Abstract
Cell fusion is a central phenomenon during the immune response that leads to formation of large elements called multinucleated giant cells (MGCs) of common occurrence at sites of granulomatous inflammation. We have previously reported on the involvement in this event of a novel receptor expressed to high level by mononuclear phagocytes, the purinergic P2X7 receptor. Herein, we show that blockade of this receptor by a specific monoclonal antibody prevents fusion in vitro. In contrast, cell fusion is stimulated by addition of enzymes that destroy extracellular ATP (i.e., apyrase or hexokinase). Experiments performed with phagocytes selected for high (P2X7 hyper) or low (P2X7 hypo) P2X7 expression show that fusion only occurs between P2X7 hyper/P2X7 hyper and not between P2X7 hyper/P2X7 hypo or P2X7 hypo/P2X7 hypo. During MGCs formation we detected activation of caspase 3, an enzyme that is powerfully stimulated by P2X7. Finally, we observed that during MGCs formation, the P2X7 receptor is preferentially localized at sites of cell-to-cell contact. These findings support the hypothesis originally put forward by our group that the P2X7 receptor participates in multinucleated giant cell formation.
INTRODUCTION
During the immune response it is frequently observed that mononuclear phagocytes fuse to generate large elements called multinucleated giant cells (MGCs) (Fais et al., 1997). It is common to find these polykarions at sites of chronic inflammation such as granulomas, whether due to bacterial pathogens or sterile foreign bodies, or as a consequence of viral infections such as acquired immunodeficiency syndrome (Cotran et al., 1994). It is known that fusion requires macrophage activation by cytokines released by T lymphocytes, among which the most important appear to be interleukin (IL)-4, interferon-γ, and tumor necrosis factor-α, but the molecular mechanism involved is still mysterious. Multinucleation also occurs in the bone during osteoclast formation under the effect of locally released cytokines (Vignery, 1989; Suda et al., 1995). Apart from osteoclasts that acquire an increased bone resorption capability after fusion, the physiological meaning of MGC formation is unknown, albeit a number of suggestions have been put forward (enhanced cytokine producing activity, a nonphagocytic pathway for antigen internalization, a mechanism for disposing infected or damaged monocytes). Likewise, the molecular mechanism that drives fusion is unknown. A few plasma membrane molecules probably involved have been identified such as intercellular adhesion molecule-1, leukocyte function–associated antigen-1, E-cadherins, CD98, CD44, or the newly cloned macrophage fusion receptor (Saginario et al., 1998; Sterling et al., 1998), but it appears that fusion does not depend on the engagement of a single receptor, but rather on the recruitement of several molecules mediating cell aggregation, establishment of close cell-to-cell contacts, and finally the actual fusion event.
Over the last years we have extensively characterized a plasma membrane receptor belonging to the subfamily of the P2X purinergic receptors, named P2X7, that is expressed to a very high level by macrophage, microglial, and dendritic cells (Ferrari et al., 1996; Chiozzi et al., 1997; Coutinho-Silva et al., 1999; Mutini et al., 1999). P2X7 is a ligand-gated receptor/channel formed by an unknown number of subunits each 595 amino acids long that upon sustained stimulation with ATP causes the formation of a nonselective pore permeable to low-molecular-weight aqueous solutes (Di Virgilio, 1995; Surprenant et al., 1996; Rassendren et al., 1997). This receptor was initially identified and characterized in immune cells (Di Virgilio et al., 1995), and eventually cloned from a rat brain library (Surprenant et al., 1996). Cloning from a brain library was not surprising because, although central neurons are basically negative for P2X7, microglia is one of the cell types that expresses P2X7 to the highest level. In mononuclear phagocytes P2X7 has been associated to cytotoxicity and to maturation and release of IL-1β (Ferrari et al., 1997a; Di Virgilio et al., 1998a), but it has been suggested that it also might participate in MGC formation (Chiozzi et al., 1997). The first indication of a role for P2X7 in macrophage fusion came from experiments performed in our laboratory showing that multinucleation of monocyte-derived human macrophages in vitro was efficiently prevented by the P2X7 blocker oxidized ATP (oATP) (Falzoni et al., 1995). Subsequently, we showed that macrophage cell clones expressing P2X7 to a very high level (P2X7 hyper) spontaneously fuse during in vitro culture, whereas clones selected for lack of P2X7 (P2X7 hypo) never do (Chiozzi et al., 1997). The step at which P2X7 takes part in cell fusion is at present unknown, but we speculate that it might be involved in the very last step of the process, the actual membrane fusion, with a mechanism reminiscent of that of the better known “fusion pore” putatively involved in the fusion of synaptic vesicles with the plasma membrane (Monck and Fernandez, 1996). In the present work we provide further evidence for the involvement of P2X7 in MGC formation.
MATERIALS AND METHODS
Cells
J774 mouse macrophages and P2X7 hyper and P2X7 hypo clones were grown in Dulbecco modified Eagle's medium (Sigma, St. Louis, MO) supplemented with 2 mM glutamine, 10% heat-inactivated horse serum, penicillin (100 units [U]/ml), and streptomycin (100 μg/ml). P2X7 hyper and P2X7 hypo variants were selected as described previously (Chiozzi et al., 1996, 1997). Fetal skin-derived dendritic cells (FSDCs) were grown in Iscove medium (Sigma) containing 50 μM 2-mercaptoethanol, 2 mM glutamine, 10% heat-inactivated fetal calf serum (Life Technologies, Paisley, Scotland), penicillin (100 U/ml), and streptomycin (100 μg/ml) as previously described (Mutini et al., 1999). Human monocytes were isolated from buffy coats by one-step Percoll gradient (Pharmacia Biotech Spa, Cologno Monzese, Italy) or by adherence on plastic Petri dishes as previously described (Falzoni et al., 1995).
Antibodies
The antihuman P2X7 monoclonal antibody (mAb) was previously characterized by Buell et al. (1998). The polyclonal anti-P2X7 Ab was previously characterized by Solini et al. (1999). Fluorescein isothiocyanate (FITC)-conjugated Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence
Cells were fixed in 2% paraformaldehyde/phosphate-buffered saline (PBS), pH 7.3, for 1 h at 4°C, and then rinsed three times with ice-cold PBS and incubated in 100 mM ammonium chloride for 20 min. At the end of this incubation, the monolayers were thoroughly rinsed with PBS, incubated with the 2.4G2 mAb (anti-Fc receptor) for a further 20 min at 4°C, and again rinsed with ice-cold PBS. Incubation with the polyclonal anti-P2X7 Ab (Solini et al., 1999) was performed overnight at 4°C at a dilution of 1:50. For immunofluorescence, the secondary Fluorescein isothiocyanate-conjugated Ab was used at a 1:200 dilution (40 min at 4°C).
Caspase Activation
Caspase 3 activation was measured fluorometrically with the EnzCheck Caspase 3 kit (Molecular Probes, Eugene, OR), as indicated by the manufacturer.
ATP Measurement
Monocyte-derived macrophages were seeded in microtiter plastic dishes in a total volume of culture medium of 100 μl in the presence or absence of concanavalin A (ConA) (10 μg/ml) and placed in a CO2 incubator at 37° for 24 h. After this time, cells were rinsed and supplemented with 100 μl of diluent buffer (FireZyme, San Diego, CA) to stabilize extracellular ATP, and placed directly in the test chamber of a luminometer (FireZyme). Then 100 μl of a luciferin–luciferase solution (FireZyme) was added, and light emission was recorded.
Microscopy
Phase contrast and fluorescence photographs were taken with an inverted fluorescence microscope (Olympus IMT-2; Olympus Optical, Tokyo, Japan) equipped with 20 and 40× objectives and fluorescein and rhodamine filters. Some images also were taken with a Nikon Eclipse TE-300 fluorescence microscope (Nikon, Tokyo, Japan) equipped with 40, 63, and 100× (oil immersion) objectives.
RESULTS
We have observed that fusion spontaneously occurs in in vitro cultures of mononuclear phagocytes clones derived from J774 macrophages as well as dendritic cells derived from mouse skin (FSDCs) that express high levels of the P2X7 receptor (P2X7 hyper clones) (Chiozzi et al., 1997; Chiozzi, Falzoni, and Di Virgilio, unpublished observations). Furthermore, fusion can be induced in primary cultures of human monocytes by incubation in the presence of ConA or phytohemagglutinin (PHA) (Takashima et al., 1993; Falzoni et al., 1995).
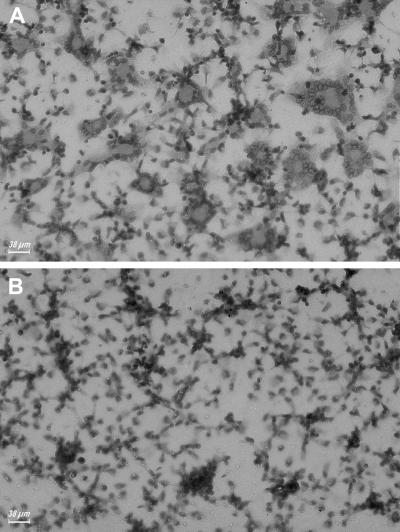
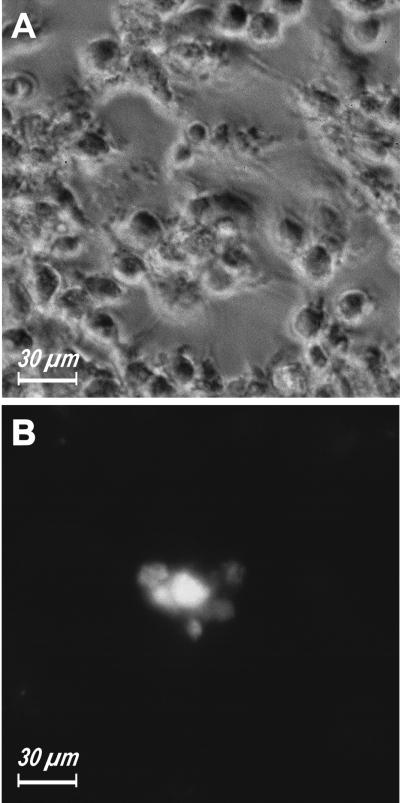
Recently, Buell et al. (1998) raised and fully characterized an inhibitory mAb directed against the outer domain of human P2X7. Pretreatment with this mAb blocked several macrophage responses dependent on P2X7 activation, including cytotoxicity and IL-1β release (Buell et al., 1998). Our preliminary evidence suggested that this mAb also could prevent polykarion formation (Di Virgilio et al., 1999). Thus, we tested more thoroughly the effect of the anti-P2X7 mAb on ConA-stimulated fusion of monocyte-derived human macrophages. Figure 1 shows that this mAb almost completely blocked MGC formation, but very interestingly, did not prevent cell aggregation, suggesting that chemotaxis and surface molecule recognition were not affected. Fusion index of six different ConA-stimulated monocyte preparations ranged from 61 to 85%, and was not affected by incubation in the presence of irrelevant mouse IgG. Pretreatment with the anti-P2X7 mAb brought the fusion index close to zero in all monolayers examined.
Figure 1.
An anti-P2X7 mAb blocks MGC formation. Monocytes were isolated from peripheral blood and plated as described in MATERIALS AND METHODS. ConA (10 μg/ml) was added soon after the plating and incubation carried on for 3 days. (B) Anti-P2X7 mAb (10 μg/ml) was added together with ConA.
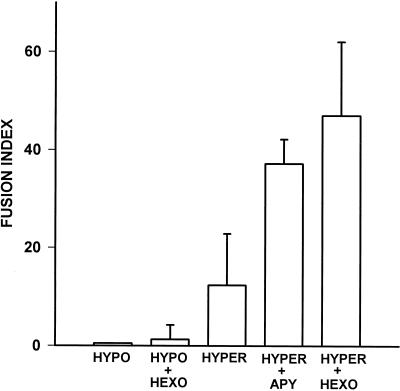
Blocking of fusion by a selective anti-P2X7 mAb is a strong indication that this receptor participates in MGC formation. However, if a functional P2X7 is needed for membrane fusion, one could expect that its activation by exogenous ATP facilitated fusion. We tested this hypothesis on J774 and FSDC P2X7 hyper clones that spontaneously undergo fusion in culture, and found that this was not the case because incubation in the presence of a range of ATP concentrations sufficient to activate the P2X7 receptor (1–5 mM) inhibited fusion. In contrast, the presence in the incubation medium of ATP-hydrolyzing enzymes such as hexokinase or apyrase powerfully enhanced MGC formation in the P2X7 hyper clones (Figure 2). Boiling abolished apyrase and hexokinase fusogenic activity. In hindsight, the effect of apyrase and hexokinase was not totally unexpected because we and others have previously shown that in some cell types P2 receptors are chronically desensitized by the continuous leak of ATP into the pericellular medium, and that hexokinase or apyrase reestablish sensitivity to stimulation by ATP (Baricordi et al., 1996; Buell et al., 1996). Phagocytes release significant amounts of ATP into the pericellular milieu, thus it is likely that their P2X7 receptor is exposed to high extracellular ATP concentrations, especially when they come in close contact before fusion. We measured bulk extracellular ATP before and during fusion in six different monocyte-derived macrophage monolayers, and found that it ranged from 250 to 592 pmol/106 cells in unstimulated cultures, and from 410 to 1010 pmol/106 cells after 24 h of stimulation with ConA. Figure 3 reports the fusion index calculated in several P2X7 hyper or P2X7 hypo J774 monolayers incubated in the absence or presence of ATP-consuming enzymes. It is noteworthy that these agents did not trigger fusion in cells lacking P2X7. Neither apyrase nor hexokinase could be used to enhance fusion of monocyte-derived macrophages because in the presence of ConA or PHA, these enzymes had a surprising toxic effect hallmarked by swelling, rounding, and vesiculation of the cells.
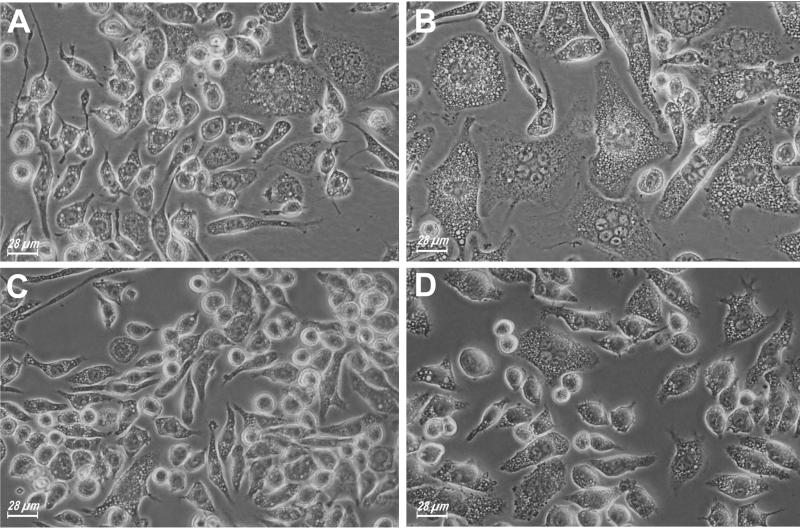
Figure 2.
Enhanced MGC formation in macrophage monolayers incubated in the presence of ATP-hydrolyzing enzymes. P2X7 hyper J774 macrophages were plated in 24-well plates and grown to confluence. At this time, hexokinase (100 μg/ml) (B) or apyrase (0.4 U/ml) (D) was added and the incubation further carried out for 24 h. A and C, parallel control cultures in the absence of the ATP-hydrolyzing enzymes.
Figure 3.
Fusion index of apyrase- or hexokinase-treated macrophage monolayers. The fusion index was determined by counting the (number of nuclei within MGCs)/(total number of nuclei counted) × 100. We identified as MGCs those cells that had more than two nuclei per cell. Three different microscopic fields were counted in three different MGC preparations. Data are average ± SD of nine determinations.
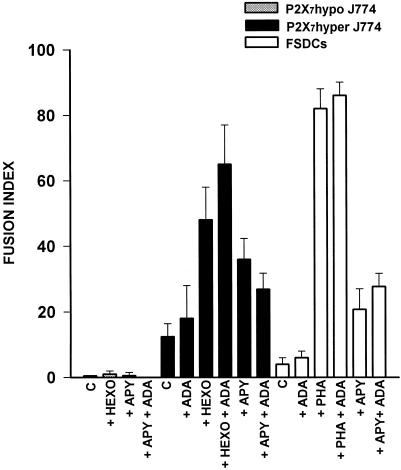
Hexokinase and apyrase, by increasing the rate of degradation of extracellular ATP, also might enhance accumulation of extracellular adenosine. Activation of A1 receptors has been shown to enhance MGC formation stimulated by phorbol 12-myristate 13-acetate in human monocyte cultures (Merril et al., 1997). Under our experimental conditions, adenosine had no effect of cell fusion over a range of concentrations from 1 to 100 μM, in fact it was inhibitory at the higher dose. We then tested the effect of adenosine deaminase, an enzyme that destroys extracellular adenosine, on two fusion models: apyrase- or hexokinase-stimulated P2X7 hyper J774 macrophages, and PHA- or apyrase-stimulated FSDCs (Figure 4). Adenosine deaminase slightly enhanced fusion by itself, and also potentiated MGC formation in the presence of the additional stimulants, but the increase never reached statistical significance, suggesting that albeit adenosine can stimulate fusion, it is not the main factor under our experimental conditions.
Figure 4.
Effect of adenosine deaminase on MGC formation. The fusion index was determined as detailed in Figure 3. P2X7 hyper and P2X7 hypo J774 cells were treated with hexokinase (HEXO) or apyrase (APY) and processed as described in Figure 2. FSDCs were incubated in the presence of PHA (10 μg/ml) or apyrase (0.4 U/ml) for 2 d before determination of fused cells. Adenosine deaminase was added at a concentration of 2 U/ml. C, control. Data are average ± SD of six determinations.
As shown in Figure 4 and by Chiozzi et al. (1997), P2X7 hypo macrophage clones are unable to fuse in culture, in striking contrast to their P2X7 hyper partners. We then asked whether expression of P2X7 is needed on both partner cells undergoing fusion, or in other words, whether a P2X7 hypo cell can fuse with a P2X7 hyper, or fusion can only occur between P2X7 hyper cells. To answer this question, we labeled P2X7 hypo and P2X7 hyper FSDCs with Texas Red and lucifer yellow, respectively, and then coincubated the two cell populations. Our anticipation was that if fusion occurred between P2X7 hyper and P2X7 hypo cells we should find MGC stained with both the red and the yellow/green stain, whereas if fusion only occurred between P2X7 hyper we should only see MGC stained in yellow/green. Figure 5 shows that by mixing Texas Red-stained P2X7 hypo and lucifer yellow-stained P2X7 hyper FSDCs, we obtained the formation of MGCs that were almost exclusively stained in yellow/green. In >10 separate, similar experiments, we calculated that ∼95–98% of MGCs were exclusively lucifer yellow positive. The residual small percentage of cells was positive for both stains due, we suggest, to the presence of some P2X7-positive cells within the P2X7 hypo population (see also Chiozzi et al., 1997).
Figure 5.
MGC formation requires P2X7 receptor expression on all cells participating to fusion. P2X7 hyper and P2X7 hypo FSDCs were allowed to pinocytose lucifer yellow (1 mg/ml) or Texas Red (0.4 mg/ml), respectively, for 3 h. At the end of this incubation time, the two batches were mixed in the well of a 24-well culture dish, and further incubated for 3 days. (A) Phase. (B) Rhodamine filter. (C) Fluorescein filter.
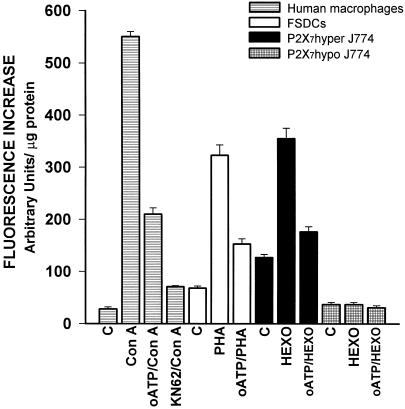
As of now, there are very few means to monitor activation of P2X7, the best and most reliable being conductance (Surprenant et al., 1996) or fluorescent dye uptake measurements (Steinberg et al., 1987). However, it is very difficult to apply these techniques to the measurement of P2X7 opening during cell fusion because patch clamp significantly perturbs the cellular microenvironment and dye uptake does not allow an easy quantification. We monitored lucifer yellow uptake in ConA-stimulated cultures of monocyte-derived macrophages and observed that cells involved in fusion showed an increased dye uptake (Figure 6, A and B). However, we also felt that dye uptake was an unsatisfactory assay for P2X7 activation under these experimental conditions because by this means we might miss opening of those receptors located on the tightly juxtapposed plasma membranes of closely juxtaposed cells (i.e., the very cells that are about to fuse), and that presumably are segregated from the extracellular milieu. Ferrari et al. (1999) showed that stimulation of P2X7 causes a large stimulation of caspase 3. We therefore asked whether this cystein protease is activated during macrophage fusion, and can thus be used as an indicator of P2X7 opening. Caspase 3 activity was measured at peak time for fusion in four different cell types: human monocyte-derived macrophages, FSDCs, P2X7 hyper, and P2X7 hypo J774 macrophages (Figure 7). Monocyte-derived human macrophages in culture were stimulated to fuse with Con A, FSDCs with PHA, and P2X7 hyper and P2X7 hypo cells with hexokinase, and were then processed for caspase 3 activation measurement. In all cell models, with the exception of the P2X7 hypo variant that is unable to fuse, there was a large caspase 3 stimulation, very likely due to P2X7 activation because it 1) was inhibited by two specific P2X7 blockers, oATP and 1-[N,O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN-62) (Murgia et al., 1993; Gargett and Wiley, 1997); and 2) did not occur in the P2X7 hypo variant.
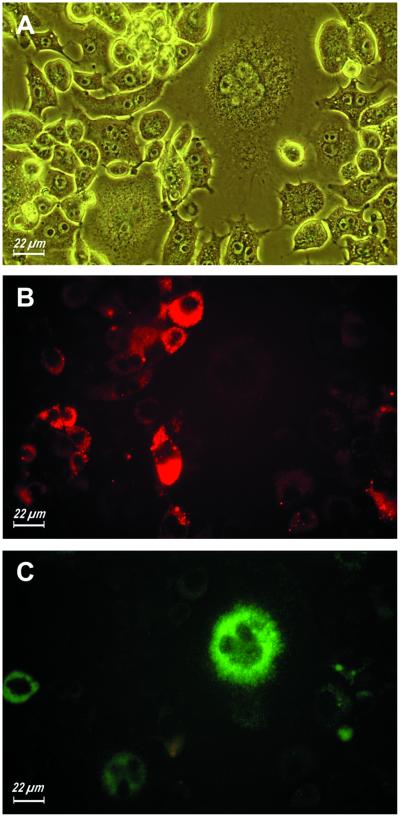
Figure 6.
Spontaneous and selective lucifer yellow uptake by fusing monocyte-derived macrophages. Monocytes were isolated from peripheral blood and plated as described in MATERIALS AND METHODS. ConA (10 μg/ml) was added soon after the plating. The picture was taken after 24 h of incubation in the presence of ConA.
Figure 7.
Caspase 3 activation during cell fusion. Cells were seeded in 24-well culture plates and incubated either in culture medium alone (C) or in culture medium supplemented with ConA (10 μg/ml) for human macrophages, PHA (10 μg/ml) for FSDCs, and hexokinase (HEXO) for J774 macrophages. In some experiments, oATP (300 μM) or KN-62 (50 nM) also were added. After 2 d, when MGC formation was maximal, the monolayers cells were rinsed several times, lysed in lysis buffer provided with the EnzCheck kit, and assayed for caspase 3 activation. Data are average ± SD of six determinations.
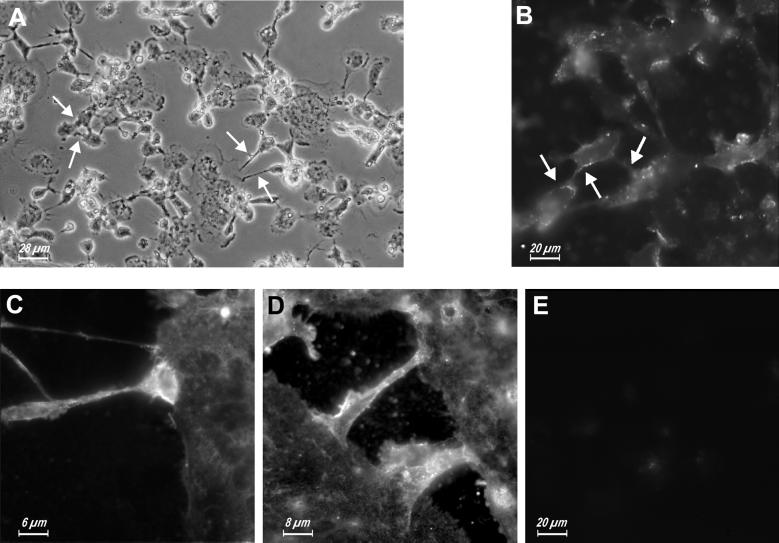
We then investigated the cellular distribution of P2X7 during the fusion process, asking whether there was an increased localization of this receptor at sites of cell-to-cell contact during MGC formation in human monocyte-derived macrophage cultures induced to fuse with ConA. Figure 8A shows the stage at which the culture was fixed and stained with the anti-P2X7 polyclonal Ab (after 1 day of culture in the presence of ConA): some MGCs were already formed and were growing in size by recruiting nearby macrophages. Podosomes projected from the cell body of the incoming macrophages (arrows) and established contact with the MGC plasma membrane. Staining with the anti-P2X7 Ab revealed discrete patches at the level of the plasma membrane (Figure 8B) that at a higher magnification appeared to be more concentrated at sites of cell-to-cell interaction, especially at the tip of the podosome (Figure 8, C and D). Figure 8E shows a control experiment performed with preimmune rabbit serum.
Figure 8.
Immunolocalization of P2X7 receptor during MGC formation. Monocyte-derived human macrophages were seeded in 24-well culture plates and stimulated with ConA (10 μg/ml). At the beginning of fusion (24 h after the seeding), cells were fixed and immunostained as described in MATERIALS AND METHODS. (A) Phase. (B–E) Fluorescence.
DISCUSSION
There is increasing awareness that mononuclear phagocytes express plasma membrane receptors for extracellular nucleotides that are likely to have a mediator role during the inflammatory reaction (Di Virgilio, 1995; Ferrari et al., 1996; Humphreys and Dubyak, 1996; Mutini et al., 1999; Sikora et al., 1999). These receptors belong to the P2Y (G-protein–coupled, seven-membrane–spanning receptors) or to the P2X (intrinsic ligand-gated ion channels) subfamilies (Burnstock, 1997; Ralevic and Burnstock, 1998). In particular, macrophage, microglial, and dendritic cells express to a high level the most peculiar member of the P2X subfamily, P2X7. This is a bifunctional receptor that although upon transient stimulation with ATP behaves as a typical cation-selective ion channel permeable to K+, Na+, and Ca2+, upon repetitive stimulation undergoes a transition into a nonselective pore that also allows transmembrane fluxes of low-molecular-mass hydrophylic molecules up to 900 Da. There is no clear-cut physiological function for such a receptor as yet, but it is clearly intriguing that it is up-regulated during monocyte-to-macrophage differentiation and by those stimuli that cause macrophage activation such as interferon-γ, and in some cases also bacterial endotoxin and tumor necrosis factor (Falzoni et al., 1995; Humphreys and Dubyak, 1996; Di Virgilio et al., 1998b). Due to the availability of a highly selective inhibitor of P2X7, the mAb originally described by Buell et al. (1998), and used in the present work, we are now able to provide strong support to our original hypothesis on the involvement of this receptor in MGC formation. This mAb specifically recognizes an epitope located on the outer domain of the P2X7 receptor, and the ability to almost completely prevent MGC formation is crucial evidence for the participation of this receptor in macrophage fusion. This is in keeping with the ability of this mAb to block other P2X7-dependent responses such as transmembrane ion fluxes and IL-1β release (Buell et al., 1998). The step in the fusion process in which P2X7 takes part is however still uncertain. We think that because macrophage clustering in the presence of the mAb is not inhibited, P2X7 does not act as a chemotactic or cell adhesion receptor, but rather intervenes in the very last phase of membrane fusion, maybe generating a “fusion pore” that establishes early bridges between the cytoplasm of the adjacent cells and drives the eventual fusion. In other words, it could be hypothesized that to form an efficient fusion pore it is necessary that at least two P2X7 receptors on opposite plasma membranes come in contact via their extracellular domains, not dissimilarly from the mechanism whereby gap-junctional communication is established by the hemi-gap junctions expressed on the membrane of adjacent cells. The finding of an oATP and KN-62 inhibitable caspase 3 activation during fusion supports the hypothesis that P2X7 transiently opens, but further experiments are needed to provide unequivocal evidence.
A related question is what turns on P2X7 during fusion. We anticipated that the trigger could be the P2X7 physiological ligand, i.e. extracellular ATP; however, this turned out not to be the case because addition of ATP to the macrophage monolayers, if anything, inhibited fusion. In hindsight, this was not entirely unexpected because it is clear that, in order to allow membrane fusion, opening of the P2X7 pore must be strictly controlled and occur only when the opposing plasma membranes are tightly juxtapposed and ready to merge. In contrast, the mere addition of ATP to a macrophage monolayer very likely activates P2X7 in an untimely manner, with an overall detrimental effect on cell fusion. Thus, we think that the trigger could be a surface molecule, maybe P2X7 itself, on the opposing membrane. This interpretation received some support from the facilitating effect of apyrase and hexokinase. These two enzymes efficiently hydrolyze extracellular ATP, and have been shown to restore sensitivity of P2X receptors desensitized by the chronic leakage of ATP that is known to occur from many cell types, macrophages included (Baricordi et al., 1996; Buell et al., 1996; Ferrari et al., 1997b). Thus, it is possible that under normal conditions P2X7 receptors are partially desensitized by the continuous leakage of ATP, or even stably occupied by this nucleotide, and therefore unavailable for fusion. Removal of ATP by apyrase or hexokinase would reestablish P2X7 sensitivity and thus accelerate fusion. Localization of P2X7 during MGC formation supports our hypothesis. In resting macrophages and MGCs P2X7 is uniformly distributed on the plasma membrane, but during fusion it concentrates in discrete membrane clusters at the site of cell-to-cell interaction.
In conclusion, our data support a role for P2X7 as a novel plasma membrane receptor involved in macrophage fusion and MGC formation.
ACKNOWLEDGMENTS
This study was supported by the Italian Ministry for Scientific Research (40 and 60%), the National Research Council of Italy (Target Project on Biotechnology), the Italian Association for Cancer Research (AIRC), the IX AIDS Project, the II Tuberculosis Project, and Telethon of Italy. A preliminary account of these results was presented at the Keystone Symposium on Macropahge Biology (Keystone, Colorado, January 22–28, 1999).
Abbreviations used:
- FSDC
fetal skin-derived dendritic cell
- MGC
multinucleated giant cell
- oATP
oxidized ATP
- P2X7 hyper
phagocytes hyperexpressing the P2X7 receptor
- P2X7 hypo
phagocytes hypoexpressing the P2X7 receptor
REFERENCES
- Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, Di Virgilio F. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- Buell GN, Chessel IP, Michel AD, Collo G, Salazzo M, Herren S, Gretner D, Grahames C, Kaur R, Kosco-Vilbois MH, Humphrey PPA. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- Buell G, Michel AD, Lewis C, Collo G, Humphrey PPA, Surprenant A. P2X1 receptor activation in HL60 cells. Blood. 1996;87:2659–2664. [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signaling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Chiozzi P, Murgia M, Falzoni S, Ferrari D, Di Virgilio F. Role of the purinergic P2Z receptor in spontaneous cell death in J774 macrophage cultures. Biochem Biophys Res Commun. 1996;218:176–181. doi: 10.1006/bbrc.1996.0031. [DOI] [PubMed] [Google Scholar]
- Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN, Collo G, Di Virgilio F. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Robbins SL. Pathologic basis of diseases. Philadelphia: WB Saunders Company; 1994. [Google Scholar]
- Coutinho-Silva R, Persechini PM, Da Cunha Bisaggio RD, Perfettini J-L, Torres de Sa Neto AC, Kanellopulos JM, Motta-Ly L, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Vishwanath V, Baricordi OR. Cytolytic P2Z purinoceptors. Cell Death Differ. 1998a;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Falzoni S, Chiozzi IP, Sanz JM, Ferrari D, Buell GN. ATP receptors and giant cell formation. J Leukocyte Biol. 1999;66:723–726. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Falzoni S, Mutini C, Sanz JM, Chiozzi P. Purinergic P2X7 receptor: a pivotal role in inflammation and immunomodulation. Drug Dev Res. 1998b;45:207–213. [Google Scholar]
- Fais S, Burgio VL, Capobianchi MR, Gessani S, Pallone F, Belardelli F. The biological relevance of polykarions in the immune response. Immunol Today. 1997;18:522–527. doi: 10.1016/s0167-5699(97)01148-1. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997a;159:1451–1458. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Los M, Bauer MKA, Vandenabeele P, Wesselborg S, Schulze-Osthoff S. P2Z purinoceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- Gargett CE, Wiley JS. The isoquinoline derivative KN-62: a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Dubyak GR. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- Merril JT, Shen C, Schreibman D, Coffey D, Zakharenko O, Fisher R, Lahita RG, Salmon J, Cronstein BN. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Monck JR, Fernandez JM. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8:524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP: an irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, Collo G, Ricciardi-Castagnoli P, Di Virgilio F. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958–1965. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor P2X7: cloning of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, Vignery A. MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol. 1998;18:6213–6223. doi: 10.1128/mcb.18.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom BR. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, miscrovesicle formation and IL-6 release. J Cell Sci. 1999;112:297–305. doi: 10.1242/jcs.112.3.297. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4− permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- Sterling H, Saginario C, Vignery A. CD44 occupancy prevents macrophage multinucleation. J Cell Biol. 1998;143:837–847. doi: 10.1083/jcb.143.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17:S87S–S91. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X (P2X7) receptor. Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Takashima T, Ohnishi K, Tsuyuguchi I, Kishimoto S. Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-γ and IL-4. J Immunol. 1993;150:3002–3007. [PubMed] [Google Scholar]
- Vignery A. Macrophage multinucleation is accompanied by the expression of new soluble and membrane antigens in mice. Am J Pathol. 1989;135:565–570. [PMC free article] [PubMed] [Google Scholar]