Abstract
The RNA helicases p68 and p72 are highly related members of the DEAD box family of proteins, sharing 90% identity across the conserved core, and have been shown to be involved in both transcription and mRNA processing. We previously showed that these proteins co-localise in the nucleus of interphase cells. In this study we show that p68 and p72 can interact with each other and self-associate in the yeast two-hybrid system. Co-immunoprecipitation experiments confirmed that p68 and p72 can interact in the cell and indicated that these proteins preferentially exist as hetero-dimers. In addition, we show that p68 can interact with NFAR-2, a protein that is also thought to function in mRNA processing. Moreover, gel filtration analysis suggests that p68 and p72 can exist in a variety of complexes in the cell (ranging from ∼150 to ∼400 kDa in size), with a subset of p68 molecules being in very large complexes (>2 MDa). The potential to exist in different complexes that may contain p68 and/or p72, together with a range of other factors, would provide the potential for these proteins to interact with different RNA substrates and would be consistent with recent reports implying a wide range of functions for p68/p72.
INTRODUCTION
The highly related p68 and p72 proteins are members of the DEAD box family of RNA helicases, which is characterised by a core segment of eight conserved motifs including a Asp–Glu–Ala–Asp (D-E-A-D) sequence (1). In vitro, DEAD box proteins share similar biochemical functions, namely RNA-dependent ATPase activity and, in several cases, RNA helicase activity. However, DEAD box proteins have been shown to be involved in a wide range of biological processes involving interaction with specific RNA substrates; examples include pre-mRNA splicing, ribosome biogenesis and translation (2,3).
p68 was initially discovered through a fortuitous cross-reaction with an antibody raised against simian virus 40 (SV40) large T antigen (4). Subsequent determination of the deduced amino acid sequence of the p68 cDNA revealed a striking homology to eIF-4A (5) and led to the definition of the ‘DEAD box’ family of proteins (1). p72 was also isolated fortuitously during screening of a HeLa cell expression library with an unrelated antibody (6). Analysis of the deduced amino acid sequence of p72 showed a remarkable similarity to p68. Over the central core, which contains the motifs conserved in the DEAD box family, the homology between p68 and p72 is 90%. However, the N- and C-terminal extensions show significant differences, with ∼60% (after introduction of appropriate gaps) and 30% homology, respectively. These observations imply that p68 and p72 may have subtly different functions in the cell, perhaps through interaction with different RNA substrates or proteins. It has been reported recently that the p72 mRNA can also be alternatively translated into an 82 kDa protein using an upstream non-AUG codon and that, in tissue culture cells, the 82 kDa species is expressed at a concentration similar to p72 (7).
p68 and p72 have been highly conserved through evolution. The mouse and human p68 proteins are 98% identical (8), while the chick homologue shares ∼90% identity with human p68, although the chick protein is missing the first 12 amino acids found in the human protein and is overall 19 amino acids shorter (9). In the case of p72, partial cDNA clones of the rat and chick homologues which include part of the conserved core also show a very high degree of homology (10). Interestingly, Saccharomyces cerevisiae only has one p68/p72 homologue (Dbp2p), which shares 55% sequence identity with the human protein, suggesting either that there is some functional redundancy between these proteins or that multicellular organisms require both proteins.
The patterns of expression of p68 and p72 mRNA in adult mammalian tissues has been shown to be different, suggesting differential expression of the p68 and p72 genes (6,11). Moreover, although expression of both p68 and p72 is developmentally regulated, their expression in development and in neuronal differentiation also appears to be different (10,11). These findings, together with the observed divergence of the N- and C-terminal regions of the p68 and p72 proteins, are consistent with these proteins having, perhaps subtly, different roles in the cell. These could arise from specialisation in the substrate specificity of the proteins and/or differential regulation of expression. Examination of the cellular localisation of the proteins has shown that p68 and p72 co-localise in the nuclei of interphase cells (6), although to date, it has been unclear whether, like p68, p72 is transiently associated with nascent nucleoli during telophase while being largely excluded from nucleoli during interphase (12,13).
p68, p72 and p82 have all been shown to exhibit the RNA-dependent ATPase and RNA helicase activities characteristic of members of the DEAD box family (6,7,14–17) while p68 and p72 have also been reported to catalyse rearrangement of RNA structure via branch migration (16).
In the last few years several biological functions have been assigned to p68 and p72. Both proteins have been shown to interact with, and act as specific co-activators for, estrogen receptor alpha (18,19). p68 has also been shown to be essential for in vitro pre-mRNA splicing, acting at the U1 snRNA-5′ splice site duplex (20), while p72 has been shown to be associated with U1 snRNP (21) and also involved in the regulation of alternative splicing (22). In addition, depletion of Dbp2p in yeast results in defects in both nonsense-mediated mRNA decay and ribosomal RNA processing with the defect in rRNA processing being rescued by human p68 (23). p68 and p72 have also been shown to be growth- and developmentally regulated (10,11,24,25) while p68 appears to be overexpressed and abnormally poly-ubiquitinated in colorectal tumours (26).
In a yeast two-hybrid screen for potential p72-interacting proteins we observed that p68 and p72 interact with each other and that both proteins can self-associate in this system. Their interaction was confirmed by co-immunoprecipitation experiments, which showed that p68 and p72 can form dimers/oligomers in the cell. Like p68, p72 can also interact with fibrillarin in the yeast two-hybrid system although the interaction appears to be weaker than that between p68 and fibrillarin. In addition, an antibody generated against a p72 C-terminal peptide cross-reacted with unrelated 105 kDa protein, NFAR-2, which can also interact with p68 and p72 in the yeast two-hybrid system. Moreover, we show by gel filtration experiments, that these proteins can exist in a multi-protein complex in the cell. These findings suggest potential regulation of p68/p72 function by altering their interaction with each other and with other proteins in the cell.
MATERIALS AND METHODS
Antibodies
p68. The antibodies used were the mouse monoclonal antibody PAb 204 and the rabbit polyclonal antibody 2906, generated against the C-terminal 15 amino acids of p68 (11). PAb 204 was originally generated against the SV40 large T antigen but it cross-reacts with p68 (4). It is specific for p68 in cells that are not infected or transformed by SV40.
p72. The antibodies used were rabbit anti-peptide polyclonal antibodies generated against amino acids 624–638 [peptide sequence ATNMIGYMGQTAYQY; antibodies 43 and 44 (our laboratory)] and 35–55 of p72 (a gift from M. Watanabe and S. Kato, University of Tokyo, Japan), respectively. Antibody 43 generally gave cleaner results in western blotting, although it also recognised NFAR-2 (see below) and was the antibody of choice for most western blotting experiments. The antibody raised against amino acids 35–55 has been reported to be specific for p72 (19) and was used for immunofluorescence. This antibody was not available in sufficient quantities for use in western blotting.
NFAR-2. A rabbit polyclonal antibody that recognises the N-terminus of NFAR-2 was used; this antibody detects both NFAR-2 and its splice variant NFAR-1 (27,28).
Myc epitope. A mouse monoclonal antibody (9E10) was used to detect proteins tagged with the myc epitope (MRQKLISEEDL).
GST. A goat polyclonal antibody (Amersham) was used to detect GST-tagged fusion proteins.
Fibrillarin. The mouse monoclonal antibody 72B9 used was kindly provided by K. M. Pollard and E. M. Tan, Department of Molecular and Experimental Medicine, Scripps Research Institute, La Jolla, CA, USA.
p68/p72 expression vectors
p72-myc. a p72 cDNA was cloned in a derivative of the mammalian expression vector pSG5 (Stratagene) containing the myc epitope at the N-terminus (6).
p82-myc. a p82 cDNA was generated by annealing oligonucleotides comprising the upstream sequence given in Uhlmann-Schiffler et al. (7) and the myc-tagged version was obtained as for p72.
p68-/p72-GST. p68 and p72 cDNAs were cloned in the vector pEBG2T (29) which allows expression of GST-tagged proteins in mammalian cells.
Yeast two-hybrid interaction
Interaction assays, using the Clontech GAL4 Matchmaker yeast two-hybrid system were carried out as described previously (13). The extent of interaction between partner proteins was estimated according to the proportion of blue colonies obtained and the time taken for the colour to develop as described in the figure legends.
Cell culture and transfections
HeLa cells and 293 cells were grown at 37°C, 5% CO2, in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (Life Technologies). Cell lysates were prepared in Igepal buffer [150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1% Igepal] or RIPA (150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1% Igepal, 0.1% SDS and 0.5% sodium deoxycholate] containing protease inhibitor cocktail (Roche). For transfections, cells were seeded at 3 × 106 per 15 cm plate 16 h prior to transfection, using the calcium phosphate precipitation method (30) and harvested 48 h later.
Co-immunoprecipitation
293 cell lysates were prepared in RIPA buffer (above) but without sodium deoxycholate. Immunoprecipitations and separation/analysis of immunoprecipitated proteins were carried out using SDS–PAGE and western blotting under standard conditions (31).
Gel filtration
293 cell lysates were prepared in RIPA buffer and fractionated on a Pharmacia Superose 6HR column in 150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 10% glycerol, 1 mM benzamidine. Fractions (0.5 ml) were collected and alternate fractions were analysed by SDS–PAGE and western blotting. Protein molecular weight standards from Pharmacia were used to calibrate the column.
Immunocytochemistry
HeLa cells were grown on coverslips and fixed in 3.7% paraformaldehyde in CSK buffer [100 mM NaCl, 300 mM sucrose, 10 mM PIPES (pH 6.8), 3 mM MgCl2, 1 mM EGTA] for 10 min at room temperature. They were permeabilised with 0.5% Triton X-100 in CSK buffer for 20 min at room temperature and stained with rabbit polyclonal antibodies for p68, p72 and NFAR-1 and -2 and monoclonal antibodies for fibrillarin and the myc epitope to detect myc-tagged p72. DNA was stained with 4,6-diamidino-2-phenylinole (DAPI). Appropriate FITC- and Texas Red-conjugated secondary antibodies were used as required. Images were captured on an Olympus IX70 microscope using a ×60 objective connected to an Improvision Openlab image processing system.
Mass spectrometry
NFAR-2 was identified by tryptic mass fingerprinting using MALDI-TOF mass spectrometry and database searching as described previously (32).
RESULTS
p68 and p72 interact in the yeast two-hybrid system
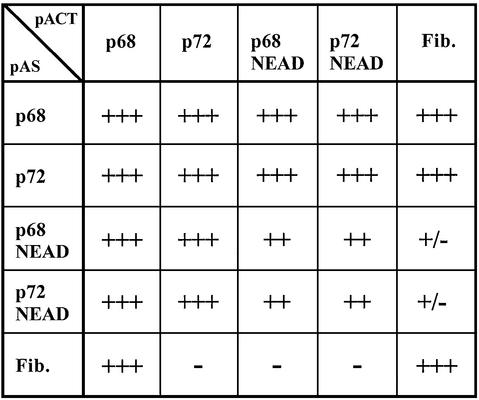
In the course of a yeast two-hybrid screen to isolate potential p72-interacting proteins we used p68 as a control protein. We observed that, in this system, p68 and p72 can interact with each other and also self-associate to yield His+ β-galactosidase-positive Y190 colonies. The initial tests were carried out with p68 as a hybrid with the GAL4 DNA-binding domain (pAS2) and p72 as a fusion with the GAL4 transactivation domain (pACT2). The interaction was subsequently confirmed by reciprocal cloning of the p68 and p72 cDNAs in the pAS2 and pACT2 vectors, and showing that co-transformation of Y190 with these plasmids still yielded His+ β-galactosidase-positive clones (Fig. 1). Since we had previously identified fibrillarin as a partner for p68 (13), and since p68 and p72 are highly homologous, we tested whether p72 can also interact with fibrillarin. Interestingly, we could only observe an interaction between these two proteins when p72 was in the pAS2 vector and fibrillarin was in the pACT2 vector (Fig. 1). This is unlikely to be due merely to hybrid stability or toxicity, since each hybrid was shown to interact with p68 and suggests that the interaction between p72 and fibrillarin is weaker or different from that between p68 and fibrillarin. We also tested p68 and p72 mutants in which the DEAD motif had been mutated to NEAD to determine whether they could interact with each other and with fibrillarin. As expected by analogy with other DEAD box proteins, these mutants are ATPase- and helicase-inactive (data not shown). Interestingly, although they can still interact with one another (albeit more weakly), they are incapable of interacting with fibrillarin (Fig. 1), suggesting that ATPase/helicase activity are required for the interaction with fibrillarin or that the mutants have an altered conformation which affects the interaction with fibrillarin, but not the interaction with one another.
Figure 1.
Interaction of p68, p72 and fibrillain in the yeast two-hybrid system. cDNAs encoding p68, p72 or fibrillarin were cloned in the yeast two-hybrid vectors (Clontech) such that they were fused either to the GAL4 DNA binding domain (pAS2) or to the transcriptional activation domain (pACT2) and checked for interaction in a standard yeast two-hybrid assay. +++, strong interaction, 100% blue colonies in <30 min; ++, intermediate interaction, >50% blue colonies in <4 h; +/–, weak interaction, <50% blue colonies in 24 h; –, no interaction, no blue colonies. Fib., fibrillarin; NEAD, p68 and p72 in which the first aspartate (D) in the D-E-A-D motif had been mutated to asparagine (N) giving ATPase and helicase inactive proteins.
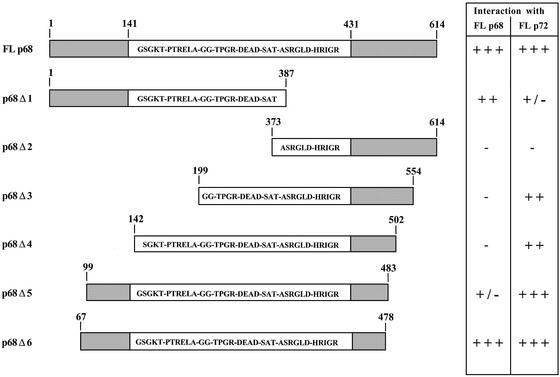
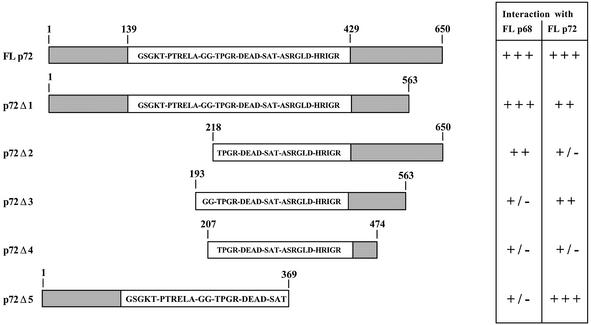
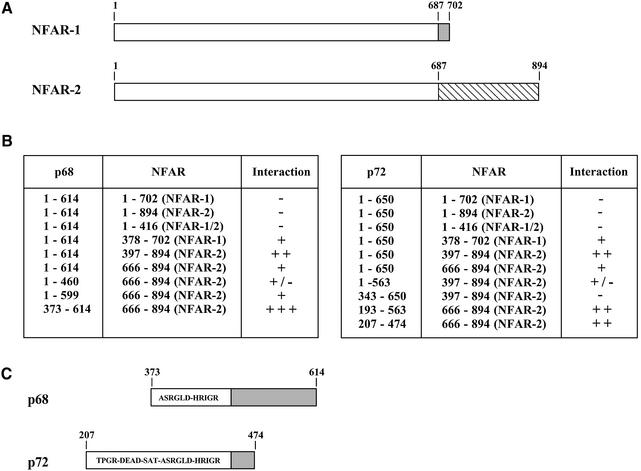
To determine the regions of p68 and p72 involved in the interaction between p68 and p72 and in the self-association of these proteins, we generated a series of p68 and p72 deletion derivatives in pACT2, and in each case tested them for interaction with both p68 and p72. Representative deletion derivatives for both proteins are shown in Figures 2 and 3. As shown in Figure 2, for p68, a large part of the conserved core is required for interaction with both p68 and p72. In addition, regions in the N-terminal extension are required for interaction with p68 (Δ1) while regions in the C-terminal extension are required for the interaction with p72 (Δ3 and Δ4). In the case of p72 (Fig. 3), again a substantial part of the conserved core is required, with N-terminal regions being required for interaction with p72 (Δ5) and C-terminal regions required for interaction with p68 (Δ2). These results imply that p68 and p72 homodimers may form in a way that is subtly different from p68/p72 heterodimers, perhaps due to differences in the N- and C-terminal extensions of these proteins, or to interaction with other factors during formation of the dimers or oligomers.
Figure 2.
Deletion mapping of the regions in p68 interacting with p68 and p72. In each case deletion derivatives were tested for interaction with the respective full-length partners in the yeast two-hybrid system. The amino acids included in each of the deletions are indicated as are the conserved ‘DEAD box’ motifs. FL, full length. +++, strong interaction, 100% blue colonies in <30 min; ++, intermediate interaction, >50% blue colonies in <4 h; +/–, weak interaction, <50% blue colonies in 24 h; –, no interaction, no blue colonies.
Figure 3.
Deletion mapping of the regions in p72 interacting with p68 and p72. In each case deletion derivatives were tested for interaction with the respective full-length partners in the yeast two-hybrid system. The amino acids included in each of the deletions are indicated as are the conserved ‘DEAD box’ motifs. FL, full length. +++, strong interaction, 100% blue colonies in <30 min; ++, intermediate interaction, >50% blue colonies in <4 h; +/–, weak interaction, <50% blue colonies in 24 h.
p68 co-immunoprecipitates with a 105 kDa protein—NFAR-2
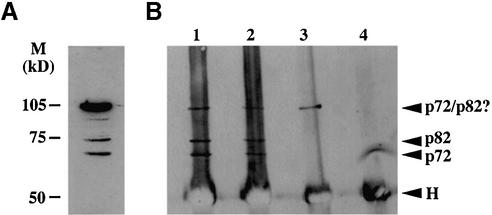
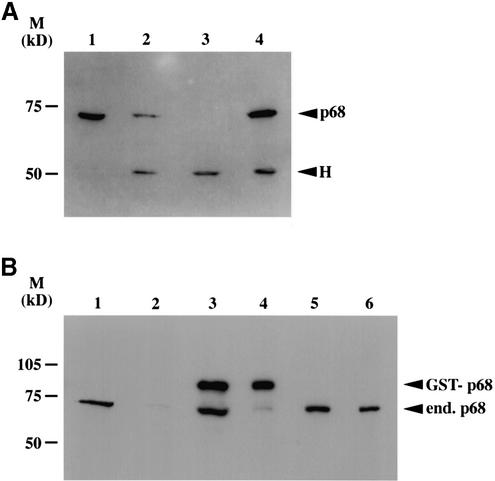
In order to analyse p72 in cell lines we generated polyclonal and monoclonal antibodies against the protein. Because of the high degree of homology between p68 and p72 and the necessity to obtain antibodies that did not cross-react with p68, we chose C-terminal regions of p72, which share only 30% homology with p68. Analysis of 293 cell lysates by western blotting using a polyclonal antibody generated against amino acids 624–638 of p72 (antibody 43) revealed the presence of three predominant species with apparent molecular weights of ∼105, ∼75 and ∼70 kDa, respectively (Fig. 4A) with the predominant species being the 105 kDa protein. Western blot analysis of a range of cell lines with antibodies 43/44, as well as an anti-peptide antibody raised against amino acids 485–498 of p72 gave similar results (data not shown). Moreover, monoclonal antibodies generated against a bacterially expressed p72 encompassing amino acids 370–650 also recognised predominantly a protein of 105 kDa (data not shown). Since we had observed that p68 interacted with p72 in the yeast two-hybrid system we decided to examine whether a p68 antibody could co-precipitate any of the p72 species. We carried out immunoprecipitation/western blotting reactions in which proteins in cell extracts were immunoprecipitated either with the p72 antibodies 43/44, with the p68 antibody 2906, or with an irrelevant antibody (as control) and then western blotted using the p72 antibody 43 (Fig. 4B). The p72 antibodies precipitated the same three protein species detected by western blotting of cell lysates using these antibodies (see Fig. 4A and B, lanes 1 and 2). The p68 antibody predominantly precipitated the 105 kDa species (Fig. 4B, lane 3). Although this could merely reflect the higher prevalence of this species, it is also possible that the epitope on the p68 molecules bound to the other p72 species is masked by virtue of the binding to p72 (see above), thus preventing these species from being precipitated. Nevertheless, taken together with the yeast two-hybrid data, this finding was consistent with p68 potentially interacting with p72 and with the 105 kDa protein being indeed a bona fide p72 species. In addition, while this study was underway, the discovery of an alternative upstream translation initiation of the p72 mRNA leading to p82 was reported (7). Analysis of exogenously expressed myc-tagged versions of p72 and p82 in a variety of cell lines, and in vitro translation of p72 and p82 cDNAs (data not shown and Fig. 7) suggested that the 70 and 75 kDa protein species recognised by the p72 antibody are indeed p72 and p82 and that the 105 kDa protein is not merely the result of post-translational modification of p72. We therefore wished to determine the identity of the 105 kDa species.
Figure 4.
Analysis of p72 species in cell lines and interaction with p68. (A) Western blotting of 293 cell lysates with an antibody generated against a p72 peptide detects three protein species. (B) Immunoprecipitation/ western blotting experiments to examine p68/p72 interactions. Proteins from 293 cell lysates were immunoprecipitated with p72 antibodies 43 and 44 (lanes 1 and 2), p68 antibody 2906 (lane 3), or an irrelevant antibody (lane 4) and then western blotted with the p72 antibody 43. p72, p82 and potential p72/p82 (p72/p82?) species and heavy chain cross-reaction (H) are indicated. The antibodies used for immunoprecipitation and western blotting were rabbit polyclonals, hence the strong cross-reaction for the heavy chain.
Figure 7.
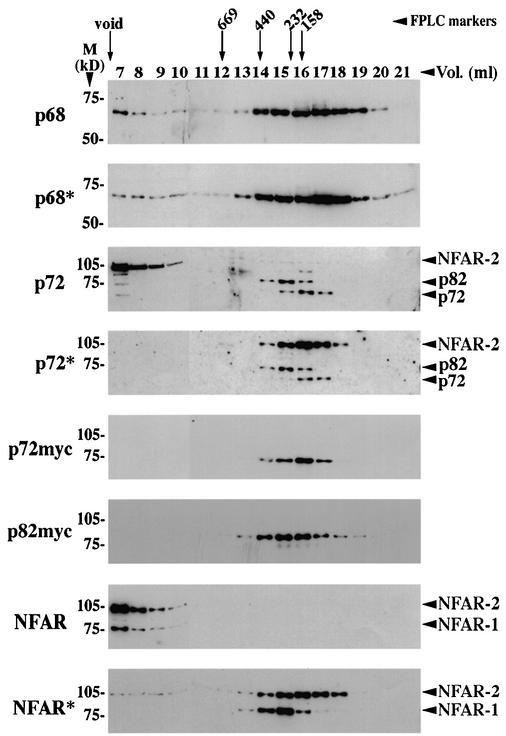
Western blots showing gel filtration elution profiles of p68, p72, p82 and NFAR. p68, p72 and NFAR in the gel filtration fractions were detected by western blotting using appropriate antibodies: 2906 for p68, 43 for p72, 9E10 for myc-tagged p72/p82 and the anti-NFAR antibody for NFAR-1 and NFAR-2. The void volume and elution position of the Pharmacia FPLC size markers are indicated, as are molecular weight markers (in kDa) for all western blots. *Lysates which had been treated with RNase A prior to gel filtration. Note that myc-tagged p72 and p82 have an electrophoretic mobility slightly slower than that of the respective endogenous proteins.
We carried out a large-scale immunoprecipitation from 293 cell lysates, using the p72 antibody 43, and the identity of the 105 kDa protein was determined by MALDI-TOF mass spectrometry analysis followed by database searching. This showed that this species was in fact NFAR-2 (nuclear factor associated with dsRNA) (27) a protein that exists as two alternatively spliced variants of ∼110 and ∼90 kDa, NFAR-2 and NFAR-1, respectively (27,28). These proteins have been shown to interact with the interferon-inducible dsRNA protein kinase (PKR) and are thought to function in pre-mRNA processing (28). Western blotting of cell lysates with an antibody that recognises NFAR-1 and NFAR-2 confirmed that the 105 kDa protein that cross-reacts with the p72 antibody has a similar electrophoretic mobility to NFAR-2 and separation of proteins from cell lysates by gel-filtration gave similar elution profiles (see Fig. 7 and below). It is unclear why the p72 antibody recognises NFAR-2 in western blots. Moreover, a different antibody generated against amino acids 485–498 of p72 (as opposed to 624–638) also cross-reacted with a 105 kDa protein in western blots. Analysis of the predicted amino acid sequence of NFAR-2 and the peptides used to generate the p72 antibodies showed no obvious homology (data not shown); however, there are precedents for such cross-reactions. Indeed p68 was originally identified through such a cross-reaction with PAb 204, an antibody raised against the SV40 large T antigen (4).
NFAR-1 and NFAR-2 have been shown to interact with pre-mRNA processing proteins (28). Given the recent reports that p68 and p72 are involved in pre-mRNA splicing (20) and the finding that p68 co-purifies with spliceosomes (33), it was important to examine their potential interaction with NFAR-2 further. To analyse this further we tested whether p68/p72 will interact with NFAR-1 and NFAR-2 in the yeast two-hybrid system as described above. We also generated a series of deletion derivatives of these proteins and tested them for interaction in this system. As shown in Figure 5A, the NFAR-1 and NFAR-2 proteins are identical for the first 687 amino acids and alternative splicing results in their having different C-termini. Analysis of the ability of p68/p72 to interact with NFAR-1/NFAR-2 showed that the full-length NFAR proteins do not interact with p68/p72 (Fig. 5B). However the C-terminal halves of NFAR-1 and NFAR-2 can interact with p68 and p72, although NFAR-2 generally interacted more efficiently. Deletion mapping of the regions required for interaction showed that the C-terminal region of NFAR-2 (amino acids 666–894), which is largely unique to NFAR-2 showed the best interaction. For p68 and p72 slightly different but overlapping regions of the proteins, incorporating parts of the conserved core and the C-terminal extensions were involved in the interaction. At present it is unclear why the full-length NFAR-2 protein does not interact with p68 or p72 in the yeast two-hybrid system. It is possible that NFAR-2 does not, in fact, interact with p68/p72 in the cell and that the co-precipitation is purely fortuitous. Alternatively it is possible that either (i) the GAL4 DNA binding/transactivation domain fusion affects the conformation and masks the binding site of the full-length NFAR-2 protein or (ii) in a mammalian cellular context, conformational changes in NFAR-2, perhaps as a result of interaction with other proteins or factors, expose the interacting regions and allow NFAR-2 to interact with p68 and/or p72.
Figure 5.
Analysis of interaction between NFAR-1/NFAR-2 and p68/p72 in the yeast two-hybrid system. (A) Diagrammatic representation of NFAR-1 and NFAR-2 coding regions highlighting identical regions (amino acids 1–687) and differences resulting from alternative splicing. (B) Interactions between full-length and deletion derivatives of NFAR-1/NFAR-2 and p68/p72. +++, strong interaction, 100% blue colonies in <30 min; ++, intermediate interaction, >50% blue colonies in <4 h; +/–, weak interaction, <50% blue colonies in 24 h; –, no interaction, no blue colonies. (C) The regions of p68 and p72 which show the strongest interaction with NFAR-2.
p68 and p72 co-immunoprecipitate from cell lysates
To confirm that p68 and p72 can indeed interact and to avoid the complication with the p72 antibody recognising several protein species we expressed myc-tagged p72 cDNA in 293 cells and examined whether it could interact with endogenous p68 by immunoprecipitating the myc-tagged p72, using an antibody directed against the myc epitope (9E10), and western blotting the precipitated proteins using a p68-specific antibody. Mock transfected 293 cells were used as a control. As shown in Figure 6A (lane 2), exogenously expressed myc-tagged p72 can co-precipitate endogenous p68 protein confirming that these proteins can indeed interact in the cell. Control immunoprecipitations using the 9E10 antibody with untransfected cells (lane 3) and a p68-specific antibody (lane 4) confirmed the specificity of the interaction. Co-immunoprecipitations using lysates which had been RNase treated gave similar results (data not shown), suggesting that RNA is not required for the interaction (see below and Fig. 7), although in our hands, treating with RNase tended to give more non-specific background.
Figure 6.
Co-immunoprecipitation of p68 and p72 from cell lysates. (A) Western blot of untransfected and myc-p72 transfected 293 cell lysates with a p68-specific antibody (2906) showing co-immunoprecipitation of exogenously expressed myc-tagged p72 with endogenous p68 (lanes 1, 2 and 4, lysates from cells transfected with myc-p72; lane 3, untransfected cells). Lane 1, endogenous p68 in cell lysate; lane 2, immunoprecipitation of myc-p72 with the anti-myc antibody 9E10; lane 3, immunoprecipitation of proteins from untransfected cells with 9E10; lane 4, immunoprecipitation of endogenous p68 from myc-p72 transfected cells with the p68-specific antibody PAb 204. H, cross-reaction of heavy chain. (B) Western blot of cells transfected with GST-tagged p68/p72 (and GST vector control) with a p68-specific antibody (2906) showing co-immunoprecipitation of exogenously expressed GST-tagged p72 with endogenous p68. In each case GST-tagged proteins were immunoprecipitated with a GST-specific antibody (lanes 1 and 2, cells transfected with GST–tagged vector control; lanes 3 and 4, cells transfected with GST-tagged p68; lanes 5 and 6, cells transfected with GST-tagged p72). Lane 1, lysate showing endogenous p68; lane 2, GST immunoprecipitation; lane 3, lysate showing endogenous and GST-tagged p68; lane 4, GST immunoprecipitation, showing immunoprecipitated GST-tagged p68 and a very low level of endogenous p68; lane 5, lysate showing endogenous p68; lane 6, GST immunoprecipitation showing endogenous p68 immunoprecipitating with GST-tagged p72.
In the yeast two-hybrid analysis (Fig. 1) we observed that p68 and p72 can, apart from interacting with each other, also self-associate. Therefore, we expressed GST-tagged versions of p68 and p72 in 293 cells and determined whether p68 and p72 could form homo- and hetero-dimers/oligomers in cells by immunoprecipitation/western blotting. (The use of GST-tagged p68/p72 allowed us to distinguish between exogenously expressed p68/p72 and the endogenous proteins.) The expressed GST-tagged p68 and p72 were immunoprecipitated using a GST-specific antibody and then probed for associated endogenous p68 using a p68-specific antibody. The expression vector expressing the GST moiety alone was used as a control. Figure 6B shows that, while GST-tagged p68 co-precipitates little endogenous p68 (lane 4) above background (lane 2), GST-tagged p72 co-precipitates a significant amount of endogenous p68 (lane 6). This again confirms that p68 and p72 interact and suggests that these proteins prefer to form hetero-dimers/oligomers in the cell. Interestingly, however, we reproducibly observed a minor increase of co-precipitation of endogenous p68 with GST-tagged p68, over the background obtained with the GST vector. Western blotting for endogenous co-precipitating p72 did not give clear results due to the low level of endogenous p72 detected and background obtained with this antibody (see Fig. 4C and data not shown). In addition, it was not possible to determine whether GST-tagged p68 and p72 interact with NFAR-2 due to non-specific binding of NFAR-2 to the GST and myc antibodies (data not shown).
p68 and p72 co-elute in a complex of >200 kDa
In order to examine further the interactions between p68 and p72 and the potential interaction between p68/p72 and NFAR-2 we subjected lysates from 293 cells to gel filtration and examined the elution profiles of p68, p72 and NFAR-1 and -2 from a Pharmacia Superose 6 column by western blotting fractions using appropriate antibodies. The lysates were prepared in RIPA buffer to avoid non-specific interactions. Examination of p68 in the fractions showed that a subset of the p68 molecules elute in very large complexes (>2 MDa) while the majority are in a heterogeneous population ranging from 440 kDa to below 100 kDa (Fig. 7). RNase treatment of the lysate prior to gel filtration results in loss of some of the p68 from the large (>2 MDa) complex and a minor shift in the heterogeneous population towards smaller, presumably monomeric p68. However, a substantial amount of p68 remains in complexes of between 150 and 400 kDa (Fig. 7). In the case of p72 and p82, the majority of the protein is present in complexes of ∼150 and ∼200 kDa, respectively, and RNase treatment does not affect this profile significantly. Fractionation of exogenously expressed myc-tagged p72/p82 gave identical elution profiles to those obtained for the endogenous proteins. It is interesting to note that the peaks of p72 and p82 have slightly different elution profiles, suggesting that they may form slightly different complexes with p68. The size of the complexes is, however, consistent with p68 and p72/p82 forming dimers in cells and with their interaction being direct rather than via RNA. Moreover, since the lysates were prepared in RIPA, in the presence of SDS and deoxycholate (see Materials and Methods) they are unlikely to be merely non-specific interactions. The use of less stringent buffers did not significantly alter the elution profiles (data not shown).
Probing of the fractions for NFAR-1 and -2 using an NFAR-specific antibody is again consistent with the 105 kDa protein detected by the p72 antibody being NFAR-2 (Fig. 7). Moreover, it shows that NFAR-1 and -2 are normally found in very large complexes (of >2 MDa) in the presence of RNA. RNase treatment, however, disrupts these complexes and results in most of the NFAR-1 and -2 proteins eluting as much smaller species. These elution profiles also suggest that if there is indeed an interaction between p68/p72 and NFAR-2, it is limited to a very small proportion of the p68 molecules that are found in the large (>2 MDa) complexes in the presence of RNA, since their presence in these complexes is abolished upon treatment with RNase (Fig. 7).
Cellular localisation of p68, p72, NFAR and fibrillarin
We also examined the cellular localisation of p68, p72, NFAR and fibrillarin by immunostaining of HeLa cells to determine whether these proteins show significant co-localisation. In each case we carried out co-staining for a pair of proteins, using appropriate specific antibodies to obtain all possible combinations. For NFAR, we used an antibody that detects both NFAR-1 and NFAR-2 (see Fig. 7) as this worked best for immunostaining; however, staining of cells expressing His-tagged NFAR-2 with a His-specific antibody gave an essentially identical staining pattern (data not shown). In the experiment to examine p72/NFAR localisation, it was not possible to use the anti-p72 and anti-NFAR antibodies together since these were both rabbit antibodies and appropriate mouse monoclonal antibodies were not available. Therefore, for these experiments, cells were transfected with a myc-tagged p72 plasmid and the expressed p72 was detected using the mouse monoclonal antibody against the myc epitope (9E10).
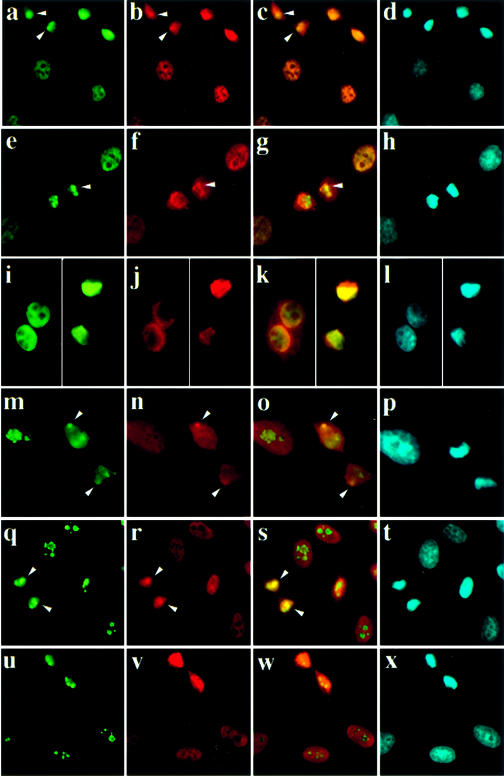
Figure 8 shows examples of the staining patterns obtained for the proteins examined. As previously shown (6), p68 and p72 [(a and b), respectively, merged image in (c)] co-localise in cells. In addition both proteins appear to be enriched in densely staining bodies during telophase, although this is less pronounced for p72, suggesting that they co-localise throughout the cell cycle [see arrows in (a, b and c)]. These findings are consistent with the p68/p72 interaction observed in the yeast two-hybrid system and in the co-immunopreciptiation studies. We had previously shown for p68 that these bodies are nascent nucleoli (13). p68 had also previously been shown to interact with and co-localise with fibrillarin during telophase [see arrows in (m, n and o)]. p72 also shows a partial co-localisation with fibrillarin in telophase [arrows in (q, r and s)]; however, as shown in (b), its localisation during telophase is more diffuse than that of p68 (a) and its enrichment in nascent nucleoli is less obvious (r). Co-staining experiments to investigate the relative localisation of p68/p72 and NFAR largely proved inconclusive (e–l). In a few cells there appeared to be a partial co-localisation of p68 and NFAR during telophase [(e and f), respectively, merged image in (g)]. However, in most cells the NFAR staining was, although largely nuclear, diffuse.
Figure 8.
Localisation of p68, p72, fibrillarin and NFAR in HeLa cells as determined by immunofluorescence microscopy. All cells were labelled with DAPI to detect DNA (d, h, i, p, t, x). The relative localisation of proteins was determined by labelling using appropriate secondary antibodies conjugated to FITC (green) and Texas Red, respectively, as follows: (a and b) p68/p72; (e and f) p68/NFAR; (i and j) p72/NFAR; (m and n) fibrillarin/p68; (q and r) fibrillarin/p72; (u and v) fibrillarin/NFAR. The respective merged images are shown in (c), (g), (k), (o), (s) and (w). Co-localisation is indicated by arrowheads. The images in (i–l) are composites of two images since in this case cells transfected with myc-tagged p72 were required. For each pair of proteins the primary antibodies were rabbit polyclonal and mouse monoclonal antibodies, respectively, thus allowing differential FITC/Texas Red staining by the secondary antibodies.
DISCUSSION
We have shown that the highly related RNA helicases p68 and p72 interact with each other and also self-associate in the yeast two-hybrid system (Fig. 1). Since we had previously shown that p68 interacts with the nucleolar protein, fibrillarin, in this system (13), we examined whether p72 also interacts with fibrillarin. Although these proteins can interact strongly when p72 was fused to the DNA binding domain and fibrillarin was fused to the transactivation domain, they did not interact in the reciprocal fashion. This was unlikely to be due to toxicity or stability because both proteins interacted strongly with p68 regardless of whether they were fused to the DNA binding or transactivation domain. This finding, therefore, suggests that the interaction between p72 and fibrillarin may be subtly different, or perhaps weaker, than that between p68 and fibrillarin. In addition, inactive p68/p72 mutants did not interact with fibrillarin, implying that ATPase and/or helicase activity may be required for interaction of these proteins with fibrillarin. These mutants were still capable of showing interaction between p68 and p72 in the yeast two-hybrid system, indicating that lack of interaction with fibrillarin was not merely due to problems with expression or toxicity in the yeast.
We also generated a series of deletion derivatives of p68 and p72 to examine further their interaction in the yeast two-hybrid system. The results obtained from these analyses (Figs 2 and 3) did not identify discrete domains required for interaction. In both cases, a large part of the conserved core of the proteins was required and, in addition, regions N-terminal to the core were needed for p68–p68 and p72–p72 interactions, while C-terminal regions were needed for p68–p72 interactions. This raises the possibility that the formation of homodimers of these proteins could occur in a somewhat different way to that of heterodimers. Further analyses of complexes of these proteins would be required to determine whether this is the case. Our earlier studies to examine the interacting regions for p68 and fibrillarin (13) suggested that regions that were discontinuous in the primary sequence were involved in the interaction. If regions relatively distant in terms of primary sequence were juxtaposed in the three-dimensional structure, it would be difficult to identify p68–p72 interacting regions accurately simply by deletion analysis, particularly if the proteins form relatively compact structures.
Co-immunoprecipitation analyses showed that p68 and p72 interact in the cell (Fig. 4A) and that while they can form both homo- and hetero-dimers/oligomers, there is a clear preference for the formation of hetero-dimers/oligomers. Fractionation of cell lysates by gel filtration showed that p68 molecules existed as a heterogeneous population with complexes of different sizes, with a small subset found in large (>2 MDa) complexes as well as monomeric forms (Fig. 7). On the other hand, p72 and p82 each gave one peak with an apparent size of ∼150 and ∼200 kDa, respectively. These coincided with a broad peak in the same fractions for p68. This would be consistent with a significant proportion of the p68 and p72/p82 molecules in the cell existing as heterodimers and is supported by the finding that exogenously expressed p68 co-immunoprecipitates endogenous p68 only to a minor extent (Fig. 6B). Interestingly, p72 and p82 do not co-elute suggesting that they do not interact with each other and that they are present in complexes (with p68), which are of different sizes, perhaps due to the presence of other factors. However, we cannot rule out that the apparent size difference is merely due to the difference between the p72 and p82 proteins.
There is a precedent for RNA helicases to exist as dimers. Studies of the crystal structures of two other members of the DEAD/DEAH family, namely the helicase domain of the NS3 protein of hepatitis C virus (34) and a DEAD box protein from Methanococcus jannaschii (MjDEAD) (35) indicate that these proteins exist as dimers in the crystal, although eIF4A exists as a monomer (36–38). However, the ability of p68 to exist both in monomeric forms and in dimers with p72/p82 adds further complexity and allows for alterations in function which may be also of relevance to other DEAD box RNA helicases. Previously, these have been thought to function either as monomers or dimers, depending on the number of dsRNA binding domains present in the polypeptide (39).
The nuclear localisation of p68, p72, fibrillarin and NFAR both in interphase and telophase, as observed by immunofluorescence (Fig. 8) supported the findings from the yeast two-hybrid, co-immunoprecipitation and gel filtration studies and was consistent with p68 and p72 having the potential to interact at all stages of the cell cycle. Like p68, p72 appears to be enriched in nascent nucleoli during telophase, although the co-localisation with fibrillarin is less defined than with p68 (Fig. 8o and s). In addition, a minor proportion of p68 molecules are present in large (>2 MDa) RNP complexes that are disrupted upon treatment of lysates with RNase A (Fig. 7) and appear to interact with NFAR-2. Although our co-immunoprecipitation and yeast two-hybrid interaction data (Figs 4 and 5) would support this idea, it will be important to investigate this potential interaction further, perhaps through the use of novel antibodies. NFAR-2 and p68 can activate/ co-activate gene expression, respectively (18,28), and associate with splicing proteins (28,33), while p68 has been shown to be essential in in vitro splicing (20). Therefore, it is interesting to speculate that the interaction of p68 and NFAR-2 may contribute to the regulation of gene expression, perhaps at the level of pre-mRNA splicing.
In our western blotting analyses of the fractions obtained from gel filtration (Fig. 7), we observe that p68 is present at a level that is apparently higher than that of p72, or its derivative p82, and exists as a more heterogeneous population. However, it is not possible to rule out relative sensitivities of the respective antibodies. Nevertheless, p72 whether endogenous or transiently expressed as a myc-tagged protein, does appear to show a more homogeneous population than p68, in terms of size as seen by gel filtration and consistently shows a weaker signal in western blotting, regardless of the antibody used (Fig. 7).
The finding that p68 and p72 can exist as dimers (with a preference for heterodimers) raises the possibility of achieving subtle alterations in p68/p72 function in the cell depending on whether these proteins are interacting with their RNA substrate(s) in monomeric, dimeric or oligomeric forms, which can be altered further depending on the relative amounts of p68 and p72 present. This might have implications in the light of our observations that, at least at the RNA level, p68 and p72 show different patterns of expression in different tissues (6,11). Moreover, the possibility of generating different p68/p72, or indeed p68/p82, complexes which may interact with a variety of other cellular factors, such as fibrillarin (13) or NFAR-2 (see Figs 4, 5 and 7), would be consistent with the various reports implying a wide range of functions for p68 and p72 in the cell. These have included transcriptional co-activation of estrogen receptor α (18,19), nonsense-mediated mRNA decay and ribosomal RNA processing in yeast (23), pre-mRNA splicing and regulation of alternative splicing (20–22) as well as potential roles in DNA demethylation as part of the 5-methylcytosine–DNA glycosylase complex (9), development and differentiation (10,11,24,25) and tumour development (26). Our data from the co-immunoprecipitation and gel filtration analyses suggest that there is the potential for a wide range of complexes that contain p68 and/or p72/p82 in the cell, some of which also contain RNA, although it is also clear that some p68 molecules are also present as monomers. Examination of the nature of such complexes and identification of the other factors present is likely to provide an important insight into the functions of p68 and p72 in the cell.
Acknowledgments
ACKNOWLEDGEMENTS
We thank E. Tan and M. Pollard (Scripps Research Institute, La Jolla) for the anti-fibrillarin antibody, and M. Watanabe and S. Kato (University of Tokyo) for the p72 antibody. This work was supported by the Medical Research Council and the Biotechnology and Biological Sciences Research Council (PhD studentship to B.J.W.).
REFERENCES
- 1.Linder P., Lasko,P.F., Leroy,P., Nielsen,P.J., Nishi,K., Schnier,J. and Slominski,P.P. (1989) Birth of the D-E-A-D box. Nature, 337, 121–122. [DOI] [PubMed] [Google Scholar]
- 2.Fuller-Pace F.V. (1994) RNA helicases: modulators of RNA structure. Trends Cell Biol., 4, 271–274. [DOI] [PubMed] [Google Scholar]
- 3.de la Cruz J., Kressler,D. and Linder,P. (1999) Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem. Sci., 24, 192–198. [DOI] [PubMed] [Google Scholar]
- 4.Lane D.P. and Hoeffler,W.K. (1980) SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature, 288, 167–170. [DOI] [PubMed] [Google Scholar]
- 5.Ford M.J., Anton,I.A. and Lane,D.P. (1988) Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature, 332, 736–738. [DOI] [PubMed] [Google Scholar]
- 6.Lamm G.M., Nicol,S.M., Fuller Pace,F.V. and Lamond,A.I. (1996) p72: a human nuclear DEAD box protein highly related to p68. Nucleic Acids Res., 24, 3739–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlmann-Schiffler H., Rossler,O.G. and Stahl,H. (2002) The mRNA of DEAD box protein p72 is alternatively translated into an 82-kDa RNA helicase. J. Biol. Chem., 277, 1066–1075. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire L. and Heinlein,U.A. (1993) High-level expression in male germ cells of murine P68 RNA helicase mRNA. Life Sci., 52, 917–926. [DOI] [PubMed] [Google Scholar]
- 9.Jost J.P., Schwarz,S., Hess,D., Angliker,H., Fuller-Pace,F.V., Stahl,H., Thiry,S. and Siegmann,M. (1999) A chicken embryo protein related to the mammalian DEAD box protein p68 is tightly associated with the highly purified protein–RNA complex of 5-MeC-DNA glycosylase. Nucleic Acids Res., 27, 3245–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip F.C., Chung,S.S., Fu,W.Y. and Ip,N.Y. (2000) Developmental and tissue-specific expression of DEAD box protein p72. Neuroreport, 11, 457–462. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson R.J., Hamilton,S.J., MacCallum,D.E., Hall,P.A. and Fuller Pace,F.V. (1998) Expression of the ‘dead box’ RNA helicase p68 is developmentally and growth regulated and correlates with organ differentiation/maturation in the fetus. J. Pathol., 184, 351–359. [DOI] [PubMed] [Google Scholar]
- 12.Iggo R.D., Jamieson,D.J., MacNeill,S.A., Southgate,J., McPheat,J. and Lane,D.P. (1991) p68 RNA helicase: identification of a nucleolar form and cloning of related genes containing a conserved intron in yeasts. Mol. Cell. Biol., 11, 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol S.M., Causevic,M., Prescott,A.R. and Fuller-Pace,F.V. (2000) The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase. Exp. Cell Res., 257, 272–280. [DOI] [PubMed] [Google Scholar]
- 14.Hirling H., Scheffner,M., Restle,T. and Stahl,H. (1989) RNA helicase activity associated with the human p68 protein. Nature, 339, 562–564. [DOI] [PubMed] [Google Scholar]
- 15.Iggo R.D. and Lane,D.P. (1989) Nuclear protein p68 is an RNA-dependent ATPase. EMBO J., 8, 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossler O.G., Straka,A. and Stahl,H. (2001) Rearrangement of structured RNA via branch migration structures catalysed by the highly related DEAD-box proteins p68 and p72. Nucleic Acids Res., 29, 2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y. and Liu,Z.R. (2002) The ATPase, RNA unwinding and RNA-binding activities of recombinant p68 RNA helicase. J. Biol. Chem., 277, 12810–12815. [DOI] [PubMed] [Google Scholar]
- 18.Endoh H., Maruyama,K., Masuhiro,Y., Kobayashi,Y., Goto,M., Tai,H., Yanagisawa,J., Metzger,D., Hashimoto,S. and Kato,S. (1999) Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol. Cell. Biol., 19, 5363–5372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Watanabe M., Yanagisawa,J., Kitagawa,H., Takeyama,K., Ogawa,S., Arao,Y., Suzawa,M., Kobayashi,Y., Yano,T., Yoshikawa,H. et al. (2001) A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J., 20, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Liu Z.R. (2002) p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5′ splice site duplex. Mol. Cell. Biol., 22, 5443–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C.G. (2002) RH70, a bidirectional RNA helicase, co-purifies with U1snRNP. J. Biol. Chem., 277, 39679–39683. [DOI] [PubMed] [Google Scholar]
- 22.Honig A., Auboeuf,D., Parker,M.M., O’Malley,B.W. and Berget,S.M. (2002) Regulation of alternative splicing by the ATP-dependent DEAD-box RNA helicase p72. Mol. Cell. Biol., 22, 5698–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond A.T., Mangus,D.A., He,F. and Jacobson,A. (2001) Absence of Dbp2p alters both nonsense-mediated mRNA decay and rRNA processing. Mol. Cell. Biol., 21, 7366–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seufert D.W., Kos,R., Erickson,C.A. and Swalla,B.J. (2000) p68, a DEAD-box RNA helicase, is expressed in chordate embryo neural and mesodermal tissues. J. Exp. Zool., 288, 193–204. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura A., Nishizuka,M., Tominaga,K., Tsuchiya,T., Nishihara,T. and Imagawa,M. (2001) Expression of p68 RNA helicase is closely related to the early stage of adipocyte differentiation of mouse 3T3-L1 cells. Biochem. Biophys. Res. Commun., 287, 435–439. [DOI] [PubMed] [Google Scholar]
- 26.Causevic M., Hislop,R.G., Kernohan,N.M., Carey,F.A., Kay,R.A., Steele,R.J. and Fuller-Pace,F.V. (2001) Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene, 20, 7734–7743. [DOI] [PubMed] [Google Scholar]
- 27.Saunders L.R., Jurecic,V. and Barber,G.N. (2001) The 90- and 110-kDa human NFAR proteins are translated from two differentially spliced mRNAs encoded on chromosome 19p13. Genomics, 71, 256–259. [DOI] [PubMed] [Google Scholar]
- 28.Saunders L.R., Perkins,D.J., Balachandran,S., Michaels,R., Ford,R., Mayeda,A. and Barber,G.N. (2001) Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem., 276, 32300–32312. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez I., Hughes,R.T., Mayer,B.J., Yee,K., Woodgett,J.R., Avruch,J., Kyriakis,J.M. and Zon,L.I. (1994) Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature, 372, 794–798. [DOI] [PubMed] [Google Scholar]
- 30.Webster G.A. and Perkins,N.D. (1999) Transcriptional cross talk between NF-kappaB and p53. Mol. Cell. Biol., 19, 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Woods Y.L., Rena,G., Morrice,N., Barthel,A., Becker,W., Guo,S., Unterman,T. and Cohen,P. (2001) The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem. J., 355, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neubauer G., King,A., Rappsilber,J., Calvio,C., Watson,M., Ajuh,P., Sleeman,J., Lamond,A. and Mann,M. (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nature Genet., 20, 46–50. [DOI] [PubMed] [Google Scholar]
- 34.Cho H.S., Ha,N.C., Kang,L.W., Chung,K.M., Back,S.H., Jang,S.K. and Oh,B.H. (1998) Crystal structure of RNA helicase from genotype 1b hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J. Biol. Chem., 273, 15045–15052. [DOI] [PubMed] [Google Scholar]
- 35.Story R.M., Li,H. and Abelson,J.N. (2001) Crystal structure of a DEAD box protein from the hyperthermophile Methanococcus jannaschii. Proc. Natl Acad. Sci. USA, 98, 1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benz J., Trachsel,H. and Baumann,U. (1999) Crystal structure of the ATPase domain of translation initiation factor 4A from Saccharomyces cerevisiae—the prototype of the DEAD box protein family. Struct. Fold Des., 7, 671–679. [DOI] [PubMed] [Google Scholar]
- 37.Johnson E.R. and McKay,D.B. (1999) Crystallographic structure of the amino terminal domain of yeast initiation factor 4A, a representative DEAD-box RNA helicase. RNA, 5, 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caruthers J.M., Johnson,E.R. and McKay,D.B. (2000) Crystal structure of yeast initiation factor 4A, a DEAD-box RNA helicase. Proc. Natl Acad. Sci. USA, 97, 13080–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson T.J. and Thompson,J.D. (1994) detection of dsRNA-binding domains in RNA helicase A and Drosophila maleless: implications for monomeric RNA helicases. Nucleic Acids Res., 22, 2552–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]