Abstract
Telomeres, the non-coding sequences at the ends of chromosomes, in the absence of telomerase, progressively shorten with each cell division. Shortening of telomeres can induce cell cycle arrest and apoptosis. The aim of this study was to investigate age- and gender-related changes in telomere length in the rat and to detect possible tissue- specific rates of telomere shortening. Changes with age in telomere lengths were assessed by Southern blotting in the kidney, pancreas, liver, lung and brain of male and female rats. We determined the percentage of telomeres in various molecular size regions rather than measuring the average telomere length. The latter was unable to detect telomere shortening in the tissues. The percentage of short telomeres increased with age in the kidney, liver, pancreas and lung of both males and females, but not in the brain. Males had shorter telomeres than females in all organs analysed except the brain, where the lengths were similar. These findings indicate that telomeres shorten in the rat kidney, liver, pancreas and the lung in an age-dependent manner. These data also provide a novel mechanism for the gender-related differences in lifespan and suggest a tissue-specific regulation of telomere length during development and ageing in the rat.
INTRODUCTION
Telomeres are the physical ends of linear chromosomes. In mammals, telomeres are composed of a variable number of tandem repeats of DNA that are made up of (TTAGGG)n repeats (1). Although the bulk of telomeric DNA is double stranded, the extreme terminus is a single-stranded G-rich 3′ overhang that serves as a template for elongation and forms a telomeric ‘T-loop’. This loop is stabilised by certain telomere-binding proteins, notably TRF1 and TRF2 (2). The functions of telomeres appear to include protection of chromosomes from illegitimate fusion, the localisation of chromosomes in the nucleus and the selective silencing of proximal subtelomeric genes (3). The telomeric repeat sequences are added on by the enzyme telomerase (4,5) which, when present, compensates for the loss of DNA from the end of chromosomes due to incomplete replication. In normal human somatic cells, because of inherent limitations in the mechanics of DNA replication, telomeres shorten at each cell division. In the absence of telomerase, when telomere shortening reaches a critical limit, cells are susceptible to chromosomal aberrations such as end-to-end fusion and aneuploidy. In such a situation, the cells cease to divide and reach replicative senescence. Telomere length in a given cell thus may serve as a marker of its replicative history and of the residual capacity for further cell division.
Although the telomeric sequence was shown to be highly conserved among eukaryotic vertebrates throughout evolution (1), the length of telomeres differs between species. In humans, telomeres are up to 20 kb in length (6). In contrast, rodent telomeres have been reported to be heterogeneous in length (7). Mus musculus has been reported to have telomeres up to 150 kb in size (8). Mus spretus, however, has telomeres with similar lengths to humans (up to 30 kb in size) (7), whereas rat telomere lengths range from 20 to 100 kb (9,10).
In humans, both in vivo and in vitro, telomere shortening appears to be a major component of cell senescence and ageing (reviewed in 11,12). Telomeres have been reported to shorten during post-natal development and ageing in liver (13,14), kidney (15) and lymphocytes (16). However, this is less apparent in mice because of the very long telomeres (30–150 kb). Telomere shortening has been extensively studied in mice, especially in telomerase-deficient knockout mice (17–19). In these mice, the critical RNA component of telomerase, mTERC, has been inactivated by homologous recombination. However, it is only after four to six generations that telomere shortening really becomes an issue (20,21). Thus, there is a need for an experimental animal which more closely mimics the human situation in order to study in more detail and under controlled conditions the role of telomere shortening in tissue and organism senescence. We have reported relationships between kidney telomere shortening and longevity in the rat (10) and a difference in longevity between male and female rats (22). Males have a shortened lifespan compared to their female counterparts. It therefore seemed important to extend these observations to a wider study of inter-tissue and inter-gender features of telomere shortening in the rat.
Therefore, the aim of this study was to investigate, in the rat, telomere changes during post-natal development and ageing in various tissues in vivo and to verify whether there is an effect of gender on the rate of telomere shortening.
MATERIALS AND METHODS
Animals
All the procedures involving animals were conducted under the British Animals (Scientific Procedures) Act (1986). Animals were maintained on standard chow. Food and water were provided ad libitum. Three age groups of male and female Wistar rats were studied (at 21 days and 3 and 15 months of age). At each time point, the males and females used were littermates.
The neonates were killed at 21 days. The remaining animals were maintained individually at 22°C on a controlled 12 h light/dark cycle. They were fed ad libitum and killed at either 3 or 15 months. Kidney, liver, pancreas, lung and brain tissues were collected and snap frozen in liquid nitrogen. Samples were kept at –80°C until the time of DNA extraction and analysis.
Telomere detection
In order to avoid shearing the genomic DNA during extraction, a commercial method (Qiagen, UK) which gave a mean molecular size of genomic DNA of 97 kb was used. Rat tissues were rapidly homogenised in a tissue lysis buffer [800 mM guanidine HCl, 30 mM Tris–HCl (pH 8.0), 5% Tween-20, 0.5% Triton X-100]. The tissue homogenate was incubated overnight at 50°C with 500 µl proteinase K and 19 µl RNase A. DNA was dissolved in 1× Tris EDTA buffer and heated at 50°C for 2 h. The isolated genomic DNA was quantified using a spectrophotometer (GeneQuant; Pharmacia Biotech, UK).
An aliquot (1.2 µg) of DNA was digested with HinfI and RsaI (16.6 U/µg DNA each) (Roche Diagnostics, Mannheim, Germany) at 37°C for 2 h. The digests (20 µl) were loaded onto a 0.8% agarose gel (Bio-Rad, Hercules, CA) and resolved by pulsed field gel electrophoresis (Chef-DR III; Bio-Rad, Hercules, CA) at 6 V/cm with a switching time of 1–30 s for 9 h to resolve DNA sizes between ∼1 kb and 1 Mb.
A Mid Range PFG Marker I (New England Biolabs, MA) as well as an undigested DNA sample were run in each gel. For gender comparison, the digested DNA samples from five tissues of one neonate, one adult (3 month) and one old (15 month) rat were run on the same gel. At each time point, a total of eight males and eight females were analysed. For the ageing part of the study, the digested DNA samples from five tissues of one male and one female were run on the same gel. Every set of samples was run in duplicate on a separate gel. A total of eight male and eight female samples were analysed.
After electrophoresis, gels were examined by ethidium bromide staining and photographed with a P/N Polaroid film for the absence of non-specific degradation and complete digestion for the undigested and digested DNA, respectively. The DNA fragments were then transferred by Southern blotting to a positively charged nylon membrane (Roche Diagnostics, Mannheim, Germany). The transfer was done overnight at room temperature using 20× SSC. The DNA was cross-linked to the nylon membrane using a UV Stratalinker (TM 2400; Stratagene).
The chemiluminescence detection of the telomeric repeats was performed using a slightly modified commercial method (Roche, Germany). The blotted DNA fragments were hybridised to a 5′-TTAGGG-3′ digoxigenin (dig)-labelled probe specific for telomeric repeats. Hybridisation was carried out at 42°C in a Techne-hybridiser HB-1D oven for 3 h and washed as recommended by the manufacturer. The membrane was then incubated with dig-specific antibody covalently coupled to alkaline phosphatase. The telomere probe was visualised by alkaline phosphatase metabolising cdp-star, a highly sensitive chemiluminescence substrate (provided with the kit). The membrane was then exposed to a hyperfilm ECL (Amersham Pharmacia). The DNA blots were analysed using Adobe PhotoShop and MAC Bas computer software.
Analysis and quantification of telomere lengths
There is increasing evidence suggesting that regardless of mean telomere length, one critical short telomere may cause a cell to enter senescence (23). Therefore, we compared telomere length measurement using the more established method based on the average telomere lengths (mean terminal restriction fragments, mean TRF) and the recently described method (10) where we determined the percentage of telomeres in four intervals of length as defined by molecular size markers. Briefly, the intensity (expressed as photo-stimulated luminescence, PSL) was quantified as follows: each telomeric sample was divided into specific grid squares according to the following molecular size ranges: 112–48.5, 48.5–8.6, 8.6–4.2 and 4.2–3.1 kb. The percentage of PSL (% PSL) in each molecular weight range was measured (% PSL = intensity of a defined region – background × 100/total lane intensity – background). For each sample, the average value of % PSL from duplicate analyses was then calculated. Using the same gels, the mean size of the TRF length was estimated in each telomeric smear. All lanes were subdivided into intervals of ∼1–2 mm. The mean size of the TRF was estimated using the formula ∑(ODi – background)/∑(ODi – background/Li) (24), where ODi is the chemiluminescent signal and Li is the length of the TRF fragment at position i.
To minimise any differences arising between gels, a DNA control sample was run on every gel. Any gel where the % PSL in any of the four telomeric regions analysed was >1.5 SD from the mean was discarded from the analysis and that gel repeated.
Statistical analysis
Statistical comparisons were made using the unpaired Student’s t-test or two-way analysis of variance (ANOVA) with age and gender as independent variables followed by Scheffe’s test where appropriate.
RESULTS
Comparison of the two methods of analysis of TRFs
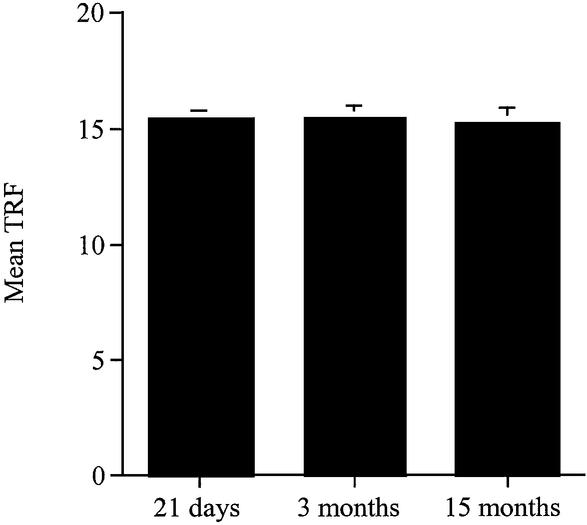
We compared two methods of assessing telomere length: first by measuring the average length and secondly by determining the percentage of the telomeres in various molecular length regions. The latter method was found to be much more sensitive in detecting changes and was therefore preferred. When we used the TRF method to analyse the renal tissue (Fig. 1), we were unable to detect any differences at the three ages studied. However, when we used the new way of analysis of TRF data on the same gels (% PSL), we showed an increase in the shorter telomeres (4.2–1.3 kb) in the male kidneys at 3 months compared to the neonates and at 15 months compared to both the neonates and the 3-month-old rats (Fig. 2B). This suggests that small changes in telomere size are masked when using the mean TRF conventional method. Moreover, the novel method allowed a greater overall interpretation of the range of various sized telomeres which exist within a tissue and helped to determine whether or not the size distribution of telomeres changed with age in male and female Wistar rats.
Figure 1.
Average telomere length (TRF) in the kidney of 21-day-old and 3- and 15-month-old male Wistar rats. Analysis was performed on the same gels used in this study. The TRF in the kidneys was calculated at 21 days and 3 and 15 months.
Figure 2.
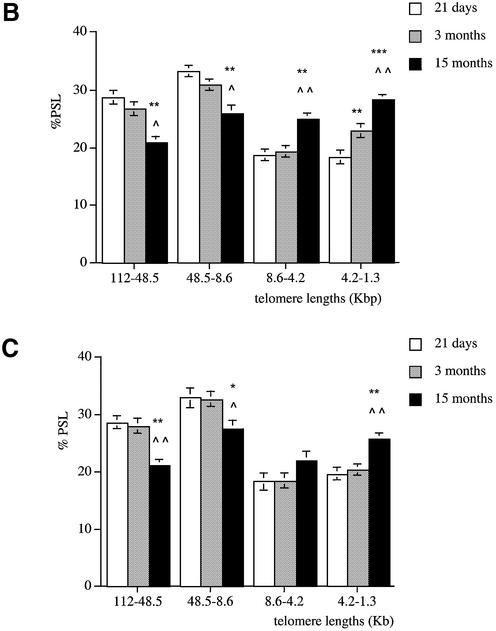
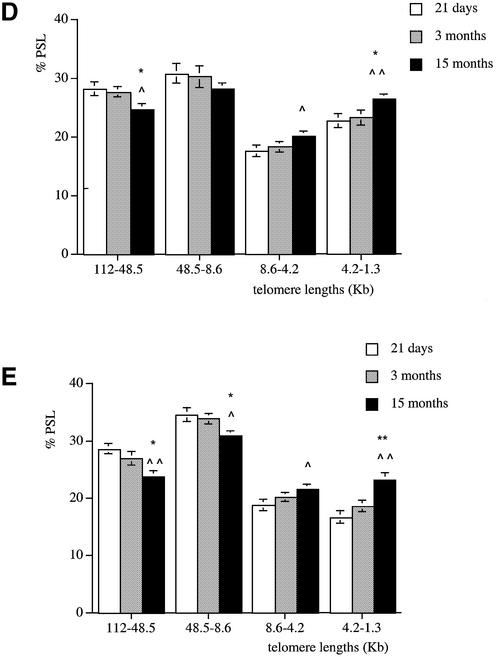
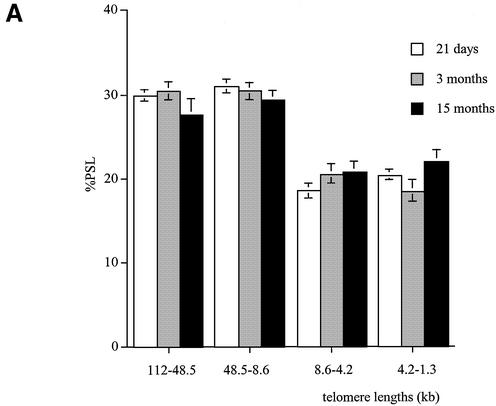
Telomere shortening with age in male Wistar rats. Genomic DNA was extracted from (A) brain, (B) kidney, (C) liver, (D) pancreas and (E) lung at 21 days (white columns), 3 months (grey columns) and 15 months (black columns). Each telomere smear was divided into four regions according to a molecular weight marker: 112–48.5, 48.5–8.6, 8.6–4.2 and 4.2–1.3 kb. Values are means of n = 8 ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the 21-day-old group; ∧P < 0.05, ∧∧P < 0.01 and ∧∧∧P < 0.001 compared to the 3-month-old group using ANOVA 2 test.
Age-related telomere shortening in male rats
We analysed telomere length changes with age in the kidney, liver, pancreas, lung and brain of male Wistar rats at 21 days and 3 and 15 months.
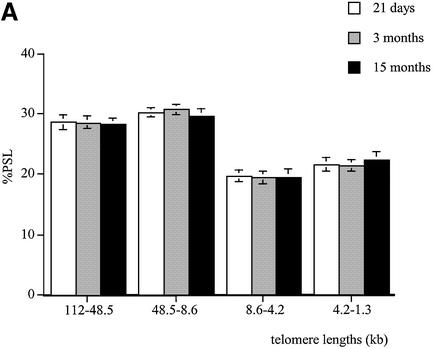
In the brain, telomere lengths remained stable with age in the four telomeric regions. No age-related telomere shortening was detected in the brain of the male rats (Fig. 2A).
In the kidney, 21-day-old males had a higher proportion of telomeres in the 112–48.5 and 48.5–8.6 kb regions compared to both 3- and 15-month-old rats (P < 0.01) (Fig. 2B). The neonates also had a lower proportion of short telomeres in the 4.2–1.3 kb size region compared to 3- and 15-month-old rats (P < 0.01 and P < 0.001, respectively). At 3 months, the kidney had more shorter telomeres in the 4.2–1.3 kb size region than the 21-day-old group (P < 0.01). At 15 months, fewer telomeres were detected in the 112–48.5 and 48.5–8.6 kb size regions compared to 21-day-old and 3-month-old rats (P < 0.01 and P < 0.05, respectively). The 15-month-old group had a higher proportion of short telomeres in the 8.6–4.2 and 4.2–1.3 kb regions when compared to both 21-day-old and 3-month-old rats.
A similar pattern was observed in the liver (Fig. 2C), pancreas (Fig. 2D) and lung (Fig. 2E). Indeed, compared to the old rats, these tissues at 21 days had more long telomeres in the 112–48.5 and 48.5–8.6 kb regions and fewer shorter ones (4.2–1.3 kb). In all the tissues analysed, at 21 days of age telomeres are mainly large in size and show a similar distribution. Apart from the brain, a significant telomere shortening occurs between 3 and 15 months of age in all the tissues with an earlier decline in telomere length observed in the kidney between 21 days and 3 months.
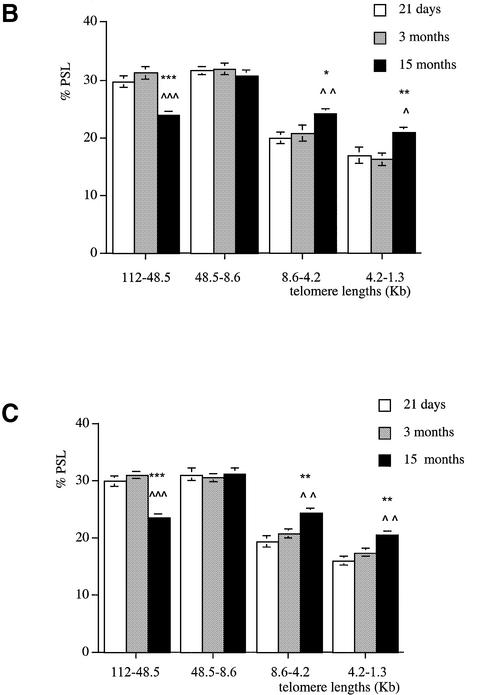
Age-related telomere shortening in females
In female brains, similarly to their male littermates, we could not detect any age-related telomere shortening in this tissue (Fig. 3A). Telomere length distribution in female brains was similar to the one found in males in the four molecular size regions and at the three ages studied.
Figure 3.
Telomere shortening with age in female Wistar rats. Genomic DNA was extracted from (A) brain, (B) kidney, (C) liver, (D) pancreas and (E) lung at 21 days (white columns), 3 months (grey columns) and 15 months (black columns). Each telomere smear was divided into four regions according to a molecular weight marker: 112–48.5, 48.5–8.6, 8.6–4.2 and 4.2–1.3 kb. Values are means of n = 8 ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the 21-day-old group; ∧P < 0.05, ∧∧P < 0.01 and ∧∧∧P < 0.001 compared to the 3-month-old group using ANOVA 2 test.
In renal tissue, the proportion of long telomeres (112–48.5 kb) was high at weaning (P < 0.01) and decreased at 15 months of age (Fig. 3B). In the shortest region (4.2–1.3 kb), 15-month-old female rats showed a significantly higher proportion of telomeres compared to 21-day-old and 3-month-old rats (P < 0.05 and P < 0.01, respectively). In the four regions analysed, kidney telomere lengths were similar in 21-day-old and 3-month-old rats.
Similar changes were observed in the liver (Fig. 3C), pancreas (Fig. 3D) and lung (Fig. 3E). Indeed, these tissues show similar distributions in telomere lengths in the four size regions. Apart from the brain, a significant telomere shortening occurred between 3 and 15 months of age in the female tissues with a greater decline in telomere length in the pancreas (P < 0.001).
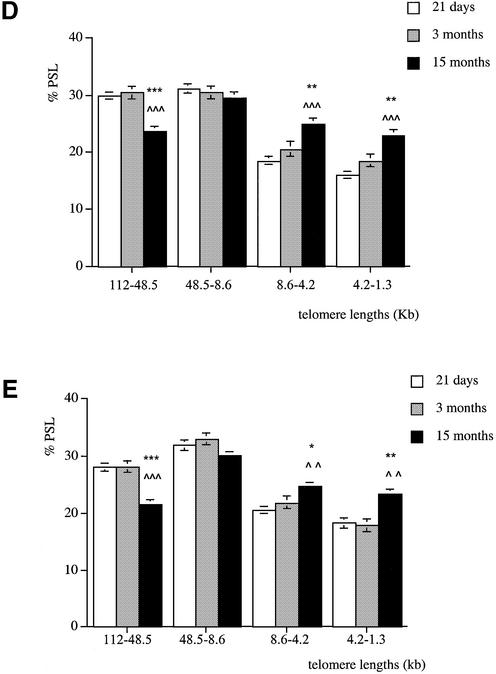
Telomere lengths in male and female tissues
At 21 days, males and females have similar telomere lengths in all the organs analysed.
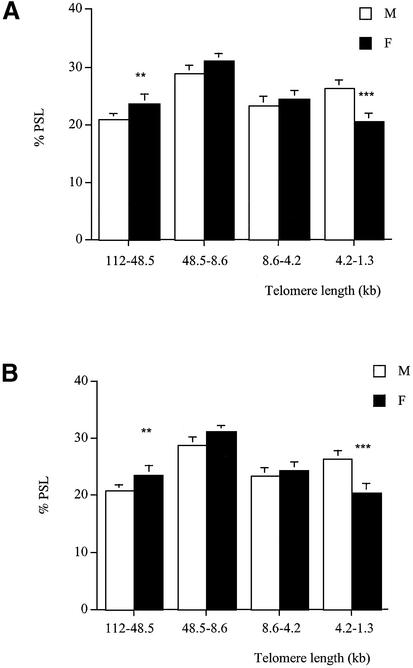
At 3 months of age, brain telomeres of males and females were of similar length. In all the other tissues analysed (kidney, liver, pancreas and lung) males had fewer long telomeres in the 112–48.6 kb size region and more shorter ones in the 4.2–1.3 kb size region compared to females (Fig. 4A–D).
Figure 4.
Telomere length analysis reflects gender differences at 3 months of age. Genomic DNA was extracted from (A) kidney, (B) liver, (C) pancreas and (D) lung of males (white columns) and females (black columns). Each telomere smear was divided into four regions according to a molecular weight marker: 112–48.5, 48.5–8.6, 8.6–4.2 and 4.2–1.3 kb. Arbitrary units (% PSL) were calculated for each size region. Values are means of n = 8 ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 using the unpaired Student’s t-test.
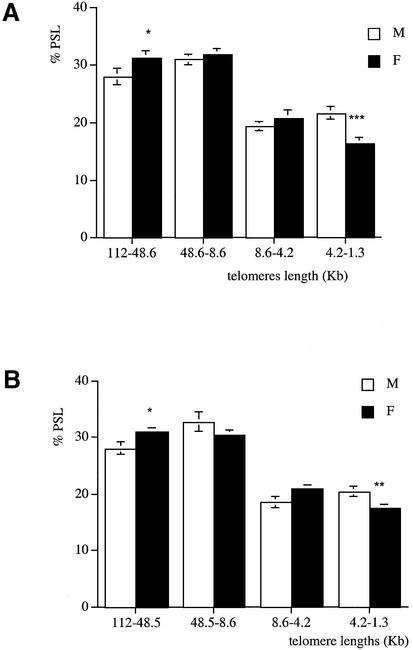
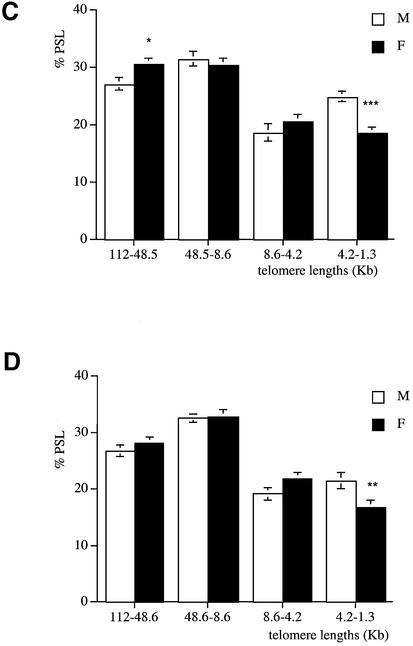
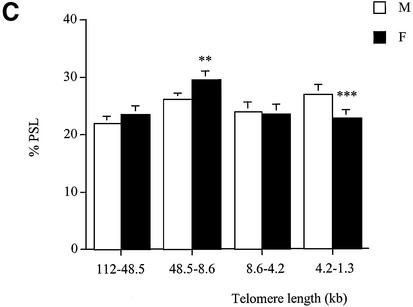
At 15 months of age, analysis of kidney, liver and pancreas (Fig. 5A–C) showed that males had a lower proportion of long telomeres (112–48.5 and 48.5–5.6 kb) (P < 0.01) and a higher proportion of short telomeres (4.2–1.3 kb) (P < 0.001) than females. However, telomere lengths were similar in the brain and the lung of both genders. In the brain, the percentage of telomeres in the 112–48.5 kb region was 25.6 ± 0.8%, whereas telomeres with a length in the 4.2–1.3 kb region were 20.5 ± 0.6%. In the lung, the percentage of telomeres in the 112–48.5 kb region was 21.2 ± 0.8%, whereas the percentage of telomeres in the 4.2–1.3 kb region was 23.6 ± 0.6%.
Figure 5.
Telomere length analysis reflects gender differences at 15 months of age. Genomic DNA was extracted from (A) kidney, (B) liver and (C) pancreas of males (white columns) and females (black columns). Each telomere smear was divided into four regions according to a molecular weight marker: 112–48.5, 48.5–8.6, 8.6–4.2 and 4.2–1.3 kb. Arbitrary units (% PSL) were calculated for each size region. Values are means of n = 8 ± SEM. **P < 0.01 and ***P < 0.001 using the unpaired Student’s t-test.
DISCUSSION
The results of the current study indicate that telomeres in the rat shorten with age in the liver, lung, kidney and pancreas, whereas the length of brain telomeres remains constant with age. We also report, for the first time in the rat, the effect of gender on telomere shortening.
We show clearly in this study that the mean TRF length method is unable to detect small changes in telomere size or to visualise the length of individual short telomeres in a distribution of TRFs. There is increasing evidence suggesting that it is not average telomere length, but rather individual critically short telomeres that trigger cellular responses to the loss of telomere function (23). We demonstrated the ability of our novel method of telomere analysis to detect small changes in the renal tissue from the three ages while the analysis of the same Southern blots based on the mean TRF length did not show any difference. A loss of a few hundred base pairs from short telomeres could be important to cellular ageing but may go undetected by traditional mean TRF analysis as the TRFs with few telomeric repeats could have been obscured by the strong signal from other TRFs with long telomeres. Studies with various strains of mice with large telomeres did not detect changes in the mean TRF lengths with age or any differences between tissues, but reported great variability (25,26). Moreover, the novel method of telomere analysis allowed a greater overall interpretation of the range of various sized telomeres which exist within a tissue and helped to determine whether or not the size distribution of telomeres changed with age in male and female Wistar rats. For these reasons, we used this novel method for all subsequent analysis.
Age-related telomere shortening that we show in the rat has been reported in several rodent tissues. We have previously shown that kidney and liver telomeres shorten with age in rats (10). In addition, telomeres in rat myocytes (27) and in lens epithelial cells (28) have also been shown to be shorter in ageing rats. Unlike humans, several rat somatic tissues express telomerase activity (29). However, the presence of telomerase does not always prevent telomere shortening (30). It has been suggested that telomerase activity might be constitutive in some rat tissues, as is the case in the rat mammary epithelium (31).
In M.spretus, telomeres have been shown to shorten with age only in the brain and spleen, whereas no age-related changes in telomere lengths were found in liver, testis or kidney (25). In the latter, telomerase activity was undetectable. However, it should be noted that the authors have based their conclusions on the mean TRF measurements.
In humans, telomere length is relatively short, highly variable between tissues and individuals and, with regard to replicating somatic cells, inversely related to donor age (32,33). The length of telomeres in most human somatic cells decreases with age (34,35). Consistent with this observation, some investigators have reported that most somatic cells do not express telomerase (36,37). However, other studies have reported telomerase activity in some somatic cells (38) and, therefore, other factors besides the presence of telomerase activity also appear to be involved in the regulation of telomere length. An alternative lengthening of telomeres (ALT) has been described recently in human cells. In the absence of any detectable telomerase activity, ALT occurs by means of homologous recombination and copy switching (39).
The tissues chosen in this study were selected in order to represent different tissue types and on the basis that they, the kidney in particular, may be important in terms of deterioration and mortality in rats. The tissue-specific differences in age-related telomere shortening that we report in this study (brain versus the other tissues) reflect differences in cell growth in the tissue types examined. Unlike human, rats have most of their rapid division after birth. The liver, kidney, pancreas and lung are expanding cell populations during post-natal life whilst in contrast, the brain is a static cell population (40).
The telomere shortening occurring between 3 and 15 months of age in both genders may not be due only to the end replication problem but may also be caused as a response to oxidative damage. In effect, oxidative damage to DNA accumulates over the lifetime of a cell or an organism and has been suggested for a long time to be a causal factor for ageing (41).
We report in this study that females have longer telomeres than males. This finding is consistent with their increased longevity compared to males. This is the first time that a gender-related telomere shortening has been reported in rat. For a given chronological age, biological ageing of males is more advanced than that of females. Similar findings have been reported in mice (25) and humans (15). In M.spretus, male telomeres in liver, kidney, spleen and brain were shorter than in females (25). Also in humans, age-adjusted telomere length was longer in white blood cells from women than in those from men. In both genders, telomere length was inversely correlated with age (15). The difference in telomere length between males and females might explain why women have a longer lifespan (42,43).
The finding of gender differences in tissue telomere lengths in the kidney, liver, lung and pancreas is one line of evidence that suggests that there is gender- and tissue-related telomere length regulation and, moreover, may point to a hormonal component of rat telomere length regulation. In effect, no gender difference in tissue telomere lengths was detected in the neonates. However, differences were noted at 3 and 15 months, suggesting the implication of the pubertal hormones in the control of telomere length. Recently, female hormones such as oestrogens have been shown to protect telomeres from shortening (44,45). The similarity of telomere lengths in the male and female brains of which the cell number is fixed at birth indicates in addition that the gender-related telomere shortening might be due to an accelerated cell division in other male tissues compared to the female.
These findings indicate that telomeres shorten in the rat tissues in an age- and gender-dependent manner. These data provide a novel mechanism for the gender-related lifespan difference and suggest a tissue-specific regulation of telomeres during development and ageing in the rat. The latter might be used as a model to help understand the biology of telomeres in the human ageing process.
Acknowledgments
ACKNOWLEDGEMENTS
We thank A. Wayman, A. Flack and D. Hutt for their technical assistance. The work was supported by the Medical Research Council, the Parthenon Trust and the Wellcome Trust.
REFERENCES
- 1.Meyne J., Ratliff,R.L. and Moysis,R.K. (1989) Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl Acad. Sci. USA, 86, 7049–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakian V.A. (1996) Structure, function and replication of Saccharomyces cerevisiae telomeres. Annu. Rev. Genet., 30, 141–172. [DOI] [PubMed] [Google Scholar]
- 3.Greider C.W. (1994) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Curr. Opin. Genet. Dev., 4, 203–211. [DOI] [PubMed] [Google Scholar]
- 4.Greider C.W. and Blackburn,E.H. (1985) Mammalian telomere dynamics: healing, fragmentation, shortening and stabilisation. Cell, 43, 405–413. [DOI] [PubMed] [Google Scholar]
- 5.Yu G.-L., Bradley,J.D., Attardi,L.D. and Blackburn,E.H. (1990) In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature, 344, 126–132. [DOI] [PubMed] [Google Scholar]
- 6.Brown W.R. (1989) Molecular cloning of human telomeres in yeast. Nature, 338, 774–776. [DOI] [PubMed] [Google Scholar]
- 7.Zijlmans J.M., Martens,U.M., Poon,S.S., Raap,A.K., Tanke,H.J., Ward,R.K. and Lansdorp,P.M. (1997) Telomeres in the mouse have large inter-chromosomal variations in the number of TA2AG3 repeats. Proc. Natl Acad. Sci. USA, 94, 7423–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prowse K.R. and Greider,C.W. (1995) Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl Acad. Sci. USA, 92, 4818–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golubovskaya V.M., Filatov,L.V., Behe,C.I., Presnell,S.C., Hooth,M.J., Smith,G.J. and Kaufmann,W.K. (1999) Telomere shortening, telomerase expression and chromosome instability in rat hepatic epithelial stem-like cells. Mol. Carcinog., 24, 209–217. [DOI] [PubMed] [Google Scholar]
- 10.Jennings B.J., Ozanne,S.E., Dorling,M.W. and Hales,C.N. (1999) Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett., 448, 4–8. [DOI] [PubMed] [Google Scholar]
- 11.Harley C.B. (1997) Human ageing and telomeres. Ciba Found. Symp., 211, 129–139. [DOI] [PubMed] [Google Scholar]
- 12.Campisi J., Dimri,G.P., Nehlin,J.O., Testori,A. and Yoshimoto,K. (1996) Coming of age in culture. Exp. Gerontol., 31, 7–12. [DOI] [PubMed] [Google Scholar]
- 13.Aikata H., Takaishi,H., Kawakami,K., Takahashi,S., Kitamoto,M., Nakanishi,T., Nakamura,Y., Shimamoto,F., Kajiyama,G. and Ide,T. (2000) Telomere reduction in human liver tissue with age and chronic inflammation. Exp. Cell Res., 256, 578–582. [DOI] [PubMed] [Google Scholar]
- 14.Takubo K. and Kaminishi,M. (2001) Diseases of the digestive tract and telomere lengths: significance and problems of telomere measurement. Nippon Shokakibyo Gakkai Zasshi, 98, 144–150. [PubMed] [Google Scholar]
- 15.Melk A., Ramassar,V., Helms,L.M., Moore,R., Rayner,D., Solez,K. and Halloran,P.F. (2000) Telomere shortening in kidneys with age. J. Am. Soc. Nephrol., 11, 444–453. [DOI] [PubMed] [Google Scholar]
- 16.Benetos A., Okuda,K., Lajemi,M., Kimura,M., Thomas,F., Skurnick,J., Labat,C., Bean,K. and Aviv,A. (2001) Telomere lengths as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension, 37, 381–385. [DOI] [PubMed] [Google Scholar]
- 17.Herrera E., Samper,E., Caballero,J.M., Flores,J.M., Lee,H.-W. and Blasco,M. (1999) Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J., 18, 2950–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artandi S.E. and Depinho,R.A. (2000) Mice without telomerase: what can they teach us about human cancer? Nature, 6, 852–855. [DOI] [PubMed] [Google Scholar]
- 19.Blasco M.A., Gasser,S.M. and Lingner,J. (1999) Telomeres and telomerase. Genes Dev., 13, 2353–2359. [DOI] [PubMed] [Google Scholar]
- 20.Blasco M.A., Lee,H.W., Handle,M.P., Samper,E., Landsdorp,P.M., DePinho,R.A. and Greider,C.W. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell, 91, 1–3. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph K.L., Chang,S., Lee,H.W., Blasco,M., Gottlieb,G.J., Greider,C. and DePinho,R.A. (1999) Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell, 5, 701–712. [DOI] [PubMed] [Google Scholar]
- 22.Hales C.N., Desai,M., Ozanne,S.E. and Crowther,N.J. (1996) Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem. Soc. Trans., 24, 341–350. [DOI] [PubMed] [Google Scholar]
- 23.Hemann M.T., Strong,M.A., Hao,L.Y. and Greider,C.W. (2001) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell, 107, 67–77. [DOI] [PubMed] [Google Scholar]
- 24.de Lange T. (1990) Structure and variability of human-chromosome ends. Mol. Cell. Biol., 10, 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gina M., Coviello-McLaughlin,G.M. and Prowse,K.R. (1997) Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res., 25, 3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipling D. and Cook,H.J. (1990) Hypervariable ultra-long telomeres in mice. Nature, 347, 400–402. [DOI] [PubMed] [Google Scholar]
- 27.Kajstura J., Pertoldi,B., Leri,A., Beltrami,C.A., Deptala,A., Darzynkiewicz,Z. and Anversa,P. (2000) Telomere shortening is an in vivo marker of myocyte replication and aging. Am. J. Pathol., 156, 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pendergrass W.R., Penn,P.E., Li,J. and Wolf,N.S. (2001) Age-related telomere shortening occurs in lens epithelium from old rats and is slowed by caloric restriction. Exp. Eye Res., 73, 221–228. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi Y., Nozawa,K., Savoysky,E., Hayakawa,N., Nimura,Y. and Yoshida,S. (1998) Change in telomerase activity of rat organs during growth and aging. Exp. Cell Res., 242, 120–127. [DOI] [PubMed] [Google Scholar]
- 30.Son N.H., Murray,S., Yanovski,J., Hodes,R.J. and Weng,N. (2000) Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J. Immunol., 165, 1191–1196. [DOI] [PubMed] [Google Scholar]
- 31.Varon D., Jiang,C., Hedican,C., Dome,J.S., Umbricht,C.B., Carey,L.A., Thompson,H.J. and Sukumar,S. (1997) Telomerase activity in the normal and neoplastic rat mammary gland. Cancer Res., 57, 5605–5609. [PubMed] [Google Scholar]
- 32.Slagboom P.E., Droog,S. and Boomsma,D.I. (1994) Genetic determination of telomere size in humans: a twin study of three age groups. Am. J. Hum. Genet., 55, 876–882. [PMC free article] [PubMed] [Google Scholar]
- 33.Jeanclos E., Schork,N.J., Kyvik,K.O., Kimura,M., Skurnick,J.H. and Aviv,A. (2000) Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension, 36, 195–200. [DOI] [PubMed] [Google Scholar]
- 34.Harley C.B., Futcher,A.B. and Greider,C.W. (1990) Telomeres shorten during ageing of human fibroblasts. Nature, 345, 458–460. [DOI] [PubMed] [Google Scholar]
- 35.Hastie N.D., Dempster,M., Dunlop,M.G., Thompson,A.M., Green,D.K. and Allshire,R.C. (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature, 346, 866–868. [DOI] [PubMed] [Google Scholar]
- 36.Counter C.M., Hirte,H.W., Bacchetti,S. and Harley,C.B. (1994) Telomerase activity in human ovarian carcinoma. Proc. Natl Acad. Sci. USA, 91, 2900–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim N.W., Piatyszek,M.A., Prowse,K.R., Harley,C.B., West,M.D., Ho,P.L., Coviello,G.M., Wright,W.E., Weinrich,S.L. and Shay,J.W. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science, 266, 2011–2015. [DOI] [PubMed] [Google Scholar]
- 38.Broccoli D., Young,J.W. and de Lange,T. (1995) Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl Acad. Sci. USA, 92, 9082–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunham M.A., Neumann,A.A., Fasching,C.L. and Reddel,R.R. (2000) Telomere maintenance by recombination in human cells. Nature Genet., 26, 447–450. [DOI] [PubMed] [Google Scholar]
- 40.Leblond C.P. (1964) Classification of cell populations on the basis of their proliferative behaviour. Natl Cancer Mongr., 14, 119–145. [PubMed] [Google Scholar]
- 41.Chen Q. and Ames,B.N. (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblasts F65 cells. Proc. Natl Acad. Sci. USA, 91, 4130–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guralnik J.M., Balfour,J.L. and Volpato,S. (2000) The ratio of older women to men: historical perspectives and cross-national comparisons. Aging (Milano), 12, 65–76. [DOI] [PubMed] [Google Scholar]
- 43.Franceschi C., Motta,L., Valensin,S., Rapisarda,R., Franzone,A., Berardelli,M., Motta,M., Monti,D., Bonafe,M., Ferrucci,L. et al. (2000) Do men and women follow different trajectories to reach extreme longevity? Italian Multicenter Study on Centenarians (IMUSCE). Aging (Milano), 12, 77–84. [DOI] [PubMed] [Google Scholar]
- 44.Kyo S., Takakura,M., Kanaya,T., Zhuo,W., Fujimoto,K., Nishio,Y., Orimo,A. and Inoue,M. (1999) Estrogen activates telomerase. Cancer Res., 59, 5917–5921. [PubMed] [Google Scholar]
- 45.Misiti S., Nanni,S., Fontemaggi,G., Cong,Y.-S., Wen,J., Hirte,H.W., Piaggion,G., Sacchi,A., Pontecorvi,A., Bacchetti,S. et al. (2000) Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol. Cell. Biol., 20, 3764–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]