Abstract
The 464-amino acid baculovirus LEF4 protein is a bifunctional mRNA capping enzyme with triphosphatase and guanylyltransferase activities. The N-terminal half of LEF4 constitutes an autonomous triphosphatase catalytic domain. The LEF4 triphosphatase belongs to a family of metal-dependent phosphohydrolases, which includes the RNA triphosphatases of fungi, protozoa, Chlorella virus and poxviruses. The family is defined by two glutamate-containing motifs (A and C), which form a metal-binding site. Most of the family members resemble the fungal and Chlorella virus enzymes, which have a complex active site located within the hydrophilic interior of a topologically closed eight stranded β barrel (the so-called ‘triphosphate tunnel’). Here we probed whether baculovirus LEF4 is a member of the tunnel subfamily, via mutational mapping of amino acids required for triphosphatase activity. We identified four new essential side chains in LEF4 via alanine scanning and illuminated structure–activity relationships by conservative substitutions. Our results, together with previous mutational data, highlight five acidic and four basic amino acids that are likely to comprise the LEF4 triphosphatase active site (Glu9, Glu11, Arg51, Arg53, Glu97, Lys126, Arg179, Glu181 and Glu183). These nine essential residues are conserved in LEF4 orthologs from all strains of baculoviruses. We discerned no pattern of clustering of the catalytic residues of the baculovirus triphosphatase that would suggest structural similarity to the tunnel proteins (exclusive of motifs A and C). However, there is similarity to the active site of vaccinia RNA triphosphatase. We infer that the baculovirus and poxvirus triphosphatases are a distinct lineage within the metal-dependent RNA triphosphatase family. Synergistic activation of the LEF4 triphosphatase by manganese and magnesium suggests a two-metal mechanism of γ phosphate hydrolysis.
INTRODUCTION
The 5′ m7G cap structure of eukaryotic cellular and viral mRNA is formed by three enzymatic reactions: (i) the 5′ triphosphate end of the pre-mRNA is hydrolyzed to a diphosphate by RNA 5′ triphosphatase; (ii) the diphosphate RNA end is capped with GMP by RNA guanylyltransferase; and (iii) the GpppN cap is methylated by RNA (guanine-N7) methyltransferase (1). Certain DNA viruses encode their own cap-forming enzymes; these include the poxviruses, African swine fever virus, infectious spleen and kidney necrosis virus (an iridovirus), Chlorella virus PBCV-1 and the baculoviruses (2).
Baculoviruses are large nuclear DNA viruses, which replicate in arthropod hosts (3). They are divided into two genera: nucleopolyhedroviruses viruses (NPVs) and granuloviruses (GVs). All baculoviruses encode a four-subunit RNA polymerase responsible for the transcription of viral genes expressed after the onset of viral DNA replication (4). The essential LEF4 subunit of the RNA polymerase of Autographa californica NPV (the prototypal baculovirus) is a bifunctional RNA capping enzyme consisting of a triphosphatase domain fused to a guanylyltransferase domain (5–8). The LEF4 triphosphatase catalyzes the cleavage of the β-γ phosphoanhydride bond of either triphosphate-terminated RNA or free NTPs in the presence of a divalent cation cofactor. The LEF4 triphosphatase belongs to a newly recognized family of metal-dependent NTPases that embraces the RNA triphosphatase components of the capping enzymes of other DNA viruses (poxviruses, African swine fever virus, Chlorella virus), and unicellular eukaryotes such as fungi, microsporidia, Plasmodia, Trypanosoma and Leishmania (1,2). The family is defined by the presence of two conserved glutamate-containing motifs (motifs A and C, Fig. 1) and the signature biochemical property of hydrolyzing NTPs to NDPs in the presence of manganese or cobalt (7,9). Motifs A and C of LEF4 are situated in the N-terminal portion of the enzyme (Fig. 1), which comprises an autonomous phosphohydrolase domain (8). The C-terminal segment includes the six conserved motifs that comprise the active site of the guanylyltransferase (1,5,7). The active site motifs of AcNPV LEF4 are conserved in the capping enzyme orthologs from other baculoviruses and in the bifunctional triphosphatase- guanylyltransferase domain of vaccinia virus capping enzyme (2,10,11). In contrast to the baculoviruses and poxviruses, Chlorella virus encodes separate RNA triphosphatase and RNA guanylyltransferase enzymes, which are more closely related to the separately encoded triphosphatase and guanylyltransferase components of the fungal capping apparatus than they are to the enzymes from other DNA viruses (2,12).
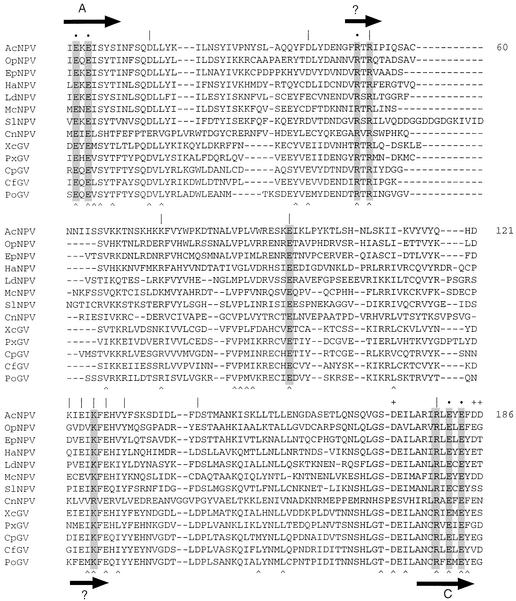
Figure 1.
Conservation of primary structure among baculovirus RNA triphosphatases. The amino acid sequence of AcNPV LEF4 from amino acids 8–186 is aligned to the orthologs encoded by other baculoviruses: Orgyia pseudotsugata NPV, Epiphyas postvittana NPV, Heliocoverpa armigera NPV, Lymantria dispar NPV, Mamestra configurata NPV, Spodoptera litura NPV, Culex nigripalpus NPV, Xestia c-nigrum GV, Plutella xylostella GV, Cydia pomonella GV, Choristoneura fumiferana GV and Phthorimaea operculella GV. Positions of side chain identity or similarity in all the aligned baculovirus proteins are denoted by a circumflex accent below the alignment. The amino acids of AcNPV LEF4 that were newly mutated to alanine in the present study are indicated by a vertical line. Residues defined previously as essential for the triphosphatase activity of LEF4 are denoted by a dot; unimportant residues are indicated by +. The nine essential side chains proposed to comprise the triphosphatase active site are highlighted in shaded boxes. The metal-binding motifs A and C are depicted as probable β strands (horizontal arrows). Two other candidate β strands are indicated by arrows and ?.
The crystal structure of the Saccharomyces cerevisiae RNA triphosphatase Cet1 revealed that its active site is located within a topologically closed hydrophilic tunnel composed of eight antiparallel β strands (13). The ‘triphosphate tunnel’ architecture is supported by an intricate network of hydrogen bonds and electrostatic interactions within the cavity, of which a high proportion are required for the catalytic activity of Cet1 (14). A single sulfate ion in the tunnel (which is proposed to mimic the γ phosphate of the substrate) is coordinated by multiple basic side chains projecting into the cavity. A single manganese ion within the tunnel cavity is coordinated with octahedral geometry to the sulfate, to the side chain carboxylates of the two glutamates in motif A and a glutamate in motif C. It is not clear at present whether the enzyme-bound divalent cation seen in the crystal structure is the sole metal cofactor for the triphosphatase activity.
Amino acid sequence comparisons and mutational analyses of the RNA triphosphatases from fungi, microsporidia, protozoa and Chlorella virus highlight the conservation of the β strands that comprise the triphosphate tunnel of yeast Cet1 (1) (see also Fig. 4 below). Thus, it is proposed that the active site folds of the fungal, microsporidian, protozoan and Chlorella virus RNA triphosphatases are conserved as β barrels, implying a common evolutionary origin for these enzymes (2). An extensive mutational analysis of the Chlorella virus RNA triphosphatase indicates that its active site architecture and catalytic mechanism adhere closely to that of yeast Cet1 (12).
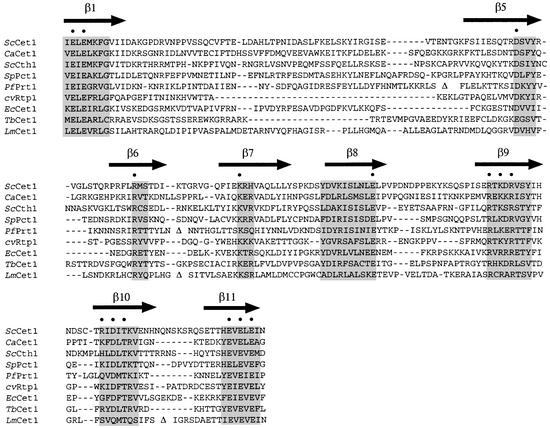
Figure 4.
The triphosphate tunnel subfamily of metal-dependent RNA triphosphatases. The amino acid sequence of the catalytic domain of S.cerevisiae RNA triphosphatase Cet1 is aligned to the sequences of Candida albicans CaCet1, S.cerevisiae Cth1, Schizosaccharomyces pombe Pct1, Plasmodium falciparum Prt1, Chlorella virus cvRtp1, Encephalitozoon cuniculi Cet1, Trypanosoma brucei Cet1 and Leishmania major Cet1. Gaps in the alignment are indicated by dashes. Non-conserved inserts in PfPrt1 and LmCet1 are omitted from the alignment and are denoted by a triangle. The β strands that form the triphosphate tunnel of ScCet1 are denoted above the sequence. Peptide segments with the highest degree of conservation in all tunnel subfamily proteins are highlighted by the shaded boxes. Essential hydrophilic amino acids of the Cet1 active site are denoted by dots above the sequence.
In contrast, the RNA triphosphatases of poxviruses and baculoviruses have little primary structure similarity to the fungal, microsporidian, protozoal and Chlorella virus enzymes. The poxvirus and baculovirus triphosphatases do contain the diagnostic metal-binding motifs A and C composed of alternating glutamate/hydrophobic side chains; indeed, mutational studies of the poxvirus and baculovirus proteins are consistent with an essential role for the conserved glutamates in metal-binding (6,8,10,11). However, the poxvirus and baculovirus enzymes do not have obvious equivalents of the other six β strands that comprise the tunnel found in the yeast enzyme and its orthologs. The structural and mechanistic relatedness among the various viral mRNA capping enzymes is of interest because it may illuminate the evolutionary connections between different families of large DNA viruses (2).
A phylogenetically guided mutational analysis of the vaccinia virus triphosphatase has identified nine essential hydrophilic amino acids that are conserved in all poxvirus mRNA capping enzymes and are likely to comprise the triphosphatase active site (11). The local sequence contexts of the catalytic residues of the poxvirus triphosphatases, and their positions relative to motifs A and C, differ from the arrangement of active site residues in triphosphate tunnel proteins. Based on the mutational evidence, it was proposed that the poxvirus triphosphatases are a distinct lineage within the metal-dependent RNA triphosphatase family (11).
To understand how the baculovirus triphosphatase fits into the scheme of viral capping enzyme structure and evolution, we sought to map the triphosphatase active site of AcNPV LEF4. The rationale was that by defining the catalytic residues of the baculovirus triphosphatase, we might either: (i) uncover otherwise hidden similarities to the active site of the tunnel subfamily; (ii) illuminate cryptic similarities to the poxvirus capping enzymes; or (iii) establish that the baculovirus triphosphatases comprise a separate lineage within the metal-dependent RNA triphosphatase family. We performed an alanine scan of 13 residues within the N-terminal segment of LEF4 that are conserved in other baculovirus capping enzymes. We thereby identified four new side chains required for triphosphatase activity: Arg53, Glu97, Lys126 and Arg179. Structure–activity relationships were clarified by conservative substitutions. These results, together with previous mutational studies, highlight an active site composed of nine hydrophilic amino acids. We can discern no pattern of clustering of the catalytic residues of the baculovirus triphosphatase similar to that of the tunnel subfamily, exclusive of motifs A and C. Possible structural similarities between baculovirus and poxvirus RNA triphosphatases are discussed. In addition, we provide evidence of synergistic activation of the LEF4 triphosphatase by manganese and magnesium, consistent with a two-metal mechanism.
MATERIALS AND METHODS
Silent diagnostic restriction sites and single alanine or conservative amino acid substitutions were introduced into the lef4 gene by PCR using the two-stage overlap extension method, with plasmid pET-LEF4 as the template for the first-stage PCR amplification (8). NdeI–BamHI restriction fragments of the PCR-amplified mutated DNAs were inserted into the T7-based expression plasmid pET16b that had been digested with NdeI and BamHI. The resulting pET-LEF4-mut plasmids contained the mutated lef4 coding sequence fused in frame with a 63 bp 5′ leader sequence that encodes 10 consecutive histidines. The presence of the desired mutations was confirmed in each case by sequencing the entire insert; the occurrence of PCR-generated mutations outside the targeted region was thereby excluded.
The His10-tagged versions of the wild type and missense mutant LEF4 proteins were expressed in Escherichia coli BL21(DE3) and purified by nickel–agarose affinity chromatography as described previously (8). The recombinant His10-LEF4 proteins were recovered in the 200 mM imidazole eluate fractions. The enzyme preparations were dialyzed against buffer containing 50 mM Tris–HCl pH 8.0, 200 mM NaCl, 2 mM DTT, 2 mM EDTA, 10% glycerol, 0.1% Triton X-100. The protein concentrations were determined by SDS–PAGE analysis of serial dilutions of the LEF4 preparations in parallel with serial dilutions of a BSA standard. The gels were stained with Coomassie blue and the staining intensities of the LEF4 and BSA polypeptides were quantitated using a Digital Imaging and Analysis System from Alpha Innotech Corporation.
RESULTS
Mutagenesis strategy
Prior mutational analyses of AcNPV LEF4 had identified the motif A and C glutamates plus a single basic residue (Arg51) as essential for the triphosphatase reaction (6,8). Alanine mutations at Glu9, Glu11, Arg51, Glu181 and Glu183 (denoted by dots in Fig. 1) abrogated the triphosphatase activity of LEF4, but not the guanylyltransferase activity. Four other acidic residues within the triphosphatase domain (Asp172, Asp185, Asp186 and Asp187) were subjected to alanine scanning and found to be unimportant for triphosphatase activity (+ in Fig. 1). Subsequent comprehensive mutational analyses of the S.cerevisiae and Chlorella virus RNA triphosphatases have shown that all of the side chains that comprise the active site are located within the polypeptide segment spanning motifs A and C (12,14). However, this region of LEF4 bears no resemblance to either yeast or Chlorella virus RNA triphosphatase. Given that the active sites of the yeast, Chlorella virus and vaccinia virus triphosphatases are composed of 9–15 essential amino acids (11–14), we suspected that the triphosphatase active site of LEF4 had not yet been fully delineated. The goal of the present study was to extend the functional map of the baculovirus triphosphatase active site by introducing new mutations into the N-terminal triphosphatase segment of LEF4. The choice of residues to be mutated was made by aligning the sequences of the LEF4 orthologs from many baculovirus isolates of the NPV or GV genera that differ in their host range (Fig. 1). The 32 positions of side-chain identity/similarity in the triphosphatase domains of all 13 proteins are denoted by a circumflex accent below the aligned sequences. The invariant positions include all of the five residues already known to be essential for triphosphatase activity.
In picking residues to target in the present study, we focused primarily on charged and polar amino acids, because such functional groups comprise the active site of Cet1 and they are most likely to be direct participants in NTP binding and catalysis of γ phosphate hydrolysis. We then used one of two additional criteria for target selection: (i) conservation in all baculovirus orthologs; and/or (ii) location within possible β-strand secondary structure, as suggested by the presence of alternating hydrophilic and hydrophobic side chains. A total of 12 new residues were subjected to alanine scanning: Asp21, Asp43, Arg53, Lys76, Glu97, Lys122, Glu124, Lys126, Glu128, Tyr131, Asp141 and Arg179. We also mutated Arg51, in order to confirm its essentiality as reported by the Guarino lab (6).
Alanine scanning identifies four new residues required for triphosphatase activity
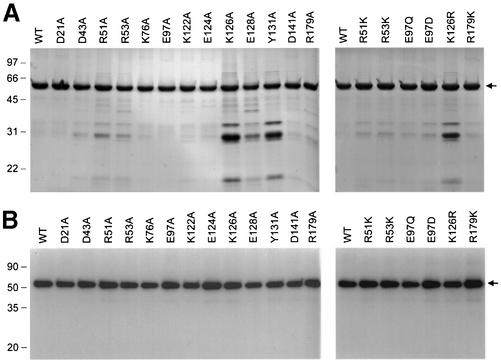
Wild-type LEF4 and the 13 Ala mutants were produced in bacteria as His10-tagged fusions and purified from soluble bacterial extracts by nickel–agarose chromatography. SDS–PAGE analysis showed that the preparations were highly enriched with respect to the 57 kDa His10-LEF4 polypeptide (Fig. 2A). The guanylyltransferase activity of each of the mutant protein preparations was demonstrated by label transfer from [α-32P]GTP to the enzyme to form a covalent enzyme–GMP complex (Fig. 2B). The findings that all of the mutant enzymes retained full guanylyltransferase activity indicated that none of the 13 Ala mutations affected the global folding of the recombinant LEF4 protein.
Figure 2.
Purification and guanylyltransferase activity of wild-type and mutant LEF4 proteins. (A) Purification. Aliquots (4 µg) of the nickel– agarose preparations of wild-type (WT) LEF4 and the indicated mutants were analyzed by SDS–PAGE. Polypeptides were visualized by staining with Coomassie blue dye. The positions and sizes (in kDa) of marker proteins are indicated on the left. The LEF4 polypeptide is denoted by an arrow on the right. (B) Guanylyltransferase activity. Reaction mixtures (10 µl) containing 50 mM Tris–HCl pH 8.0, 5 mM DTT, 20 mM MgCl2, 2 mM [α-32P]GTP, and 1 µg of WT or mutant LEF4 as specified were incubated for 15 min at 30°C. The reactions were quenched with SDS and the products were analyzed by SDS–PAGE. Autoradiographs of the dried gels are shown. The positions and sizes of marker polypeptides are indicated on the left. The LEF4–GMP complex is denoted by an arrow on the right.
The triphosphatase activities of wild-type LEF4 and the LEF4-Ala mutants were assayed by the release of 32Pi from [γ-32P]ATP. The ATPase specific activities were determined with three different divalent cation cofactors (Mg, Mn or Co) at their respective pH optima (8) and the activities were normalized to that of wild-type LEF4 (Table 1). For the purpose of stratifying the mutational effects, we defined a threshold of significance as a 10-fold decrement in ATPase specific activity.
Table 1. Effects of alanine mutations on LEF4 phosphohydrolase activity.
| LEF4 mutant | ATPase (% of wild-type) | ||
|---|---|---|---|
| |
MnCl2 |
CoCl2 |
MgCl2 |
| D21A | 73 | 160 | 97 |
| D43A | 42 | 39 | 7 |
| R51A | 0.4 | 0.1 | 0.3 |
| R53A | 0.6 | 0.3 | 0.1 |
| K76A | 130 | 120 | 140 |
| E97A | 0.4 | 0.2 | <0.1 |
| K122A | 130 | 130 | 140 |
| E124A | 110 | 97 | 6 |
| K126A | 1 | 0.2 | 0.1 |
| E128A | 35 | 16 | 11 |
| Y131A | 87 | 140 | 90 |
| D141A | 110 | 150 | 120 |
| R179A | 13 | 9 | 2 |
The purified wild-type and mutant LEF4 proteins were titrated for ATPase activity in reaction mixtures (10 µl) containing either: (i) 1 mM MnCl2, 50 mM Tris–HCl pH 7.5 and 0.2 mM [γ-32P]ATP; (ii) 2 mM CoCl2, 50 mM Tris–HCl pH 7.5 and 0.2 mM [γ-32P]ATP; or (iii) 20 mM MgCl2, 50 mM Tris–HCl pH 9.0 and 2 mM [γ-32P]ATP. All reaction mixtures were incubated for 15 min at 30°C. ATPase specific activities were determined in the linear range of protein dependence and are expressed as percentages of the specific activity of wild-type LEF4. Each value is the average of at least two independent titration experiments. The ATPase turnover numbers for wild-type LEF4 were 5.1 s–1 for Mn, 4.7 s–1 for Co and 4.8 s–1 for Mg.
We observed three distinct classes of Ala-mutation effects. (i) Mutations at six of the targeted positions failed to elicit a significant decrement in ATPase activity with any of the three divalent cation cofactors (D21A, K76A, K122A, E128A, Y131A, D141A). (ii) Mutations at four positions resulted in drastic reductions in activity (to ≤1% of wild-type) with each of the three divalent cation cofactors (R51A, R53A, E97A, K126A). (iii) Mutations at three positions caused a significant loss of function in a cofactor-dependent fashion, either exclusively in the presence of magnesium, but not manganese or cobalt (D43A, E124A) or jointly with magnesium and cobalt, but not manganese (R179A).
We interpreted the results of the alanine scan as follows. Residues Arg51, Arg53, Glu97 and Lys126 are candidates to play direct roles in catalysis, in light of the magnitude of the mutational defects and their independence of the choice of metal. Thus, these residues were subject to further analysis by conservative substitution (see below). Residues Asp43 and Glu124 are candidates to play a specific role in forming a binding site for magnesium, insofar as the removal of either side chain selectively reduced Mg-ATPase activity to 6–7% of the wild-type value. Whereas the D43A mutations caused a modest reduction in Mn-ATPase and Co-ATPase (to ∼40% of wild-type), the E124A change had no effect on Mn-ATPase or Co-ATPase activity. Specific abrogation of LEF4 triphosphatase activity in magnesium, with sparing of manganese-dependent activity, was described previously for Asp mutants of metal-binding residues Glu9 and Glu181 (8). It was suggested then that the smaller atomic radius of magnesium may impose more stringent requirements for its binding than what is needed to coordinate the larger manganese ion (8). In any event, it is clear that neither Asp43 nor Glu124 can be regarded as constitutively essential for catalysis. These positions were not subjected to additional mutations.
The R179A mutation reduced Mg-ATPase to 2% of wild-type, Co-ATPase to 9%, and Mn-ATPase to 13%. Although the latter value exceeded our threshold for significance, the relatively small differential between the cobalt and manganese activities suggested a general role for Arg179 in some aspect of the phosphohydrolase reaction; therefore we tested the effects of a conservative mutation at this position. Given that Ala mutations at Asp21, Lys76, Lys122, Tyr131 and Asp141 had no discernible effects on phosphohydrolase activity, we conclude that these side chains are not involved in catalysis and thus did not subject these positions to further analysis. The modest effects of the E128A change on triphosphatase activity (11–35% of wild-type, depending on the cofactor) did not meet the significance threshold that would warrant additional mutagenesis, although the kinetic parameters of the E128A mutant were investigated (see below).
Effects of conservative mutations of Arg51, Arg53, Glu97, Lys126 and Arg179
To better evaluate the contributions of Arg51, Arg53, Glu97, Lys126 and Arg179 to the triphosphatase reaction, we tested the effects of conservative substitutions. Arginine was replaced by lysine, glutamate by glutamine and aspartate, and lysine by arginine. The R51K, R53K, E97Q, E97D, K126R and R179K proteins were purified from soluble bacterial extracts by nickel–agarose chromatography (Fig. 2A). All of the conservative mutants retained guanylyltransferase activity (Fig. 2B).
The triphosphatase specific activities with Mg, Mn or Co cofactors were determined by enzyme titration and normalized to the wild-type values (Table 2). Conservative replacement of Glu97 with Asp or Gln elicited a severe catalytic defect comparable to that seen with E97A (≤2% of wild-type activity). These data establish a requirement for a carboxylate residue at position 97 and they indicate a minimum distance requirement from the main-chain to the carboxylate that is met by glutamate but not aspartate. Note that glutamate is strictly conserved at this position in all other baculovirus capping enzymes (Fig. 1). We found that R53K was just as defective as the R53A mutant, with ≤2% of wild-type triphosphatase activity. Thus, arginine is specifically required at position 53. In contrast, replacement of the nearby Arg51 by lysine resulted in a significant gain of function compared to R51A, such that Co-ATPase and Mn-ATPase were 40–45% of wild-type (versus 0.1–0.4% for R51A) and Mn-ATPase was 6% of wild-type (versus 0.3% for R51A). Positive charge appears to be the critical feature at position 51, although Mg-ATPase remains significantly impaired when this residue is a lysine. Arg51 is strictly conserved in other baculovirus Lef4 orthologs (Fig. 1).
Table 2. Effects of conservative mutations on LEF4 phosphohydrolase activity.
| LEF4 mutant | ATPase (% of wild-type) | ||
|---|---|---|---|
| MnCl2 | CoCl2 | MgCl2 | |
| R51K | 45 | 40 | 6 |
| R53K | 2 | 0.7 | 0.7 |
| E97D | 1 | 0.8 | 0.4 |
| E97Q | 2 | 1.4 | 0.4 |
| K126R | 96 | 56 | 48 |
| R179K | 14 | 39 | 4 |
ATPase specific activities were determined as described in Table 1 and expressed as percentages of the specific activity of wild-type LEF4. Each value is the average of at least two independent titration experiments.
Replacing Lys126 by arginine revived Mn-ATPase to wild-type level (compared with 1% for the K126A mutant) and restored Co-ATPase and Mg-ATPase to about half the respective wild-type activities. We surmise that a positive charge is critical for function and that the bulkier arginine side chain can be accommodated at position 126 with no detrimental effect. Note that the equivalent position is naturally occupied by arginine in the Culex nigripalpus NPV capping enzyme (Fig. 1). The R179K mutant displayed the same decrements in Mn-ATPase and Mg-ATPase as R179A, although the lysine substitution resulted in an increase in Co-ATPase to 39% of wild-type, compared with 9% for R179A. Thus, arginine is specifically required for the function of this side chain in the presence of manganese or magnesium. Arg179 is strictly conserved in the various baculovirus capping enzymes (Fig. 1).
Mutational effects on steady-state kinetic parameters
Kinetic parameters for manganese-dependent ATP hydrolysis were determined for wild-type LEF4 (Km 41 µM ATP; kcat 5.3 s–1) and five of the mutants that displayed modestly reduced ATPase activity: D43A, R51K, E128A, R179A and R179K (Table 3). The R51K, E128A, R179A and R179K mutations increased the Km for ATP to 120–140 µM (∼3-fold higher than the wild-type Km), while reducing kcat to 58, 37, 6 and 11% of the wild type value, respectively. The D43A mutation had little effect on Km (61 µM) and lowered kcat to 48% of the wild-type value.
Table 3. Mutational effects on kinetic parameters.
| LEF4 protein | Mn-ATPase | |
|---|---|---|
| Km (µM) | kcat (s–1) | |
| Wild-type | 41 | 5.3 |
| D43A | 62 | 2.6 |
| R51K | 140 | 3.2 |
| E128A | 130 | 2.0 |
| R179A | 140 | 0.3 |
| R179K | 140 | 0.6 |
Reactions mixtures (10 µl) containing 50 mM Tris–HCl pH 7.5, 2 mM MnCl2, various concentrations of [γ-32P]ATP, and LEF4 were incubated for 15 min at 30°C. Kinetic parameters were calculated from double-reciprocal plots for each titration series. Each value is the average of at least two independent titration experiments.
Evidence for two-metal catalysis
Initial studies of the divalent cation specificity of LEF4 showed that the hydrolysis of mononucleoside 5′ triphosphates was activated by manganese (or cobalt), but not by magnesium, whereas the hydrolysis of RNA 5′ triphosphatase ends was supported by either manganese or magnesium (6,7). The strong bias for manganese over magnesium for the LEF4 NTPase activity echoed the findings for the NTPase activity of the yeast RNA triphosphatase Cet1 (9). Subsequent studies of LEF4 showed that the early failure to detect a magnesium-dependent ATPase activity stemmed from the use of unfavorable pH and substrate concentrations for the activity measurements. The Mg-ATPase activity of LEF4 is optimal at pH 9.0 (rather than pH 7.5, the optimum for Mn-ATPase) and requires high concentrations of both ATP (Km 4 mM) and magnesium (optimum 10–40 mM MgCl2) (8). Thus, there is no intrinsic difference in the spectrum of metal cofactors that can support hydrolysis of NTP versus RNA substrates by LEF4, which, after all, entails seemingly identical chemistry. Instead, there is a poorly understood disparity in the optimal reaction parameters for one metal versus another. [It is noteworthy that the divalent cation specificity of vaccinia virus capping enzyme for NTP hydrolysis is also pH dependent (10)].
To explain the distinctive ATPase reactions conditions required for manganese versus magnesium, we considered the possibility that LEF4 may exploit a two-metal mechanism requiring occupancy of distinct metal-binding sites, M1 and M2, on the enzyme and/or the NTP substrate. A simple model would be that one site prefers manganese while the other prefers magnesium. A prediction of such a model is that a mixture of suboptimal levels of magnesium and manganese would elicit a synergistic activation of LEF4 ATPase activity.
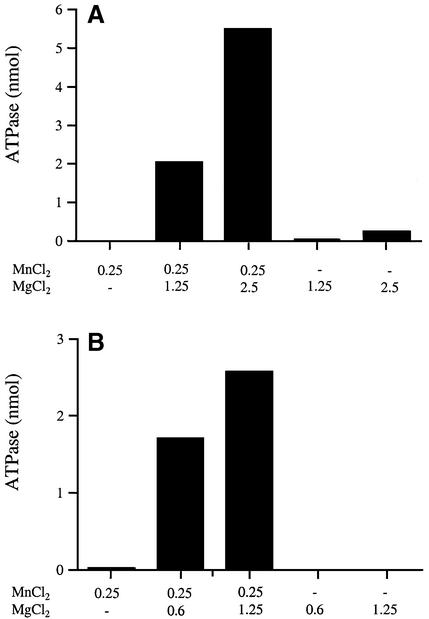
Indeed, we observed profound synergy between manganese and magnesium at either pH 7.5 or 9.0 (Fig. 3). Whereas there was minimal detectable hydrolysis of 1 mM ATP in the presence of 0.25 mM manganese alone or 0.6–2.5 mM magnesium alone, the combination of 0.25 mM Mn and 0.6 or 1.25 mM Mg (at pH 9.0, Fig. 3B) or 0.25 mM Mn and 1.25 or 2.5 mM Mg (at pH 7.5, Fig. 3A) restored vigorous ATPase activity. The extent of ATP hydrolysis at pH 9.0 with 0.25 mM Mn plus 0.6 or 1.25 mM Mg at pH 9.0 was 86- and 130-fold greater than that seen with 0.25 mM Mn alone (Fig. 3B). At pH 7.5, the ATPase activity with 0.25 mM Mn plus 1.25 or 2.5 mM Mg was 55- and 23-fold greater than the activity seen with 1.25 or 2.5 mM Mg alone (Fig. 3A). The amount of ATP hydrolyzed by LEF4 at pH 7.5 in the presence of 0.25 mM Mn plus 2.5 mM Mg (5.48 nmol) exceeded the amount hydrolyzed at the optimal concentration of 1 mM Mn alone (3.92 nmol) (Fig. 3 and data not shown). These results are consistent with a two-metal mechanism for the LEF4 triphosphatase.
Figure 3.
Synergy between manganese and magnesium suggests two-metal catalysis. Reaction mixtures (10 µl) containing either 50 mM Tris–HCl (A) pH 7.5 or (B) pH 9.0, 1 mM [γ-32P]ATP, MnCl2 and/or MgCl2 as specified, and either (A) 100 ng or (B) 20 ng of LEF4 were incubated for 15 min at 30°C. Each datum is the average of three experiments.
DISCUSSION
By performing a mutational analysis of 13 residues of the AcNPV LEF4 protein, we have identified four new amino acids that are essential for triphosphatase activity. These results, together with previous mutational studies (6,8), highlight nine charged amino acids that are likely to comprise the triphosphatase active site: Glu9, Glu11, Arg51, Arg53, Glu97, Lys126, Arg179, Glu181 and Glu183. The nine essential residues are conserved in all baculovirus mRNA capping enzymes (Fig. 1). We hypothesize that these residues participate directly in the catalysis of γ phosphate hydrolysis.
The baculovirus triphosphatase active site consists of five acidic and four basic functional groups. The LEF4 motif A and C glutamates (Glu9, Glu11, Glu181 and Glu183) are implicated in metal binding on the basis of previous mutational studies (8) and their similarity to the metal-binding β strands of Cet1 (β1 and β11 in Fig. 4). We posit a role for the essential Glu97 side chain as a general base catalyst to activate the nucleophilic water for its attack on the γ phosphorus, analogous to the general base function postulated for the essential glutamate in the β8 strand of the tunnel enzymes (13,14) (Fig. 4). We suggest that the side chains Arg51, Arg53, Lys107 and Arg126 interact with the γ phosphate and stabilize the transition state.
Capping enzyme evolution
The LEF4 active site is similar in complexity to the active site of vaccinia RNA triphosphatase (which also has nine essential side chains; Fig. 5) but not as complex as the active site of yeast RNA triphosphatase Cet1, which consists of fifteen essential hydrophilic side chains that emanate from the eight β strands that form the triphosphate tunnel (13,14). A structure-based alignment of the sequences of the members of the tunnel family of RNA triphosphatases is shown in Figure 4, with the β strands of the tunnel denoted by arrows and the essential side chains of Cet1 indicated by dots. Ten of the fifteen essential side chains of Cet1 make direct or water-mediated contacts with the enzyme-bound metal cofactor, the γ phosphate, or the putative nucleophilic water. These ten residues include four glutamates and an aspartate that bind the metal cofactor either directly or via water, two arginines and a lysine that contact the γ phosphate directly, and one aspartate and one glutamate that coordinate phosphate-interacting waters, one of which is the putative nucleophile in γ phosphate hydrolysis. The other five essential residues appear to stabilize the tunnel architecture and are not implicated directly in catalysis (13,14).
Figure 5.
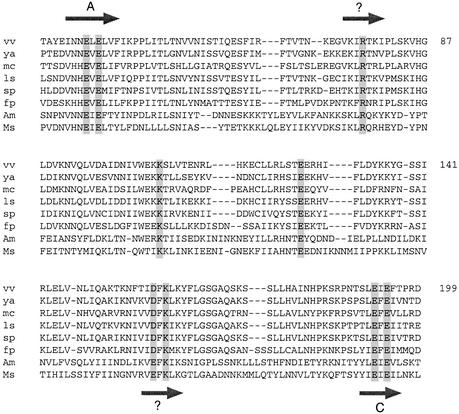
Poxvirus RNA triphosphatases. The amino acid sequence of the capping enzyme large subunit of vaccinia virus from amino acid 30–199 is aligned to the related polypeptides encoded by other poxviruses: Yaba-like virus (ya); molluscum contagiosum virus (mc); lumpy skin disease virus (ls); swinepox virus (sp); fowlpox virus (fp); Amsacta moorei entomopoxvirus (Am); and Melanoplus sanguinipes entomopoxvirus (Ms). The nine essential side chains proposed to comprise the triphosphatase active site are highlighted in shaded boxes. The metal-binding motifs A and C are depicted as probable β strands (horizontal arrows). Two other candidate β strands are indicated by arrows and ?.
Are any of the essential constituents of baculovirus triphosphatase (other than those in motifs A and C) likely to be located in β strands? Our inspection of the sequences surrounding the essential LEF4 triphosphatase residues hints that the peptide motifs 50FRTRI54 and 123IEIKFE128, which are conserved in the other baculovirus LEF4 orthologs (Fig. 1), are likely candidates to adopt a β secondary structure. The pattern of alternating hydrophobic and hydrophilic side chains could allow the projection of the hydrophilic functional groups into the enzyme’s active site, while the hydrophobic residues on the opposite face could project into the enzyme’s core. Whereas the pattern of alternating basic side chains in the LEF4 50FRTRI54 motif is similar to that of the β9 motif of the tunnel subfamily enzymes (Fig. 4), they are unlikely to be structurally equivalent, insofar as the location of the basic motif relative to motifs A and C is quite different in the baculovirus and tunnel subfamily triphosphatases. The sequence of LEF4 candidate β motif 123IEIKFE128 bears no resemblance to any of the conserved β strands of the tunnel enzyme subfamily (Fig. 4). Our inference is that the baculovirus triphosphatases do not adopt a topologically closed tunnel architecture. Although the LEF4 active site may well retain a significant component of β secondary structure, we are unable to discern any amino acid sequence similarity to the Chlorella virus, protozoan and fungal enzymes, exclusive of metal-binding motifs A and C.
In contrast, we do discern similarities between active sites of the baculovirus and poxvirus RNA triphosphatases beyond simple conservation of metal-binding motifs A and C. For example, the LEF4 50FRTRI54 peptide, which contains two essential arginines, resembles the vaccinia peptide 74VKIRTKI80, which also includes a catalytically essential arginine. The spacing between these basic motifs (depicted as β strands in Figs 1 and 5) and metal-binding motif A is nearly identical in the baculovirus and poxvirus capping enzymes. Also, the baculovirus and poxvirus enzymes each contain an essential glutamate (Glu97 in LEF4; Glu126 in vaccinia capping enzyme) located midway between motifs A and C. The sequences immediately upstream of the glutamate are similar in both virus families, with a consensus motif Rx(S/N)xE. The other candidate LEF4 β motif, 123IEIKF127, which contains the essential Lys126 residue, resembles the vaccinia peptide motif 158IDFKL162 motif, which includes the essential residue Lys161. The baculovirus and poxvirus enzymes both contain acidic residues located two positions upstream of this essential lysine; Glu124 in LEF4 and Asp159 in vaccinia capping enzyme. Mutating Glu124 to Ala reduced Mg-dependent triphosphatase activity to 6% of wild-type LEF4 (Table 2); similarly, the D159A mutation of vaccinia capping enzyme reduced triphosphatase activity to 11% of the wild-type value (11). The conservation of sequence in this (D/E)xK motif, the concordance of mutational effects, and the similar distances separating the (D/E)xK motif from the upstream catalytic glutamate and the downstream metal-binding motif C, convey the strong impression that this putative β strand is a conserved component of the active sites of baculovirus and poxvirus triphosphatases. A key distinction between the two groups of viral enzymes is the occurrence of a conserved and functionally important arginine two positions upstream of the first glutamate of baculovirus motif C, which is lacking in all of the poxvirus enzymes.
Our inference from mutational analyses is that baculovirus and poxvirus RNA triphosphatases are structurally related, even though their overall amino acid sequences are not well conserved. Whether the emergence of distinct baculovirus and poxvirus branches of the metal-dependent triphosphate family reflects divergence from a common viral ancestor, or convergence toward a common metal-binding and substrate-binding site, cannot be surmised at present. However, given that the baculovirus and poxvirus capping enzymes both consist of an N-terminal triphosphatase domain fused to a downstream guanylyltransferase domain, we favor the idea that they share an evolutionary history.
Two-metal catalysis
The structure of yeast Cet1 revealed a single enzyme-bound manganese ion with three of the six octahedral coordination sites being occupied by motif A and C glutamates, one site being taken by an enzyme-bound water, and a fifth site by a water not engaged by the enzyme. The sixth contact is to a lone sulfate ion in the tunnel cavity that likely mimics the hydrolyzed γ phosphate in the product complex. The structure suggests that the enzyme-bound metal promotes catalysis by stabilizing the negative charge developed on the γ phosphate in the transition state, a task that is also aided by the numerous contacts between the phosphate and essential lysine and arginine side chains projecting from the tunnel walls.
Transition state stabilization is one of several potential catalytic roles invoked for divalent cations in phosphoryl transfer chemistry (15). Others include: activation of the nucleophile, e.g. by lowering the pKa of a metal-coordinated water; protonation of the leaving group via a metal-coordinated water; or ensuring optimal substrate conformation for in-line chemistry. In the case of the tunnel triphosphatases, we have suggested a role for the β8 glutamate in activating the water nucleophile based on the crystal structure and have extended this inference to the conserved essential glutamate between motifs A and C of the baculovirus and poxvirus triphosphatases. However, we cannot exclude an alternative model in which a metal ion assists in this step. The low pKa of the bridging oxygen of diphosphate leaving group in the RNA triphosphatase reaction suggests that a metal ion (or any general acid) may not be needed to promote its expulsion. That leaves substrate coordination as a likely step at which the posited second metal might function during the triphosphatase reaction.
The demonstration of synergy between magnesium and manganese in the LEF4 triphosphatase reaction is consistent with a two-metal mechanism. One metal is most probably coordinated by motifs A and C, as seen in the Mn-Cet1 crystal. The binding site of the second metal cannot be surmised, but may entail contacts primarily with the α and β phosphates of the substrate, rather than with side chains on the enzyme. Metal binding to the NTP substrate could stabilize the conformation of the triphosphate for optimal binding and orientation with respect to the catalytic side chains on the enzyme. We can rationalize the presence of only one metal in the crystal of the putative Pi product complex of yeast Cet1, because it lacks the residual 5′ nucleoside diphosphate (13). Additional structures of metal-dependent RNA triphosphatase family members in various functional states will be needed to elucidate the requirements for substrate recognition and to obtain structural confirmation of the two-metal hypothesis.
Acknowledgments
ACKNOWLEDGEMENT
Supported by NIH grant GM42498.
REFERENCES
- 1.Shuman S. (2001) The mRNA capping apparatus as drug target and guide to eukaryotic phylogeny. Cold Spring Harb. Symp. Quant. Biol., 66, 301–312. [DOI] [PubMed] [Google Scholar]
- 2.Shuman S. (2002) What mRNA capping tells us about eukaryotic evolution. Nature Rev. Mol. Cell Biol., 3, 619–625. [DOI] [PubMed] [Google Scholar]
- 3.Herniou E.A., Olszewski,J.A., Cory,J.S. and O’Reilly,D.R. (2003) The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol., 48, 211–234. [DOI] [PubMed] [Google Scholar]
- 4.Guarino L.A., Xu,B., Jin,J. and Dong,W. (1998) A virus-encoded RNA polymerase purified from baculovirus-infected cells. J. Virol., 72, 7985–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarino L.A., Jin,J. and Dong,W. (1998) Guanylyltransferase activity of the LEF-4 subunit of baculovirus RNA polymerase. J. Virol., 72, 10003–10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin J., Dong,W. and Guarino,L.A. (1998) The LEF-4 subunit of baculovirus RNA polymerase has RNA 5′-triphosphatase and ATPase activities. J. Virol., 72, 10011–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross C.H. and Shuman,S. (1998) RNA 5′-triphosphatase, nucleoside triphosphatase and guanylyltransferase activities of baculovirus LEF-4 protein. J. Virol., 72, 10020–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins A. and Shuman,S. (2001) Mutational analysis of baculovirus capping enzyme Lef4 delineates an autonomous triphosphatase domain and structural determinants of divalent cation specificity. J. Biol. Chem., 276, 45522–45529. [DOI] [PubMed] [Google Scholar]
- 9.Ho C.K., Pei,Y. and Shuman,S. (1998) Yeast and viral RNA 5′ triphosphatases comprise a new nucleoside triphosphatase family. J. Biol. Chem., 273, 34151–34156. [DOI] [PubMed] [Google Scholar]
- 10.Ho C.K., Martins,A. and Shuman,S. (2000) A yeast-based genetic system for functional analysis of viral mRNA capping enzymes. J. Virol., 74, 5486–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong C. and Shuman,S. (2003) Mapping the active site of vaccinia virus RNA triphosphatase. Virology, in press. [DOI] [PubMed] [Google Scholar]
- 12.Gong C. and Shuman,S. (2002) Chlorella virus RNA triphosphatase: mutational analysis and mechanism of inhibition by tripolyphosphate. J. Biol. Chem., 277, 15317–15324. [DOI] [PubMed] [Google Scholar]
- 13.Lima C.D., Wang,L.K. and Shuman,S. (1999) Structure and mechanism of yeast RNA triphosphatase: an essential component of the mRNA capping apparatus. Cell, 99, 533–543. [DOI] [PubMed] [Google Scholar]
- 14.Bisaillon M. and Shuman,S. (2001) Structure-function analysis of the active site tunnel of yeast RNA triphosphatase. J. Biol. Chem., 276, 17261–17266. [DOI] [PubMed] [Google Scholar]
- 15.Galburt E.A. and Stoddard,B.L. (2002) Catalytic mechanisms of restriction and homing endonucleases. Biochemistry, 41, 13851–13860. [DOI] [PubMed] [Google Scholar]