Abstract
The septins are a conserved family of proteins that are involved in cytokinesis and other aspects of cell-surface organization. In Drosophila melanogaster, null mutations in the pnut septin gene are recessive lethal, but homozygous pnut mutants complete embryogenesis and survive until the pupal stage. Because the completion of cellularization and other aspects of early development seemed likely to be due to maternally contributed Pnut product, we attempted to generate embryos lacking the maternal contribution in order to explore the roles of Pnut in these processes. We used two methods, the production of germline clones homozygous for a pnut mutation and the rescue of pnut homozygous mutant flies by a pnut+ transgene under control of the hsp70 promoter. Remarkably, the pnut germline-clone females produced eggs, indicating that stem-cell and cystoblast divisions in the female germline do not require Pnut. Moreover, the Pnut-deficient embryos obtained by either method completed early syncytial development and began cellularization of the embryo normally. However, during the later stages of cellularization, the organization of the actin cytoskeleton at the leading edge of the invaginating furrows became progressively more abnormal, and the embryos displayed widespread defects in cell and embryo morphology beginning at gastrulation. Examination of two other septins showed that Sep1 was not detectable at the cellularization front in the Pnut-deficient embryos, whereas Sep2 was still present in normal levels. Thus, it is possible that Sep2 (perhaps in conjunction with other septins such as Sep4 and Sep5) fulfills an essential septin role during the organization and initial ingression of the cellularization furrow even in the absence of Pnut and Sep1. Together, the results suggest that some cell-division events in Drosophila do not require septin function, that there is functional differentiation among the Drosophila septins, or both.
INTRODUCTION
The septins are a family of proteins that were first discovered in Saccharomyces cerevisiae but now appear to be ubiquitous in fungi and animals (for review, see Cooper and Kiehart, 1996; Longtine et al., 1996; Longtine and Pringle, 1999). Each organism investigated has two or more family members, with levels of sequence identity ranging from 30 to 80%. All of the known septins have in common an apparent GTP-binding domain near the NH2 terminus, and most have a predicted coiled-coil domain at the COOH terminus. The septins form multimeric complexes, and those purified from S. cerevisiae (Frazier et al., 1998) and Drosophila (Field et al., 1996) have been shown to form filaments in vitro. However, it is not clear that the septins' filament-forming properties are essential for their function (Frazier et al., 1998; Longtine et al., 1998).
In all cell types examined to date, the septins become concentrated at the site of cytokinesis (Haarer and Pringle, 1987; Neufeld and Rubin, 1994; Fares et al., 1995; Hime et al., 1996; Longtine et al., 1996; Kinoshita et al., 1997; Xie et al., 1999), and septin mutants of S. cerevisiae (Hartwell, 1971; Longtine et al., 1996) and Drosophila (Neufeld and Rubin, 1994) are defective in cytokinesis. In addition, microinjection of antiseptin antibodies can cause cleavage-furrow regression in mammalian cells (Kinoshita et al., 1997). Septins also have roles in processes other than cytokinesis. For example, in S. cerevisiae, they are required for proper localization of the bud-site-selection markers Bud3p and Bud4p (Chant et al., 1995; Sanders and Herskowitz, 1996) and of the subunits of the chitin synthase III complex (DeMarini et al., 1997). These and other data have led to the hypothesis that the septins function as a scaffold on which other proteins assemble at the cell surface (Longtine et al., 1998; Longtine and Pringle, 1999). In both Drosophila and mammals, the septins are also localized in ways that suggest noncytokinesis functions. For example, they are highly concentrated in nondividing neuronal cells (Neufeld and Rubin, 1994; Fares et al., 1995; Caltagarone et al., 1998; Trimble, 1999; Valencik and Pringle, unpublished data), where they may be involved in vesicle trafficking and/or exocytosis (Hsu et al., 1998; Beites et al., 1999).
In Drosophila, five septins have been identified to date, named Pnut, Sep1, Sep2, Sep4, and Sep5 (Neufeld and Rubin, 1994; Fares et al., 1995; Field et al., 1996; Al-Awar, Peifer, and Pringle, unpublished observations; Hawley, personal communication; our unpublished observations). In wild-type flies, Pnut, Sep1, and Sep2 colocalize in all situations examined (Fares et al., 1995; Al-Awar, Peifer, and Pringle, unpublished observations 2000). In addition, these three septins all interact with each other in the yeast two-hybrid system (Al-Awar, Shih, Peifer, and Pringle, unpublished observations), and the filament-forming septin complex isolated from embryos appears to consist largely, if not entirely, of these three proteins in stoichiometric amounts (Field et al., 1996). Moreover, in homozygous pnut mutant embryos, cells of the embryonic central nervous system, which have no detectable Pnut protein (the maternal contribution having been depleted by that time), also have no detectable Sep1 or Sep2 (Fares et al., 1995; Al-Awar, Peifer, and Pringle, unpublished observations). Together, these data have suggested that Pnut, Sep1, and Sep2 always function as part of a complex with each other. The localization and function of Sep4 and Sep5 and their relationships to the other septins have not yet been characterized in detail.
In addition to ordinary cytokinesis events, Drosophila development involves a special cytokinesis event known as cellularization (for review, see Schejter and Wieschaus, 1993). Early development occurs in a syncytium in which 13 nuclear divisions (9 in the interior, followed by 4 after the migration of most nuclei to the embryo cortex) result in the production of a monolayer of ∼6000 closely packed nuclei just beneath the surface of the embryo. The four cortical nuclear divisions are accompanied by partial invaginations of plasma membrane (the “pseudocleavage furrows”) between the nuclei. Cellularization then occurs in the interphase after the 13th nuclear division, with each nucleus becoming enclosed to form a cell by invagination of the embryo plasma membrane. Initially, a hexagonal cytoskeletal network outlining the nuclei organizes under the plasma membrane. This network contains a variety of proteins, including actin, myosin, the actin-binding protein anillin, Pnut, Sep1, and Sep2 (Schejter and Wieschaus, 1993; Neufeld and Rubin, 1994; Field and Alberts, 1995; Fares et al., 1995; Al-Awar, Peifer, and Pringle, unpublished observations). Next, this cytoskeletal network and the overlying membrane invaginate between all of the cortical nuclei simultaneously during the “slow phase” of cellularization. As the leading edges of the system (the “cellularization front”) pass the bases of the nuclei, the cytoskeletal network resolves into discrete rings. These rings contract beneath the nuclei during the “fast phase” of cellularization, while the cellularization front continues to invaginate toward the interior of the embryo, thus lengthening the forming cells. The cells do not close completely at their bases during the fast phase; instead, each cell retains a membrane-bounded channel (the “yolk plug”) between its cytoplasm and the underlying yolk cytoplasm. The yolk plug maintains actin, myosin, anillin, and the septins in a surrounding ring. After cellularization, the cell-shape changes associated with gastrulation begin. The yolk plugs are present through much of the early part of gastrulation, finally closing off during the stage of germ-band extension (Rickoll and Counce, 1980).
We wanted to investigate the roles of the septins in cellularization, subsequent embryonic cytokinesis events, and the other embryonic events in which roles for the septins had been suggested by protein localization data. However, a problem was that homozygous pnut mutant embryos survive until pupation. This survival seemed likely to result from a maternal contribution of Pnut mRNA and/or protein that was deposited in the oocyte during oogenesis. Thus, we used two different methods to deplete this maternal contribution. Surprisingly, the results suggest that some cell-division events in Drosophila do not require septin function, that there is functional differentiation among the Drosophila septins, or both.
MATERIALS AND METHODS
The wild-type D. melanogaster stock used in this study was Canton S. Other stocks and genetic crosses used are described in Table 1.
Table 1.
Drosophila melanogaster stocks used in this study
| Stock | Genotype | Source/Reference |
|---|---|---|
| 1 | pnutrN498/CyO; rya | Neufeld and Rubin, 1994 |
| 2 | w; P[mini w+, FRT]2R-G13, L/CyO | Chou and Perrimon, 1996b |
| 3 | w; P[mini w+, FRT]2R-G13, pnutrN498/CyO | 1 × 2 |
| 4 | w, P[ry+, HS-FLP]1; Adv1/CyO | Chou and Perrimon, 1996b |
| 5 | w, P[ry+, HS-FLP]1; P[mini w+, FRT]2R-G13, pnutrN498/CyO | 3 × 4 |
| 6 | P[mini w+, FRT]2R-G13, P[mini w+, ovoD1-18]2R1, P[mini w+, ovoD1-18]2R2/Dp(?;2), bwD, S1, Sp1, Ms(2)M/CyO | Chou and Perrimon, 1996b |
| 7 | w, P[ry+, HS-FLP]1/+; P[mini w+, FRT]2R-G13, pnutrN498/P[mini-w+, FRT]2R-G13, P[mini w+, ovoD1-18]2R1, P[mini w+, ovoD1-18]2R2 | 5 × 6 |
| 8 | w; pnutxp/CyO; P[w+, HS-pnut+]c | Neufeld and Rubin, 1994 |
| 9 | w; pnutxp; P[w+, HS-pnut+]d | 8 × 8 |
| 10 | w; P[w+, pnut+], ee | Neufeld and Rubin, 1994 |
| 11 | w; Noc[Sco]/CyO | b |
| 12 | w; Noc[Sco]/CyO; P[w+, pnut+] | 10 × 11 |
| 13 | w; pnutrN498; P[w+, pnut+] | 1 × 12 |
| 14 | w; pnutxp; P[w+, pnut+] | 8 × 12 |
pnutrN498 is P[lacZ, ry+]rN498; it carries the P[lacZ, ry+] element inserted at nucleotide 75 of the pnut coding region (Neufeld and Rubin, 1994). This lethal mutation is recessive to the wild-type pnut+ allele carried on the CyO balancer chromosome.
This stock was obtained from the Bloomington Stock Center (Bloomington, IN).
pnutxp was generated by excision of the P element of the pnutrN498 allele, leaving a small deletion that removes most of the pnut coding region (Neufeld and Rubin, 1994). P[w+, HS-pnut+] is a transgene carrying a wild-type pnut+ coding region under the control of the hsp70 promoter (Neufeld and Rubin, 1994).
Mature adults of this genotype were produced by intermittently inducing transcription from the HS-pnut+ transgene by a 2-h heat shock (at 37°C) once per day.
P[w+, pnut+] is a transgene inserted into chromosome 3; it contains 10 kb of genomic sequence, including the full pnut+ gene and flanking sequences.
To examine living embryos and cuticles, embryos were dechorionated in 50% bleach, washed three times in 0.1% Triton X-100, and submerged in halocarbon oil. Embryos at syncytial blastoderm stage were selected and mounted in defined locations on a Petriperm dish (Bachofer, Reutlingen, Germany) at 23°C. The embryos were then photographed at 10- to 15-min intervals for ∼3 h. After 2 to 3 days, the coverslip was removed, and cuticles were prepared by washing each embryo individually in 0.1% Triton X-100, mounting on a slide with 1:1 Hoyer's reagent:lactic acid (Wieschaus and Nüsslein-Volhard, 1986), and heating at 65°C overnight. Embryos and cuticles were observed and photographed with a Nikon Optiphot-2 microscope.
For fluorescent staining, embryos were allowed to develop at 25°C for 1 to 4 h. They were then dechorionated in 50% bleach, washed three times with 0.1% Triton X-100 in PBS, and fixed wing one of the following protocols. For visualization of F-actin and nuclei by the use of BODIPY FL-phallacidin and propidium iodide (Molecular Probes, Eugene, OR), embryos were resuspended in a vial containing 1 ml of heptane and 1 ml of 37% formaldehyde and swirled for 5 min. The heptane and formaldehyde were replaced with 0.1% Triton X-100 in PBS, and embryos were devitellinized by hand (Theurkauf, 1992) and processed as described by Orsulic and Peifer (1994). For visualization of Pnut and Sep1 by immunofluorescence, embryos were fixed as described by Cox et al. (1996), devitellinized by shaking in 1:1 methanol:heptane, and stained as described by Peifer et al. (1993). For visualization of Sep2, embryos were fixed by use of a modification of the procedure described by Miller et al. (1989): they were immersed for 5 s in boiling 70 mM NaCl, 0.03% Triton X-100, cooled by resuspending in the same solution at 0°C, devitellinized by shaking in 1:1 methanol:heptane, incubated in methanol for several hours at room temperature, and stained as described by Cox et al. (1996). For visualization of anillin, embryos were fixed as described above for visualization of F-actin, devitellinized either by hand or in 1:1 methanol:heptane, incubated for 2 h in 1% Triton X-100 in PBS, and stained as described by Field and Alberts (1995). For visualization of Pnut, anillin, and tubulin in ovaries, isolated ovaries were fixed for 20 min in 1% formaldehyde in PEM [0.1 M piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.9, 2 mM MgSO4, 1 mM EDTA] containing 1% NP-40, washed once and then incubated for 1 h in block solution (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% NP-40, 5 mg/ml BSA), washed once with wash solution (like block solution except with 1 mg/ml BSA), incubated for 6 h at 22°C with primary antibodies diluted in wash solution, washed three times (5 min per wash) in wash solution, incubated for 2 h at 22°C with secondary antibodies diluted in wash solution, and washed three more times (5 min per wash) in wash solution. Monoclonal antibodies 4C9, which recognizes the NH2-terminal third of Pnut (Neufeld and Rubin, 1994), and E7, which recognizes β-tubulin, were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and used at dilutions of 1:3 and 1:500, respectively. The KEKK rabbit polyclonal antibody, which recognizes the COOH terminus of Pnut (Field et al., 1996), was used at 0.005 mg/ml. Affinity-purified anti-Sep1 (Fares et al., 1995), anti-Sep2 (Field et al., 1996), and antianillin (Field and Alberts, 1995) rabbit polyclonal antibodies were used at dilutions of 1:50, 1:20, and 1:5000, respectively. Fluorescein isothiocyanate- and rhodamine-conjugated secondary antibodies (Boehringer Mannheim, Indianapolis, IN) were used at a dilution of 1:500; Cy2- and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used at a dilution of 1:1000. After staining, embryos or ovaries were mounted in AquaPolymount (Polysciences, Warrington, PA) and observed and photographed with a Zeiss LSM 410 confocal microscope.
For Western-blot analyses, embryos were dechorionated in 50% bleach, washed three times in 0.1% Triton X-100, crushed in 1.2× Laemmli buffer (Laemmli, 1970) by the use of a Teflon pestle and microfuge tube, boiled for 5 min, and centrifuged in a microfuge. The supernatants were resolved on 10% SDS-polyacrylamide gels, blotted to a nitrocellulose membrane (Schleicher & Schuel, Keene, NH), and stained with antibody 4C9 (used at 1:30), KEKK (used at 0.0005 mg/ml), or monoclonal anti-Bicaudal D (used at 1:50; Suter and Steward, 1991) as described by Peifer (1993). Detection was by the ECL chemiluminescence system (Amersham, Arlington Heights, IL).
RESULTS
Occurrence of Stem-cell and Cystoblast Divisions in the Absence of Pnut
Homozygous null pnut mutant embryos survive until the pupal stage (Neufeld and Rubin, 1994). To ask whether such embryos survive early development because of maternally contributed pnut product, and thus to explore septin function during embryogenesis, we used the FLP-DFS system (Chou and Perrimon, 1996) to generate germline clones homozygous for a pnut null mutation. Females were produced that were homozygous for a centromere-proximal FRT site and trans-heterozygous for the pnutrN498 allele and a transgene containing the dominant female sterility mutation ovoD (Table 1, stock 7). Induction of the FLP site-specific recombinase in second-instar larvae of such females results in a high frequency of mitotic recombination at the FRT site, producing twin clones homozygous either for pnutrN498 or for ovoD. ovoD does not affect somatic cells, but when homozygous or heterozygous in the female germline, it blocks development at an early stage. Thus, such flies should not produce eggs unless homozygosis of the pnutrN498 chromosome arm occurred in the stem cells, so that no copies of ovoD remained in the developing syncytial germ cells. However, if Pnut were required for stem-cell divisions, the cystoblast divisions that generate the oocyte and nurse cells, or both, no eggs would be produced. Surprisingly, we found that these flies efficiently produced eggs for at least 10 d after eclosion of the adults. Thus, in contrast to several larval cell types (Neufeld and Rubin, 1994), female germline stem cells and developing germ cells appear to be able to divide without Pnut.
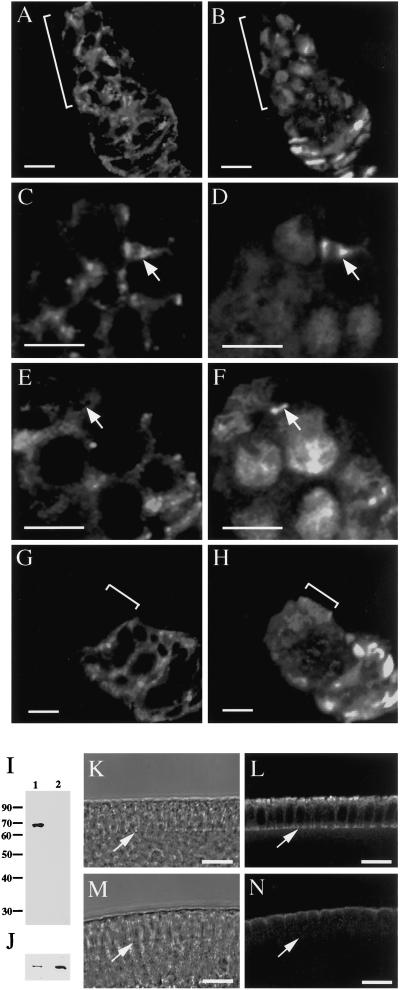
One possibility raised by these results was that Pnut is normally not expressed in the female germline stem cells and cystoblasts. However, staining of wild-type ovarioles showed that Pnut is present in these cells (Figure 1, A and B) and is indeed present in their cleavage furrows (Figure 1C and our unpublished observations), where it colocalizes with anillin (Figure 1D). Female germline stem cells do not complete division during telophase but instead maintain a cytoplasmic connection (the “stem-cell ring canal”) between the stem cell and its daughter cystoblast during part of the subsequent interphase (de Cuevas and Spradling, 1998). Interestingly, despite its consistent presence in stem-cell cleavage furrows, Pnut was not always detectable in the stem-cell ring canals (Figure 1, E and F, arrows), and, when it was detectable, it did not precisely colocalize with anillin (our unpublished observations).
Figure 1.
Presence of Pnut in wild-type stem cells and absence of Pnut in pnut germline clones. (A–H) Immunofluorescence micrographs of wild-type (A–F) and pnut germline-clone (G and H) ovarioles, showing germaria that were double-stained with the 4C9 anti-Pnut antibody (A, C, E, and G) and with antianillin (B, D, F, and H) antibody. (A and B) Presence of Pnut in cells throughout the region of germline proliferation (indicated by brackets) in wild-type germaria. (C and D) Presence of Pnut in stem-cell cleavage furrows (arrows). (E and F) Absence of Pnut from some stem-cell ring canals (arrows). In F, note the presence of anillin in the nuclei, showing that the cells have progressed into interphase (Field and Alberts, 1995; de Cuevas and Spradling, 1998). (G and H) Absence of Pnut from the region of germline proliferation (brackets) in germline-clone germaria. The Pnut-expressing cells in G are the somatic follicle cells that surround the germline cells after germline proliferation is complete. (I–N) Absence of Pnut from germline-clone embryos. Embryos were collected from the wild-type stock and from germline-clone females that had been allowed to mate with their siblings. (I and J) Western blots of extracts of 0–3-h embryos of wild-type (lane 1) and pnut germline clones (lane 2) stained with the KEKK anti-Pnut antibody (I) or with anti-Bicaudal D antibody as a loading control (J). (K–N) Transmitted-light (K and M) and immunofluorescence (L and N) micrographs of cellularizing wild-type (K and L) and germline-clone (M and N) embryos stained with the KEKK antibody. Arrows indicate the position of the cellularization front. A weak apical background staining is seen in germline-clone embryos with this antibody. The 4C9 anti-Pnut monoclonal antibody also showed no Pnut staining at the cellularization front but had a different background pattern (our unpublished observations). Bars, 5 μm (A–H), 10 μm (K–N).
A second possibility was that cell divisions could occur in pnut germline-clone stem cells and cystoblasts simply because of perdurance of Pnut protein. However, Pnut was not detectable in the germline cells of pnut germline-clone ovarioles from 4-d-old females (Figure 1, G and H). Staining of such ovarioles with antitubulin and antianillin antibodies (our unpublished observations) showed that at least some stem cells were still dividing actively in females of this age. Interestingly, the morphology of the germarium was often abnormal, with region 1 (where germline proliferation occurs) being shorter than normal (compare the bracketed region in Figure 1, G and H, with that in Figure 1, A and B), suggesting that although Pnut is dispensable for cell division, it may play some role in the morphogenesis or cell cycle of these cells. Perhaps related to this, the eggs derived from germline clones were ∼70% the length of wild-type eggs and were abnormally round.
To confirm that the embryos derived from germline-clone eggs were indeed devoid of Pnut, we performed both Western blots and immunofluorescence staining. In extracts of wild-type embryos, we observed a polypeptide of ∼65 kDa, close to the predicted molecular mass of Pnut (Figure 1I, lane 1). Neither this polypeptide nor smaller cross-reacting polypeptides were observed in extracts from germline-clone embryos (Figure 1I, lane 2), even though staining with antibodies to the unrelated protein Bicaudal D showed that there was considerably more protein in this lane (Figure 1J, lanes 1 and 2). Moreover, Pnut was readily detectable at the cellularization front in wild-type embryos (Figure 1, K and L, arrows) but not in germline-clone embryos (Figure 1, M and N, arrows). Thus, as expected, the germline-clone embryos appeared to be devoid of Pnut protein.
Abnormal Morphogenesis in Pnut-depleted Embryos
Germline-clone females were crossed to either pnutrN498/CyO or wild-type males. In both crosses, all embryos died without hatching to larvae. Thus, maternally contributed Pnut appears to be essential for embryogenesis, and a zygotic copy of the gene cannot rescue the lethality due to depletion of the maternal supply. Flies homozygous for the pnutrN498 chromosome could be rescued to full adult viability and fertility by a pnut+ transgene (Table 1, stock 13), confirming that the lethality was due to the pnut mutation.
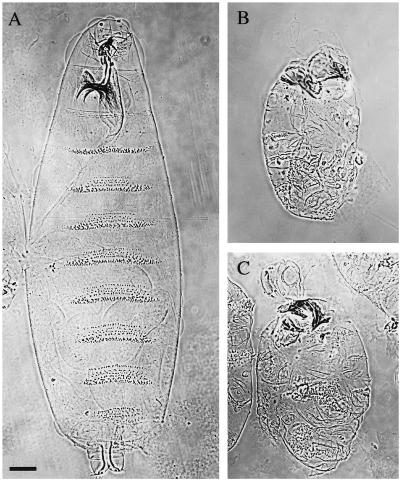
As a first step in evaluating embryonic morphogenesis in the Pnut-depleted embryos, we examined the cuticles produced by the embryos prior to their death. The cuticle is an exoskeleton deposited by epidermal cells of embryos shortly prior to hatching; its structure reflects the differentiated identities of the underlying epidermal cells. Wild-type cuticles typically fill the eggshell, possess distinguishable head and tail structures, and have discrete bands of denticle-containing and denticle-free cuticle (corresponding to the larval segments) along the ventral surface (Figure 2A). pnut germline-clone embryos displayed a characteristic but variable cuticle phenotype. The cuticles were always much smaller than those from wild type and did not usually fill the eggshell (Figure 2, B and C; our unpublished observations). This suggests that the Pnut-deficient embryos develop or maintain fewer epidermal cells than do normal embryos. The head and tail structures also were severely defective (Figure 2, B and C). In addition, although some denticles could be identified and were usually grouped into clusters that might be called bands, these bands varied considerably in number (from 0 to 5, with 3 about average), shape, size, and position. Thus, the pnut germline-clone embryos experienced major (albeit somewhat variable) defects in morphogenesis.
Figure 2.
Abnormal cuticle phenotype of pnut germline-clone embryos. In A–C, the anterior of the embryo is up. (A) Wild-type embryo. (B and C) Representative embryos from a mating of germline-clone females to their siblings. Bar, 50 μm.
To ask if there were any rescue of these defects by zygotically supplied Pnut, we compared the ranges of cuticle phenotypes in the crosses of germline-clone females to pnutrN498/CyO and wild-type males. The types and severities of defects were similar in the two crosses, suggesting that at least some of the developmental processes that cause these defects occur early, prior to activation of the zygotic transcriptional machinery.
As an alternative method to analyze the Pnut-deficient phenotype, we examined embryos derived from females whose only functional pnut gene was under the control of a heat–shock promoter (HS-pnut+; Table 1, stock 9). By a regimen of daily heat shocks during larval and pupal development, adults homozygous for the pnutxp null allele (Neufeld and Rubin, 1994) could be produced. (Flies homozygous for this allele were rescued to full viability and fertility by a transgene in which pnut+ was under the control of its own promoter [Table 1, stock 14], demonstrating that the phenotypes described below were due to the pnut mutation.) The heat shocks were ended upon eclosion or at various times after eclosion of the adult flies to limit and vary the maternal Pnut contribution to the germline. This method was expected to allow generation of embryos only partially depleted of Pnut, and thus observation of defects in later stages of embryonic development. The HS-pnut+ females efficiently produced eggs. However, in most crosses either to wild-type or to pnutrN498/CyO males, all of the embryos died without hatching, even when the heat shocks were continued for 1 to 5 d after eclosion of the adult flies, and even when we focused on the first eggs laid, which should have had the greatest maternal Pnut contribution. (In two early experiments, 4 and 16% of the fertilized eggs hatched, but this probably resulted from the presence of contaminating heterozygous females.) In contrast, wild-type females subjected to heat shocks in parallel displayed efficient hatching (and subsequent development) of fertilized eggs. We compared the cuticle and cytoskeletal (see below) phenotypes of embryos from HS-pnut+ females crossed to either wild-type or pnutxp/CyO males with those of germline-clone embryos. The types and severities of the defects in the embryos that failed to hatch were similar in all crosses, suggesting that embryos derived from HS-pnut+ females exhibit developmental defects as early as do germline-clone embryos. This is probably not surprising given that the hsp70 promoter cannot drive gene expression during late oogenesis or early blastoderm development (Wang and Lindquist 1998). We were therefore unable to analyze the role of Pnut in late developmental processes.
Abnormal Cellularization and Gastrulation in Pnut-depleted Embryos
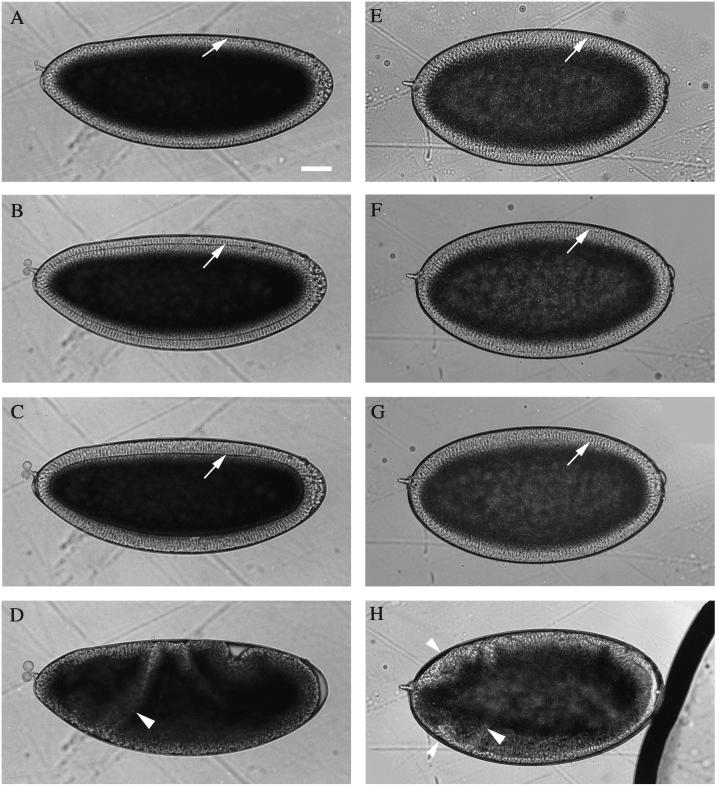
Because Pnut and other septins are concentrated at the leading edge of the furrow during cellularization, we examined the effect of Pnut depletion on this process. Similar results were obtained with germline-clone embryos and with embryos derived from HS-pnut+ females. To look for possible global defects, we observed the development of living Pnut-depleted embryos. Events prior to gastrulation usually appeared to occur normally: a layer of cleared cytoplasm like that in wild-type embryos (Figure 3A) formed at the cortex of mutant embryos (Figure 3E), indicating that nuclei arrived and were maintained at the cortex, and cellularization began and proceeded evenly over the entire embryo at a normal speed and to a normal depth (Figure 3, B, C, F, and G), although the cellularization front typically appeared somewhat less well defined than in wild-type embryos. Global defects were generally not evident until gastrulation onset, when characteristic morphogenetic features associated with gastrulation were observed to be abnormal; for example, an ectopic fold (Figure 3H, small arrowheads) was often seen anterior to the cephalic furrow (Figure 3, D and H, large arrowheads). In addition, the posterior midgut plate often migrated to the embryo's left or right instead of dorsally (our unpublished observations). The apparently successful completion of cellularization suggested either that Pnut is dispensable for this process or that it is required for some aspect(s) of furrow structure or function that is not visible in living embryos, where only the overall progress of the leading edge can be observed. In the latter case, the defects in gastrulation might be (at least in part) consequences of the cellularization defect(s).
Figure 3.
Time-lapse observations on living embryos during cellularization and early gastrulation. (A–D) A wild-type embryo. (E–H) An embryo from the mating of HS-pnut+ flies. Anterior is to the left and dorsal is up. The dark area filling most of the interior of each embryo is the yolk-containing cytoplasm; the transparent layer contains the cortical nuclei and yolk-free cytoplasm; the cellularization front (arrows) is visible as a thin line running parallel to the surface. (A and E) Early cellularization; (B and F) slow phase; (C and G) fast phase; (D and H) early gastrulation. Large arrowheads, cephalic furrows; small arrowheads, an ectopic fold (see text). Bar, 50 μm.
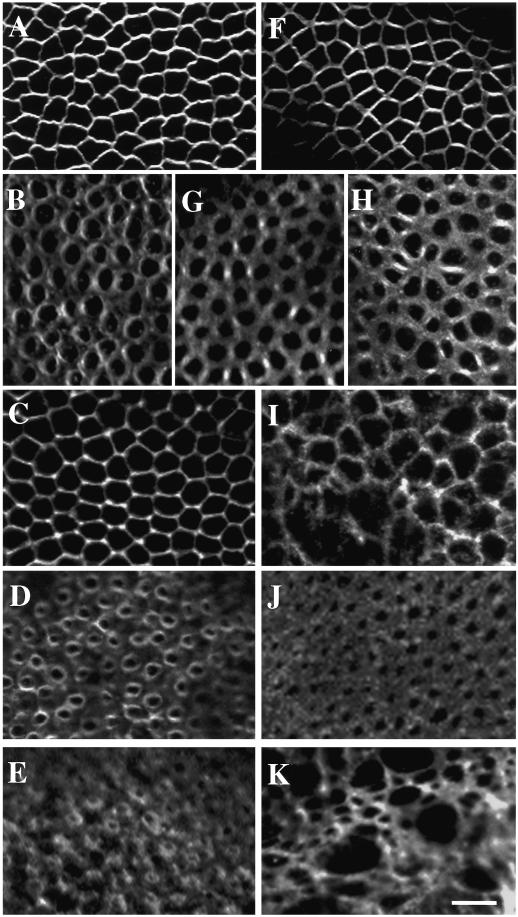
To investigate these possibilities, we stained Pnut-depleted embryos at different stages for F-actin. Actin organization during events prior to cellularization, such as the syncytial blastoderm divisions and formation of the pole cells (the future germ cells), was approximately normal (our unpublished observations). In addition, the embryos were able to organize a normal-looking actin cytoskeleton at the beginning of cellularization and retained normal-looking actin organization through the slow phase of this process (Figure 4, A and F; and our unpublished observations). However, during the subsequent fast phase, although most embryos appeared grossly normal (Figure 5, A and G), close examination of the leading edge of the furrow revealed defects in the organization of F-actin at the bases of the forming cells. At this stage in wild-type embryos, actin is concentrated in a ring surrounding the base of each cell, with smaller amounts present elsewhere in the plane of the leading edge (Figures 4B and 5B). In contrast, in Pnut-deficient embryos, the actin did not resolve into discrete rings and was more uniformly distributed in the plane of the leading edge, except for the presence of concentrated “bars” of actin between some pairs of cell bases (Figure 4, G and H; Figure 5H). About 10% of the germline-clone embryos exhibited regions where the bases of cells were unevenly closed (Figure 4H). In the abnormal regions of such embryos, there was an associated disruption of the actin cytoskeleton along the lateral surfaces of the forming cells (Figure 4I; cf. the wild-type embryo in Figure 4C) and occasional abnormal displacement of nuclei from the cortex (Figure 5H, arrow). Later in the fast phase, at the stage when a wild-type embryo has actin organized in a discrete ring around the yolk plug at each cell base (Figure 4D), the abnormality of actin organization around the bases of Pnut-deficient cells was more consistent and more conspicuous, with the actin displaying a punctate distribution throughout the plane of the leading edge and actin bars no longer visible between pairs of cell bases (Figure 4J). In summary, it appeared that the cellularization machinery was initially assembled normally but did not maintain its organization as cellularization proceeded.
Figure 4.
Apparently normal F-actin organization during the slow phase of cellularization and abnormal F-actin organization during the fast phase of cellularization and early gastrulation in pnut germline-clone embryos. Wild-type (A–E) and germline-clone (F–K) embryos were stained with BODIPY FL-phallacidin and viewed at the level of the cellularization front (A, B, D–H, J, and K) or apical to the front (C and I). (A and F) Slow phase; (B, C, and G–I) early fast phase; (D and J) late fast phase; (E and K) early gastrulation. (G and J) Representatives of the majority class (∼90%) of pnut germline-clone embryos; (H and I) representatives of the minority class (∼10%) of pnut germline-clone embryos. Bar, 5 μm.
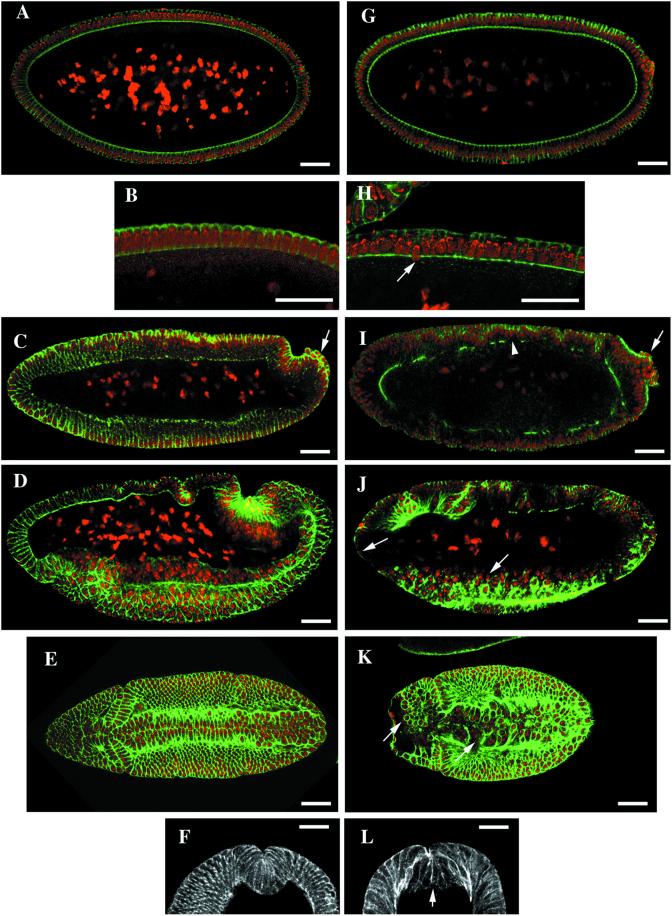
Figure 5.
Largely normal cellularization and grossly defective gastrulation in pnut germline-clone embryos. Wild-type (A–F) and pnut germline-clone (G–L) embryos were stained with BODIPY FL-phallacidin (green) and propidium iodide (red) (A–E and G–K) or with BODIPY FL-phallacidin alone (F and L). (A–D and G–J) Sagittal optical sections; anterior is to the left and dorsal is up. (A, B, G, and H) Embryos in the fast phase of cellularization. Arrow, a nucleus positioned inappropriately with respect to the cortex. (C and I) Embryos at gastrulation onset. Arrows, pole cells; arrowhead, a disruption in the cytoskeleton at the cell bases. (D and J) Embryos during early gastrulation. Arrows, examples of nuclei not surrounded by an actin cytoskeleton at the anterior end and in the ventral furrow. (E and K) Surface sections at the time of ventral-furrow formation: anterior is to the left, ventral face is shown. Arrows, nuclei not surrounded by an actin cytoskeleton. (F and L) Cross sections showing the ventral furrow. Arrow, base of a ventral-furrow cell. Bars, 50 μm.
Because the yolk plugs persist well into gastrulation in wild-type embryos, one might expect that the disruption of the associated cytoskeleton in Pnut-deficient embryos would produce problems during gastrulation. Indeed, gross morphological abnormalities were typically first seen at the beginning of gastrulation, when the bases of many cells appeared to reopen (Figure 4K; cf. the wild-type embryo in Figure 4E), the distribution of actin along the cellularization front became much more patchy than in wild-type embryos (Figure 5, C and I), cell integrity appeared to be disrupted over large parts of the embryo (e.g. Figure 5I, arrowhead), and germ band extension was delayed, as indicated by the position of the pole cells and the morphology of the underlying epithelial cells (Figure 5, C and I, arrows). In slightly older embryos, nuclei could be seen that were no longer surrounded by an actin cytoskeleton (Figure 5, J and K, arrows; cf. the wild-type embryos in Figure 5, D and E), and small areas at the anterior of the embryo appeared to be devoid of nuclei (Figure 5K). In addition, the actin cytoskeleton in the ventral furrow was grossly disorganized (Figure 5, K and L; cf. the wild-type embryos in Figure 5, E and F); cross sections showed that the cell bases were compromised and contained less (and seemingly less well organized) actin than normal (Figure 5L).
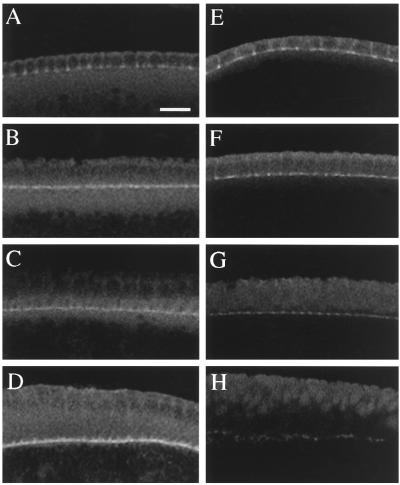
The interpretations based on actin staining were supported by examination of the actin-associated protein anillin. The localization of anillin appeared normal during the slow phase of cellularization (Figure 6, A and E) but became progressively more abnormal as cellularization proceeded, with progressively more anillin seen in the cytoplasm (Figure 6F), and later in the nucleus (Figure 6, G and H), than in wild-type embryos at the same stages (Figure 6, B–D). Anillin was still detectable at the leading edge even during early gastrulation, but its concentration there was greatly reduced (Figure 6H) compared with that in wild-type embryos (Figure 6D).
Figure 6.
Progressive loss of anillin localization from the cellularization front in pnut germline clones. Wild-type embryos (A–D) and pnut germline-clone embryos (E–H) were stained with antianillin antibody. (A and E) Slow phase of cellularization; (B and F) early fast phase; (C and G) late fast phase; (D and H) early gastrulation. Bar, 10 μm.
Localization of Other Septins in Pnut-deficient Embryos
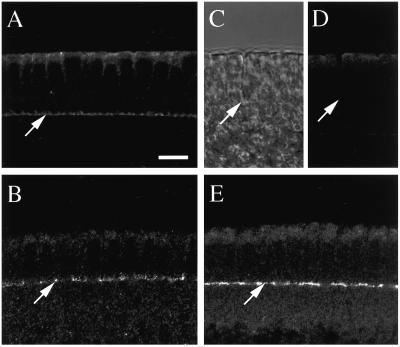
Previous observations had suggested that localization of the fly septins was interdependent (see INTRODUCTION). To test this hypothesis, the localizations of Sep1 and Sep2 to the cellularization front were analyzed in the pnut germline-clone embryos. Remarkably, although Sep1 was not detectable at the cellularization front in such embryos (Figure 7, A, C, and D), Sep2 appeared to be present in approximately normal levels throughout cellularization (Figure 7, B and E).
Figure 7.
Localization of Sep2, but not of Sep1, to the cellularization front in pnut germline-clone embryos. Wild-type (A and B) and pnut germline-clone (C–E) embryos were stained with anti-Sep1 (A and D) or anti-Sep2 (B and E) antibodies. (C) Transmitted-light image of the region shown in D. Arrows indicate the cellularization fronts. Bar, 10 μm.
DISCUSSION
The Role of the Septins in Ordinary Cytokinesis
In all fungal and animal cell types examined to date, the septins localize to the site of cytokinesis, and genetic and antibody-microinjection experiments have indicated that the septins are indeed essential for cytokinesis in S. cerevisiae and mammalian cells (see INTRODUCTION). Moreover, Drosophila mutants homozygous for a pnut null mutation are apparently defective in cytokinesis in several larval tissues, including the brain, lymph glands, and imaginal disks, and HS-pnut+ females held at noninducing temperatures for several days exhibit cytokinesis defects in ovarian follicle cells (Neufeld and Rubin, 1994). Thus, it seemed likely that other cytokinesis events in Drosophila would also be septin dependent. Because the available data further suggested that Pnut functions as part of a complex with at least two other septins (see INTRODUCTION), it also seemed likely that cytokinesis events in Drosophila would all depend on Pnut itself. Thus, we expected that failure of cytokinesis in the germline stem cells and/or in the derived cystoblasts would preclude the production of eggs by germline clones homozygous for a pnut null mutation. However, we found that germline-clone females produced by induction of mitotic recombination in second-instar larvae could produce Pnut-depleted eggs for at least 10 d after the eclosion of adults. By this time, the original germline cells that had become homozygous for the pnut mutation should have divided many times (Lin, 1997). This appears to eliminate the possibility that perdurance of Pnut protein or mRNA could account for the continued divisions of the germline cells, and indeed we detected no Pnut protein in the germline cells of such females or in the germline-clone embryos. Thus, we must conclude either that these divisions do not depend on the septins at all or that an essential septin role can be filled by a subset of the septins that does not include Pnut (or, presumably, Sep1, given the demonstrated dependence of Sep1 localization on Pnut in other contexts, as discussed below).
Such conclusions seem particularly surprising for the stem-cell divisions. In contrast, because the cystoblast divisions do not normally go to completion (the developing nurse cells and oocyte remain permanently connected by cytoplasmic channels, the “ring canals” [Cooley and Robinson, 1996]), their ability to proceed in the absence of Pnut might mean simply that the septins are normally required only for a late stage in cytokinesis. However, the localization of Pnut in wild-type germaria complicates this interpretation. The completion of stem-cell divisions occurs only after a delay (until the beginning of the next S phase), during which the stem cell remains connected to its cystoblast daughter by the stem-cell ring canal (de Cuevas and Spradling, 1998). Although Pnut was found in the stem-cell cleavage furrows during cytokinesis, it was usually not detected in the stem-cell ring canals and is therefore presumably absent during the completion of division. Thus, stem cells in the female germline must use a Pnut-independent mechanism to complete division (consistent with the implications of the germline-clone data). Moreover, Pnut is dispensable for the early stages of stem-cell cytokinesis even though it is present in the cleavage furrow. Distinguishing unambiguously among the possible interpretations of these results will presumably require genetic analysis of other septins, which is in progress.
The Role of the Septins in Cellularization
In wild-type embryos, Pnut, Sep1, and Sep2 are all localized to the cellularization front throughout cellularization. We therefore expected that Pnut would be essential for the entire process, so that depleting the maternal contribution would result in severe defects early in cellularization. Instead, we observed that the initial slow phase was apparently normal and that even the subsequent fast phase was only subtly abnormal. As in the case of cytokinesis in the female germline, this suggests either that the septins are not essential for these processes or that Pnut (and thus Sep1) is nonessential because an essential septin role can be filled by another subset of the septins. Consistent with the latter possibility, Sep2 appeared to localize normally to the cellularization front in the Pnut-deficient embryos, as discussed further below.
Cellularization in the absence of Pnut did not result in normal cells, indicating that Pnut is indeed required for some aspect(s) of cellularization. Analysis of actin and anillin organization at the leading edge of the cellularization furrow indicated that Pnut is required for maintenance of actin organization, and probably generally for maintenance of the cellularization machinery, at the leading edge during the fast phase. This subtle defect was followed by gross morphological defects upon gastrulation onset, when the cytoskeleton appeared to disintegrate beginning at the basal ends of some cells, such as those of the ventral furrow. One possible interpretation of these observations is that when actin organization at the leading edge is disrupted, ring closure proceeds with reduced efficiency, so that it is sensitive to the competing forces that come into play in cells undergoing movements and/or shape changes during gastrulation. This interpretation may also explain the observation that defects in overall cell structure were sometimes observed during the fast phase of cellularization, because a process occurring with reduced efficiency might occasionally exhibit defects more severe than the norm.
Functional Differentiation among the Drosophila Septins
Previously available data had suggested that Pnut, Sep1, and Sep2 function as a complex, so that the absence of Pnut was expected to ablate the localization and function of all three septins. However, the observation that Sep2 remained at the cellularization front in the absence of Pnut, although Sep1 did not, suggests that the reality is not so simple, and that there may exist alternative septin complexes and/or cell-type–specific differences in septin expression or function. It should be noted in this context that the available Sep2 antibodies cross-react, albeit poorly, with Sep5 (our unpublished observations), reflecting the fact that the two proteins are ∼70% identical in sequence. Thus, the cellularization front may contain Sep5 in addition to (or conceivably instead of) Sep2. However, this would not alter the central conclusion that some septin(s) is able to localize to the cellularization front in the absence of Pnut and Sep1. It is also possible that assembly of the septin complex at the cellularization front is hierarchical, with Sep2 (and/or Sep5) nucleating the complex and then recruiting Pnut and hence Sep1. In this case, the Sep2 observed at the cellularization front could be nonfunctional in the absence of Pnut and Sep1. However, even on this model, Sep2 would be showing a functional differentiation from the other septins.
The possibility of functional differentiation among the Drosophila septins has some precedents. For example, in S. cerevisiae, two of the seven septins are expressed only in sporulating cells (De Virgilio et al., 1996; Fares et al., 1996). Moreover, although the five S. cerevisiae septins involved in vegetative growth all localize to the mother-bud neck (Longtine et al., 1996; Carroll et al., 1998; Mino et al., 1998) and appear to be present in a complex (Carroll et al., 1998; Frazier et al., 1998), only Cdc3p and Cdc12p appear to be essential under all growth conditions (Longtine et al., 1996). In contrast, deletion of SEP7 produces little effect in a wild-type background (Carroll et al., 1998; Mino et al., 1998), and cells deleted for CDC10 or CDC11 also survive although they are temperature sensitive and morphologically abnormal (Flescher et al., 1993; Longtine et al., 1996; Frazier et al., 1998). In the absence of either Cdc10p or Cdc11p, a complex containing the other vegetatively expressed septins still forms and is apparently at least partially functional (Frazier et al., 1998). In addition, in mammals, there appear to be marked differences in the expression of different septins in different cell types (Yagi et al., 1998; Xie et al., 1999; Kinoshita, personal communication; Valencik and Pringle, unpublished data), and some septins, but not others, colocalize with actin stress fibers in interphase cells (Kinoshita et al., 1997; Xie et al., 1999; Kinoshita, personal communication).
The Relationship between the Septins and the Actomyosin Contractile Ring
In S. cerevisiae, intact septin structures at the neck are required for the formation of the actomyosin contractile ring (Bi et al., 1998; Lippincott and Li, 1998). However, actomyosin-ring assembly in this organism is unusual in that the septin-dependent assembly of a myosin ring occurs very early in the cell cycle (prior to bud emergence), whereas the myosin-dependent assembly of the associated actin does not occur until just prior to cytokinesis. Thus, it is not clear whether actomyosin-ring assembly in other cell types should be expected to be septin dependent, and there are as yet no published data that address this point decisively. In the studies reported herein, we have shown that germline-stem-cell and cystoblast divisions, which presumably require successful actomyosin-ring assembly and function (Miller and Kiehart, 1995), can occur without Pnut (and thus, presumably, without assembled Sep1). Moreover, we have shown directly that the actin network of the cellularization front assembles and begins to function apparently normally in the absence of Pnut and Sep1. However, as noted above, it remains possible that assembly of germline-cell contractile rings and the cellularization machinery depends on a different subset of the septins. On this model, the progressive loss of organization of the actin network late in cellularization in the Pnut-depleted embryos would reflect a partial dependency of this network on Pnut and Sep1, and loss of additional (or other) septins would produce earlier and more severe effects on the formation and/or maintenance of this network. Our observations on cellularization also could be explained if the initial organization of the actomyosin network (which has no close parallel in ordinary cytokinesis) were indeed septin independent, and the later reorganization of this network into discrete rings (perhaps more similar to those that function during ordinary cytokinesis) were septin dependent.
Nonetheless, it is worth considering the possibility that the conserved role of the septins in cytokinesis does not relate to actomyosin-ring assembly. In S. cerevisiae, the actomyosin ring is not essential for cytokinesis, as deletion of the MYO1 gene ablates actin-ring formation but does not block cytokinesis (Bi et al., 1998; Lippincott and Li, 1998). In contrast, loss of septin function completely blocks cytokinesis (Hartwell, 1971; Longtine et al., 1996). Thus, there must be an alternative, septin-dependent mechanism by which cytokinesis can be completed in this organism. The nature of this alternative mechanism is not known, but it seems likely to involve the localized extension of plasma membrane and/or deposition of extracellular material (cell wall). Such a role for the septins would be consistent with other evidence for their involvement in localized cell-wall deposition in yeast (DeMarini et al., 1997) and in vesicle-trafficking and/or exocytosis in mammalian cells (Hsu et al., 1998; Beites et al., 1999; Trimble, 1999), as well as with other evidence for the importance of localized membrane addition in cytokinesis in various cell types (for review, see Hales et al., 1999). If the septins' role in cytokinesis in animal cells is of this type, then the progressive loss of organization of the actin network during cellularization in the Pnut-depleted embryos could be viewed as secondary to other defects in the assembly or progression of the cellularization front.
ACKNOWLEDGMENTS
We thank Tom Neufeld, Gerald Rubin, and the Bloomington Stock Center for stocks; Christine Field for antibodies, stimulating discussions, and communication of results prior to publication; Makoto Kinoshita, William Sullivan, and Daniel Kiehart for valuable discussions and communication of results prior to publication; Beat Suter for antibodies; Tony Perdue and Susan Whitfield for help with confocal microscopy and photography; and members of the Pringle and Peifer laboratories for encouragement, discussion, and comments on the manuscript. This work was supported by National Institutes of Health grant GM 52606 to J.R.P. and M.P.
REFERENCES
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltagarone J, Rhodes J, Honer WG, Bowser R. Localization of a novel septin protein, hCDCrel-1, in neurons of human brain. NeuroReport. 1998;9:2907–2912. doi: 10.1097/00001756-199808240-00042. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Altman R, Schieltz D, Yates JR, III, Kellogg D. The septins are required for the mitosis-specific activation of the Gin4 kinase. J Cell Biol. 1998;143:709–717. doi: 10.1083/jcb.143.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Mischke M, Mitchell E, Herskowitz I, Pringle JR. Role of Bud3p in producing the axial budding pattern of yeast. J Cell Biol. 1995;129:767–778. doi: 10.1083/jcb.129.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L, Robinson DN. Stable intercellular bridges in development, the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6:474–479. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Kiehart DP. Septins may form a ubiquitous family of cytoskeletal filaments. J Cell Biol. 1996;134:1345–1348. doi: 10.1083/jcb.134.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AEM, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C, DeMarini DJ, Pringle JR. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology. 1996;142:2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- Fares H, Goetsch L, Pringle JR. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J Cell Biol. 1996;132:399–411. doi: 10.1083/jcb.132.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol Biol Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Al-Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer BK, Pringle JR. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol Cell Biol. 1987;7:3678–3687. doi: 10.1128/mcb.7.10.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales KG, Bi E, Wu J-Q, Adam JC, Yu I-C, Pringle JR. Cytokinesis: an emerging unified theory for eukaryotes? Curr Opin Cell Biol. 1999;11:717–725. doi: 10.1016/s0955-0674(99)00042-3. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Hsu S-C, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin H. The tao of stem cells in the germline. Annu Rev Genet. 1997;31:455–491. doi: 10.1146/annurev.genet.31.1.455. [DOI] [PubMed] [Google Scholar]
- Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Fares H, Pringle JR. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Pringle JR. Septins. In: Kreis T, Vale R, editors. Guidebook to the Cytoskeletal and Motor Proteins. New York: Oxford. University Press; 1999. pp. 359–363. [Google Scholar]
- Miller KG, Field CM, Alberts BM. Actin-binding proteins from Drosophila embryos: a complex network of interacting proteins detected by F-actin affinity chromatography. J Cell Biol. 1989;109:2963–2975. doi: 10.1083/jcb.109.6.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Kiehart DP. Fly division. J Cell Biol. 1995;131:1–5. doi: 10.1083/jcb.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino A, Tanaka K, Kamei T, Umikawa M, Fujiwara T, Takai Y. Shs1p: a novel member of septin that interacts with Spa2p, involved in polarized growth in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1998;251:732–736. doi: 10.1006/bbrc.1998.9541. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. A method to stain nuclei of Drosophila for confocal microscopy. BioTechniques. 1994;16:441–447. [PubMed] [Google Scholar]
- Peifer M. The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J Cell Sci. 1993;105:993–1000. doi: 10.1242/jcs.105.4.993. [DOI] [PubMed] [Google Scholar]
- Peifer M, Orsulic S, Sweeton D, Wieschaus E. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- Rickoll WL, Counce SJ. Morphogenesis in the embryo of Drosophila melanogaster - germ band extension. Wilhelm Roux's Arch. 1980;188:163–177. doi: 10.1007/BF00849045. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Herskowitz I. The Bud4 protein of yeast, required for axial budding, is localized to the mother/bud neck in a cell cycle-dependent manner. J Cell Biol. 1996;134:413–427. doi: 10.1083/jcb.134.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter ED, Wieschaus E. Functional elements of the cytoskeleton in the early Drosophila embryo. Annu Rev Cell Biol. 1993;9:67–99. doi: 10.1146/annurev.cb.09.110193.000435. [DOI] [PubMed] [Google Scholar]
- Suter B, Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991;67:917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Behavior of structurally divergent α-tubulin isotypes during Drosophila embryogenesis: evidence for post-translational regulation of isotype abundance. Dev Biol. 1992;154:205–217. doi: 10.1016/0012-1606(92)90060-t. [DOI] [PubMed] [Google Scholar]
- Trimble WS. Septins. A highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J Membr Biol. 1999;169:75–81. doi: 10.1007/s002329900519. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lindquist S. Developmentally regulated nuclear transport of transcription factors in Drosophila embryos enable the heat shock response. Development. 1998;125:4841–4850. doi: 10.1242/dev.125.23.4841. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts DB, editor. Drosophila, A Practical Approach. Oxford: IRL Press; 1986. pp. 199–227. [Google Scholar]
- Xie H, Surka M, Howard J, Trimble WS. Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other septin proteins. Cell Motil Cytoskeleton. 1999;43:52–62. doi: 10.1002/(SICI)1097-0169(1999)43:1<52::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Yagi M, Zieger B, Roth GJ, Ware J. Structure and expression of the human septin gene HCDCREL-1. Gene. 1998;212:229–236. doi: 10.1016/s0378-1119(98)00146-2. [DOI] [PubMed] [Google Scholar]