Abstract
Recent work in our laboratory showed that products formed by the antibody-catalyzed water-oxidation pathway can kill bacteria. Dihydrogen peroxide, the end product of this pathway, was found to be necessary, but not sufficient, for the observed efficiency of bacterial killing. The search for further bactericidal agents that might be formed along the pathway led to the recognition of an oxidant that, in its interaction with chemical probes, showed the chemical signature of ozone. Here we report that the antibody-catalyzed water-oxidation process is capable of regioselectively converting antibody-bound benzoic acid into para-hydroxy benzoic acid as well as regioselectively hydroxylating the 4-position of the phenyl ring of a single tryptophan residue located in the antibody molecule. We view the occurrence of these highly selective chemical reactions as evidence for the formation of a short-lived hydroxylating radical species within the antibody molecule. In line with our previously presented hypothesis according to which the singlet-oxygen (1O*2) induced antibody-catalyzed water-oxidation pathways proceeds via the formation of dihydrogen trioxide (H2O3), we now consider the possibility that the hydroxylating species might be the hydrotrioxy radical HO , and we point to the remarkable potential of this either H2O3- or O3-derivable species to act as a masked hydroxyl radical (HO•) in a biological environment.
, and we point to the remarkable potential of this either H2O3- or O3-derivable species to act as a masked hydroxyl radical (HO•) in a biological environment.
Antibodies, regardless of origin or antigenic specificity, can convert singlet dioxygen (1O*2) into hydrogen peroxide (H2O2) (1) via a process that we have postulated to involve dihydrogen trioxide (H2O3) (2). We have shown that, regardless of antibody specificity, this process can be used to kill bacteria, thus linking recognition and killing within the same molecule of the immune system (3). A close analysis of the bactericidal process revealed that hydrogen-peroxide production by antibodies was necessary, but not sufficient, for the observed killing, suggesting that more aggressive oxidants may be involved. Analytical studies showed that an additional oxidant is generated in the process that possesses a chemical signature of ozone. Thus, we had hypothesized that the bactericidal potential of the antibody-catalyzed water-oxidation pathway is due to the generation of a complex cascade of redox processes that may include the formation of ozone as well as other toxic trioxygen species. The chemistry of this hypothesis has found support in quantum chemical calculations by Goddard and coworkers (4).
Ozone itself is known to be highly bactericidal. A formation of ozone from the trioxygen species H2O3 by either disproportionation or further oxidation by 1O*2 is expected to proceed via or be accompanied by the formation of the hydrotrioxy radical (HO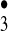 ), a species deserving special consideration in the context of radicals that display the chemical signature of the notorious hydroxyl radical (HO•) (4). Because the end product of the antibody-catalyzed water-oxidation pathway is H2O2, its interaction with ozone could also act as a source of the very same trioxygen radical. Ozone is known to decompose in the presence of H2O2 (5) to form oxidants that include species with the signature of HO• (6, 7).
), a species deserving special consideration in the context of radicals that display the chemical signature of the notorious hydroxyl radical (HO•) (4). Because the end product of the antibody-catalyzed water-oxidation pathway is H2O2, its interaction with ozone could also act as a source of the very same trioxygen radical. Ozone is known to decompose in the presence of H2O2 (5) to form oxidants that include species with the signature of HO• (6, 7).
Here we report the regioselective hydroxylation of benzoic acid as an antigen when it is bound within the combining site of an antibody during activation of the water-oxidation pathway. Further, we show by x-ray analysis that a single tryptophan residue is hydroxylated at the 4-position of the indole ring only when the antibody-catalyzed water-oxidation pathway is activated. Both chemical events bear the signature of HO•.
Materials and Methods
Murine IgG 4C6, WD1-6G6, and the Fab fragment of 4C6 were obtained in-house as described (1). Bovine catalase was obtained from Sigma.
Benzoic-Acid Hydroxylation Assay.
In a typical experiment, a solution of benzoic acid (2 mM) in PBS (pH 7.4) is irradiated with UV (312 nm, 0.8 μW⋅cm−2) at room temperature in the presence or absence of antibody (4C6 or WD1-6G6, 20 μM), H2O2 (1 mM), or catalase (13 milliunits/ml). At certain times, aliquots were removed (10 μl) and diluted 1:3 into acetonitrile/water (1:1). Hydroxylation of benzoic acid was followed by reversed-phase HPLC (Hitachi D-7000) using a Spherisorb RP-C18 column and a mobile phase of acetonitrile and water (0.1% trifluoroacetic acid) (30:70) at a flow rate of 1 ml/min. Localization was performed by UV detection at 254 nm (RT benzoic acid = 6.35 min, RT ortho-hydroxy benzoic acid = 8.57 min, RT meta-hydroxy benzoic acid = 4.02 min, and RT para-hydroxy benzoic acid = 3.72 min). Peak areas were converted to concentration by comparison to standard curves.
UV Irradiation and X-Ray Analysis of Murine 4C6 Fab.
Purified 4C6 Fab (200 μl, 26 mg/ml in 0.1 M sodium-acetate buffer, pH 5.5) was irradiated on a transilluminator with UV light (312 nm, 0.8 mW⋅cm−2) for 30 min. Crystals of the UV-irradiated 4C6 Fab were grown as described (8). A data set (2.51-Å resolution) was collected from a single crystal of the UV-irradiated 4C6 Fab by using 25% glycerol as a cryoprotectant. Diffraction data were recorded on a MAR Research (Hamburg) image plate detector (30 cm) mounted on a Rigaku (Tokyo) generator (100 kV, 50 mA). The data were integrated and scaled by using HKL2000 (9). The structure of the UV-irradiated 4C6 Fab was determined by standard refinement with the program CNS (10), starting from the refined native 4C6 Fab crystal structure (8). TrpL163 was refined as an alanine residue to avoid potential bias in the electron-density maps.
Results and Discussion
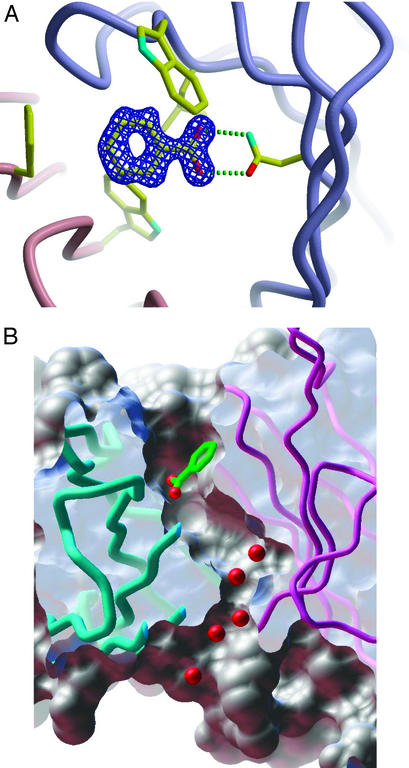
Among our large collection of x-ray structures of antibodies, murine monoclonal antibody 4C6 (11) turned out to be of special interest in the context of the search for radicals generated during the antibody-mediated water-oxidation pathway, because it was discovered to bind one molecule of the widely used hydroxyl-radical probe, benzoic acid, within the antibody-combining site (Fig. 1A). Interestingly, the crystal structure of 4C6 Fab also contains a unique water channel that links the interfacial region of the constant and variable domains, where we have speculated that the chemistry of the water-oxidation pathway occurs (2), to the combining site that contains the bound benzoic acid (Fig. 1B). It was considered that the antibody-bound benzoic acid, being so sequestered in this “molecular reaction chamber,” may be expected to react in situ with short-lived reactive species that, because of their low diffusion radius and high reactivity, might otherwise not be detected by the use of external probes in solution.
Figure 1.
(A) Rendering of the combining site of 4C6 Fab bound to the benzoic-acid ligand. (B) Molecular surface representation showing a putative channel that links the interfacial variable and constant domains with the antigen-binding site of murine 4C6 Fab and the benzoic-acid ligand bound directly above this channel. The images were generated by using APPLICATION VISUALIZATION SYSTEM (AVS) software (23), and the molecular surfaces were computed by using msms software (24).
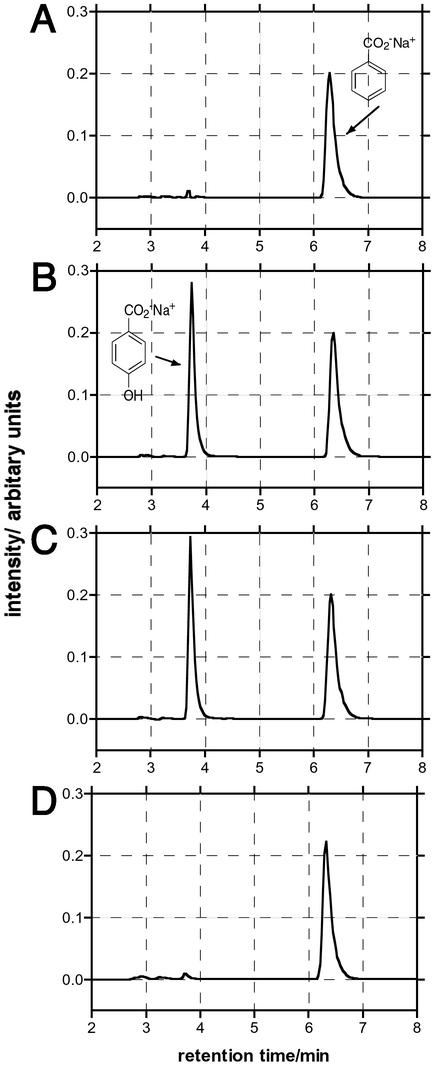
When a solution of sodium benzoate (2 mM) and antibody 4C6 (20 μM) in PBS is irradiated with UV light (312 nm, 0.8 μW⋅cm−2), regioselective hydroxylation of benzoic acid yields exclusively para-hydroxy benzoic acid (Fig. 2B). No ortho- or meta-isomers were detected. The observed initial rate of formation of the para-isomer is ≈0.8 μM/min, and 4C6 Fab generates >10 mol equivalents (10% of substrate) of para-hydroxy benzoic acid without a significant decrease in this initial rate. The addition of catalase (13 units/ml) during the irradiation of 4C6 does not affect the hydroxylation reaction (Fig. 2C). The product conversion is 10% (relative to the starting concentration of benzoic acid) after 3 h of irradiation. By contrast, no hydroxylation of sodium benzoate occurs either by irradiation in PBS or when exposed to 1O*2, generated by irradiation of hematoporphyrin IX (40 μM) with visible light (2.7 mW⋅cm−2) (data not shown). Similarly, no hydroxylation is observed when benzoic acid is irradiated with UV light (312 nm, 0.8 μW⋅cm−2) in the presence of H2O2 (1 mM) (Fig. 2A). Significantly, when this same experiment is repeated with an antibody that does not bind benzoic acid, murine IgG 6G6 (12), no hydroxylation of benzoate is observed (Fig. 2D).
Figure 2.
HPLC analysis of sodium benzoate hydroxylation. RT benzoic acid, 6.4 min; RT 4-hydroxybenzoic acid, 3.8 min. (A) H2O2 (1 mM) + UV irradiation + benzoic acid (2 mM). (B) 4C6 (20 μM) + UV irradiation + benzoic acid (2 mM). The peak area of para-hydroxy benzoic acid is equivalent to ≈200 μM. (C) 4C6 + UV irradiation + benzoic acid (2 mM) + catalase (13 milliunits/ml). The peak area of para-hydroxy benzoic acid is equivalent to ≈220 μM. (D) 6G6 (20 μM) + UV irradiation + benzoic acid (2 mM).
The combined experimental observations that catalase does not affect the 4C6-catalyzed hydroxylation process, and that hydroxylation by nonspecific antibodies such as 6G6 is not detectable under our assay conditions, suggest that the hydroxylating species is being generated within the antibody fold and is sequestered and/or very short-lived. The reaction of the HO• radical with benzoic acid in aqua has been investigated intensively and is known to lead to an isomeric mixture of hydroxy isomers, the constitution of which depends on the method of formation of the radical species, the pH of the media, and the species that oxidizes the intermediary cyclohexadienyl radical. For example, Klein et al. (13) observed that at pH 7.0, with O3 as the oxidant, the observed ortho/meta/para ratio was 1:0.6:0.5. Therefore, the fact that the hydroxylation reaction of benzoic acid with 4C6 is highly regioselective is consistent with a process that occurs when the benzoic acid is bound within the antibody-combining site.
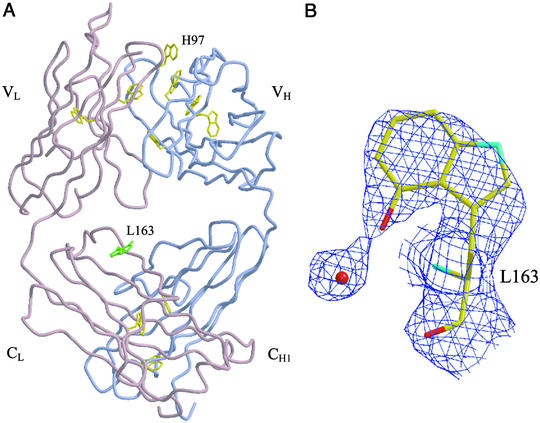
Our postulate that highly reactive intermediates are generated by the antibody-catalyzed water-oxidation process raises the question of whether the actual antibody molecule might be modified at or near the sites where they are generated. An x-ray structural analysis of the 4C6 Fab crystallized after activation of the water-oxidation pathway by UV irradiation provides a unique insight into this issue. The analysis revealed that, remarkably, only one tryptophan residue, TrpL163, within the antibody molecule was modified (Fig. 3A). TrpL163 is located in the constant region of the 4C6 Fab light chain, where its side chain protrudes into the interfacial region between the constant and variable domains. Only the 4-position of the indole nucleus is hydroxylated, and the hydroxyl group appears to make a hydrogen bond to a proximate water molecule (Fig. 3B). Although TrpL163 is the most solvent-accessible tryptophan residue of the 4C6 Fab (with a solvent-accessible area of 113 Å2 for a 1.41-Å probe radius), it is unlikely that this should be the sole reason for it being uniquely oxidized because no oxidation of TrpH97, which possesses a nearly identical solvent-accessible area (100 Å2) is observed. A more plausible explanation for the regioselective oxidation is that TrpL163 is located in close proximity to where the short-lived intermediates are generated. Under aerobic conditions in solution, it is known that “hydroxyl-radical-mediated” hydroxylation of tryptophan leads to a mixture of 4-, 5-, 6-, and 7-hydroxy tryptophan in addition to N-formylkynurenine (NFK) (14).
Figure 3.
(A) Standard view of the 4C6 Fab with the light (L) and heavy (H) chains colored in pink and blue, respectively. The locations of all tryptophans (highlighted in yellow) within the Fab are also shown. The hydroxylated tryptophan, L163, is highlighted in green. (B) Electron-density view of the hydroxylated tryptophan L163 and a proximate water molecule. A 2Fo-Fc electron-density map was calculated to 2.5 Å from a crystal structure with the tryptophan refined as alanine and contoured at 0.8 σ. The figure was generated in BOBSCRIPT (25) and rendered in RASTER3D (26).
The signature of the two highly regioselective hydroxylation reactions observed in this study clearly corresponds to one conventionally expected of the hydroxyl radical. In our view, however, these two reactions, when coupled with our previous evidence for the generation of ozone and H2O2 during the antibody-catalyzed water-oxidation pathway, strongly imply that transient trioxygen species are being generated that can act as hydroxyl-radical equivalents. This hypothesis is supported further by quantum chemical delineations of possible reaction paths for the disproportionation and/or oxidation of H2O3 to radical species such as the [HO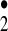 HO
HO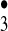 ] complex (15).
] complex (15).
The most interesting while at the same time experimentally most elusive species that we consider to occur along the antibody-mediated water-oxidation pathway is the hydrogen trioxide radical, HO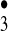 . The hydrotrioxide radical has been postulated to be an important intermediate in atmospheric processes (16), but it was not clear whether after formation it would dissociate immediately into 3O2 and HO• (17, 18). However, HO
. The hydrotrioxide radical has been postulated to be an important intermediate in atmospheric processes (16), but it was not clear whether after formation it would dissociate immediately into 3O2 and HO• (17, 18). However, HO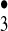 has recently been detected experimentally by both Speranza (19) and Cacace and coworkers (20, 21) using Fourier-transform ion cyclotron resonance mass spectrometry and neutralization-reionization mass spectrometry, respectively, and its lifetime has been calculated to be of the order of microseconds. Our own qualitative chemical reasoning as well as quantum chemical calculations point to the potential of the HO
has recently been detected experimentally by both Speranza (19) and Cacace and coworkers (20, 21) using Fourier-transform ion cyclotron resonance mass spectrometry and neutralization-reionization mass spectrometry, respectively, and its lifetime has been calculated to be of the order of microseconds. Our own qualitative chemical reasoning as well as quantum chemical calculations point to the potential of the HO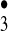 or the [HO
or the [HO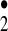 HO
HO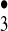 ] complex to simulate the chemistry of the hydroxyl radical. Thus, HO
] complex to simulate the chemistry of the hydroxyl radical. Thus, HO may undergo OH transfer to substrates via either an associative or dissociative pathway, both being driven by the extrusion of 3O2 as the “leaving group” of a substitution reaction at the hydroxyl oxygen (Fig. 4). This presumed property conveys to the HO
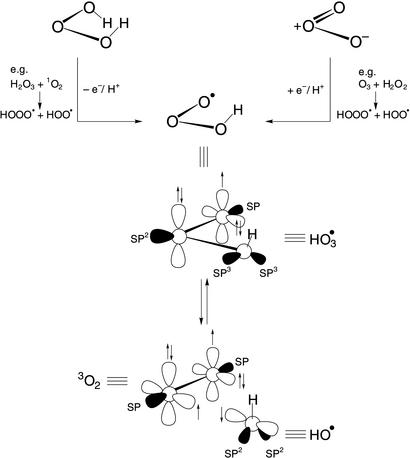
may undergo OH transfer to substrates via either an associative or dissociative pathway, both being driven by the extrusion of 3O2 as the “leaving group” of a substitution reaction at the hydroxyl oxygen (Fig. 4). This presumed property conveys to the HO species the potential to act either as a masked hydroxyl radical or, via the dissociative pathway, as a source of the “free” hydroxyl radical. It constitutes a species through which long-lived hydroxyl-radical reactivity can be generated from dihydrogen trioxide or ozone (Fig. 4) (19–21), from H2O3 by either disproportionation or single-electron oxidation (e.g., from 1O*2), or from O3 by a single-electron reduction (e.g., from H2O2). It is from this perspective that an underlying important aspect of the antibody-mediated formation of H2O3 from O2 and H2O becomes apparent, namely, that it constitutes a low-energy pathway connecting the realms of dioxygen and trioxygen chemistry.
species the potential to act either as a masked hydroxyl radical or, via the dissociative pathway, as a source of the “free” hydroxyl radical. It constitutes a species through which long-lived hydroxyl-radical reactivity can be generated from dihydrogen trioxide or ozone (Fig. 4) (19–21), from H2O3 by either disproportionation or single-electron oxidation (e.g., from 1O*2), or from O3 by a single-electron reduction (e.g., from H2O2). It is from this perspective that an underlying important aspect of the antibody-mediated formation of H2O3 from O2 and H2O becomes apparent, namely, that it constitutes a low-energy pathway connecting the realms of dioxygen and trioxygen chemistry.
Figure 4.
Chemical formulae illustrating the central position of the hydrotrioxide radical between H2O3 and O3 and its presumed conversion into 3O2 and the hydroxyl radical.
It is tempting to consider that such a mechanistically unifying position of the HO radical may extend to other types of oxidation processes, most notably to the biological oxidation reactions catalyzed by iron-containing enzymes. Given the possibility that the iron complexes of H2O3, for instance, might form from H2O2 and iron-oxene intermediates, it seems surprising that their occurrence in biological oxidation mechanisms seem not to have been considered. One of the more obvious among a number of questions to be raised in this connection refers to the mechanism of catalase action. Does that process involve a catalase–Fe(III) complex of H2O3 as the central intermediate, the formation of which would involve the nucleophilic addition of H2O2 to the oxenoid catalase species and decomposition of which to the catalase–Fe(III)–aqua complex and triplet oxygen would constitutionally (but not with regard to spin multiplicity) correspond to the known decomposition of H2O3 to H2O and singlet oxygen (22)?
radical may extend to other types of oxidation processes, most notably to the biological oxidation reactions catalyzed by iron-containing enzymes. Given the possibility that the iron complexes of H2O3, for instance, might form from H2O2 and iron-oxene intermediates, it seems surprising that their occurrence in biological oxidation mechanisms seem not to have been considered. One of the more obvious among a number of questions to be raised in this connection refers to the mechanism of catalase action. Does that process involve a catalase–Fe(III) complex of H2O3 as the central intermediate, the formation of which would involve the nucleophilic addition of H2O2 to the oxenoid catalase species and decomposition of which to the catalase–Fe(III)–aqua complex and triplet oxygen would constitutionally (but not with regard to spin multiplicity) correspond to the known decomposition of H2O3 to H2O and singlet oxygen (22)?
Although it is usually difficult to obtain direct evidence for the existence of such short-lived radical species in biological systems, we were able to do so because of the special circumstances of the high effective molarity, and perhaps activation, of the benzoic-acid probe offered by its sequestration within the antibody 4C6-combining site. The regioselective hydroxylation of benzoic acid is an observation that, beside its mechanistic significance in terms of the antibody-catalyzed water-oxidation pathway, is also important, because it demonstrates unequivocally that antibodies can catalyze reactions that chemically modify their antigens.
Acknowledgments
We thank Mike Pique for rendering of the 4C6 Fab molecular surface in Fig. 1B and several I.A.W. laboratory members for data collection and processing. This work was supported by National Institutes of Health Grants GM43858 (to K.D.J.) and PO1CA27489 (Program Project Grant, to K.D.J., I.A.W., and R.A.L.) and by The Skaggs Institute for Chemical Biology.
References
- 1.Wentworth A D, Jones L H, Wentworth P J, Janda K D, Lerner R A. Proc Natl Acad Sci USA. 2000;97:10930–10935. doi: 10.1073/pnas.97.20.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wentworth P, Jr, Jones L H, Wentworth A D, Zhu X, Larsen N A, Wilson I A, Xu X, Goddard W A, III, Janda K D, Eschenmoser A, Lerner R A. Science. 2001;293:1806–1809. doi: 10.1126/science.1062722. [DOI] [PubMed] [Google Scholar]
- 3.Wentworth P, Jr, McDunn J, Wentworth A D, Takeuchi C, Nieva J, Janda K D, Eschenmoser A, Lerner R A. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 4.Datta D, Nagarajan V, Xu X, Goddard W A., III Proc Natl Acad Sci USA. 2002;99:2636–2641. doi: 10.1073/pnas.052709399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss J. Trans Faraday Soc. 1935;31:668–681. [Google Scholar]
- 6.Hoigne J, Bader H. Water Res. 1976;10:377–386. [Google Scholar]
- 7.Bray W C. J Am Chem Soc. 1938;60:82–87. [Google Scholar]
- 8. Zhu, X., Heine, A., Monnat, F., Houk, K. N., Janda, K. D. & Wilson, I. A. (2003) J. Mol. Biol., in press. [DOI] [PubMed]
- 9.Otwinowsky Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 10.Brünger A T, Adams P D, Clore G M, Delano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Janda K D, Ashley J A, Lerner R A. Science. 1994;264:1289–1293. doi: 10.1126/science.8191282. [DOI] [PubMed] [Google Scholar]
- 12.Lo C-H L, Wentworth P, Jr, Jung K W, Yoon J, Ashley J A, Janda K D. J Am Chem Soc. 1997;119:10251–10252. [Google Scholar]
- 13.Klein G W, Bhatia K, Madhavan V, Schuler R H. J Phys Chem. 1975;79:1767–1774. [Google Scholar]
- 14.Maskos Z, Rush J D, Koppenol W H. Arch Biochem Biophys. 1992;296:514–520. doi: 10.1016/0003-9861(92)90605-v. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Muller P R, Goddard W A., III Proc Natl Acad Sci USA. 2002;99:3376–3381. doi: 10.1073/pnas.052710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder D. Angew Chem Int Ed Engl. 2002;41:573–576. [Google Scholar]
- 17.Jungkamp T P W, Seinfeld J H. Chem Phys Lett. 1996;257:15–22. [Google Scholar]
- 18.Lay T H, Bozzelli J W. J Phys Chem A. 1997;101:9505–9510. [Google Scholar]
- 19.Speranza M. Inorg Chem. 1996;35:6140–6151. [Google Scholar]
- 20.Cacace F, dePetris G, Pepi F, Troiani A. Science. 1999;285:81–82. doi: 10.1126/science.285.5424.81. [DOI] [PubMed] [Google Scholar]
- 21.Cacace F, Speranza M. Science. 1994;265:208–209. doi: 10.1126/science.265.5169.208. [DOI] [PubMed] [Google Scholar]
- 22.Koller J, Plesnicar B. J Am Chem Soc. 1996;118:2470–2471. [Google Scholar]
- 23.Upson C, Faulhaber T, Jr, Kaims D, Laidlaw D, Schlegel D, Vroom J, Gurwitz R, van Dam A. IEEE Comp Graphics Appl. 1989;9:30–42. [Google Scholar]
- 24.Sanner M, Olson A J, Spehner J C. Biopolymers. 1996;38:305–320. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Esnouf R M. Acta Crystallogr D. 1999;55:938–940. doi: 10.1107/s0907444998017363. [DOI] [PubMed] [Google Scholar]
- 26.Merritt E A, Murphy M E P. Acta Crystallogr D. 1994;50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]