of the night. By contrast, these data show a decline in theta amplitude over REM sleep in all groups. The meaning of such an outcome is not clear, although Corsi-Cabrera et al30 suggest that theta oscillations are under the same gonadal influence as delta and, therefore, should mirror the temporal characteristics of the latter.
Investigating the temporal organization of sleep EEG across successive REM periods may provide a sensitive means of discriminating between depressed and healthy individuals and between the sexes. It remains to be demonstrated, nonetheless, whether temporal organization of EEG microarchitecture in REM sleep falls under homeostatic regulation and shows the same response to sleep deprivation and extending prior wakefulness as slow-wave activity in non-REM sleep. Certainly, there is some evidence that this may be the case in healthy individuals. Our future work will evaluate this possibility in individuals with MDD. If the findings of this preliminary report are confirmed, it suggests that the temporal dynamics of both REM and non-REM sleep EEG are both disease- and sex-dependent.
Acknowledgments
We thank Kenneth Z. Altshuler, MD, former Chair, for administrative support, the staff of the Sleep Study Unit, Darwynn D. Cole, Supervisor, Irma Aguilar, PhD, and Kimberly Yonkers, MD, for assistance in recruiting and evaluating patients and clinical follow-up.
Footnotes
Supported in part by the Sarah M. and Charles E. Seay Center for Brain and Applied Research in Psychiatric Illness, and NIMH MH46886.
Competing interests: None declared.
Correspondence to: Dr. Roseanne Armitage, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas TX 75390-9070; fax 214 648-7359; Roseanne.Armitage@UTSouthwestern.edu
Submitted Jan. 31, 2001 Revised Jul. 4, 2001 Accepted Jul. 19, 2001
References
- 1.Reynolds CF III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215. [DOI] [PubMed]
- 2.Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biol Psychiatry 1995;37:72-84. [DOI] [PubMed]
- 3.Armitage R, Hoffmann R. Sleep electrophysiology of major depressive disorders. Curr Rev Mood Anxiety Disord 1997;1:139-51.
- 4.Armitage R, Hoffmann RF, Rush, AJ. Biological rhythm disturbance in depression: temporal coherence of ultradian sleep EEG rhythms. Psychol Med 1999;29:1435-48. [DOI] [PubMed]
- 5.Armitage R, Hoffmann R, Fitch T, Trivedi M, Rush AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep 2000;23(5):607-17. [PubMed]
- 6.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res 2000;95:201-13. [DOI] [PubMed]
- 7.Borbély AA, Tobler I, Loepfe M, Kupfer DJ, Ulrich RF, Grochocinski V, et al. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res 1984;12:27-33. [DOI] [PubMed]
- 8.Kupfer D J, Ulrich RF, Coble PA, Jarrett DB, Grochocinski VJ, Doman J, et al. Application of an automated REM and slow wave sleep analysis: I. Normal and depressed subjects. Psychiatry Res 1984;13:325-34. [DOI] [PubMed]
- 9.Kupfer DJ, Ulrich RF, Coble PA, Jarrett DB, Grochocinski VJ, Doman J, et al. Application of an automated REM and slow wave sleep analysis. II. Testing the assumptions of the two-process model of sleep regulation in normal and depressed subjects. Psychiatry Res 1984;13:335-43. [DOI] [PubMed]
- 10.Reynolds CF III, Kupfer DJ, Thase ME, Frank E, Jarrett DB, Coble PA. Sleep, gender, and depression: an analysis of gender effects on the electroencephalographic sleep of 302 depressed outpatients. Biol Psychiatry 1990;28:674-84. [DOI] [PubMed]
- 11.Manber R, Armitage R. Sex steroids and sleep: a review [erratum appears in Sleep 1999;23(2):145-9]. Sleep 1999;22:540-55. [PubMed]
- 12.Parry BL. Hormonal basis of mood disorders in women. In: Frank E, editor. Gender and its effects on psychopathology. Washington: American Psychiatric Press; 2000. p. 3-21.
- 13.Spitzer M, Williams JBW, Gibbons M. The structured clinical interview for DSM-III-R (SCID). New York: New York Psychiatric Research Institute; 1986.
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington: American Psychiatric Association; 1994.
- 15.Nunez P. Electrical fields of the brain. New York: Oxford Press; 1981.
- 16.Hoffmann RF, Moffitt AR, Shearer JC, Sussman PS, Wells RB. Conceptual and methodological considerations towards the development of computer-controlled research on the electro-physiology of sleep. Waking Sleeping 1979;3:1-16. [PubMed]
- 17.Armitage R, Roffwarg HP, Rush AJ. Digital period analysis of sleep EEG in depression. Biol Psychiatry 1992;31(1):52-68. [DOI] [PubMed]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington: Public Health Service; 1968. National Institutes of Health publication no 204.
- 19.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev 2001;5(3):237-46. [DOI] [PubMed]
- 20.Antonijevic IA, Murck H, Frieboes RM, Holsboer F, Steiger A. On the gender differences in sleep-endocrine regulation in young normal humans. Neuroendocrinology 1999;70:280-7. [DOI] [PubMed]
- 21.Antonijevic IA, Murck H, Frieboes R-M, Barthelmes J, Steiger A. Sexually dimorphic effects of GHRH on sleep-endocrine activity in patients with depression and normal controls. Part I: The sleep EEG. Sleep Res Online 2000;3(1):5-13. [PubMed]
- 22.Rubin RT, O'Toole SM, Rhodes ME, Sekula LK, Czambel RK. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: sex differences between major depressives and matched control subjects. Psychiatry Res 1999;89:1-20. [DOI] [PubMed]
- 23.Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Rev 1999;30:135-52. [DOI] [PubMed]
- 24.Dijk DJ, Brunner DP, Beersma DGM, Borbély AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep 1990;13:430-40. [DOI] [PubMed]
- 25.Dijk DJ, Brunner DP, Borbély AA. Time course of EEG power density during long sleep in humans. Am J Physiol 1990;258: R650-61. [DOI] [PubMed]
- 26.Dijk DJ, Brunner DP, Borbély AA. EEG power density during recovery sleep in the morning. Electroencephalogr Clin Neurophysiol 1991;78:203-14. [DOI] [PubMed]
- 27.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res 1993;626:190-9. [DOI] [PubMed]
- 28.Aeschbach D, Borbély AA. All-night dynamics of human sleep EEG. J Sleep Res 1993;2:70-81. [DOI] [PubMed]
- 29.Dijk DJ. Circadian variation of EEG power spectra in NREM and REM sleep in humans: dissociation from body temperature. Sleep Res 1999;8:189-95. [DOI] [PubMed]
- 30.Corsi-Cabrera M, Guevara MA, Del Rio-Portilla Y, Arce C, Villanueva-Hernández Y. EEG bands during wakefulness, slow-wave and paradoxical sleep as a result of principal component analysis in man. Sleep 2000;23(6):738-44. [PubMed]


