Abstract
The binary Cre-lox conditional knockout system requires an essential part of the target gene to be flanked by loxP sites, enabling excision in vivo upon Cre expression. LoxP sites are introduced by homologous recombination, together with a selectable marker. However, this marker can disturb gene expression and should be removed. The marker is therefore often prepared with a third, flanking loxP site (tri-lox construct), facilitating its selective removal by partial Cre-lox recombination. We have shown that this excision can be achieved in vivo in the germline using EIIaCre transgenic mice, and have described the advantages of in vivo over in vitro removal. We show here that MeuCre40, a new transgenic mouse, more reliably and reproducibly generates an optimal partial mosaic Cre-lox recombination pattern in the early embryo. This mosaicism was transmitted to the germline and to many other tissues. Alleles with partial deletions, in particular floxed alleles from which the selectable marker was removed, were readily recovered in the next generation, after segregation from the transgene. Segregation via paternal or maternal transmission led to successful recovery of the alleles of interest. We also obtained total deletion of the floxed regions in the same experiment, making this transgene a polyvalent Cre-lox tool. We rigorously tested the ability of MeuCre40 to solve tri-lox problems, by using it for the in vivo removal of neoR- and hprt-expression cassettes from three different tri-lox mutants.
INTRODUCTION
Conditional gene targeting is increasingly used to create loss-of-function mouse models, and a standard approach involving the Cre-lox recombination system has emerged [1,2, see also Genesis, special issue February 2002, 32(2)]. This approach involves creating a floxed conditional allele, designed to remain functionally equivalent to the wild-type, and then inactivating it in vivo in a cell type-specific and temporally controlled fashion, using transgenes expressing Cre recombinase (3,4). For this, an essential part of the gene, one or several exons in general, is flanked with loxP sites by homologous recombination. It is however necessary to ensure that the loxP sites do not interfere with the wild-type pattern of gene expression. Current technology for homologous recombination in embryonic stem (ES) cells requires the concomitant insertion of selectable markers—expression cassettes conferring e.g. antibiotic resistance such as neoR, hygR or puroR—into the target gene. These cassettes contain strong promoters and prokaryotic DNA and their presence can disturb the expression of the targeted and sometimes also surrounding genes in unpredictable ways. These cassettes may therefore lead to hypomorphic phenotypes (5–8). To prevent the possibility of unwanted effects on target genes the removal of the selectable marker is recommended in state-of-the-art conditional gene knockout (1). Existing strategies for removal of the marker include the elegant, yet not very often used double replacement knock-in procedure, where the markers are removed via a second homologous recombination event (9,10). Or the removal of the marker is implemented, like conditional inactivation of the gene itself, using site-specific recombination (4,11,12). Whether based on the use of an additional loxP site (4,13) (tri-lox strategy), or nesting the Cre-lox with the analogous Flp-FRT recombination system (14,15), these latter strategies are simple to realize at the level of the targeting construct. However, in the often-used tri-lox strategy, partial removal is not yet straightforward. This is particularly surprising because gene targeting in ES cells, clonal selection, mutant identification and chimera production by microinjection, are all well established, efficient procedures (16).
Originally, typical tri-lox configurations (Fig. 1A) were converted into final floxed conditional alleles by the transient transfection of targeted ES cell clones in vitro with Cre-expressing plasmids, prior to chimera production (11,13,17). The difficulties associated with this approach were summarized by Kaartinen and Nagy (18), who proposed an adenovirus-based alternative. We and others have shown that it is possible and advantageous to eliminate the floxed marker fragment selectively in vivo, using the recombinase expression of EIIaCre transgenic mice (19,20). EIIaCre transgenic mice produce the necessary partial Cre-lox recombinations, but two drawbacks were encountered in the use of these mice (19). The first was variation in the degree of Cre-lox recombination between individuals. This variation makes it necessary to produce a larger number of double transgenic mice, and to select a mouse with an optimal level of mosaicism for segregation breeding. The second problem is that females express EIIaCre strongly in the oocytes, resulting in the excision of all floxed segments in these cells (19). Thus, only male mosaic double transgenics can be used for segregation breeding, resulting in only a small fraction of the initially screened mice being useful mosaics. These problems led us to screen other transgenic mice, with a view to identifying a strain with a higher level of performance.
Figure 1.
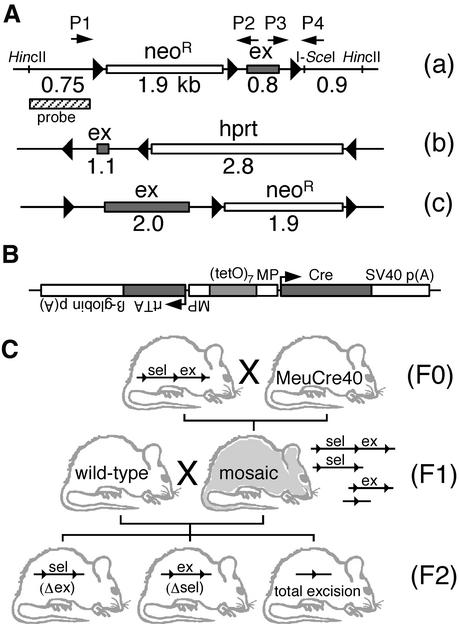
Tri-lox alleles, transgene construction and breeding strategy. (A) Three tri-lox configurations concerning the genes encoding IGF-1R (a), APC (b) and Doublecortin (c) were tested. Triangles indicate loxP sites, white boxes indicate selectable expression cassettes and shaded boxes indicate exons (ex). The probe and restriction sites used for Southern analysis of the IGF-1Rtri-lox alleles are indicated; neoR designates the neomycin resistance expression cassette, hprt designates the hypoxanthine phosphoribosyl transferase mini-gene (13). The I-SceI meganuclease site was introduced together with the loxP sites. Arrows indicate primers P1–P4 (see Materials and Methods for details). The size of the floxed exonic regions and of the floxed cassettes is indicated in kilobases without taking into account the small loxP insertions. (B) The DNA construct used to generate MeuCre40 had a total size of 4.3 kb. Cre, wild-type Cre cDNA; MP, minimal CMV promoter; p(A), polyadenylation signal; rtTA, reverse tetracycline-dependent transactivator (26); tetO, tetracycline resistance operon; arrows indicate transcription start sites; further details are provided in a previous paper (25). (C) Heterozygous F0 tri-lox mice were bred to obtain double transgenics in the F1 generation, all of which proved to be mosaic. The crossing of these mosaic mice with wild-type mice resulted in the offspring having a 50% probability of inheriting one of the several mutant alleles and a 50% probability of receiving MeuCre40. F2 animals were screened for MeuCre40 and mutant allele segregation. sel, selectable expression cassette; Δsel, deletion of the selectable marker neoR or hprt; Δex, deletion of the floxed exon.
We report here the identification of a new transgenic mouse, MeuCre40, which displayed efficient and reliable partial Cre-lox recombination in all tissues, including the germline. We used the MeuCre40 transgene to remove the selection cassettes from the conditional tri-lox alleles of three different target genes. This enabled us to establish a simple and generally applicable experimental protocol. This transgene may also be useful for other strategies requiring ubiquitous embryonic mosaicism (21–23).
MATERIALS AND METHODS
Transgenic production
Mice with targeted tri-lox conditional alleles (Fig. 1A) were produced at the gene targeting facility of the Institut Cochin in Paris, according to published procedures (16,24). The Cre transgenic DNA construct has been described elsewhere (25). Briefly, a bi-directional promoter was used to control the transcription of Cre and rtTA (Fig. 1B) (26,27). We used standard protocols for pronuclear DNA injection and embryo transplantation (16). Transgenic offspring were identified by PCR and identification was confirmed by Southern blotting. Although the construct was designed to give transgene auto-inducibility in the presence of doxycycline and minimal leakage in its absence, four strains, presented here, displayed a pattern of mosaic, early embryonic, ubiquitous (Meu) Cre expression that was not dependent on nor affected by doxycycline treatment. This pattern of expression probably resulted from non-specific transcriptional activation, through positional effects.
Animals
Tri-lox conditional alleles (Fig. 1A) were present in the 129/Sv genetic background, MeuCre40 transgenic mice (Fig. 1B) in C57Bl/6, and all these mice were heterozygotes. Mice were housed in standard conditions, at 23°C, with a 14/10 h light/dark cycle, and food and water supplied ad libitum. For the partial excision of floxed cassettes, MeuCre40 transgenic mice were mated with mice carrying the IGF-1R, APC and Doublecortin tri-lox alleles. The F1 generation was screened for the presence of MeuCre40 and partial recombination of the tri-lox allele, by Southern blotting and PCR. Mice with partial and mosaic patterns were then mated with wild-type C57Bl/6 mice for segregation of the Cre-recombined alleles from MeuCre40 in the F2 generation. Segregation was monitored by Southern blotting or PCR (breeding scheme in Fig. 1C). For some experiments (see following paragraph), MeuCre40 mice were mated with IGF-1Rflox mice, which carried the final conditional allele with only two loxP sites.
DNA analysis
DNA extraction and Southern analysis have been described elsewhere (19). We extracted DNA for PCR and genotyped Cre transgenes by PCR as previously described (28). Partial recombination of the IGF-1R (29) conditional tri-lox allele was analyzed by Southern blotting and PCR. For PCR, we used four oligonucleotides for the simultaneous detection of wild-type (WT), floxed (flox), selectively removed neoR (Δneo) and totally excised (Δtotal) alleles. The forward oligonucleotide primer P1 (5′-CCATGGGTGTTAAATGTTAATGGC-3′; all primers were from Genset) annealed upstream from the left loxP site (see Fig. 1A), and the reverse primer P4 (5′-ATGAATGCTGGTGAGGGTTGTCTT-3′) annealed downstream from the right loxP site. The forward primer P3 (5′-ATCTTGGAGTGGTGGGTCTGTTTC-3′) annealed upstream from the right loxP-site, and the reverse primer P4 (5′-AGCTGCCCAGGCACTCCG-3′) annealed immediately downstream from the neoR cassette, within the floxed region containing the exon. The expected sizes of the products (corresponding allele and primer pair in parenthesis) were 256 bp (WT, P3P4), 312 bp (flox, P3P4), 204 bp (Δtotal, P1P4), and 362 bp (Δneo, P1P2). The amplification conditions were as follows: 94°C for 5 min for initial strand separation and polymerase activation (Platinum Taq, Invitrogen), then 40 cycles of 94°C, 50°C and 72°C, 30 s each segment, followed by a final 7 min elongation step at 72°C, in an Applied Biosystems 2400 PCR system. DNA fragments larger than 800 bp would not amplify efficiently under these conditions. The APC and Doublecortin tri-lox alleles were analyzed in analogous PCR assays and Southern blot analyses, the details of which can be requested from the authors. The data presented in Figures 2B, C and 4 were obtained using the IGF-1Rflox mutant allele as a di-lox reporter gene, solely to facilitate the semi-quantitative evaluation of PCR results (28).
Figure 2.
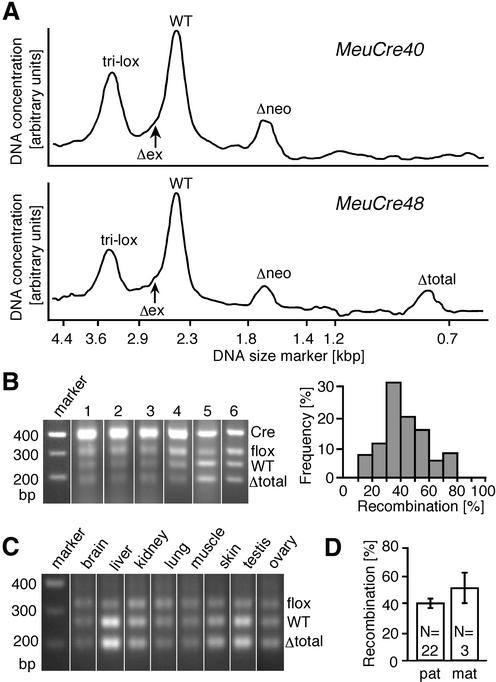
MeuCre40-induced Cre-lox recombination. (A) Scanned Southern blot analysis (double digest HincII/I-SceI; probe as indicated in Fig. 1A) of partial recombination in a MeuCre40/IGF-1Rtri-lox animal showed that Δneo alleles (1.7 kb) were present in considerable quantity compared with unrecombined IGF-1Rtri-lox alleles (3.5 kb) and the corresponding wild-type (WT) IGF-1R allele (2.4 kb). The profile generated by the related strain MeuCre48 showed a shift towards stronger recombination, with fewer Δneo alleles and a peak corresponding to total excisions (Δtotal; 0.8 kb). Signals indicating selective deletions of the floxed exon region (Δex; 2.6 kb) were superimposed over those of the wild-type (arrows), and could not be differentiated in this Southern blot analysis. Nevertheless, the partial Cre-lox excision pattern seen in APC and Doublecortin (data not shown) indicated that the final recombination profile depended to some degree on the particular interaction between MeuCre40 and the respective tri-lox alleles. (B) Partial Cre-lox recombination patterns were assayed by a multiplex PCR that detected relative amounts of unrecombined and recombined (Δtotal) IGF-1R alleles in the same reaction (28). The flox band and the WT band amplified with primer pair P3P4, the Δtotal band amplified with primer pair P1P4 (see Fig. 1A). Shown here for six representative MeuCre40/IGF-1Rflox animals, this test revealed little inter-individual variability, and most double transgenics displayed a recombination of 30–60% (bar graph, n = 25). (C) We observed only minor differences in the Cre-lox recombination pattern between major tissues. Male and female gonads (two lanes on the right) showed similar patterns of recombination to other tissues. (D) We investigated whether maternal (mat) MeuCre40 transmission affected the frequency of recombination, which was not the case (pat, paternal).
Figure 4.
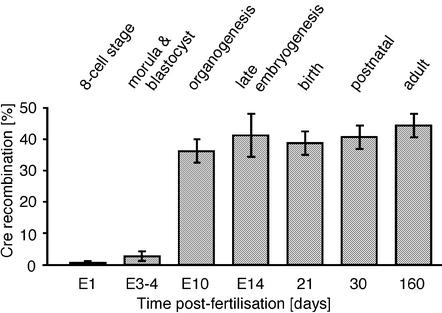
Developmental kinetics of MeuCre40-induced recombination in double transgenic (MeuCre40/IGF-1Rflox) animals. The percentage of recombined IGF-1Rflox reporter gene was determined from the eight-cell stage to the adult animal by a multiplex PCR assay (primers P1, P3 and P4; Fig. 1A) that detected relative amounts of unrecombined versus recombined allelic forms of the floxed IGF-1R gene (28). DNA was extracted from whole embryos, or postnatally from skin biopsies. Recombination was first observed during morula and blastocyst stages. Few changes were observed after E10. No Cre-lox recombination was detected in eight-cell embryos. Error bars indicate the SEM; total n = 52.
For quantification, Southern blots were placed against phosphorimager screens, with detection of the signal by Storm 850 (Molecular Dynamics), and analysis with ImageQuant software. PCR products were separated by electrophoresis in 2% agarose gels pre-stained with ethidium bromide. Video images of the gels, under UV transillumination, were quantitatively analyzed with NIH imaging software as described previously (28).
Histology
LacZ staining was performed according to standard protocols (16). Briefly, parasagittal cryostat sections from newborn mice, double transgenic for Rosa26lacZ (30) and MeuCre40 or MeuCre48, were post-fixed by incubation in 4% paraformaldehyde at 4°C for 10 min, rinsed in detergent solution and stained with X-gal overnight. The sections were then counterstained with 1% orange G, mounted and observed under a light microscope (Olympus BX51) equipped with an electronic camera (Olympus DP50). Rosa26lacZ positive-Cre negative littermates served as negative controls. Newborns double transgenic for Rosa26lacZ and an early embryonic Cre deleter transgene (M.Holzenberger, unpublished strain) were used as positive control.
RESULTS
We obtained seven independent Cre transgenic strains by pronuclear microinjection. We characterized these mice by crossing offspring of the seven strains with floxed reporter mice and screened for Cre-lox recombination by PCR and Southern blotting. Four strains generated mosaic loxP recombination pattern. We then investigated whether partial recombination occurred in these four lines by crossing them with mice with the IGF-1Rtri-lox allele (24). MeuCre44 displayed only a low level of recombination in a subset of tissues, and partial recombination was undetectable (data not shown). MeuCre17 displayed mosaic and partial recombination in all tissues (31), but the degree of individual mosaicism differed considerably between animals, possibly indicating variegated transgene expression. MeuCre40 displayed a particularly high level of partial Cre-lox recombination (Fig. 2A) in a mosaic distribution in all tissues examined. The pattern induced in MeuCre40 was much more reproducible (Fig. 2B) than that in MeuCre17. MeuCre48 displayed a mixture of partial and total Cre-lox recombination (Fig. 2A) in all tissues. Thus, both MeuCre40 and MeuCre48 had the characteristics required for efficient in vivo cassette removal. Further tests showed that MeuCre40 transmitted partial excisions to the next generation at reasonable frequencies, whereas the efficiency of MeuCre48 was much lower. Therefore, in subsequent experiments, we focused on the analysis of the performance of strain MeuCre40. We present here the pattern of MeuCre40-induced loxP recombination in more detail, and then summarize data from experiments in which we solved three tri-lox problems using this transgene.
Spatiotemporal distribution of MeuCre40-induced loxP recombination
We expressed the degree of MeuCre40-induced mosaicism as a frequency distribution, and most individuals were found to have recombination of 30–60% (Fig. 2B). All double transgenic mice were mosaic and potentially useful for the transmission of partial recombination to the F2 progeny. Recombination patterns were analyzed from tail biopsy samples, consisting mostly of skin and several other tissues of different developmental origins. We found that there was little variation between tissues (Fig. 2C), implying that results from skin and tail biopsy samples can indicate the degree of recombination in other tissues of the animal with sufficient accuracy. The ubiquitous pattern also suggested that recombination probably occurred some time very early in embryonic development (see below), and that the mosaicism was thereafter transmitted to all developing tissues and their component cell types. As early embryonic Cre-lox activity may depend on the mode of transgene transmission (19), we compared the pattern of Cre-lox recombination in individuals in which the transgene was paternally or maternally inherited; we observed no significant difference between these two types of mosaic mice (Fig. 2D).
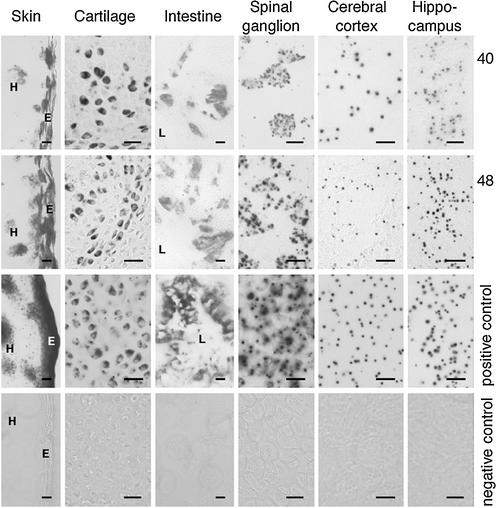
We investigated the distribution of recombinant cells within tissues by crossing MeuCre40 mice with a Cre-lox reporter mouse (30), and carrying out lacZ histological staining in newborn mice. In all tissues with sufficiently high levels of reporter gene expression, recombinant cells were observed to be evenly distributed throughout the tissue or to be present in small clusters (Fig. 3). We noticed that there were differences in reporter gene expression between tissues, which could explain why in several tissues the percentage of LacZ-positive cells differed from the percentage of Cre recombined cells, as determined by Southern blot and PCR using the IGF-1Rlox allele as reporter. Nevertheless, a MeuCre48-induced LacZ pattern indicated that this line produced a slightly more extended recombination compared with MeuCre40, which is in line with the results presented in Figure 2A. These results also suggested that recombination events occurred early in embryonic development, but probably not thereafter. We determined the kinetics of MeuCre40 excision by analyzing double transgenic (MeuCre40/IGF-1Rflox) animals from the eight-cell stage to the adult by a multiplex PCR assay that simultaneously detected relative amounts of unrecombined versus recombined allelic forms of the floxed IGF-1R gene (28) (Fig. 4). The Cre-lox recombination indicated that significant MeuCre40 expression started probably during the morula and the blastocyst stages, and that the degree of recombination varied little after day 10 of embryonic development. It is unclear whether the slight increase in Cre-lox recombination after organogenesis reflects the late action of MeuCre40 or is due to the method used for estimating the recombination.
Figure 3.
Histology of MeuCre40- and MeuCre48-induced loxP recombination. Newborn mice harboring MeuCre40 or MeuCre48 and a reporter gene indicating Cre-lox recombination by lacZ activity (29), were killed and tissue sections stained for lacZ activity. Shown here in six representative tissues, the patterns were clearly mosaic and very similar in the two strains. Black spots indicate Cre-lox recombinant cells. The positive control panels show lacZ activity in sections from newborns with a constitutively activated Rosa26 lacZ reporter gene. The negative control panels contain sections from mice with an unrecombined reporter gene, that produces no staining at all. Negative controls are recorded as phase contrast micrographs. Size bars indicate 20 µm. E, skin epithelium; H, hair follicle; L, intestinal lumen.
Producing partial excisions from tri-lox alleles
We assessed the efficiency of MeuCre40 for generating partial Cre-lox excision and the probability of recovering discrete partial deletions in the next generation. For this the MeuCre40 strain was crossed with mice containing three different target alleles, each corresponding to a particular tri-lox problem (Fig. 1A). Analysis of partial recombination and segregation was performed by three different teams and all three desired mutants were rapidly obtained. Thus, the strategy described was reproducible in various contexts and the performance of MeuCre40 was robust. As the pattern of recombination varied little between the double transgenic F1 mosaics, only a few were required to solve each tri-lox problem (Table 1). We used between one (IGF-1R) and six (Doublecortin) mosaic individuals per gene to produce a small population of F2 mice (Table 1). Among the F2 progeny and without selecting for optimal mosaicism, on average one tenth of the individuals (10 out of 96) was Δneo (or Δhprt) and negative for MeuCre40 (Table 1). We used both male and female F1 mosaic mice for the production of the F2 generation, and found that Δneo alleles were readily recovered, whether segregation occurred in the male or female germline. Thus, supposedly no significant MeuCre40 expression occurred during oogenesis or meiotic cell division, as previously observed for EIIaCre (19,22,32). Among F2 individuals, a significant proportion showed complete excision of the floxed regions (Δtotal, see Table 1), which for the three genes presented here was the equivalent of a classical, constitutive knockout. A number of F2 animals inherited the intact tri-lox allele. Finally, the APC gene illustrated particularly well how important the elimination of the selection cassette can be, because mice heterozygous for the APC tri-lox allele (still with the floxed hprt cassette) developed the same phenotype of intestinal polyposis as the heterozygous classical knockout. This phenotype was reversed after selective elimination of hprt, conditio sine qua non of any subsequent conditional APC knockout (S. Colnot and M. Giovannini, unpublished results).
Table 1. Efficiency of MeuCre40-mediated selection cassette removal from three tri-lox allelesa.
| Target gene | F1 mosaic mice testedb | F2 mice screened | Cre-negative heterozygotes | |||
|---|---|---|---|---|---|---|
| Tri-lox | Δselc | Δexon | Δtotal | |||
| IGF-1R | 1 | 11 | 2 | 1 | 0 | 1 |
| APC | 1 | 38 | 0 | 3 | 0 | 5 |
| Doublecortin | 6 | 47 | 5 | 6 | 0 | 6 |
| Total | 7 | 10 | 0 | 12 | ||
aComparative data for MeuCre48, which produced only one Δsel among 35 F2 mice, and for EIIaCre can be obtained from the authors.
bThe frequency of transmission of MeuCre40 from heterozygotes to their offspring was 56% (n = 78), which is close to the expected Mendelian frequency.
cΔneo in the case of IGF-1R and Doublecortin, Δhprt for APC.
Based on the here reported results we estimate that, for a typical tri-lox problem and using only heterozygous animals, approximately 30 mice (F0, F1 and F2 together) are required for the successful production of Δsel mutants. Two F2 litters of normal size should in most cases be enough to generate Δsel and Δtotal mutants. If F1 mosaic mice are selected for optimal representation of the Δsel allele, this efficiency can be further increased. We backcrossed MeuCre40 with inbred C57Bl/6 and 129/Sv mice, and we currently maintain the transgene in both genetic backgrounds. Maintenance of the original 129/Sv background (from the ES cell lines used for homologous recombination) may be important in some studies, whereas the C57Bl/6 background is preferred for a number of approaches, such as behavioral studies. MeuCre40, available in the C57Bl/6 background, can be used to eliminate the transiently inserted floxed selection cassette and to start (or continue) backcrossing with the C57Bl/6 strain.
DISCUSSION
The transgenic construct used here was designed for doxycycline-dependent Cre-lox recombination. However, four of the seven established lines produced mosaic, ubiquitous recombination that occurred early in embryonic development and was not dependent on doxycycline administration. Cre recombinase, produced from gene constructs under the control of CMV promoters (MeuCre40 contains only the minimal CMV promoter), can generate widespread mosaic recombination (33,34), and has also been reported to induce early embryonic and partial recombination in the germline of mice (see below). The pattern of recombination described here was very useful for the selective excision of tri-lox alleles, an essential step in many conditional Cre-lox projects. We estimated the number of copies of the transgene in each line (data not shown) and found that the degree of recombination depended on copy number, with strains with many copies showing the strongest recombination. MeuCre44, which showed the lowest levels of recombination, had approximately 10 copies; the intermediate MeuCre40 strain had approximately 20 copies, and the strongest mosaic deleter strain, MeuCre48, had approximately 30 copies. The observed pattern of recombination presumably also depends, at least partly, on the site of transgene integration and the ability of this construct to trap non-specific transcriptional activation from nearby regulatory elements.
Although based on no more than circumstantial evidence, it seems to us that partial deletions of tri-lox alleles are strongly biased towards one of the two possible forms, whether EIIaCre (19) or MeuCre40 is used. In the cases presented here the Δsel mutant was predominant, but the contrary may also occur. To efficiently address this important issue, more systematic in vivo experiments are required. The analysis of the present tri-lox alleles indicates so far that the relative and absolute distances between loxP sites, at least for the ranges used here (see Fig. 1A), or the orientation of the loxP sites relative to the order of the floxed regions, are rather unlikely to influence the partial Cre-lox deletion. Proximity to prokaryotic DNA, however, the natural environment for loxP sites, or the nucleotide sequences immediately surrounding the loxP sites may potentially modify the likelihood of recombination. Differences in the susceptibility of loxP sites to recombine should also lead to variable Cre-lox recombination depending on the target gene. This is because recombinase expressed from MeuCre40 must act at a certain threshold of efficiency that assures the mosaic and especially partial recombinations essential for this strategy. Nevertheless, MeuCre40 proved efficient for cassette removal in all three cases presented here.
In vivo marker removal to protect the genetic background of mutations
The proposed in vivo strategy is based on crosses between conditional knockout mice and the MeuCre40 strain, raising important questions about genetic background. Genetic background is known to influence the phenotype of many mutations and must be taken into consideration when planning knockout studies. Homologous recombination experiments in mice are currently performed mostly in ES cells originating from 129 substrains, such as 129/Ola and 129/Sv (35). Once established, targeted mutations may be maintained in the original 129 background, or may be transferred to other genetic backgrounds, such as C57Bl/6, by backcrossing. It is important to ensure that mice used for backcrosses are correctly inbred. Any targeted mutation or transgene, even after backcrossing for 10 or more generations, will contain, around the mutated locus or the transgene integration site, at least several hundred genes that are linked to the locus and correspond genetically to the targeted chromosome or the chromosome into which the transgene originally inserted. Thus, the genetic origin of these sequences is uncertain.
MeuCre40, backcrossed into both the C57Bl/6 and 129/Sv strains, is no exception to this rule. However, it is unlikely that the chromosomal locus containing MeuCre40 ever contaminates the so-treated tri-lox mutant because we selected against MeuCre40 in the F2 generation, and thus against the entire region around its integration site. Segregation breeding should therefore eliminate all foreign DNA of unknown genetic origin linked to MeuCre40. The proposed method is thus neutral from a genetic point of view, and indeed, backcrossing provides an opportunity to eliminate the marker cassette whilst advancing one generation.
Other in vivo and in vitro methods are possible. The alternative partial deletor EIIaCre exists as backcrosses in the C57Bl/6 and 129/Sv backgrounds (see www.jax.org) and therefore, as discussed above, does not present a genetic problem. The alternative in vivo method, involving the adenoviral infection of 16-cell embryos (18), efficiently removes floxed genomic segments, but the possibility of DNA from virus preparations stably integrating into the genome of early embryonic cells cannot be excluded. The problem of inadvertently introduced mutations is even greater in classical protocols. In such protocols, tri-lox targeted ES cell clones are electroporated with Cre expression plasmids. These clones are derived from cells that have already been electroporated with the tri-lox targeting construct. However, whereas Southern screens with internal probes are undertaken after the initial electroporation, to eliminate clones with random integrations, this is generally not done after Cre plasmid electroporation. Circular plasmids are used, but this does not entirely prevent the risk of contamination. Once integrated, it may take several generations of backcrosses to eliminate this material. In the meantime, it introduces genetic and potentially phenotypic heterogeneity into the experimental populations. Moreover, the karyotype of ES cells appears to be intrinsically less stable than that of live mice (36), and ES cell clones may lose their ability to colonize the germline during prolonged maintenance in culture and repeated electroporation. Thus, the in vivo approach proposed here is an original way of efficiently protecting the targeted mutation and its genetic background during the recommended step of selection cassette excision.
As only a few double transgenics are required for this approach, it is possible to directly cross hetero- or homozygous MeuCre40 females with efficient tri-lox chimeras to generate mosaic offspring. One generation later, heterozygous floxed mice are available and can be used for breeding with mice carrying other, tissue-specific, Cre transgenes. Thus, a single additional mouse generation (9–10 weeks) is required to accomplish selection cassette excision.
Alternative transgenic lines
As previously reported, EIIaCre, which contains a viral promoter, can also delete floxed segments from tri-lox alleles, but some limitations must be taken into account if EIIaCre mice are used (19,32,37). These arise from the considerable variability of Cre-lox recombination (obliging to select mice with optimal levels of mosaicism for segregation breeding) and from the fact that EIIaCre is strongly expressed in the oocytes (resulting in the excision of all floxed segments). Hence, using EIIaCre, only certain male mosaics will effectively transmit bi-lox alleles (19). To selectively eliminate a floxed cassette from a tri-lox strain using EIIaCre, close to 100 mice are necessary (19,20). Using MeuCre40, on average 30 mice should be sufficient to accomplish the same task, making this strategy three times more efficient in terms of mice needed. Other Cre transgenic lines expressing from CMV promoters have been successfully employed to selectively delete floxed genomic segments (38–40), and expression from viral promoters thus seems adequate to produce partial Cre-lox recombination events. The ubiquitous nature of MeuCre40- and EIIaCre-induced partial recombination is of value because mosaic individuals can be identified from readily available tail biopsy samples. However, a ubiquitous distribution of partial recombination is not essential, and a pan-endothelial Cre expression has been used recently (41). It would even be sufficient for partial mosaicism to occur in the germline only. Transgenes expressing Cre specifically in the germline have been described, and Sycp1-Cre (42), which expresses Cre during male meiosis, has been used to generate partial excision. Recently, however, the same authors reported that loxP sites were methylated after co-segregation with Sycp1, resulting in the possible permanent loss of function of these sites (43). It may therefore be prudent to use other strains for partial loxP recombination, until this important phenomenon has been investigated further. In contrast, Tcp10bt-Cre, a new transgenic mouse established by the same group, has been used successfully for the selective excision of marker cassettes, yielding two Δsel mice among the 38 F2 mice screened (E. Taillebourg and M. Rassoulzadegan, personal communication). The drawback of using germline-specific Cre-lox recombination is that mosaicism cannot be monitored, so double transgenics must be generated to reveal the presence of partial recombination in the genotype of their offspring, 6 or more weeks later.
In conclusion, we created and characterized a Cre transgenic mouse capable of inducing partial loxP recombination in vivo, and we used this mouse to develop an optimized method highly useful for creating Cre-lox-inducible mutant alleles from tri-lox targeted genes. This approach is a significant amelioration of a formerly cumbersome experimental procedure.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Julie Sappa for expert language revision, Pierre Casanovas, Marie-Cécile Samson and Yoann Saillour for the maintenance of mouse colonies, Christophe Houbron for embryo preparation, and Vincent Abramowski for mouse genotyping. This work was sponsored by INSERM, University of Paris 6, MENRT (M.H.), European Commission (QLG3CT2000-00158), Human Frontier Science Program (RG0283/1999-B), Ligue Nationale Française contre le Cancer (M.G.) and Association pour la Recherche sur le Cancer (M.G.).
REFERENCES
- 1.Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. [DOI] [PubMed] [Google Scholar]
- 2.Kwan K.M. (2002) Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis, 32, 49–62. [DOI] [PubMed] [Google Scholar]
- 3.Nagy A. (2000) Cre recombinase: the universal reagent for genome tailoring. Genesis, 26, 99–109. [PubMed] [Google Scholar]
- 4.Kulkarni R.N., Holzenberger,M., Shih,D.Q., Ozcan,U., Stoffel,M., Magnuson,M.A. and Kahn,C.R. (2002) β-cell-specific deletion of the IGF-1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nature Genet., 31, 111–115. [DOI] [PubMed] [Google Scholar]
- 5.Jacks T., Shih,T.S., Schmitt,E.M., Bronson,R.T., Bernards,A. and Weinberg,R.A. (1994) Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genet., 7, 353–361. [DOI] [PubMed] [Google Scholar]
- 6.Meyers E.N., Lewandoski,M. and Martin,G.R. (1998) An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nature Genet., 18, 136–141. [DOI] [PubMed] [Google Scholar]
- 7.Nagy A., Moens,C., Ivanyi,E., Pawling,J., Gertsenstein,M., Hadjantonakis,A.K., Pirity,M. and Rossant,J. (1998) Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr. Biol., 8, 661–664. [DOI] [PubMed] [Google Scholar]
- 8.Fiering S., Bender,M.A. and Groudine,M. (1999) Analysis of mammalian cis-regulatory DNA elements by homologous recombination. Methods Enzymol., 306, 42–66. [DOI] [PubMed] [Google Scholar]
- 9.Gschwind M. and Huber,G. (1998) Introduction of hereditary disease-associated mutations into the beta-amyloid precursor protein gene of mouse embryonic stem cells: a comparison of homologous recombination methods. Mol. Cell. Biol., 18, 4651–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson P., Smith,S.N., Webb,S., Kilanowski,F.M., Campbell,I.J., Taylor,M.S., Porteous,D.J., Willemsen,R., de Jonge,H.R., Farley,R. et al. (2002) The severe G480C cystic fibrosis mutation, when replicated in the mouse, demonstrates mistrafficking, normal survival and organ-specific bioelectrics. Hum. Mol. Genet., 11, 243–251. [DOI] [PubMed] [Google Scholar]
- 11.Tronche F., Kellendonk,C., Kretz,O., Gass,P., Anlag,K., Orban,P.C., Bock,R., Klein,R. and Schütz,G. (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genet., 23, 99–103. [DOI] [PubMed] [Google Scholar]
- 12.Mantamadiotis T., Lemberger,T., Bleckmann,S.C., Kern,H., Kretz,O., Martin Villalba,A., Tronche,F., Kellendonk,C., Gau,D., Kapfhammer,J. et al. (2002) Disruption of CREB function in brain leads to neurodegeneration. Nature Genet., 31, 47–54. [DOI] [PubMed] [Google Scholar]
- 13.Giovannini M., Robanus-Maandag,E., van der Valk,M., Niwa-Kawakita,M., Abramowski,V., Goutebroze,L., Woodruff,J.M., Berns,A. and Thomas,G. (2000) Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev., 14, 1617–1630. [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X., Lewandoski,M., Meyers,E.N., Liu,Y.H., Maxson,R.E.,Jr and Martin,G.R. (2000) Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nature Genet., 25, 83–86. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez C.I., Buchholz,F., Galloway,J., Sequerra,R., Kasper,J., Ayala,R., Stewart,A.F. and Dymecki,S.M. (2000) High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nature Genet., 25, 139–140. [DOI] [PubMed] [Google Scholar]
- 16.Hogan B., Beddington,R., Constantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo, 2nd Edn. CSHL Press, Cold Spring Harbor, NY, pp. 296–298.
- 17.Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- 18.Kaartinen V. and Nagy,A. (2001) Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis, 31, 126–129. [DOI] [PubMed] [Google Scholar]
- 19.Holzenberger M., Lenzner,C., Leneuve,P., Zaoui,R., Hamard,G., Vaulont,S. and Le Bouc,Y. (2000) Cre-mediated germ-line mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res., 28, e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Li,C., Garret-Beal,L., Larson,D., Wynshaw-Boris,A. and Deng,C.X. (2001) Direct removal in the mouse of a floxed neo gene from a three-loxP conditional knockout allele by two novel approaches. Genesis, 30, 1–6. [DOI] [PubMed] [Google Scholar]
- 21.Tremml G., Dominguez,C., Rosti,V., Zhang,Z., Pandolfi,P.P., Keller,P. and Bessler,M. (1999) Increased sensitivity to complement and a decreased red blood cell life span in mice mosaic for a nonfunctional Piga gene. Blood, 94, 2945–2954. [PubMed] [Google Scholar]
- 22.Keller P., Tremml,G., Rosti,V. and Bessler,M. (1999) X inactivation and somatic cell selection rescue female mice carrying a Piga-null mutation. Proc. Natl Acad. Sci. USA, 96, 7479–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J.L., Grinberg,A., Westphal,H., Sauer,B., Accili,D., Karas,M. and LeRoith,D. (1998) Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol. Endocrinol., 12, 1452–1462. [DOI] [PubMed] [Google Scholar]
- 24.Holzenberger M., Leneuve,P., Hamard,G., Ducos,B., Périn,L., Binoux,M. and Le Bouc,Y. (2000) A targeted partial invalidation of the insulin-like growth factor I receptor gene in mice causes a postnatal growth deficit. Endocrinology, 141, 2557–2566. [DOI] [PubMed] [Google Scholar]
- 25.Holzenberger M., Zaoui,R., Leneuve,P., Hamard,G. and Le Bouc,Y. (2000) Ubiquitous postnatal LoxP recombination using a doxycycline auto-inducible Cre-transgene (DAI-Cre). Genesis, 26, 157–159. [PubMed] [Google Scholar]
- 26.Gossen M., Freundlieb,S., Bender,G., Müller,G., Hillen,W. and Bujard,H. (1995) Transcriptional activation by tetracyclines in mammalian cells. Science, 268, 1766–1769. [DOI] [PubMed] [Google Scholar]
- 27.Baron U., Freundlieb,S., Gossen,M. and Bujard,H. (1995) Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res., 23, 3605–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leneuve P., Zaoui,R., Monget,P., Le Bouc,Y. and Holzenberger,M. (2001) Genotyping of Cre-lox mice and detection of tissue-specific recombination by multiplex PCR. Biotechniques, 31, 1156–1162. [DOI] [PubMed] [Google Scholar]
- 29.Blakesley V.A., Butler,A.A., Koval,A.P., Okubo,Y. and Le Roith,D. (1999) IGF-I receptor function: transducing the IGF-I signal into intracellular events. In Rosenfeld,R.and Roberts,C.,Jr (eds), The IGF System. Humana Press, NJ, USA, pp. 143–164.
- 30.Mao X., Fujiwara,Y. and Orkin,S.H. (1999) Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl Acad. Sci. USA, 96, 5037–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzenberger M., Hamard,G., Zaoui,R., Leneuve,P., Ducos,B., Beccavin,C., Périn,L. and Le Bouc,Y. (2001) IGF-I receptor gene dosage generates a sexually dimorphic pattern of organ-specific growth deficits, affecting fat tissue in particular. Endocrinology, 142, 4469–4478. [DOI] [PubMed] [Google Scholar]
- 32.Williams-Simons L. and Westphal,H. (1999) EIIaCre—utility of a general deleter strain. Transgenic Res., 8, 253–254. [DOI] [PubMed] [Google Scholar]
- 33.Feil R., Brocard,J., Mascrez,B., Lemeur,M., Metzger,D. and Chambon,P. (1996) Ligand-activated site-specific recombination in mice. Proc. Natl Acad. Sci. USA, 93, 10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baskar J.F., Smith,P.P., Ciment,G.S., Hoffmann,S., Tucker,C., Tenney,D.J., Colberg-Poley,A.M., Nelson,J.A. and Ghazal,P. (1996) Developmental analysis of the cytomegalovirus enhancer in transgenic animals. J. Virol., 70, 3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson E.M., Linder,C.C., Sargent,E.E., Davisson,M.T., Mobraaten,L.E. and Sharp,J.J. (1997) Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nature Genet., 16, 19–27. [DOI] [PubMed] [Google Scholar]
- 36.Eggan K., Rode,A., Jentsch,I., Samuel,C., Hennek,T., Tintrup,H., Zevnik,B., Erwin,J., Loring,J., Jackson-Grusby,L. et al. (2002) Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat. Biotechnol., 20, 455–459. [DOI] [PubMed] [Google Scholar]
- 37.Lakso M., Pichel,J.G., Gorman,J.R., Sauer,B., Okamoto,Y., Lee,E., Alt,F.W. and Westphal,H. (1996) Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl Acad. Sci. USA, 93, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arango N.A., Lovell-Badge,R. and Behringer,R.R. (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell, 99, 409–419. [DOI] [PubMed] [Google Scholar]
- 39.Wiebel F.F., Rennekampff,V., Vintersten,K. and Nordheim,A. (2002) Generation of mice carrying conditional knockout alleles for the transcription factor SRF. Genesis, 32, 124–126. [DOI] [PubMed] [Google Scholar]
- 40.Su H., Mills,A.A., Wang,X. and Bradley,A. (2002) A targeted X-linked CMV-Cre line. Genesis, 32, 187–188. [DOI] [PubMed] [Google Scholar]
- 41.Koni P.A., Joshi,S.K., Temann,U.A., Olson,D., Burkly,L. and Flavell,R.A. (2001) Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med., 193, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vidal F., Sage,F., Cuzin,F. and Rassoulzadegan,M. (1998) Cre expression in primary spermatocytes: a tool for genetic engineering of the germline. Mol. Reprod. Dev., 51, 274–280. [DOI] [PubMed] [Google Scholar]
- 43.Rassoulzadegan M., Magliano,M. and Cuzin,F. (2002) Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J., 21, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]