Abstract
Objective
To examine if atypical depression may be associated with hypersuppression of the hypothalamic-pituitary-adrenal (HPA) axis.
Method
Eight women with atypical major depression and 11 controls with no history of psychiatric illness, matched on age and body mass index, were challenged with low-dose dexamethasone (0.25 mg and 0.50 mg in random order and 1 week apart). Dexamethasone was self administered at 11 pm, and plasma cortisol samples were drawn at 8 am and 3 pm on the following day.
Results
After the 0.50-mg dexamethasone challenge, mean suppression of morning cortisol was significantly greater in patients with atypical depression (91.9;, standard deviation [SD] 6.8%) than in the controls (78.3%, SD 10.7%; p < 0.01).
Conclusion
These preliminary data add to the growing body of literature that suggests atypical depression, in contrast to classic melancholia, may be associated with exaggerated negative feedback regulation of the HPA axis.
Medical subject headings: corticotropin-releasing hormone; depression, atypical, dexamethasone; hormones; hydrocortisone; mood disorders; stress
Abstract
Objectif
Déterminer s'il est possible d'établir un lien entre la dépression atypique et l'hypersupression de l'axe hypothalamo-hypophyso-surrénalien (HPS).
Méthode
Huit femmes atteintes de dépression majeure atypique et 11 sujets témoins sans antécédent de maladie psychiatrique, jumelées selon l'âge et l'indice de masse corporelle, ont fait l'objet d'une provocation à la dexaméthasone à faible dose (0,25 mg et 0,50 mg, dans un ordre aléatoire et à une semaine d'intervalle). Les patientes se sont en outre administré elles-mêmes la dexaméthasone à 23 h et l'on a prélevé des échantillons de cortisol plasmatique à 8 h et à 15 h le lendemain.
Résultats
Après la provocation à la dexaméthasone à 0,50 mg, la suppression moyenne du cortisol matinal était plus élevée chez les patientes atteintes d'une dépression atypique (91,9 %, écart type [ET] 6,8 %) que chez les sujets témoins (78,3 %, ET 10,7 %; p < 0,01).
Conclusion
Ces données préliminaires s'ajoutent à la masse croissante de documents indiquant que, contrairement à ce qui se passe dans le cas de la mélancolie classique, il est possible d'établir un lien entre la dépression atypique et la régulation par rétroaction négative exagérée de l'axe HPS.
Introduction
Major depression with atypical features (MD-AF) is a subtype of mood disorder characterized by mood reactivity, profound fatigue with “leaden paralysis,” reversed neurovegetative symptoms including hypersomnia, increased appetite and weight gain, and increased sensitivity to interpersonal rejection.1 Compared with classic melancholic depression, MD-AF is associated with an earlier age of onset,2 a more chronic recurrent course,3 and a preferential responsiveness to MAO inhibitors relative to tricyclic antidepressants.4 These clinical findings suggest that the pathophysiology of MD-AF might differ from that of melancholic depression.
Consistent with this hypothesis, there is emerging evidence for marked differences in stress hormone production in MD-AF versus classic depression. Classic depression has been associated with overactivity of the hypothalamic-pituitary-adrenal (HPA) axis and hypersecretion of central corticotropin-releasing hormone (CRH),5 whereas these same systems may be underactive in atypical subtypes of depression.6,7,8,9 It has been speculated that hypoactivity of the HPA axis, central CRH or both may contribute to the profound fatigue and reversed neurovegetative symptoms which characterize atypical depression.7,10
To further explore HPA axis activity in MD-AF, we conducted a pilot study with a low-dose dexamethasone suppression test (DST) in women with MD-AF and normal controls. The DST, which uses dexamethasone in the 0.25–0.5 mg range, is designed to assess possible hypersuppression of the HPA axis.11 This contrasts with the standard 1 mg DST, which assesses overactivity and lack of feedback sensitivity of this system.12 The low-dose DST was used because our working hypothesis was that, compared with a matched normal control group, women with MD-AF would exhibit hypersuppression of cortisol after low doses of dexamethasone.
Method
Subjects in the MD-AF group were 8 consecutive female outpatients presenting to the Depression Clinic of the Clarke Division of the Centre for Addiction and Mental Health who met the following DSM-IV criteria for MD-AF: mood reactivity and at least 2 of increased appetite or weight gain, hypersomnia, leaden paralysis or interpersonal rejection sensitivity.1
Eleven normal controls were recruited via posters and newspaper advertisements at the University of Toronto. They had no history of psychiatric illness and were matched as closely as possible to the depressed patients on age and body mass index (BMI).
None of the subjects were pregnant, and all had regular menstrual cycles in the 3 months before the study. Menstrual phase was documented by self-report and was defined as: day 0–5, menstrual; day 5–14, follicular; and day 14 to menses, luteal. To control for possible effects of menstrual cycle on cortisol measures, all subjects were tested during the follicular phase. Subjects were excluded if they were medically ill, taking corticosteroids or actively abusing substances. None of the study subjects were taking antidepressants at the time of the study.
Each subject was given an oral and written summary of the purposes, procedures and potential risks of the project and each gave informed consent. Ethics approval was obtained from the University of Toronto.
Procedure
As it was not known which dose of dexamethasone would be optimal to demonstrate differences in cortisol suppression, each subject was challenged twice — once with 0.25 mg and once with 0.5 mg of dexamethasone, in random order.
Before undergoing the first dexamethasone challenge, subjects in the atypical depression group were administered the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale (SIGH-SAD).13 This version of the HDRS includes an 8-item subscale to assess atypical symptoms of depression.
On the day of each challenge, a baseline plasma cortisol sample was drawn by venipuncture at 8 am. Dexamethasone was self-administered by the study subjects at 11 pm, and postchallenge plasma cortisol samples were drawn at both 8 am and 3 pm the next day. The morning sample was taken before breakfast to avoid the confounding effect of food intake on plasma cortisol levels. The second dexamethasone challenge was completed 1 week after the first.
All blood samples were drawn at the hospital's clinical laboratory. To standardize blood drawing, subjects arrived 15 minutes before each procedure. Plasma cortisol levels were measured via radioimmunoassay by a technician blind to the nature of the study.
Data analysis
Demographic variables of the 2 groups were compared with unpaired t-tests. Across all subjects, the 2 prechallenge plasma cortisol levels taken 1 week apart were highly correlated (r = 0.72, p < 0.01). To assess the relation of baseline cortisol levels to key demographic and clinical variables, we calculated a mean overall baseline cortisol level (i.e., mean CORT-0) for each subject by averaging the 2 prechallenge values. The relation of mean CORT-0 to key demographic and clinical variables was then assessed using Pearson correlations.
Because of the small sample size and high degree of variability in plasma cortisol levels, nonparametric statistics (i.e., Mann-Whitney U tests) were used to compare cortisol levels and cortisol percent change scores across the 2 study groups. Individual prechallenge baseline measures (not the mean CORT-0 described above) were used for these analyses. Percent change scores were calculated for each postchallenge time point as: ([postchallenge value – prechallenge value]/[prechallenge value]) х 100. Where applicable, correlations between percent change scores and other study variables were assessed.
Results
The study groups were not significantly different with respect to age (28.6 yr [standard deviation (SD) 8.1 yr] for controls v. 34.4 yr [SD 6.9 yr] for MD-AF) or BMI (23.5 [SD 4.6 ] for controls v. 28.6 [SD 8.02] for MD-AF). The MD-AF group had a mean HDRS-29 score of 36.6 (SD 5.6) and a mean HDRS-8 (atypical) score of 15.0 (SD 4.1).
There was no significant difference between groups with respect to overall mean CORT-0 (controls 156.7 [SD 40.1] nmol/L, MD-AF 140.7 [SD 72.7] nmol/L). There were no significant correlations between mean CORT-0 and either age or BMI across the 2 study groups or within each group. Within the MD-AF group, there were no significant correlations between mean CORT-0 and either total HAM-29 or HAM-8 scores.
Table 1 compares pre- and post-dexamethasone plasma cortisol levels and cortisol percent change scores across the 2 study groups. After the 0.5-mg dose of dexamethasone, the MD-AF group had significantly lower 8 am plasma cortisol levels and significantly greater percentage cortisol suppression compared with baseline than did the control group. Fig. 1 shows the individual cortisol percent change scores for the 0.5-mg 8 am levels and demonstrates the relative consistency within each group. Post hoc, these change scores were correlated with demographic and clinical variables including age, BMI and depression scores. The only statistically significant result was a negative correlation between age and percent cortisol suppression in the MD-AF group (r = –0.71, p = 0.048).
Table 1
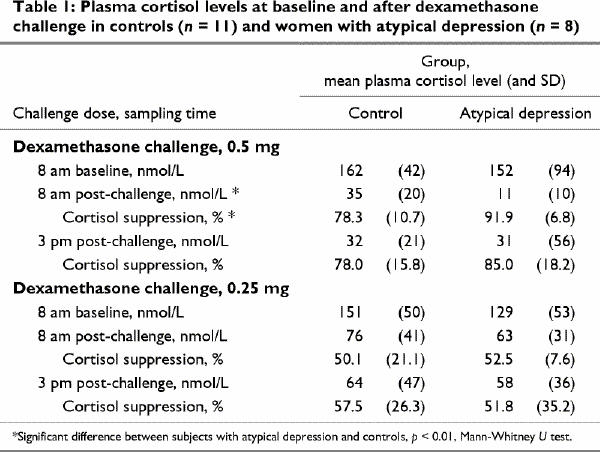
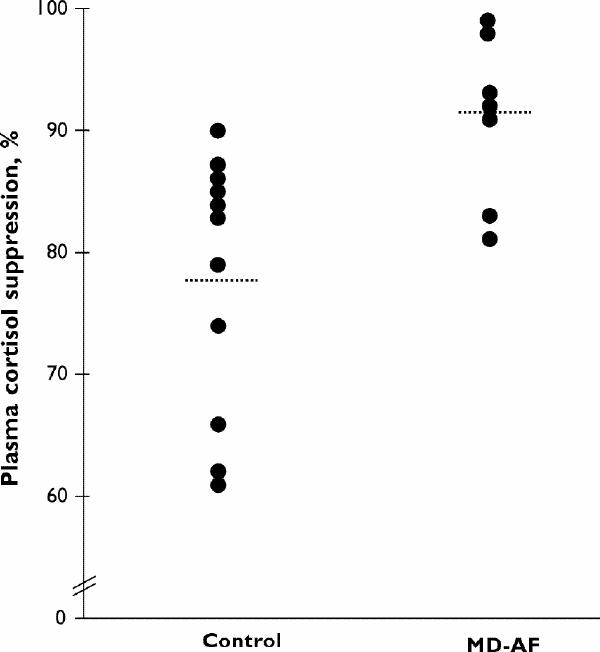
Fig. 1: Percent suppression of plasma cortisol at 8 am after the 11 pm, 0.5 mg dexamethasone challenge in 11 control subjects and 8 women with major depression with atypical features (MD-AF).
Discussion
Although highly preliminary and in need of replication in larger samples, this pilot study suggests that women with MD-AF exhibit hypersuppression of early morning cortisol secretion in response to a 0.5-mg dexamethasone challenge. This strongly contrasts with DST findings in classic melancholic depression12 and suggests that atypical depression may be a biologically distinct mood disorder.
Our findings add to a growing literature pointing to underactivity of the HPA axis and central CRH neurons in atypical subtypes of depression. Most relevant to the current data, low plasma cortisol levels, in the face of elevated corticotropin levels, have been reported in subjects with MD-AF relative to normal controls.6 Furthermore, individuals with atypical depressive symptoms and low cortisol levels may respond clinically to the exogenous administration of corticosteroids.7 Regarding other psychiatric disorders characterized by atypical depressive symptoms, a significant negative correlation between atypical neurovegetative symptoms of depression and plasma cortisol levels has been reported in bulimia nervosa,8 whereas in seasonal affective disorder, delayed and reduced responses to exogenous CRH have been found.9
Taken as a whole, these various results point to underactivity of the HPA axis and central CRH neurons in subtypes of depression with atypical or reversed features.
Several limitations of the study merit consideration. The sample size was modest, and only the higher dose of dexamethasone at the early morning sampling time produced significant differences across groups. We speculate that the lack of effect at other sampling times may have been due to insufficient plasma levels of dexamethasone with afternoon sampling and with the 0.25-mg dose of dexamethasone. Plasma dexamethasone levels would have been helpful in this regard, but were not possible because of funding limitations.
A potential confound for this study is the reported link between atypical symptoms of depression and early childhood trauma14 — childhood trauma itself has been associated with cortisol hypersuppression after low-dose DST.15 In future studies of stress hormone production in atypical depression, it would thus be important to include an interpersonal trauma questionnaire to assess whether low cortisol levels are limited to a previously traumatized subgroup.
Notwithstanding, our results point to possible hypersuppression of the HPA axis in women with MD-AF. Further studies of HPA axis functioning in atypical depression are needed and should include larger samples, plasma dexamethasone measures, direct comparisons with melancholic depression and detailed assessment of early traumatic experiences.
Acknowledgments
We acknowledge Dr. Audrey Brown for her significant contribution to this work. Dr. Brown passed away during the course of this project. The authors also thank Dr. Charles Nemeroff for his recommendations regarding the assessment of the HPA axis.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Robert D. Levitan, Clarke Division of the Centre for Addiction and Mental Health, 250 College St., Rm. 1135, Toronto ON M5T 1R8; fax 416 979-6821; Robert_Levitan@camh.net
Submitted Mar. 5, 2001 Revised Aug. 16, 2001 Accepted Sept. 11, 2001
References
- 1.Quitkin FM, Harrison W, Liebowitz M, McGrath P, Rabkin JG, Stewart J, et al. Defining the boundaries of atypical depression. J Clin Psychiatry 1984;45:19-21. [PubMed]
- 2.Davidson JR, Miller RD, Turnbull CD, Sullivan JL. Atypical depression. Arch Gen Psychiatry 1982;39:527-34. [DOI] [PubMed]
- 3.Stewart JW, McGrath PJ, Rabkin JG, Quitkin FM. Atypical depression. A valid clinical entity? Psychiatr Clin North Am 1993;16:479-95. [PubMed]
- 4.Quitkin FM, Stewart JW, McGrath PJ, Tricamo E, Rabkin JG, Ocepek-Welikson K, et al. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry Suppl 1993;16:30-4. [PubMed]
- 5.Nemeroff CB. Clinical significance of psychoneuroendocrinology in psychiatry: focus on the thyroid and adrenal. J Clin Psychiatry 1989;50(Suppl):13-20. [PubMed]
- 6.Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry 1999;4:182-8. [DOI] [PubMed]
- 7.Bouwer C, Claassen J, Dinan TG, Nemeroff CB. Prednisone augmentation in treatment-resistant depression with fatigue and hypocortisolaemia: a case series. Depress Anxiety 2000;12:44-50. [DOI] [PubMed]
- 8.Levitan RD, Kaplan AS, Brown GM, Joffe RT, Levitt AJ, Vaccarino FJ, et al. Low plasma cortisol in bulimia nervosa patients with reversed neurovegetative symptoms of depression. Biol Psychiatry 1997;41:366-8. [DOI] [PubMed]
- 9.Joseph-Vanderpool JR, Rosenthal NE, Chrousos GP, Wehr TA, Skwerer R, Kasper S, et al. Abnormal pituitary-adrenal responses to corticotropin-releasing hormone in patients with seasonal affective disorder: clinical and pathophysiological implications. J Clin Endocrinol Metab 1991;72:1382-7. [DOI] [PubMed]
- 10.Gold PW, Licinio J, Wong ML, Chrousos GP. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci 1995;771:716-29. [DOI] [PubMed]
- 11.Yehuda R, Boisoneau D, Lowy MT, Giller EL. Dose–response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995;52:583-93. [DOI] [PubMed]
- 12.Carroll BJ. Use of the dexamethasone suppression test in depression. J Clin Psychiatry 1982;43:44-50. [PubMed]
- 13.Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured interview guide for the Hamilton Depression rating Scale, Seasonal Affective Disorders Version (SIGH-SAD). New York: New York Psychiatric Institute; 1988.
- 14.Levitan RD, Parikh SV, Lesage AD, Hegadoren KM, Adams M, Kennedy SH, et al. Major depression in individuals with a history of childhood physical or sexual abuse: relationship to neurovegetative features, mania, and gender. Am J Psychiatry 1998;155:1746-52. [DOI] [PubMed]
- 15.Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 1997;42:680-6. [DOI] [PubMed]


