Abstract
The crystal structure of the complex formed between the anthracycline antibiotic 3′-deamino-3′- hydroxy-4′-(O-l-daunosaminyl)-4-demethoxydoxo rubicin (MEN 10755), an active disaccharide analogue of doxorubicin, and the DNA hexamer d(CGATCG) has been solved to a resolution of 2.1 Å. MEN 10755 exhibits a broad spectrum of antitumor activities, comparable with that of the parent compound, but there are differences in the mechanism of action as it is active in doxorubicin-resistant tumors and is more effective in stimulating topoisomerase DNA cleavage. The structure is similar to previously crystallised anthracycline– DNA complexes. However, two different binding sites arise from drug intercalation so that the two halves of the self-complementary duplex are no longer equivalent. In one site both sugar rings lie in the minor groove. In the other site the second sugar protrudes out from the DNA helix and is linked, through hydrogen bonds, to guanine of a symmetry-related DNA molecule. This is the first structure of an anthracycline–DNA complex where an interaction of the drug with a second DNA helix is observed. We discuss the present findings with respect to the relevance of the amino group for DNA binding and to the potential role played by the second sugar in the interactions with topoisomerases or other cellular targets.
INTRODUCTION
Anthracycline antibiotics represent a major class of antitumor drugs and one of the most widely used in chemotherapy for over 30 years (1). Although doxorubicin remains one of the most effective agents for the treatment of solid tumours, there is still large interest in new anthracyclines that might display improved antitumor properties and reduced toxicity (2).
Anthracyclines, like many other antitumor agents, work by DNA intercalation, a specific mode of binding in which the drug chromophore is inserted between adjacent DNA base pairs (3). The binding of anthracyclines to DNA has been extensively studied by a variety of biochemical and biophysical techniques (4); a number of crystallographic studies of drug–DNA complexes have provided a detailed picture of the molecular interactions (5). The drug intercalates at a pyrimidine–purine step with a preference for the CG sequence, and also acts as a groove binder since the sugar moiety interacts with the minor groove (6,7). DNA binding is a necessary but not sufficient condition for drug activity. There is no simple relationship between DNA binding affinity and cytotoxicity; other molecular interactions may play an important role as well. Though the exact mechanism of action is not fully understood, anthracyclines exert their cytotoxic action primarily by interfering with topoisomerases, enzymes that govern the topological interconversion of DNA during the DNA cellular processes. Such interference results in DNA strand breaks and formation of a drug–DNA–enzyme ternary complex in which the enzyme is covalently linked to the broken DNA strand (8,9). A number of different mechanisms have been proposed for anthracyclines, but inhibition of topoisomerases is likely to be the one most closely associated with their cytoxicity (10). Thus, in the development of new anthracyclines, much attention is given to chemical modifications that may influence the interactions with other cellular targets but do not alter the overall molecular architecture and the associated DNA binding capacity.
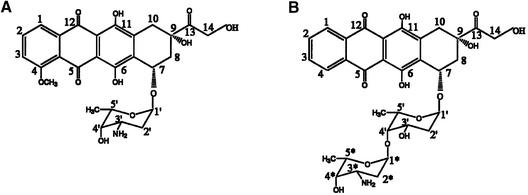
A number of innovative anthracyclines with original structural motifs have been prepared and tested in recent years. Among these a disaccharide anthracycline analogue 3′-deamino-3′-hydroxy-4′-(O-l-daunosaminyl)-4-demethoxydoxo-rubicin (MEN 10755) (Fig. 1), with a broader spectrum of antitumor activity than doxorubicin, has recently entered phase 2 clinical trials (11). Experiments with radiolabelled anthracyclines showed that MEN 10755, compared to doxorubicin, exhibits reduced cellular accumulation and a different subcellular distribution, with lower nuclear uptake (12). In spite of this, MEN 10755 is as potent as doxorubicin in eliciting DNA single- and double-strand breaks, G2/M cell arrest and apoptosis (13). The extent of drug-induced topoisomerase II cleavage of DNA is greater for MEN 10755 than for doxorubicin; thus, a more stable interaction of MEN 10755 in the ternary complex has been invoked. These observations have raised great interest in the elucidation of MEN 10755 binding to DNA and in the structural characterisation of the complex.
Figure 1.
Molecular formula of (A) doxorubicin and (B) MEN10755.
We report here a study of the interactions of MEN 10755 with the DNA hexamer d(CGATCG) analysed by X-ray crystallography.
MATERIALS AND METHODS
The self-complementary DNA hexamer d(CGATCG) was purchased from Integrated DNA Technologies, USA. MEN 10755 was prepared according to the reported method (11).
Crystals were grown at 4°C by the vapour diffusion method using the hanging drop technique. Orange-red crystals appeared after 5–10 days from a solution of 40 mM sodium cacodilate, pH 6.5; 0.9 mM DNA, 4.2 mM MgCl2, 8.3 mM spermine hydrocloride, 2 mM MEN 10755 and 20% (by vol.) 2,4-methyl-pentane-diol (MPD) equilibrated against a reservoir containing a solution of 60% MPD. X-ray diffraction data were collected on the XRD1 beamline of the Italian Synchroton facility ELETTRA, Trieste, Italy. Crystals were mounted in nylon loops and exposed to a cold (100°K) nitrogen stream (Oxford Cryosystem Cryocooler, Oxford, UK). A total of 100 frames (125 121 reflections) were collected at a wavelength of 1.0300 Å using a 165 mm CCD detector from MarResearch. Data were processed and scaled with the software DENZO and SCALEPACK (14), respectively, resulting in 2621 unique reflections with a completeness of 94.3% to a resolution of 2.1 Å. The unit cell dimensions were determined to be a = b = 37.81 Å c = 63.02 Å in the tetragonal space group P43212. The structure was solved by the molecular replacement methods with AMoRe (15), using as starting model the coordinates of the complex MAR70–d(CGATCG) (C.Temperini, M.Cirilli, A.Ettorre and G.Ughett, unpublished data). Rigid body refinement of the best rotation-translation solution yielded an R value of 43.5% and a correlation coefficient of 0.749. The refinement of the structure was carried out with the program NUCLSQ (16). Electron density maps (2Fo – Fc) and (Fo – Fc), calculated with the program CNS (17) and displayed using the graphic program O (18), were used at subsequent stages of the refinement to fit the second sugar ring of the model and to locate solvent molecules. The structure was refined to a final R factor of 22.4%, R-free 23.3%, for 2321 reflections at Fo > 2σ (Fo) in the resolution range 8.0–2.1 Å with an rms deviation from standard geometry of 0.013 Å in bond lengths and 1.1° in angles. In the asymmetric unit there are two DNA strands forming one duplex, two bound drugs and 35 solvent molecules. The temperature factors are in the range of 16– 30 Å2 for DNA base pairs and drug aglycones, 23–39 Å2 for DNA backbone and drug glycosides side chains. Neither ions nor spermine molecules could be identified. Data collection statistics and refinement parameters are summarised in Table 1. Torsion angles and geometrical DNA helical parameters are given in Table S1, Supplementary Material. The coordinates and structure factors have been deposited with the Nucleic Acid Database: NDB ID DD0054, PDB ID 1NAB, RCSB ID RCSB017715.
Table 1. Crystallographic parameters and refinement statistics.
| Parameter | Value |
|---|---|
| X-ray source | Synchroton radiation |
| Wavelength (Å) | 1.03 |
| Cell Parameters | a = b = 37.81 Å; c = 63.02 Å |
| α = β = γ = 90° | |
| Space group | P43212 |
| No. of unique reflections | 2621 |
| Completeness (%) | 95.4 (75.6) |
| No. of reflections [> 2σ (Fo)] | 2321 |
| <I/σ(I)>a | 5.4 (1.37) |
| Resolution range (Å) | 8–2.15 |
| R-merge (%)b | 11.8 (53.1) |
| R-factor (%) | 22.4 |
| R-free (%)c | 23.3 |
| Rmsd of bonds from ideality (Å) | 0.013 |
aValues in parenthesis relate to the highest resolution shell (2.17–2.15 Å).
bR-merge = Σ |I – <I>| /Σ<I>.
cCalculated using 10% of data.
RESULTS AND DISCUSSION
Binding assay. In a previous study we analysed the interactions of MEN 10755 with calf thymus DNA in solution. Spectrophotometric and fluorescence titrations of MEN 10755 with calf thymus DNA revealed spectral patterns very similar to those obtained with doxorubicin implying that the binding mechanism and the stability of the resulting complexes are nearly the same (19).
Thus, spectrophotometric and fluorescence studies were repeated to characterise the interaction of MEN 10755 with the d(CGATCG) hexamer (C.Temperini, M.Cirilli, A.Ettorre and G.Ughett, unpublished results). These studies point out that MEN 10755 binds tightly to the oligonucleotide; complex formation results in large hypochromic effects of the characteristic anthracycline visible bands analogous to doxorubicin. Characteristic quenching of the intrinsic fluorescence of MEN 10755 is observed as well.
Molecular structure of the complex. The asymmetric unit of the crystal structure contains one duplex of the self-complementary hexamer d(CGATCG), that here we denote from C1 to G6 in one strand and from C7 to G12 in the complementary strand, and two drug molecules, D13 and D14. Therefore the two strands are not in symmetrycally related positions. However, despite this non-equivalence, the DNA molecule retains the symmetry of the self-complementary DNA. This is not true for the drug molecules that appear to have a different conformation.
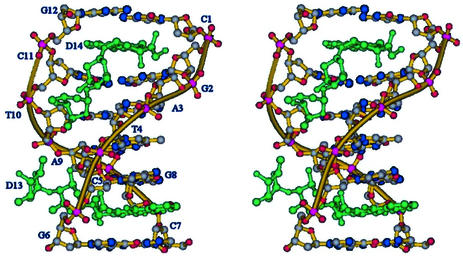
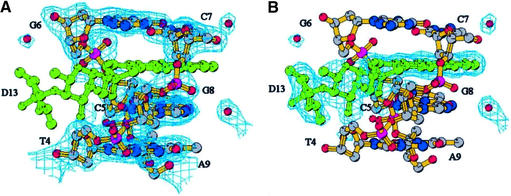
The overall structure of the complex is similar to that of the other complexes of anthracyclines with DNA hexamers (Fig. 2). Intercalation occurs at each end of the DNA duplex in the CpG steps with the drug chromophore perpendicular to the long axis of stacked base pairs. The drug–DNA complex has a distorted B-type helical conformation. There are large buckles associated with the base pairs above and below the intercalator and a small unwinding in the helical twist next to the intercalation site. DNA-backbone distortions, to accommodate the drug molecule, and DNA helical parameters do not differ substantially from those observed in other anthracycline–DNA complexes (5). However, the two binding sites show large differences. In one site both sugar rings lie in the minor groove while in the other site the second sugar of the disaccharide chain protrudes outside the double helix into the solvent region. MEN 10755 lacks the 4-methoxy group on the aglycone moiety, like the anthracycline analogue idarubicin. The other differences with respect to doxorubicin (Fig. 1) are the substitution of the amino group for a hydroxyl group on the 3′ position and the presence of a second daunosamine linked to the 4′ position, giving rise to a 2-deoxy-fucosyl-4′-daunosamyl disaccharide residue. The mode of intercalation of MEN 10755 is not affected by the removal of the 4-methoxy group, as in this complex the orientation of the chromophore between the base pairs in both sites is almost identical to that observed in other anthracycline–DNA complexes. The 3′ amino group on daunosamine has been found to form hydrogen bonds with the DNA molecule in the minor groove, directly or through bridging water molecules, depending on the sequence context (6). This group is also involved in the formation of formaldehyde-mediated cross-links with N2 of a guanine or a 2-aminoadenine residue (21,22). Its substitution with a hydroxyl group in MEN 10755 results in less tight interactions in the minor groove that may affect the orientation of the sugar moiety. The arrangement of the disaccharide chain of the drug intercalated at C5pG6/C7pG8 is such that the first sugar, directly linked to the aglycone, lies in the minor groove. However, with respect to the position observed in doxorubicin complexes, a rotation of ∼45°around the O7-C1′ bond brings it away from the base pairs. The sugar is in chair conformation as in doxorubicin complexes, with the hydroxyl group in the 3′ position still in van der Waals contact with the guanine residue. As a result the second sugar protrudes out into the solvent region where the amino and the hydroxyl groups interact with two water molecules. In addition the amino group forms strong hydrogen bonds with a guanine residue of symmetry related DNA chain (see below). The electron density of a composite omit map around this region is depicted in Figure 3. On the contrary, the disaccharide moiety of the drug intercalated at the C1pG2/C11pG12 step lies entirely in the minor groove. A similar arrangement was also observed in the crystal structure of the complexes formed by the anthracycline disaccharide MAR70 with the DNA hexamers d(CGATCG) (C.Temperini, M.Cirilli, A.Ettorre and G.Ughett, unpublished data) and d(CGTACG) (21). In this site the disaccharide chain does not form any direct interaction with the DNA molecule. The second sugar is in the boat conformation while the fucose ring is in the chair conformation with the hydroxyl group on the 3′ position in van der Waals contact with a cytosine residue.
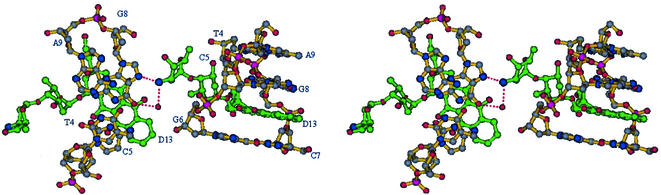
Figure 2.
A stereo view of the crystal structure of the MEN 10755–d(CGATCG) complex. In the DNA molecule the nitrogen atoms are blue, the oxygen atoms are red, the phosphorous atoms are purple and the carbon atoms are grey. The drug molecule is green. Stick and ribbon representation is from Bobscript (20).
Figure 3.
Composite omit map at 1.0 σ covering the part of the complex where the drug interacts with a second DNA molecule: (A) displayed around the DNA molecule, (B) displayed around the drug molecule. The map was obtained by accumulation of partial maps calculated with the program CNS (17) omitting 5% of the structure. Representation program and atom colours are as in Figure 2.
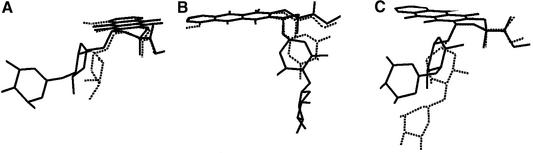
In both sites the glicosyl linkage between the two sugars adopts the preferred conformation observed in disaccharide chains. The torsion angles around C4′-O4′ and O4′-C1* are within the range most frequently observed in disaccharide structures, in a way that makes the two sugars nearly perpendicular to each other (21). The differences in the orientation of the sugar moiety observed in this complex with respect to doxorubicin and between the two MEN 10755 molecules are illustrated in Figure 4.
Figure 4.
Comparison of the conformations of MEN 10755 in this complex with that of doxorubicin in the complex with d(CGATCG)2 (6). (A) Doxorubicin (dashed lines) and MEN 10755 intercalated at the C1pG2 step (filled lines). (B) Doxorubicin (dashed lines) and MEN 10755 intercalated at the C5pG6 step (filled lines). (C) MEN 10755 intercalated at the C1pG2 step (dashed lines) and at the C5pG6 step (filled lines). The drug molecules are superimposed by least square fitting of the common atoms on the chromophore.
Crystal packing and interaction between duplexes. In the crystal lattice the drug–DNA complexes are stacked end-over-end to form continuous pseudo-helices. In the most frequent packing arrangement observed for the crystallised anthracycline– DNA complexes the columns formed by the stacked duplexes run parallel to each other, and there are direct or water-mediated intermolecular hydrogen bonds involving the phosphate backbone. Here the complex is oriented in the cell so that the helix axis of the duplex is aligned along the diagonal of the a-b plane, and the columns formed by the pseudo-helices do not run parallel but are crossing each other at right angles along the tetragonal c-axis. This leads to the formation of two layers of stacked duplexes mutually perpendicular to one another (Fig. S1, Supplementary Material). An arrangement of this type results in fewer side to side packing interactions, and was also observed in the crystal structures of nogalamycin (23) and WP401 (24) complexed with DNA hexamers. The packing contacts occur in the region where the complexes are end-to-end stacked. The duplexes associate in the packing where the minor groove faces the major groove of the crossing complex. The interactions in this region involve three unique direct intermolecular hydrogen bonds. A phosphate oxygen of the T10pC11 sequence is hydrogen bonded to the terminal O3′ of the symmetry related G12 (1/2 + x, 1/2 – y, 1/4 – z). The other two hydrogen bonds involve the drug molecule. The hydroxyl group O14 on the aglycon moiety is hydrogen bonded to the phosphate oxygen of C7pG8 (1/2 + x, 1/2 – y, 1/4 – z) and the N3 amino group on the second sugar is directly hydrogen bonded to N7 and through a bridging water to O6 of the symmetry related guanine G8 (1/2 + y, 1/2 – x, 1/4 + z) (Fig. 5). Therefore a tight interaction takes place between the amino group of the disaccharide moiety and a second DNA molecule, different from that where the drug is intercalated. To our knowledge this is the first structure of an anthracycline–DNA complex where a hydrogen bond is formed between the anthracycline and a second DNA helix. These tight contacts are likely to have produced the unusual packing observed here. There is only one other anthracycline disaccharide, MAR70 that has been previously co-crystallised with a DNA sequence (20). The crystals in this case adopt the ‘normal’ tetragonal lattice with the parallel alignment of the DNA helices, and in the complex both sugar rings lie in the minor grove. The presence in MAR70, as in natural disaccharide anthracyclines, of an amino sugar in the first carbohydrate moiety directly linked to the chromophore, can account for the different structural behaviour. A number of studies have shown the important role of the amino group at the 3′ position of the sugar moiety for drug DNA binding affinity and antitumor activity (9). In the crystallised anthracycline–DNA complexes the conformation of the amino sugar has been found to vary from structure to structure, depending on DNA sequence. In these structures, the charged amino group has always been observed approaching the edges of base pairs contributing directly or via water mediated hydrogen bonding to the stabilisation of the sugar moiety in the minor groove. Analogues bearing the substitution of the charged amino group for a hydroxyl group, exhibit appreciably reduced DNA binding affinity, yet retain a comparable cytotoxic and antitumor activity (25). In MEN 10755 this substitution has the effect of weakening the interactions in the minor groove and makes the disaccharide moiety less tightly bound and more flexible. As a result we observe in this crystal structure that the first sugar in the disaccharide moiety can lie in two different positions, flipping from the usual position in the minor groove to a position shifted away from the base pairs. This shift in the binding site at the C5pG6 step permits the second sugar to protrude out enough from the helix to interact strongly with a second DNA duplex. The amino group seems to be the critical substituent, as the strong hydrogen bonds that it forms when located on the second sugar balance the interactions lost in the minor groove. Two well ordered tetra-coordinated water molecules occupy the cavity created by the shifting of the fucose moiety and form a network of hydrogen bonds in the minor groove of the T4pC5/A9pT10 sequence. In the other binding site both sugars fill the minor groove and the base pairs are not accessible to solvent molecules.
Figure 5.
A stereo close up view of the packing interactions between the second sugar ring of the drug bound to one duplex and the symmetry-related duplexes (1/2 – y, 1/2 + x, 3/4 + z). The dotted lines represent hydrogen bonds. Representation program and atom colours are as in Figure 2.
CONCLUSION
Our results reveal that binding of MEN 10755 to d(CGATCG)2 is characterised by the classical features of anthracycline–DNA interactions. Thus, the substitution at the N3′ position of the amino sugar and the presence of a second sugar ring does not strongly influence DNA binding. This is in agreement with observations from monosaccharide anthracyclines where the substitution of the amino group with a hydroxyl group does not have important consequences for drug cytotoxicity and antitumor activity. The first sugar moiety of MEN 10755 lies in the minor groove like in the other anthracycline–DNA complexes. The slightly different conformation observed in one site, especially on the O7-C1′ torsion angle, is mainly dictated by the interactions of the second sugar ring. Two different binding modes arise from the spatial arrangement of the second sugar moiety. It is more flexible than the first one and can either lie in the minor groove or protrude out into the solvent region, interacting with a second symmetry-related DNA molecule through hydrogen bonds. This peculiar behaviour suggests that the second sugar is not strictly required for DNA binding; in contrast, it may govern the interactions with other cellular targets such as topoisomerases. All substituents in this moiety are accessible and may have a critical influence on the formation of the ternary complex or on the sequence specificity of DNA cleavage. On the basis of this structural study and due to the lack of detailed knowledge of the ternary complex structure, it is not possible to identify which positions may be important for more favourable modifications. However, it appears that disaccharide anthracyclines offer a wider range of options in designing new drugs, as the possible substitutions on the second sugar moiety are not expected to affect DNA binding while affecting the potential interactions with topoisomerases or other cellular targets.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENT
We thank Antonello Ranieri for his helpful technical support.
NDB no. DD0054, PDB no. 1NAB, RCSB no. RCSB017715
REFERENCES
- 1.Arcamone F. (1981) Doxorubicin Anticancer Antibiotics. Academic Press, New York, NY.
- 2.Arcamone F. (1998) From the pigments of the actinomycetes to third generation antitumor anthracyclines. Biochimie, 80, 201–206. [DOI] [PubMed] [Google Scholar]
- 3.Wang A.H.J. (1992) Intercalative drug binding to DNA. Curr. Opin. Struct. Biol., 2, 361–368. [Google Scholar]
- 4.Lown J.W. (ed.) (1988) Anthracycline and Anthracenedione-based Anticancer Agents. Elsevier, Amsterdam, The Netherlands.
- 5.Berman H.M., Olson,W.K., Beveridge,D.L., Westbrook,J., Gelbin,A., Demeny,T., Hsich,S.H., Srinivasan,A.R. and Schneider,B. (1992) The nucleic acid database: a comprehensive relational database of three-dimensional structure of nucleic acids. Biophys. J., 63, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frederick C.A., Williams,L.D., Ughetto,G., van der Marel,G.A., van Boom,J.H., Rich,A. and Wang,A.H.J. (1990) The structural comparison of anticancer drug–DNA complexes: adriamycin and daunomycin. Biochemistry, 29, 2538–2549. [PubMed] [Google Scholar]
- 7.Chaires J.B., Herrera,J.E. and Waring,J.M. (1990) Preferential binding of daunomycin to 5′ at CG and 5′ at GC sequences revealed by footprinting titration experiments. Biochemistry, 29, 6145–6153. [DOI] [PubMed] [Google Scholar]
- 8.Tewey K.M., Rowe,T.C., Yang,L., Halligan,B.D. and Liu,L.F. (1984) Adriamycin induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 226, 466–468. [DOI] [PubMed] [Google Scholar]
- 9.Zunino F. and Capranico,G. (1990) DNA topoisomerase II as the primary target of anti-tumor anthracyclines. Anticancer Drug Des., 34, 307–317. [PubMed] [Google Scholar]
- 10.Gerwitz D.A. (1992) A critical evaluation of the mechanism of action proposed for the antitumor effects of the anthracyclines antibiotics adriamycin and daunorubicin. Biochem. Pharmacol., 57, 727–741. [DOI] [PubMed] [Google Scholar]
- 11.Animati F., Arcamone,F., Berettoni,M., Cipollone,A., Franciotti,M. and Lombardi,P. (1996) New anthracycline disaccharides. Synthesis of l-daunosaminyl-α(1→4)-2-deoxy-l-rhamnosyl and of l-daunosaminyl-α(1→4)-2-deoxy-l-fucosyl daunorubicin analogues. J. Chem. Soc. Perkin Trans. I, 1327–1329. [Google Scholar]
- 12.Arcamone F., Animati,F., Berettoni,M., Bigioni,M., Capranico,G., Casazza,AM., Caserini,C., Cipollone,A., De Cesare,M., Franciotti,M., Lombardi,P., Madami,A., Manzini,S., Monteagudo,E., Polizzi,D., Pratesi,G., Righetti,S.C., Salvatore,C., Supino,R. and Zunino,F. (1997) Doxorubicin disaccharide analogue: apoptosis-related improvement of efficacy in vivo. J. Natl Cancer Inst., 89, 1217–1223. [DOI] [PubMed] [Google Scholar]
- 13.Pratesi G., De Cesare,M., Caserini,C., Perego,P., Dal Bo,L., Polizzi,P., Supino,R., Bigioni.M., Manzini,S., Iafrate,E., Salvatore,C., Casazza,A., Arcamone,F. and Zunino,F. (1998) Improved efficacy and enlarged spectrum of activity of a novel anthracycline disaccharide analogue of doxorubicin against human tumor xenografts. Clin Cancer Res., 4, 2833–2839. [PubMed] [Google Scholar]
- 14.Otwinowski Z. (1993) DENZO: An Oscillation Data Processing Program for Macromolecular Crystallography. Yale University Press, New Haven, CT.
- 15.Navaza J. (1994) An automatic package for molecular replacement. Acta Crystallogr. A, 50, 157–163. [Google Scholar]
- 16.Westhof E., Dumas,P. and Moras,D. (1985) Crystallographic refinement of yeast aspartic acid transfer RNA. J. Mol. Biol., 184, 119–145. [DOI] [PubMed] [Google Scholar]
- 17.Brunger A.T., Adams,P.D., Clore,G.M., Delano,W.L., Gros,P., Grosse-Kunsteleve,R.W., Fiang,J., Kuszewsky,J., Niles,M., Pannu,N.S., Read,R.J., Rice,L.M., Simmonson,T. and Warren,G.L. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 18.Jones T.A., Zhou,J.Y., Cowan,S.W. and Kjeldoard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- 19.Messori L., Temperini,C., Piccioli,F., Orioli,P., Di Bugno,C. and Animati,F. (2001) Solution chemistry and DNA binding properties of MEN 10755, a novel disaccharide analogue of doxorubicin. Bioorg. Med. Chem., 9, 938–941. [DOI] [PubMed] [Google Scholar]
- 20.Esnouf R.M. (1999) Further addition to Molscript, version 1.4, including reading and contouring of electron density map. Acta Crystallogr. D, 55, 938–941. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y., Liaw,Y., Li,Y., van der Marel,G.A., van Boom,J.H. and Wang,A.H.J. (1991) Facile formation of a crosslinked adduct between DNA and the daunorubicin derivative MAR70 mediated by formaldehyde: Molecular structure of the MAR70-d(CGTnACG) covalent adduct. Proc. Natl Acad. Sci. USA, 88, 4845–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A.H.J., Gao,Y., Liaw,Y. and Li,Y. (1991) Formaldehyde cross-links daunorubicin and DNA efficiently: HPLC and X-ray diffraction studies. Biochemistry, 30, 3812–3815. [DOI] [PubMed] [Google Scholar]
- 23.Smith C.K., Davies,G.J., Dodson,E.J. and Moore,M.H. (1995) DNA–nogalamycin interactions: the crystal structure of d(TGATCA) complexed with nogalamycin. Biochemistry, 34, 415–425. [DOI] [PubMed] [Google Scholar]
- 24.Dutta R., Gao,Y., Priebe,W. and Wang,A.H.J. (1998) Binding of the modified daunorubicin WP401 adjacent to a T-G base pair induces Watson–Crick conformation: crystal structures of the WP401-TGGCCG and WP401-CGG[br5C]CG complexes. Nucleic Acids Res., 26, 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zunino F., Pratesi,G. and Perego,P. (2001) Role of the sugar moiety in the pharmacological activity of anthracyclines: development of a novel series of disaccharide analogs. Biochem. Pharmacol., 61, 933–938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.