Abstract
COPI, a protein complex consisting of coatomer and the small GTPase ARF1, is an integral component of some intracellular transport carriers. The association of COPI with secretory membranes has been implicated in the maintenance of Golgi integrity and the normal functioning of intracellular transport in eukaryotes. The regulator of G protein signaling, RGS4, interacted with the COPI subunit β′-COP in a yeast two-hybrid screen. Both recombinant RGS4 and RGS2 bound purified recombinant β′-COP in vitro. Endogenous cytosolic RGS4 from NG108 cells and RGS2 from HEK293T cells cofractionated with the COPI complex by gel filtration. Binding of β′-COP to RGS4 occurred through two dilysine motifs in RGS4, similar to those contained in some aminoglycoside antibiotics that are known to bind coatomer. RGS4 inhibited COPI binding to Golgi membranes independently of its GTPase-accelerating activity on Giα. In RGS4-transfected LLC-PK1 cells, the amount of COPI in the Golgi region was considerably reduced compared with that in wild-type cells, but there was no detectable difference in the amount of either Golgi-associated ARF1 or the integral Golgi membrane protein giantin, indicating that Golgi integrity was preserved. In addition, RGS4 expression inhibited trafficking of aquaporin 1 to the plasma membrane in LLC-PK1 cells and impaired secretion of placental alkaline phosphatase from HEK293T cells. The inhibitory effect of RGS4 in these assays was independent of GTPase-accelerating activity but correlated with its ability to bind COPI. Thus, these data support the hypothesis that these RGS proteins sequester coatomer in the cytoplasm and inhibit its recruitment onto Golgi membranes, which may in turn modulate Golgi–plasma membrane or intra-Golgi transport.
INTRODUCTION
RGS (regulator of G protein signaling) proteins inhibit signaling pathways induced by heterotrimeric G proteins by acting as GTPase-accelerators for Gα subunits of the Gi and Gq classes, resulting in faster deactivation and a decreased lifetime for activated Gα to interact with effectors (Berman et al., 1996b; Watson et al., 1996; Berman and Gilman, 1998). Signaling pathways such as MAPK activation induced by stimulation of G protein–coupled receptors are inhibited by RGS protein expression (Druey et al., 1996; Huang et al., 1997; Yan et al., 1997). In earlier studies, we found that although RGS4 is membrane-associated, the protein is mainly localized to the cytoplasm in the neuronal cell line NG108 (Druey et al., 1998). In addition, a closely related RGS protein, GAIP (Gα-interacting protein), was shown to localize in the cytosol and with Golgi-derived vesicles (both clathrin-coated and other) in rat liver and Madin-Darby canine kidney cells (De Vries et al., 1998; Wylie et al., 1999). The intracellular localization of these proteins suggests a broader physiological role for RGS4 and RGS-GAIP in the regulation of processes in addition to plasma membrane–associated signaling events.
To identify proteins that might interact with RGS4 to modulate novel pathways, we performed yeast two-hybrid interaction cloning (Hollenberg et al., 1995). We screened a mouse embryonic stem cell library with a full-length RGS4–LexA fusion protein and repeatedly isolated a cDNA encoding a portion of β′-COP, a subunit of coatomer. The majority of coatomer exists as a cytosolic protein complex of seven subunits (α-, β-, β′-, γ-, δ-, ε-, and ζ-COP) that, along with the monomeric GTP-binding protein ARF1 (ADP-ribosylation factor), is recruited onto secretory membranes to form COPI coats (Serafini et al., 1991; Waters et al., 1991; Lowe and Kreis, 1995; Cosson and Letourneur, 1997). Defects in coatomer subunits lead to severely disrupted intracellular transport in both yeast and mammalian cells (Duden et al., 1994; Guo et al., 1994, 1996; Schekman and Orci, 1996), and microinjection of antibodies to β-COP disrupts Golgi integrity and blocks forward membrane trafficking of viral stomatitis virus glycoprotein (Pepperkok et al., 1993; Peter et al., 1993).
The precise physiological function of the COPI coat is controversial. Some evidence suggests that COPI mediates forward trafficking of membrane-bound components upon exit from the endoplasmic reticulum (ER) by facilitating their concentration and sorting from resident proteins in transport carriers (TCs) (Klausner et al., 1992; Peters et al., 1995; Gaynor and Emr, 1997; Presley et al., 1997; Scales et al., 1997). An equal body of evidence supports a role for ARF1/COPI in the recycling of resident Golgi enzymes back to the ER (Letourneur et al., 1994; Pelham, 1994; Schekman and Mellman, 1997). Recent imaging studies of Green Fluorescent Protein (GFP)-tagged coatomer subunits in living cells suggest that COPI predominates in TCs, shuttling anterograde cargo from the ER to the Golgi. Furthermore, this forward transport appears to be accompanied by progressive segregation of COPI and anterograde cargo-rich domains in TCs, suggesting that COPI may sequester cargo bound for ER retrieval (Shima et al., 1999).
The recruitment of coatomer onto Golgi membranes is stimulated by the activation (GTP binding) and membrane association of ARF1, which may interact directly with the β- and γ-COP subunits (Donaldson et al., 1992; Palmer et al., 1993; Zhao et al., 1997, 1999). Inactivation of ARF1 by ARFGAP-initiated GTPase activity promotes COPI membrane dissociation. Coatomer has also been shown to bind to a C-terminal dilysine motif, KKXX (where X is any amino acid), contained in the cytoplasmic tail of a family of ER-resident type I transmembrane proteins (p23 and p24 family) (Cosson and Letourneur, 1994; Fiedler et al., 1996; Sohn et al., 1996). The KKXX–coatomer interaction is mediated by the γ-COP subunit (Fiedler et al., 1996; Sohn et al., 1996; Harter and Wieland, 1998). Two recent studies have established that coatomer can be precipitated by some aminoglycoside antibiotics such as neomycin that contain two closely spaced amino groups (Hudson and Draper, 1997) and that such compounds interfere with COPI binding to Golgi membranes and coatomer function (Hu et al., 1999). These studies suggest that the compounds may interact with a dilysine-binding site on coatomer, because the effects can be reproduced by dilysine itself.
In this report, we describe and characterize the novel interaction between coatomer and at least two RGS proteins, RGS4 and RGS2. We found that RGS4 and RGS2 inhibited COPI binding to Golgi membranes and that these effects depended on two internal dilysine motifs in RGS4 similar to the COPI-binding motifs in some aminoglycoside antibiotics. Although the β′-COP binding site on RGS4 mapped partially inside the conserved RGS (G protein–binding) box, these effects were independent of the enzymatic GTPase-accelerating (GAP) activity of RGS4 on Giα, implying a novel and perhaps unrelated role for this G protein regulatory molecule in intracellular membrane trafficking.
MATERIALS AND METHODS
Yeast Two-Hybrid Screening
RGS4 was used as the bait to screen a mouse embryonic stem cell library constructed as described (Hollenberg et al., 1995). A PCR fragment containing the coding region of rat RGS4 was subcloned in-frame with the LexA coding region into the vector pLexA. This plasmid was transformed into L40 yeast, which contain HIS3 driven by four LexA operators. One liter of RGS4/L40 was transformed with 0.5 mg of a mixture of mouse embryonic libraries (d 9.5 and 10.5) by the lithium acetate method. After overnight recovery in yeast complete medium (Trp−Leu−Ura−), transformants were plated on medium to select for histidine prototrophy (Trp−Leu−Ura−Lys−His−). After 5 d, histidine-positive colonies were lysed in liquid nitrogen and assayed for β-galactosidase activity on filters. Positive colonies were further analyzed after loss of bait plasmid (by plating on low-adenine–containing medium). These yeast were mated with an opposite mating phenotype strain (AMR70) containing either pLexA-RGS4 or pLexA-lamin as a negative control. Plasmid DNA from colonies that were repeatedly β-galactosidase positive in the mating assay specifically with RGS4 was isolated and transformed into HB101 bacteria by electroporation. These plasmids were sequenced with a pLEXA-specific primer by automated sequencing.
Plasmids and Proteins
GFP–wild-type RGS4 and truncation mutant inserts were generated by PCR and subcloned in-frame with the C terminus of GFP into the vector pEGFP-C1 (Clontech, Palo Alto, CA). The preparation of His-tagged RGS4 and mutagenesis have been described previously (Watson et al., 1996; Druey and Kehrl, 1997). Recombinant His-tagged MEK1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant truncated thioredoxin–RGS4 fusion proteins were generated by subcloning PCR products in-frame with thioredoxin into the BamHI–XbaI sites of pHis-Thio A (Invitrogen, Carlsbad, CA). Plasmids were transformed into BL21(DE3)pLysS Escherichia coli and induced with 1 mM isopropylthio-β-galactoside. Thio-fusion proteins were purified on Thio-bond resin according to the manufacturer's instructions. GST–β′-COP was constructed by excision of full-length β′-COP from pBluescript as a blunt (filled in EcoRI site)–XhoI fragment, which was ligated into blunt (filled in BamHI site)–XhoI-digested pGEX5X-1 (Amersham Pharmacia Biotech, Arlington Heights, IL) to create an in-frame GST fusion. This plasmid was transformed into BL21(DE3)pLysS bacterial strain and induced with 1 mM isopropylthio-β-galactoside at 30°C for 1.5 h., and recombinant protein was purified on glutathione–Sepharose as described elsewhere (Rohman and Harrison-Lavoie, 2000).
Coimmunoprecipitations
Immunoprecipitation of coatomer with antibody 23C was carried out essentially as described (Harrison-Lavoie et al., 1993) from 293T cells transfected with GFP (3 μg) or GFP–RGS4 plasmids for 12 h with the use of Lipofectamine (Life Technologies-BRL, Grand Island, NY). Forty-eight hours after transfection, cells were lysed in buffer containing 50 mM HEPES, pH 7.4, 90 mM KCl, 0.5% Triton X-100, and protease inhibitors (Roche Molecular, Basel, Switzerland). Clarified lysates were immunoprecipitated with 3 μg of 23C and 20 μl of goat anti-rat immunoglobulin G (IgG) Dynabeads (Dynal, Lake Success, NY). Immunoprecipitates were washed three times with buffer A (Watson et al., 1996) before addition of Laemmli buffer, boiling, and separation by SDS-PAGE. Gels were transferred to nitrocellulose filters and blotted for β′-COP or GFP (Santa Cruz Biotechnology). Blots were stripped and sequentially reprobed with antibodies against β-COP (M3A5 antibody; Sigma Chemical, St. Louis, MO) or γ- or δ-COP (a kind gift of Cordula Harter, Ruprecht-Karls-Universitat, Heidelberg, Germany). For affinity precipitations, recombinant GST–β′-COP was incubated with 6His-RGS4 protein and 20 μl of Ni-nitriloacetic acid beads (Qiagen, Chatsworth, CA) for 1 h at 4°C. The beads were then pelleted and washed twice with buffer A (Watson et al., 1996). Bound proteins were solubilized in SDS sample buffer and separated on 12% gels before transfer and immunoblotting with antibodies against β′-COP or hexahistidine (Santa Cruz Biotechnology).
Gel Filtration
NG108 or HEK293T cells were lysed in 50 mM Tris, pH 8, 5 mM EDTA plus a protease inhibitor cocktail (Roche Molecular). The cells were homogenized by Dounce pestle and passage through a 25-gauge needle 10 times. After a low-speed spin to pellet nuclei, cells were centrifuged at 75,000 × g to pellet membranes. The cytosolic fraction (supernatant) was concentrated by ultrafiltration and loaded onto a Synchropack GPC 100 HPLC (NG108) or Superdex 200 fast performance liquid chromatography (FPLC) (HEK293T) column (Amersham Pharmacia Biotech). Standards were run as shown, and 0.5-ml fractions were collected, separated on 12% SDS gels, and immunoblotted as indicated. Antibodies against an N-terminal RGS4 peptide have been described previously (Druey et al., 1998). RGS2-specific polyclonal antibodies were raised in rabbits against a C-terminal RGS2 peptide (CKKPQITTEPHAT). To estimate the amount of β-COP–associated RGS4 in the fractions, we used Kodak (Rochester, NY) one-dimensional image-analysis software to quantitate the amount of specific protein on immunoblots.
Precipitation of Coatomer
The precipitation of coatomer was performed exactly as described by Hudson and Draper (1997). Briefly, 75–100 μg of rat liver cytosol was incubated in 25 μl of buffer containing 25 mM HEPES, pH 7.4, 50 mM KCl, and 2.5 mM Mg(OAc)2. Varying amounts of neomycin or RGS4 were then added. An equal portion of each reaction was removed to measure the input for each condition. The remaining reaction was incubated at 4°C for 2 h and then centrifuged at 100,000 × g for 30 min. Supernatants were removed, and the pellets were washed once with incubation buffer before solubilization in Laemmli buffer and separation on 12% SDS gels. Proteins were transferred to polyvinylidene difluoride membranes and blotted for β′-COP as above.
Golgi Membrane–binding Assays
Rat liver Golgi membranes and cytosol were prepared and binding assays were performed essentially as described previously (Stow et al., 1991). Golgi membrane protein (20 μg), saturating amounts of cytosolic protein (1 mg), and recombinant 6His-RGS2, -RGS4, or -β-galactosidase (5 μg) were incubated for 10 min at 37°C in the presence or absence of GTPγS (25 μM). A portion of the total mixture of cytosol plus membranes was removed for electrophoresis and immunoblotting with His antibody (BAbCO, Richmond, CA). Membranes were then isolated by centrifugation at 14,000 × g and solubilized in SDS sample buffer. Samples were separated on 6–15% gradient gels and immunoblotted for β-COP (M3A5). The mean intensity of the bands was determined with the use of NIH Image software. Bar graphs represent the mean ± SEM of five experiments measuring binding compared with control conditions (no additions, 100%).
Immunofluorescence
LLC-PK1 epithelial cells were transfected with GFP or GFP–RGS4 constructs with the use of the Superfect reagent (Qiagen). Twenty-four hours after transfection, cells were fixed in 4% paraformaldehyde/5% sucrose/PBS and permeabilized with either 0.1% SDS/PBS (for β-COP, ARF1, and AQP1) or 0.1% SDS/PBS followed by 1% Triton X-100/PBS (for giantin). Cells were then incubated for 1 h with antibodies against β-COP (M3A5, Sigma; 1:50), ARF1 (1:250), or giantin (1:100). Donkey anti-mouse IgG or anti-rabbit IgG Cy3-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were applied in a second 1-h incubation. The stable expression of AQP1 in LLC-PK1 epithelial cells and the specificity of AQP1 antibodies have been described previously (Katsura et al., 1995). The characterization and specificity of anti-ARF1 antibodies have also been described elsewhere (Marshansky et al., 1997). Polyclonal anti-giantin antisera were the kind gift of Dr. M. Renz (Institute of Immunology and Molecular Genetics, Karlsruhe, Germany).
Measurement of Alkaline Phosphatase Secretion
To determine the effect of RGS4 on secretion, we used the Great EscAPe SEAP (secretion of placental alkaline phosphatase) assay (Clontech) as described elsewhere (Andreev et al., 1999). One microgram of SEAP-control plasmid and hemagglutinin (HA)-RGS4 constructs (4 μg unless indicated otherwise) was transfected into HEK293TT cells in six-well plates by the calcium phosphate method. A total of 250 ng of cytomegalovirus–β-galactosidase plasmid per point was cotransfected to normalize for transfection efficiency. Forty-eight hours after transfection, cells were washed twice with PBS, and fresh medium was added for 4 additional hours. SEAP in the supernatant was measured as luciferase activity according to the manufacturer's instructions. Standard amounts of alkaline phosphatase were assayed to determine the linear range of luciferase values. After supernatant removal, cells were lysed in 100 μl of Reporter lysis buffer (Promega, Madison, WI) plus a protease inhibitor cocktail (Roche Molecular), and SEAP activity was measured in cell lysates. β-Galactosidase activity was determined as described previously (Beadling et al., 1999). Secretion was expressed as the ratio of SEAP activity in culture supernatants and the sum of SEAP activity in supernatants and cell lysates. Luciferase values were normalized by β-galactosidase values to account for transfection variations. The value of SEAP in the presence of empty vector was set at 100%, and the remaining values were expressed as a percentage of the control value. Amounts of RGS4 expressed were determined by immunoblotting cell lysates with a monoclonal anti-HA antibody (BAbCO; our unpublished results).
RESULTS
Yeast Two-Hybrid Screening Identifies β′-COP as a Binding Partner for RGS4
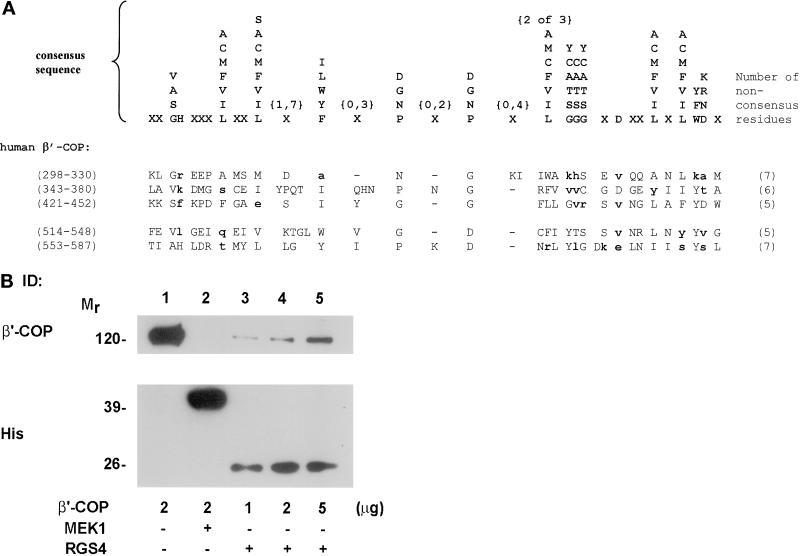
We used yeast two-hybrid interaction cloning to isolate proteins that interacted with RGS4. An expression vector that encodes full-length RGS4 fused to the DNA-binding domain of the transcription factor LexA was used as the bait to screen a mouse embryonic stem cell cDNA library. From approximately two million transformants, four clones were identified that interacted reproducibly with RGS4. Two of these encoded a portion (residues 455–576) of the protein β′-COP (Harrison-Lavoie et al., 1993; Stenbeck et al., 1993). None of the other clones encoded a coatomer subunit. The interaction of RGS4 and β′-COP was confirmed initially by a mating assay and by reintroduction of the partial β′-COP clone and RGS4 into yeast (our unpublished results). This region of β′-COP encodes two copies of what appear to be more divergent versions of the WD40 repeat motifs (amino acids 514–548 and 553–587), similar to those found in Gβ subunits and other proteins contained in macromolecular complexes (Figure 1A) (Van Der Voorn and Ploegh, 1992; Neer et al., 1994; Wall et al., 1995; Garcia-Higuera et al., 1998; Smith et al., 1999). We have also identified three other potential divergent “WD40 repeat–like” motifs between residues 298 and 452. All five of these are shown in Figure 1A. Six other WD40 repeats and one other WD40 repeat–like motif have already been identified in β′-COP (Harrison-Lavoie et al., 1993; Stenbeck et al., 1993; Csukai et al., 1997). In Gβ, the only WD40 repeat protein for which the crystal structure has been resolved to date, the WD40 repeats form the blades of a toroidal propeller structure (Wall et al., 1995). It has been suggested that all WD40 repeat–containing proteins are likely to form propeller structures, which may facilitate interaction with multiple protein partners (Garcia-Higuera et al., 1998). Therefore, it is possible that β′-COP may contain one or more such propeller structures.
Figure 1.
Interaction between RGS4 and the COPI subunit β′-COP. (A) A cDNA encoding a portion of β′-COP was isolated by yeast two-hybrid screening with RGS4 as the bait. Shown are five new potential divergent versions of the WD40 repeat found in β′-COP, aligned with the WD40 consensus sequence from Neer et al. (1994). Most of the residues of the two bottom repeats (514–548 and 553–587) are contained within the cDNA clone isolated in the RGS4 two-hybrid screen. In the consensus sequence, any single residue in a vertical column may occur in that position. Positions marked with the letter X can contain any amino acid. The numbers within braces above the symbols indicate the range of positions over which the symbol may be repeated. Residues that vary from the consensus are indicated in bold lowercase type. The numbers of residues in each repeat that do not fit the consensus are indicated in parentheses to the right of each repeat. (B) Binding of purified RGS4 to GST–β′-COP. Hexahistidine-tagged RGS4 (5 μg; lanes 3–5) and His-MEK1 (5 μg; lane 2) were bound to nickel/nitriloacetic acid beads and incubated with increasing amounts of GST–β′-COP (1–5 μg; lanes 3–5). In lane 1, 2 μg of β′-COP was loaded as an input control for lane 4. After extensive washing, protein that remained bound to the beads was eluted in Laemmli buffer by boiling for 5 min. Proteins were then separated on SDS gels and immunoblotted with antibodies against β′-COP (top panel) or His (bottom panel) as indicated.
To confirm that the RGS4–β′-COP interaction was direct, we incubated various amounts of purified, recombinant GST–β′-COP with hexahistidine-tagged RGS4 (or a control cytoplasmic protein, 6His-MEK1) bound to nickel beads, washed away unbound proteins, and subjected affinity-purified proteins to electrophoresis and immunoblotting with antibodies directed against either β′-COP or hexahistidine (Figure 1B). In this assay, β′-COP bound the immobilized RGS4 in a concentration-dependent manner, with a maximum retrieval of ∼14% of the input. Similar binding affinities were observed for two RGS4 mutant proteins that do not bind Giα and have no measurable GAP activity, RGS4(L159F) and RGS4(1–158) (Druey and Kehrl, 1997; Srinivasa et al., 1998), and for 6His-RGS2 (our unpublished results). These studies demonstrate that RGS4 and RGS2 bind β′-COP directly and that the N-terminal half of RGS4 is sufficient for the interaction.
RGS4 Binds the Coatomer Complex through Conserved Dilysine Motifs
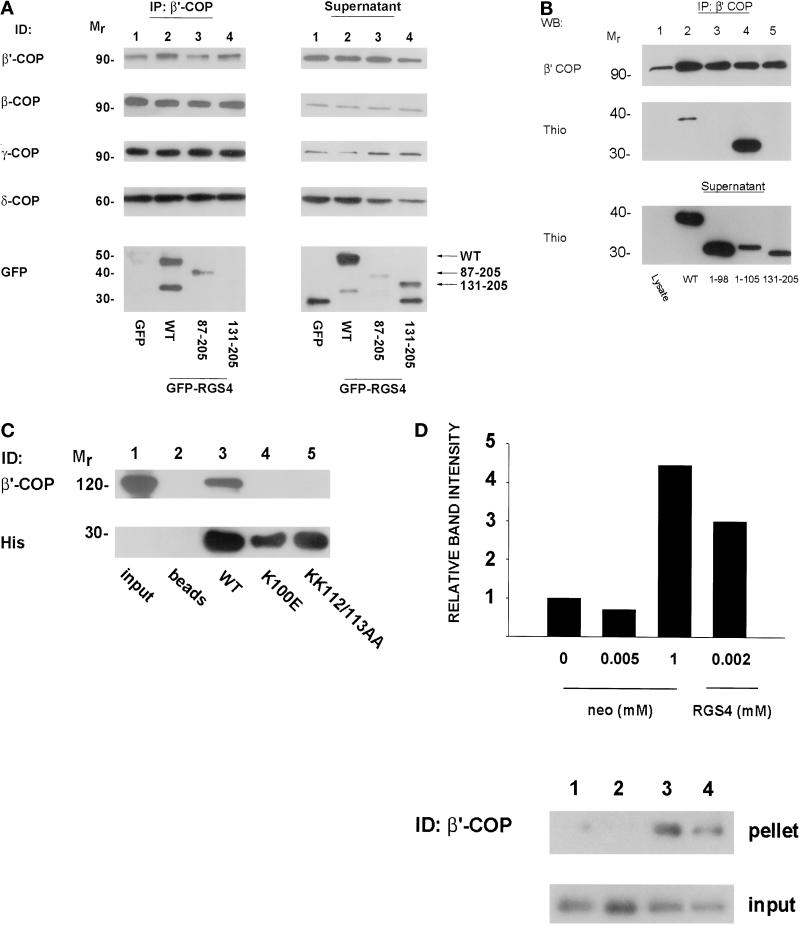
To determine whether RGS4 associates with intact coatomer complex as opposed to free cytosolic β′-COP and to confirm that this interaction occurs in intact cells, we used an antibody directed against β′-COP (Harrison-Lavoie et al., 1993) to immunoprecipitate endogenous coatomer from lysates of HEK293T cells that were transfected with either control (GFP) or GFP–RGS4 constructs encoding the full-length protein or various N-terminal deletions. The immunoprecipitations were then analyzed for the presence of various coatomer subunits by immunoblotting with available COPI antibodies and were also analyzed for GFP–RGS proteins by immunoblotting with anti-GFP antibodies. As shown in Figure 2A (IP: β′-COP), the β′-COP antibody immunoprecipitated β-, β′-, γ-, and δ-COP as well as full-length GFP–RGS4 and GFP–RGS4(87–205), but it failed to immunoprecipitate GFP–RGS4(131–205) or the GFP-only control. The four COPI subunits could also be detected in postimmunoprecipitation lysates, as could the GFP-only control and all of the GFP–RGS4 fusion proteins, including GFP–RGS4(131–205) (Figure 2A, Supernatant). The identities of the lower-molecular-weight proteins detected with anti-GFP antibodies are uncertain, but because they are detectable with the GFP antibody, they are likely to be proteins prematurely truncated at their C termini or proteolytic cleavage products. These experiments suggest that RGS4 associates with the entire COPI complex or at least a subcomplex containing β-, β′-, γ-, and δ-COP rather than free β′-COP. They also indicate that the β′-COP binding site(s) on RGS4 lies between residues 87 and 130, which encompasses the N-terminal half of the RGS domain.
Figure 2.
(A) Coimmunoprecipitation of RGS4 and coatomer from HEK293TT cells. GFP–RGS4 full-length and deletion (87–205 and 131–205) fusion proteins were expressed in HEK293TT cells and immunoprecipitated with antibody to β′-COP as described in MATERIALS AND METHODS. The entire amount of each immunoprecipitation was separated on SDS gels, transferred to nitrocellulose membranes, and blotted with antibodies against GFP or β-, β′-, γ-, or δ-COP as shown in the left panel (IP: β′-COP). Equal amounts of protein from postimmunoprecipitation lysates (quantitated by Bradford assay) were then separated by SDS-PAGE and immunodetected (ID) as labeled (Supernatant). (B) Mapping of the β′-COP binding region of RGS4. Truncated RGS4 proteins fused to thioredoxin were expressed and purified from E. coli. Coatomer from rat liver cytosol was immunoprecipitated with anti-β′-COP antibody, and immunoprecipitates were then incubated with thioredoxin fusion proteins. After washing away unbound proteins, each immunoprecipitate was electrophoresed and immunodetected as labeled. Postimmunoprecipitation supernatants (bottom panel) were incubated with Thio-bond resin, washed, fractionated, and immunoblotted with an anti-thioredoxin antibody to ensure that the amounts of thioredoxin fusion protein added were not limiting. (C) Mapping of RGS4 residues involved in β′-COP binding. Hexahistidine-tagged wild-type or mutant RGS4 proteins (5 μg) were incubated with nickel beads and GST–β′-COP (2 μg) and processed as in Figure 1B. (D) Relative affinity of COPI for RGS4 and neomycin. Rat liver cytosol was incubated with varying concentrations of either neomycin sulfate or 6His-RGS4 before centrifugation at 100,000 × g. Supernatants were removed, and the pellets were solubilized by boiling in Laemmli buffer before electrophoresis on SDS gels. The relative amounts of precipitated β′-COP in the pellet were assessed by immunoblotting (top panel). An equal amount of supernatant was removed before centrifugation to assess the amount of input protein for each condition (bottom panel). The bar graph represents the relative amount of β′-COP precipitated in each condition for the immunoblot shown as measured by densitometry. Similar results were observed in three independent experiments.
Amino acids 87–130 in RGS4 contain several important regions for contact with its known substrate, Gα. Based on crystallographic data for RGS4 complexed with AlF4−-Giα1, residues 84–88 and 128–130 form direct contacts with the G protein (Tesmer et al., 1997). However, β′-COP did not inhibit the GAP activity of RGS4 toward Giα1 (our unpublished results). Thus, we hypothesized that because RGS4 binding to β′-COP did not block G protein binding allosterically, the binding site(s) probably involved residues that do not contact the G protein directly. We noted in this region (99/100 and 112/113) the presence of two dilysine-containing motifs reminiscent of those found in some aminoglycoside antibiotics known to precipitate COPI. Because these positively charged regions represent candidate contact sites with the negatively charged WD40 repeat–like motifs of β′-COP, we sought to determine the precise residues involved in binding by two methods. First, we generated truncated RGS4 proteins fused to thioredoxin, incubated them with rat liver cytosol as a source of coatomer, and immunoprecipitated β′-COP as before. Immunoprecipitates were separated on SDS gels and blotted with antibodies against β′-COP or thioredoxin. Although full-length Thio-RGS4 and Thio-RGS4(1–105) coimmunoprecipitated with β′-COP, neither Thio-RGS4(1–98) nor Thio-RGS4(131–205) bound (Figure 2B). These results show that RGS4 residues 99–105 are necessary to bind β′-COP. Second, we generated 6His-RGS4 proteins with mutations in the dilysine motif of the β′-COP binding region (K100E) or a proximate dilysine motif as a control (KK112/113AA), bound them to nickel beads, and incubated them with GST–β′-COP. After washing away unbound proteins, we separated bound proteins by SDS-PAGE and blotted for β′-COP or hexahistidine. Surprisingly, although the wild-type protein bound as before, either mutation in RGS4 abolished β′-COP binding (Figure 2C). The inability of either mutant protein to bind β′-COP was probably not due to misfolding, because both mutants demonstrated enzymatic GAP activity similar to the wild-type protein and proteins containing mutations of residues that surround the downstream dilysine motif (KA110/111AS or IY114/115AA) bound β′-COP as well as wild-type RGS4 (our unpublished results). Based on these results, although RGS4 contains two internal KK motifs in other regions of the protein, either of the two dilysine-containing sites involving residues 99/100 or 112/113 are required for β′-COP binding, and perhaps both synergize to bind β′-COP.
To estimate the affinity of β′-COP for the internal KK-containing sequences, we compared the ability of neomycin, an aminoglycoside antibiotic, and RGS4 to precipitate COPI from rat liver cytosol (Figure 2D). Cytosol was incubated with various concentrations of either neomycin or 6His-RGS4 and then centrifuged at 100,000 × g. The pellets were washed, and proteins were solubilized and separated by SDS-PAGE. Gels were transferred and immunoblotted for β′-COP. As has been shown previously, high concentrations of neomycin (1 mM) precipitated COPI (Figure 2D, lane 3). However, micromolar concentrations of RGS4 precipitated a similar amount of coatomer as 1 mM neomycin, whereas neomycin in micromolar concentrations had little effect (Figure 2D, lanes 2 and 4, respectively). These studies suggest that the affinity of COPI for RGS4 is substantially greater than its affinity for neomycin; thus, high concentrations of neomycin or other dilysine compounds could have an RGS4-like effect in vitro.
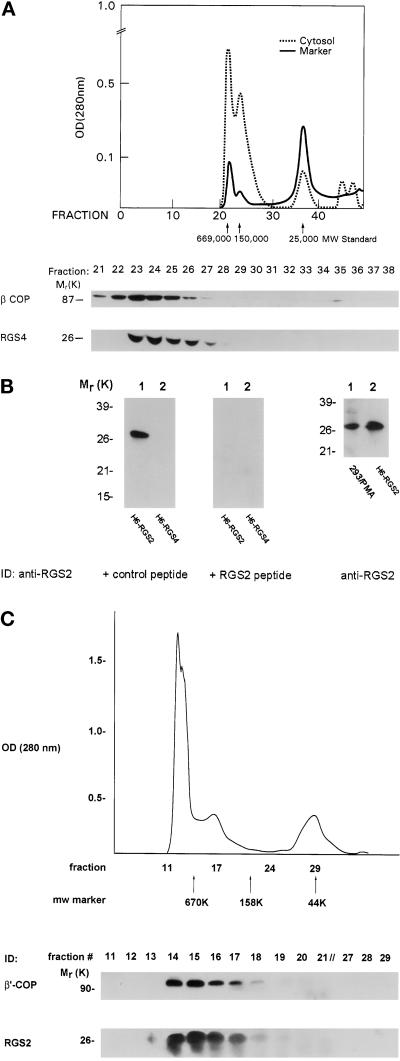
To determine the physiological relevance of the RGS–COPI interaction, we sought to demonstrate an association of endogenous RGS proteins with native coatomer in vivo. Although NG108 cells contain detectable amounts of cytosolic RGS4 protein, we have not been able to localize the protein ultrastructurally in these cells because of technical difficulties related to cell preservation. Instead, we gel filtered NG108 cytosol and blotted the fractions for RGS4 and β-COP (Figure 3A). Although purified, bacterially expressed RGS4 eluted from an HPLC column with a profile consistent with its monomeric molecular weight of ∼24,000 (Berman et al., 1996a), whereas virtually the entire cellular complement of RGS4 in NG108 cells migrated with an apparent molecular weight of >150,000. Approximately 74% of the cytosolic β-COP in these cells overlapped with fractions containing RGS4. Similarly, we investigated whether RGS2, which also contains the conserved dilysine motifs, associated with COPI. Although RGS2 is relatively ubiquitously expressed, we did not detect this protein in NG108 cells, so we fractionated HEK293T cells, which contain endogenous RGS2 mRNA (our unpublished results). RGS2 protein was detected with a polyclonal antiserum raised against a C-terminal RGS2 peptide. This antiserum was specific for RGS2 in that it recognized only 6His-RGS2 and not 6His-RGS4 (Figure 3B, left panel). In addition, blocking studies showed that the RGS2 signal was abrogated by preincubating the antibody with immunizing peptide (but not a control peptide) before immunoblotting (Figure 3B, middle panel). The antibody also detected a protein band of similar mobility to RGS2 in HEK293T cells, and this protein was markedly up-regulated by treatment of the cells with phorbol 12-myristate 13-acetate (PMA) (Figure 3B, right panel; our unpublished results). Therefore, we fractionated PMA-treated HEK293T cytosol by FPLC and blotted for β′-COP (Figure 3C, top panel) and RGS2 (bottom panel). Virtually all of the cytosolic RGS2 was contained in the high-molecular-weight fractions containing β′-COP. Thus, the comigration of cytosolic RGS4 and RGS2 with COPI on a size fractionation column is compatible with the notion that at least some native RGS4 and RGS2 associates with COPI complexes in these two cell types.
Figure 3.
Cofractionation of endogenous RGS proteins and COPI. (A) Endogenous RGS4 cofractionates with β-COP in NG108 cells by gel filtration. Protein molecular weight standards were loaded onto a Synchropack GPC 100 HPLC column; the elution profile is shown in the chromatograph for thyroglobulin (669,000), aldolase (150,000), and chymotrypsinogen (25,000) molecular weight standards (solid line). NG108 cytosol was loaded onto the column, and 0.5-ml fractions were collected. The entire amount of protein in peak fractions was separated on 12% gels, transferred to nitrocellulose, and probed with specific antibodies against β-COP or RGS4 as indicated. (B) Characterization of RGS2 antibody. 6His-RGS2 or -RGS4 (50 ng) was electrophoresed and immunoblotted with RGS2 antiserum. The antiserum was preincubated with either a control peptide (FLAG; left panel) or immunizing peptide (middle panel) (both at 10 μg/ml) for 30 min at room temperature before immunodetection. Right panel shows lysate from HEK293T cells (100–150 μg of protein) treated with PMA (100 ng/ml for 2 h) (lane 1) or 6His-RGS2 as a positive control (lane 2) electrophoresed and blotted with RGS2 antiserum. (C) Endogenous RGS2 cofractionates with β′-COP in HEK293T cells. The cytosolic fraction from PMA-treated HEK293T cells was loaded onto a Superdex 200 FPLC column and fractionated as in A. Molecular weight marker migrations are indicated by arrows (thyroglobulin, 669,000; γ-globulin, 158,000; and ovalbumin, 44,000). Peak protein-containing fractions were separated by SDS-PAGE and immunodetected with antibodies for β′-COP (top panel) or RGS2 (bottom panel).
RGS4 Inhibits COPI Binding to Golgi Membranes
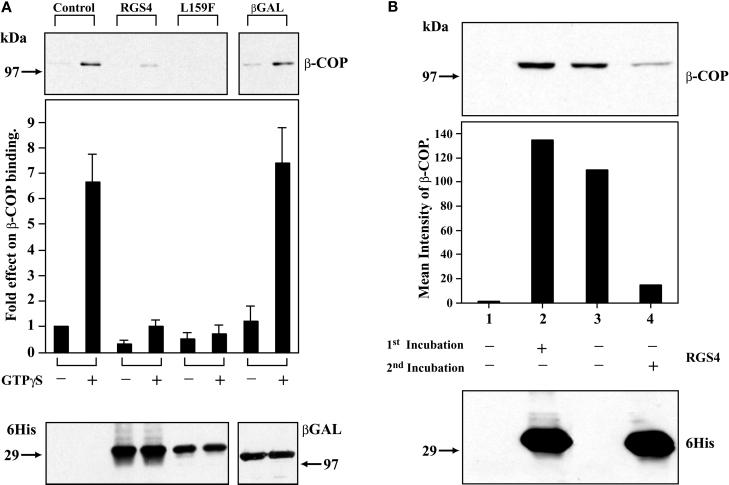
The overlap in distribution of RGS4 with COPI coupled with the interaction of RGS4 with coatomer via the β′-COP subunit led us to hypothesize that RGS4 may affect COPI recruitment onto Golgi membranes. To test this possibility, we incubated isolated Golgi stacks with cytosolic coatomer and recombinant 6His-RGS4 and measured the amount of membrane binding of β-COP by immunoblotting. Under control conditions, COPI bound to Golgi membranes, and GTPγS, as shown previously, enhanced binding (Stow et al., 1991). When RGS4 (but not a control protein, 6His-β-galactosidase, which also contains a dilysine motif) was added to cytosol before addition to Golgi membranes, COPI binding was dramatically inhibited (Figure 4A). In a similar experiment, the addition of recombinant 6His-RGS2 also inhibited COPI Golgi binding (our unpublished results). Like the direct binding of RGS4 to β′-COP, the inhibitory effect of RGS4 on COPI binding to Golgi membranes did not depend on binding of RGS4 to Giα or its GAP activity, because RGS4(L159F) inhibited COPI binding. However, when RGS4 was incubated with Golgi membranes before the addition of cytosolic COPI, RGS4 bound to Golgi membranes but failed to inhibit subsequent COPI binding (Figure 4B). These results suggest that the consequential COPI–RGS interaction occurs in the cytosol before COPI is recruited onto membranes.
Figure 4.
Inhibition of COPI binding to Golgi membranes by RGS4. (A) Both purified, wild-type RGS4 and RGS4(L159F) inhibit binding of COPI to Golgi membranes in vitro. Golgi membranes were incubated with equal amounts of 6His-RGS4 (wild type), 6His-RGS4 (L159F), or 6His-β-Gal (5 μg) in the presence of rat liver cytosol (1 mg of protein). After extensive washing, membranes were solubilized in Laemmli buffer, electrophoresed on SDS gels, and immunoblotted for bound β-COP (top panel). Before washing, aliquots of the total mixture (membranes plus cytosol) were removed, electrophoresed, and immunoblotted with an anti-His antibody (bottom panel). The bar graph represents means ± SEM of bound β-COP intensity under various conditions (five independent experiments). (B) Membrane-bound RGS4 does not inhibit β-COP Golgi membrane binding. 6His-RGS4 was incubated with Golgi membranes before addition of cytosol (bottom panel, lane 2); all other lanes contained only buffer in the first incubation. Membranes were pelleted by centrifugation, and unbound protein was removed by washing. Pellets were then incubated with cytosol, GTPγS (25 μM), and either buffer (lane 2) or 6His-RGS4 (lane 4) in a second incubation before recentrifugation, solubilization in Laemmli buffer, and electrophoresis and immunoblotting with a β-COP antibody (top panel). The bar graph represents the relative β-COP intensity for each condition in the experiment shown; a subsequent similar experiment gave equivalent results.
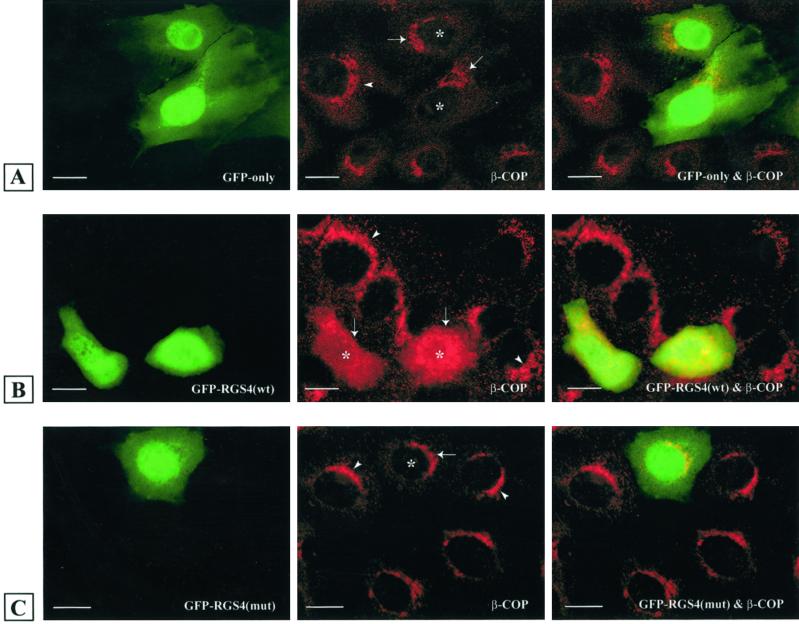
RGS4 Expression Modifies the Golgi Localization of COPI
To determine the effect of RGS expression on COPI localization in intact cells, we transfected LLC-PK1 epithelial cells with plasmids encoding GFP (Figure 5A) or GFP–RGS4 (Figure 5B), stained the cells with β-COP antibodies to localize the COPI complex, and examined them by indirect immunofluorescence microscopy. Control cells showed punctate Golgi β-COP localization, whereas cells expressing RGS4 showed a marked loss of perinuclear β-COP staining. Treatment of cells with GDP–magnesium aluminum fluoride (AMF), which increased β-COP staining in control cells, did not stimulate recruitment of β-COP to Golgi membranes in cells expressing RGS4 (our unpublished results). To determine if the effect of RGS4 on β-COP localization was due to its ability to bind β′-COP, we transfected cells with a plasmid encoding GFP–RGS4, which lacks the β′-COP binding site (amino acids 131–205), and stained for β-COP (Figure 5C). Like GFP alone, GFP–RGS4(131–205) did not have any detectable effect on the Golgi localization of β-COP.
Figure 5.
Cytosolic dispersion of β-COP and reduction in COPI association with Golgi in RGS4-expressing cells. Kidney LLC-PK1 epithelial cells were transfected with plasmids encoding GFP alone (A), GFP–RGS4 (wild type) (B), or GFP–RGS4(131–205) (C) and stained with antibodies against β-COP. Left column represents GFP-, GFP–RGS4 (wild type)-, and GFP–RGS4(131–205)-expressing cells; middle column shows endogenous β-COP distribution; and right column is the merge of the two images in each row. Arrowheads indicate the Golgi complex of nontransfected cells (control), and arrows indicate the Golgi complex of transfected LLC-PK1 cells in the same cell culture. Asterisks represent mistargeted and dispersed β-COP in RGS4-transfected cells. Bars, 5 μm.
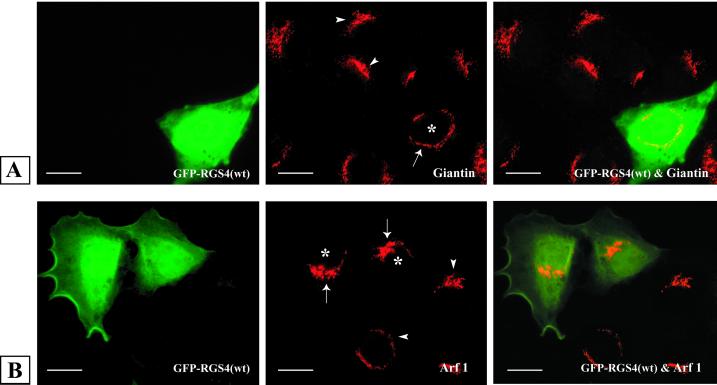
In contrast to the specific effect of RGS4 expression on COPI localization, the distribution of endogenous ARF1 or giantin, an integral membrane protein of the cis-Golgi (Seelig et al., 1994), appeared to be unaffected by RGS4 expression (Figure 6). These studies suggest that Golgi morphology is not disrupted in RGS4-expressing cells and that there is a selective effect of RGS4 on COPI association with Golgi membranes.
Figure 6.
Intact Golgi morphology in RGS4-expressing cells. LLC-PK1 cells were transiently transfected with plasmids encoding either GFP or GFP–RGS4 before staining with giantin (A) or ARF1 (B) antibodies and visualization by immunofluorescence microscopy. Left column represents GFP–RGS4-expressing cells; middle column shows endogenous giantin (A) or ARF1 (B) distribution; and right column is the merge of the two images in each row. Arrowheads indicate the Golgi region of nontransfected (control) cells, and arrows delineate the Golgi region of cells in the same culture transfected with GFP–RGS4 (wild type). Asterisks indicate the nucleus of GFP–RGS4 (wild type)-transfected cells (middle column). Bars, 5 μm.
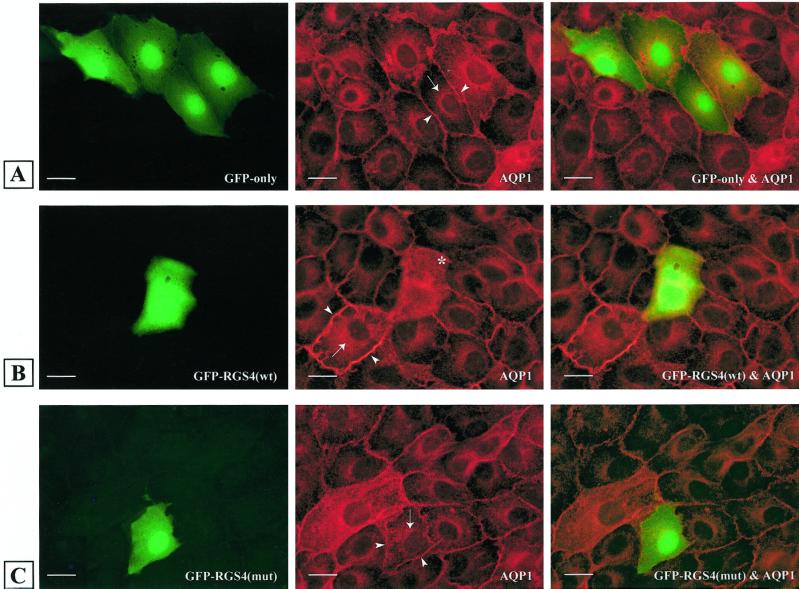
RGS4 Impairs Intracellular Transport
To determine whether intracellular transport was altered in RGS4-expressing cells, we examined the localization of AQP1, which is constitutively targeted to the plasma membrane (PM), in stably transfected LLC-PK1 cells (Katsura et al., 1995) (Figure 7). In contrast to the PM localization of AQP1 in cells transfected with a GFP construct (cell perimeter staining) (Figure 7A), cells expressing GFP–RGS4 (wild type) showed accumulation of AQP1 in the cytosolic and perinuclear areas, indicating that constitutive transport of the protein to the PM was inhibited in these cells (Figure 7B). This effect was not dependent on the GAP activity of RGS4, because RGS4(L159F) had a similar effect on AQP1 (our unpublished results). Lastly, the inhibition of AQP1 trafficking by RGS4 correlated with its ability to bind β′-COP, because GFP–RGS4(131–205) had no detectable effect on the transport of AQP1 (Figure 7C).
Figure 7.
Expression of RGS4 (wild type) but not RGS4(131–205) impairs transport of AQP1 to the plasma membrane in LLC-PK1 cells. Cells stably transfected with AQP1 were transiently transfected with plasmids encoding GFP (A), GFP–RGS4 (wild type) (B), or GFP–RGS4(131–205) (mut) (C). Left column represents GFP-, GFP–RGS4 (wild type)-, and GFP–RGS4(131–205)-expressing cells; middle column shows AQP1 distribution; and right column is the merge of the two images in each row. Arrows indicate AQP1 distribution in the perinuclear region, and arrowheads indicate AQP1 localization in the plasma membrane of LLC-PK1 cells. The asterisk indicates a GFP–RGS4 (wild type) transfected cell with mistargeted and dispersed AQP1. Plasma membrane staining is not detectable in this cell, in contrast to surrounding cells. Bars, 5 μm.
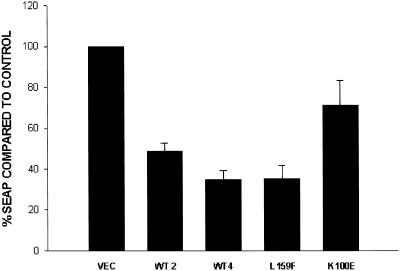
Finally, as an overall measure of transport, we examined the SEAP in HEK293T cells by expressing SEAP under the control of a constitutive promoter and measuring alkaline phosphatase protein levels in supernatants and cell lysates of RGS4-transfected cells, as described previously (Andreev et al., 1999) (Figure 8). Approximately 60% of the alkaline phosphatase was secreted into the medium after 4 h. Compared with cells transfected with an empty vector, the secretion of SEAP was inhibited ∼65% by wild-type RGS4. As in previous assays, this effect did not depend on GAP activity, because the GAP-dead mutant (L159F) inhibited secretion of alkaline phosphatase as well as wild-type RGS4. However, a mutant unable to bind β′-COP (K100E) did not inhibit alkaline phosphatase secretion significantly, suggesting that the effect of RGS4 correlated with its ability to bind the β′-COP in this assay. From these two independent measures of intracellular trafficking, we conclude that RGS4 expression impairs intracellular transport.
Figure 8.
RGS4 inhibits secretion of placental alkaline phosphatase in HEK293T cells. Cells were transfected with plasmids encoding SEAP cytomegalovirus–β-galactosidase and a control vector or various HA–RGS4 plasmids. Forty-eight hours after transfection, medium was washed out and replaced with fresh medium for 4 h. Alkaline phosphatase was measured from the supernatant and cell lysate as luciferase activity and divided by β-galactosidase values to obtain normalized SEAP levels. The bar graph represents means ± SEM of two independent experiments (assayed in triplicate) expressed as a percentage of control vector alone (ratios of SEAP in supernatant and the sum of alkaline phosphatase in supernatant and cell lysate).
DISCUSSION
In this study, we have characterized a novel and direct interaction between two members of the RGS family of signaling molecules, RGS4 and RGS2, and the coat protein β′-COP. These RGS proteins are likely to associate with the intact COPI complex, as demonstrated by the ability of RGS4 to coimmunoprecipitate with several coatomer subunits from detergent lysates of HEK293T cells and by cofractionation of native RGS4 and RGS2 with COPI in two different cell lines. The most significant result of the RGS–COPI interaction is the ability of RGS4 and RGS2 to inhibit cytoplasmic COPI binding to purified Golgi membranes in vitro and to decrease Golgi-associated COPI in intact LLC-PK1 epithelial cells. Moreover, RGS4 protein expression impaired constitutive trafficking of AQP1 to the plasma membrane in these cells and decreased secretion of alkaline phosphatase in HEK293T cells.
Although an established biochemical mechanism of RGS protein action is to accelerate GTP hydrolysis by Giα and Gqα by stabilizing the transition state conformation of the G protein during the hydrolysis reaction (mimicked by AMF) (Berman et al., 1996a; Druey and Kehrl, 1997; Tesmer et al., 1997; Srinivasa et al., 1998), the binding of RGS4 to β′-COP and its effect on COPI membrane localization is independent of a G protein. A mutation in RGS4, L159F, which abolishes its binding to Giα, does not diminish its ability to interact with coatomer or its inhibition of COPI Golgi association both in vitro and in intact cells. Moreover, neither Golgi membrane stimulation with AMF nor GTPγS appears to have any bearing on the inhibitory effects of RGS4 on COPI binding. These results also suggest that the enzymatic GAP activity of RGS4 is not required for its effect on COPI localization. We mapped the region in RGS4 involved in β′-COP binding to two internal dilysine-containing sequences in the RGS domain. Based on the crystal structure of RGS4-Giα1-AMF, this region forms a linker between two α-helices (α4 and α5), and the downstream motif (KAKK110–114) is partially contained within the “top” of the α5 helix (where the “bottom” of the helix is defined as the G protein binding surface) (Tesmer et al., 1997). Because these regions lie at opposite sides of the RGS–Gα interface, it appears that RGS4 would be able to contact β′-COP and Gα simultaneously. Notably, these motifs are conserved in many RGS proteins, implying that several of them may serve a similar function depending on the cell type in which they are expressed.
Although the mechanism of coatomer recruitment to membranes is uncertain, it is thought to involve at least two mechanisms. First, the small GTP-binding protein ARF1, which is found on Golgi membranes, controls COPI binding through its GTPase cycle. GTP–ARF1 can be photo–cross-linked to β-COP and γ-COP, suggesting that direct binding of the two subunits by ARF1 may be partially responsible for COPI membrane binding (Zhao et al., 1997, 1999). In addition, the GAP-initiated GTPase activity of ARF1 may be enhanced by coatomer in an in vitro assay, implying that COPI stimulates its own membrane dissociation by augmenting the deactivation of ARF1 (Goldberg, 1999).
Second, coatomer has been shown to bind dilysine motifs in the C-terminal tails of a family of type I Golgi membrane–associated proteins (Sohn et al., 1996). Dilysine motifs in the cytoplasmic domain of membrane-associated proteins are associated with retrieval to and residence in the ER during membrane trafficking (Kreis et al., 1995). Recent studies showed that the γ-COP subunit mediates the interaction between the coatomer complex and KKXX-containing p24 proteins (Harter et al., 1996; Harter and Wieland, 1998; Zhao et al., 1999).
Although the binding of coatomer to the KKXX motif via γ-COP is similar to our results in that both involve interaction between a COPI subunit and a dilysine motif, these two interactions appear to be mediated by different COPI subunits (γ- and β′-COP). In addition, the KKXX retrieval motif must be near the C terminus for efficient coatomer-mediated retrieval to occur (Cosson and Letourneur, 1994), whereas the dilysine motifs in RGS4 and RGS2 are internal. Our results are more consistent with recent studies that showed that some aminoglycoside antibiotics such as neomycin could precipitate COPI, which requires the presence of two closely spaced amino groups in the antibiotic that mimic a dilysine motif (Hudson and Draper, 1997). Subsequent experiments revealed that the compound 1,3-cyclohexanebis(methylamine) (CBM), which also contains two closely spaced amino groups, caused dispersion of COPI from the Golgi and inhibited Golgi-to-PM transport, as shown by the perinuclear accumulation of vesicular stomatitis virus G protein in CBM-treated cells (Hu et al., 1999). We compared the relative affinities of COPI for neomycin and RGS4 and found that its affinity for RGS4 was significantly higher than for neomycin. Thus, these results suggest that chemicals such as neomycin and CBM at higher concentrations may act in a manner similar to RGS4 to prevent recruitment of cytosolic coatomer to Golgi membranes.
Our results suggest that RGS4, as well as several other RGS proteins with conserved internal dilysine motifs such as RGS2, may bind or aggregate COPI via β′-COP and act as a cytosolic “sink” to prevent the interaction of COPI with membrane receptors. This hypothesis is consistent with the result that the functional RGS–COPI interaction occurs in the cytosol, because membrane-bound RGS4 does not affect subsequent recruitment of COPI. We hypothesize that when RGS4 was incubated with Golgi membranes before the addition of coatomer from cytosol, it may have bound to the preexisting COPI on the membranes, which was no longer susceptible to disruption by RGS4, possibly because of stabilization by interactions between COPI subunits and ARF1 and/or p24 proteins. In addition, the effect of RGS4 on COPI membrane association appears to be independent of ARF1, because it inhibited COPI binding to Golgi membranes even in the presence of GTPγS, which causes irreversible ARF1 activation. Thus, RGS4 may sequester cytosolic COPI and prevent it from binding to a putative receptor simply by allosteric inhibition, or it may induce a conformational change in COPI that prevents binding. Conversely, association of RGS4 and RGS2 with COPI in the cytoplasm may serve as a docking site for these RGS proteins, from which they may translocate to the plasma membrane to regulate G protein–mediated signaling, as has been shown previously for RGS3 and RGS4 (Druey et al., 1998; Dulin et al., 1999).
Because it is unlikely that RGS proteins exert a direct GAP effect on a monomeric GTPase such as ARF1 (Watson et al., 1996), the possibility remains that Gα proteins could also be involved in the regulation of COPI membrane recruitment or in the concentration or sorting of cargo in transport carriers. COPI binding to Golgi membranes may be sensitive to modulators of heterotrimeric G protein activation such as mastoparan or pertussis toxin (Donaldson et al., 1991; Ktistakis et al., 1992). Previously, we found that expression of Giα3 in LLC-PK1 cells inhibited secretion of heparan sulfate proteoglycan and that pertussis toxin reversed this effect (Stow et al., 1991). Recently, Gβγ proteins have been implicated in potential signaling events associated with Golgi disassembly/reassembly during mitosis (Jamora et al., 1997). In addition, Gα subunits have been found on intracellular organelle membranes, where it is unclear whether they are associated with either heptahelical receptors or Gβγ (Leyte et al., 1992; Maier et al., 1995; Denker et al., 1996; Helms et al., 1998). RGS proteins could also be targeted to intracellular organelle membranes by virtue of their interaction with Gα and thus be poised to regulate other aspects of the secretory trafficking process in addition to the G protein–independent COPI membrane recruitment described here. Thus, the potential interplay between COPI, RGS proteins, and heterotrimeric G proteins may not strictly follow the paradigm of signaling events that occur at the PM. Our results suggest that COPI binding to Golgi membranes is highly regulated and provide an impetus to further study the role of heterotrimeric G proteins and RGS proteins in membrane trafficking.
ACKNOWLEDGMENTS
We thank Peter Sun, Julie Donaldson, and Jennifer Lippincott-Schwartz for reagents and helpful advice, Cindy Pagonis for editorial assistance, and Dean Metcalfe for his support. B.M.S., V.M., H.Y.L., D.A.A., and D.B. are supported by National Institutes of Health grant DK38452. K.J.H.-L. is supported by the Cancer Research Campaign.
Abbreviations used:
- AMF
magnesium aluminum fluoride
- ER
endoplasmic reticulum
- FPLC
fast performance liquid chromatography
- GAP
GTPase-activating protein
- GFP
Green Fluorescent Protein
- HA
hemagglutinin
- HEK293T
human embryonic kidney 293
- IgG
immunoglobulin G
- PM
plasma membrane
- RGS
regulator of G protein signaling
- SEAP
secretion of human placental alkaline phosphatase
- TCs
transport carriers
REFERENCES
- Andreev J, Simon J-P, Sabatini DD, Kam J, Plowman G, Randazzo PA, Schlessinger J. Identification of a new Pyk2 target protein with ARF-GAP activity. Mol Cell Biol. 1999;19:2338–2350. doi: 10.1128/mcb.19.3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Druey KM, Richter G, Kehrl JH, Smith KM. Regulators of G. protein signaling exhibit distinct patterns of gene expression and target G protein specificity in human lymphocytes. J Immunol. 1999;162:2677–2682. [PubMed] [Google Scholar]
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- Berman DM, Kozasa T, Gilman AG. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem. 1996a;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996b;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer (COPI)-coated vesicles: role in intracellular transport and protein sorting. Curr Opin Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- Csukai M, Chen CH, De Matteis MA, Mochly-Rosen D. The coatomer protein β′-COP, a selective binding protein (RACK) for protein kinase Cε. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of α subunits and βγ subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries L, Elenko E, McCaffery JM, Fischer T, Hubler L, McQuistan T, Watson N, Farquhar MG. RGS-GAIP, a GTPase-activating protein for Gαi heterotrimeric G proteins, is located on clathrin-coated vesicles. Mol Biol Cell. 1998;9:1123–1134. doi: 10.1091/mbc.9.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Kahn RA, Lippincott-Schwartz J, Klausner RD. Binding of ARF and β-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991;254:1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Druey KM, Blumer KJ, Kang VH, Kehrl JH. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- Druey KM, Kehrl JH. Inhibition of regulator of G protein signaling function by two mutant RGS4 proteins. Proc Natl Acad Sci USA. 1997;94:12851–12856. doi: 10.1073/pnas.94.24.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druey KM, Sullivan BM, Brown D, Fischer ER, Watson N, Blumer KJ, Gerfen CR, Scheschonka A, Kehrl JH. Expression of GTPase-deficient Giα2 results in translocation of cytoplasmic RGS4 to the plasma membrane. J Biol Chem. 1998;273:18405–18410. doi: 10.1074/jbc.273.29.18405. [DOI] [PubMed] [Google Scholar]
- Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R. Yeast β- and β′-coat proteins (COP) J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- Dulin NO, Sorokin A, Reed E, Elliott S, Kehrl JH, Dunn MJ. RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Gα11. Mol Cell Biol. 1999;19:714–723. doi: 10.1128/mcb.19.1.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I, Gaitatzes C, Smith TF, Neer EJ. Folding a WD repeat propeller: role of highly conserved aspartic acid residues in the G protein β subunit and Sec13. J Biol Chem. 1998;273:9041–9049. doi: 10.1074/jbc.273.15.9041. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent. anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;24:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Penman M, Trigatti BL, Krieger M. A single point mutation in ε-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant. J Biol Chem. 1996;271:11191–11196. doi: 10.1074/jbc.271.19.11191. [DOI] [PubMed] [Google Scholar]
- Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Lavoie KJ, Lewis VA, Hynes GM, Collison KS, Nutland E, Willison KR. A102 kDa subunit of a Golgi-associated particle has homology to β subunits of trimeric G proteins. EMBO J. 1993;12:2847–2853. doi: 10.1002/j.1460-2075.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, Tschochner H, Wieland F. Nonclathrin coat protein γ, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway. Proc Natl Acad Sci USA. 1996;93:1902–1906. doi: 10.1073/pnas.93.5.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter C, Wieland FT. A single binding site for dilysine retrieval motifs and p23 within the γ subunit of coatomer. Proc Natl Acad Sci USA. 1998;95:11649–11654. doi: 10.1073/pnas.95.20.11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Helms-Brons D, Brugger B, Gkantiragas I, Eberle H, Nickel W, Nurnberg B, Gerdes HH, Wieland FT. A putative heterotrimeric G protein inhibits the fusion of COPI-coated vesicles. J Biol Chem. 1998;273:15203–15208. doi: 10.1074/jbc.273.24.15203. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Kao C-Y, Hudson RT, Chen A, Draper RK. Inhibition of secretion by 1,3 cyclohexanebis(methylamine), a dibasic compound that interferes with coatomer function. Mol Biol Cell. 1999;10:921–933. doi: 10.1091/mbc.10.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi-, and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RT, Draper RK. Interaction of coatomer with aminoglycoside antibiotics: evidence that coatomer has at least two dilysine binding sites. Mol Biol Cell. 1997;10:1901–1910. doi: 10.1091/mbc.8.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Takizawa PA, Zaarour RF, Denesvre C, Faulkner DJ, Malhotra V. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- Katsura T, Verbavatz J-M, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA. 1995;92:7212–7216. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Lowe M, Pepperkok R. COPs regulating membrane traffic. Annu Rev Cell Dev Biol. 1995;11:677–706. doi: 10.1146/annurev.cb.11.110195.003333. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Linder ME, Roth MG. Action of brefeldin A blocked by activation of a pertussis-toxin-sensitive G protein. Nature. 1992;356:344–346. doi: 10.1038/356344a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Leyte A, Barr FA, Kehlenbach RH, Huttner WB. Multiple trimeric G-proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO J. 1992;11:4795–4804. doi: 10.1002/j.1460-2075.1992.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Kreis TE. In vitro assembly and disassembly of coatomer. J Biol Chem. 1995;270:31364–31371. doi: 10.1074/jbc.270.52.31364. [DOI] [PubMed] [Google Scholar]
- Maier O, Ehmsen E, Westermann P. Trimeric G protein α subunits of the Gs and Gi families localized at the Golgi membrane. Biochem Biophys Res Commun. 1995;208:135–143. doi: 10.1006/bbrc.1995.1315. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Bourgoin S, Londono I, Bendayan M, Vinay P. Identification of ADP-ribosylation factor-6 in brush border membrane and early endosomes of human kidney proximal tubules. Electrophoresis. 1997;18:538–547. doi: 10.1002/elps.1150180334. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Helms JB, Beckers CJM, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- Pelham HRB. About turn for the COPs? Cell. 1994;79:1125–1127. doi: 10.1016/0092-8674(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Peter F, Plutner H, Zhu H, Kreis TE, Balch WE. β-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi in vitro. J Cell Biol. 1993;122:1155–1167. doi: 10.1083/jcb.122.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rohman, M., and Harrison-Lavoie, K.J. (2000). Separation of co-purifying GroEL from GST fusion proteins. Protein Expression and Purification (in press). [DOI] [PubMed]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schekman R, Mellman I. Does COPI go both ways? Cell. 1997;90:197–200. doi: 10.1016/s0092-8674(00)80326-8. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Seelig HP, Schranz P, Schroter H, Wiemann C, Griffiths G, Renz M. Molecular genetic analyses of a 376-kilodalton Golgi complex membrane protein (giantin) Mol Cell Biol. 1994;14:2564–2576. doi: 10.1128/mcb.14.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Shima DT, Scales SJ, Kreis TE, Pepperkok R. Segregation of COPI-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr Biol. 1999;9:821–824. doi: 10.1016/s0960-9822(99)80365-0. [DOI] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasa SP, Watson N, Overton MC, Blumer KJ. Mechanism of RGS4, a GTPase-activating protein for G protein α subunits. J Biol Chem. 1998;273:1529–1533. doi: 10.1074/jbc.273.3.1529. [DOI] [PubMed] [Google Scholar]
- Stenbeck G, Harter C, Brecht A, Herrmann D, Lottspeich F, Orci L, Wieland FT. β′-COP, a novel subunit of coatomer. EMBO J. 1993;12:2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow JL, de Almeida JB, Narula N, Holtzman EJ, Ercolani L, Ausiello DA. A heterotrimeric G protein, Gαi-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991;114:1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJG, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4-activated Giα1: stabilization of the transition state for nucleotide hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- Van Der Voorn L, Ploegh HL. The WD-40 repeat. FEBS Lett. 1992;307:131–134. doi: 10.1016/0014-5793(92)80751-2. [DOI] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase activating proteins for heterotrimeric G-protein α-subunits. Nature. 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- Wylie F, Heimann K, Tam LL, Brown D, Rabnott G, Stow JL. GAIP, a Gαi3 binding protein, is associated with Golgi-derived vesicles and protein trafficking. Am J Physiol. 1999;276:C497–C506. doi: 10.1152/ajpcell.1999.276.2.C497. [DOI] [PubMed] [Google Scholar]
- Yan Y, Chi PP, Bourne HR. RGS4 inhibits Gq-mediated activation of mitogen-activated protein kinase and phosphoinositide synthesis. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- Zhao L, Helms JB, Brugger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland FT. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Helms JB, Brunner J, Wieland FT. GTP-dependent binding of ADP-ribosylation factor to coatomer in close proximity to the binding site for dilysine retrieval motifs and p23. J Biol Chem. 1999;274:14198–14203. doi: 10.1074/jbc.274.20.14198. [DOI] [PubMed] [Google Scholar]