Abstract
Podocalyxin is a major membrane protein of the glomerular epithelium and is thought to be involved in maintenance of the architecture of the foot processes and filtration slits characteristic of this unique epithelium by virtue of its high negative charge. However, until now there has been no direct evidence for podocalyxin's function. Podocalyxin is a type 1 transmembrane sialoprotein with an N-terminal mucin-like domain. To assess its function, we cloned rat podocalyxin and examined the effects of its expression on the cell adhesion properties of stably transfected Chinese hamster ovary (CHO)-K1 and Madin-Darby canine kidney (MDCK) cells and inducible ecdysone receptor–expressing (EcR)-CHO cells. In a cell aggregation assay, CHO-K1 cells expressing high levels of podocalyxin showed complete inhibition of cell aggregation, and MDCK transfectants showed greatly reduced aggregation (∼60–80%) compared with parental cells. In EcR-CHO cells, the expression level of podocalyxin induced by increasing levels of ecdysone analogue correlated closely with the antiadhesion effect. The inhibitory effect of podocalyxin was reversed by treatment of the cells with Arthrobacter ureafaciens sialidase, indicating that sialic acid is required for inhibition of cell adhesion. Overexpression of podocalyxin also affected transepithelial resistance and the distribution of junctional proteins in MDCK cells by an unknown mechanism that may involve interaction with the actin cytoskeleton. These results provide direct evidence that podocalyxin functions as an antiadhesin that maintains an open filtration pathway between neighboring foot processes in the glomerular epithelium by charge repulsion.
INTRODUCTION
Cells regulate their attachment to other cells and to the extracellular matrix by regulating the expression of adhesion molecules, such as cadherins and integrins, or of “antiadhesins,” such as N-CAMs and membrane-associated mucins (Wesseling et al., 1996). Membrane-associated mucins such as glycophorin, episialin, and leukosialin are heavily sialylated, O-linked glycoproteins that have been shown to interfere with cell adhesion by steric hindrance or charge repulsion. Podocalyxin (PC), another membrane-associated mucin (Kershaw et al., 1995, 1997), was originally identified as the major sialoprotein on the apical surface of the podocytes or glomerular epithelium (Kerjaschki et al., 1984). It is also expressed on vascular endothelia (Kerjaschki et al., 1984; Horvat et al., 1986), including high endothelial venules (Sassetti et al., 1998), and, most recently, was found to be expressed in hematopoietic stem cells, megakaryocytes, and thrombocytes (McNagny et al., 1997). With the exception of high endothelial venules, PC has been postulated to serve an antiadhesion function in all of these locations.
The predicted amino acid sequences of rabbit (Kershaw et al., 1995), human (Kershaw et al., 1997), and chicken (McNagny et al., 1997) PC indicate that it is a type 1 transmembrane protein with features typical of membrane-associated mucins, including ectodomains that are serine-, threonine-, and proline-rich, heavily sialylated, and extensively O-glycosylated (Hilkens et al., 1992). The transmembrane and cytoplasmic domains of rabbit, human, and chicken PC are remarkably conserved, but their ectodomains are more heterogeneous, sharing only the mucin-like structure and location of four conserved cysteines.
Based on its properties, particularly its high net negative charge, we suggested that PC corresponds to the previously identified glomerular polyanion (Michael et al., 1970) and that it functions in the maintenance of the unique foot process and filtration slit architecture of the glomerular epithelium (Kerjaschki et al., 1984). Considerable indirect evidence suggests that this is the case. For example, during kidney development, the glomerular epithelium develops from a typical polarized epithelium with apical junctional complexes that differentiates into a characteristic arborized layer with foot processes and a modified junctional structure allowing passage of the glomerular filtrate (Schnabel et al., 1989, 1990). The expression of PC during glomerular development is closely coupled to the appearance of open intercellular spaces (filtration slits) bridged by slit diaphragms (Schnabel et al., 1989, 1990). Also, in experimental nephrosis, loss of the foot process organization is associated with a reduction in the sialic acid content of PC to one-third of normal (Kerjaschki et al., 1985), and neutralization of the glomerular epithelial cell surface charge causes a dramatic collapse of the filtration slits and reorganization of the foot processes (Seiler et al., 1977; Kurihara et al., 1992b). However, direct evidence of the role of PC in glomerular organization and function is still lacking.
In this study, we cloned rat PC and investigated its antiadhesion functions in Chinese hamster ovary (CHO) and Madin-Darby canine kidney (MDCK) cells by examining the effects of PC expression on cell aggregation. Here we demonstrate that overexpression of PC inhibits cell–cell adhesion in an expression level–dependent manner and that this inhibitory effect is due to charge repulsion, because it can be reversed by sialidase treatment. We also made the surprising observation that expression of PC affects the transepithelial electrical resistance (TER) and the distribution of adherens junction (AJ) and tight junction (TJ) proteins in MDCK cells. Thus, we provide direct evidence that PC functions as an antiadhesin to maintain open filtration slits between neighboring foot processes by charge repulsion of adjacent cell membranes.
MATERIALS AND METHODS
Materials
Polyclonal rabbit anti-PC (0601) was raised against a synthetic peptide corresponding to the C-terminal 12 amino acids (aa) (KDDLDEEEDTHL) of the cytoplasmic tail of rabbit PC and was affinity purified with the use of Affi-Gel 10 Gel (Bio-Rad, Hercules, CA). Anti-PC mAbs 1A and 5A were prepared as described (Miettinen et al., 1990). Polyclonal anti-E-cadherin, which recognizes the cytoplasmic domain of type 1 cadherins (anti-pan-cadherin), was kindly provided by Dr. James Nelson (Stanford University, Stanford, CA) (Marrs et al., 1993). Polyclonal anti-occludin and anti-ZO-1 were purchased from Zymed (South San Francisco, CA). Cross-absorbed FITC-conjugated donkey anti-rabbit or Texas red–conjugated donkey anti-mouse F(ab′)2 were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA); affinity-purified goat anti-rabbit and anti-mouse immunoglobulin G (IgG) (H+L) conjugated to HRP were from Bio-Rad. Enhanced chemiluminescent substrate (SuperSignal) was obtained from Pierce Chemical (Rockford, IL). [35S]EasyTag Express protein-labeling mix (∼1000 Ci/mmol) and [32P]dCTP were from DuPont–New England Nuclear (Boston, MA). Primers were synthesized at Life Technologies/BRL (Gaithersburg, MD), and restriction enzymes were purchased from New England Biolabs (Beverly, MA). Chemical reagents were obtained from Sigma–Aldrich (St. Louis, MO) except as indicated.
Cell Culture
CHO-K1 cells, obtained from the American Type Culture Collection (Rockville, MD), were maintained in Ham's F12 medium supplemented with 10% (vol/vol) FCS (Life Technologies/BRL). Ecdysone receptor–expressing (EcR)-CHO cells were purchased from Invitrogen (Carlsbad, CA) and maintained in Ham's F12 medium supplemented with 10% FCS and 250 μg/ml zeocin (Invitrogen). MDCK type I cells were maintained in MEM Earles supplemented with 10% FCS. All culture media contained 100 U/ml penicillin G and 100 μg/ml streptomycin sulfate (Life Technologies/BRL).
Cloning of Rat PC cDNA
To obtain the rat partial PC cDNA, degenerate primers (5′-CMYRCCSCTCATMATCACCAT-3′ and 5′-ATCCARCTGTCCCYCAGCTC-3′) were designed from homologous sequences in the cytoplasmic tail of rabbit and human PC. A degenerate PCR product, obtained from a λZAP II rat kidney cDNA library (Saito et al., 1994), was amplified and subcloned into pGEM-T Easy vector (Promega, Madison, WI). Both strands were sequenced by automated DNA sequencing. To obtain the complete rat cDNA sequence, 5′ and 3′ rapid amplification of cDNA ends (RACE) was performed on a Marathon Ready rat kidney cDNA library (Clontech, Palo Alto, CA) with the use of primers (5′-GAGTCTCCATCACTTCCAAGGTTGGG-3′ and 5′-GCATCCTTCCTGCTCCTCGTTGC-3′) designed from the obtained sequence. The RACE products were ligated into pGEM-T Easy vector. Three clones of each RACE product were sequenced on both strands, and sequence analysis was performed with MacVector version 6 software (Oxford Molecular, Madison, WI).
Northern Blotting
Total RNA was isolated from rat glomeruli, kidney, heart, lung, brain, and cultured cells. Samples were electrophoresed on 1% formaldehyde–agarose gel and transferred to nitrocellulose filters (Schleicher & Schuell, Keene, NH). A cDNA fragment was labeled with [32P]dCTP by the random labeling method. Prehybridization, hybridization, and washings were carried out according to the ExpressHyb protocol (Clontech).
Construction of Stable or Inducible Expression Vectors
The complete coding sequence of PC was amplified by PCR using 5′-CAGCCACCTGCTCCGAGTCC-3′ and 5′-TCGGAGTGGCTGGCGGACTG-3′ as primers. PCR was carried out using 10 pmol of each primer, 0.5 ng of Marathon Ready rat kidney cDNA, 200 μM dNTP, 2.5 U Pfu Turbo polymerase (Stratagene, La Jolla, CA), and PCR reaction buffer in a total volume of 50 μl. PCR products were purified with a QIAquick gel-purification kit (Qiagen, Valencia, CA), followed by dATP tailing with Taq polymerase (Life Technologies/BRL), and subcloned into pGEM-T Easy vector (Promega). The fidelity of the construct was verified by sequencing on both strands. The cDNA insert was excised with NotI or NotI–SpeI and subcloned into the pIRES1neo expression vector (Clontech) at NotI restriction sites or the pIND-inducible vector (Invitrogen) at NotI–XbaI restriction sites to make expression vectors pIRES-PC or pIND-PC, respectively.
Immunocytochemistry
For immunofluorescence, cells on glass coverslips were fixed with 2% paraformaldehyde in 0.2 M phosphate buffer, pH 7.4, for 45 min, permeabilized with 0.1% Triton X-100 in PBS for 10 min, and incubated for 2 h at room temperature with anti-PC mAb 5A (1:50), anti-pan-cadherin (1:400), anti-occludin (1:200), or anti-ZO-1 (1:200) in PBS containing 1% BSA. Detection was with FITC- or Texas red–conjugated donkey anti-rabbit or anti-mouse F(ab′)2 (1:250) for 1 h at room temperature. For double labeling, cells were incubated simultaneously with mouse mAb 5A and rabbit polyclonal anti-occludin, anti-ZO-1, or anti-pan-cadherin, followed by incubation with appropriate anti-rabbit and anti-mouse conjugates. Cells were mounted in 75% glycerol/PBS containing 1 mg/ml paraphenylinediamine and examined with either a Zeiss Axiophot microscope equipped for epifluorescence (Carl Zeiss, Thornwood, NY) or a Bio-Rad confocal microscope (MRC 1024) equipped with Lasersharp 3.1 software (Bio-Rad) and a krypton–argon laser. The X-Z (vertical) sections were generated with a 0.1-μm motor step. Images from Axiophot or confocal microscopy were processed with Scicon Image version 1.59 (Scicon, Frederick, MA) or Adobe Photoshop version 5 software (Adobe Systems, San Jose, CA), respectively.
SDS-PAGE and Immunoblotting
Cells were lysed in TDU buffer (1.6% Triton X-100, 2% dodecyl sulfate, 7.2 M urea, and 0.8% DTT). Proteins were separated on 6 or 7.5% SDS gels under reducing conditions and transferred to polyvinylidene difluoride Immobilon-P membranes (Millipore, Bedford, MA) using a wet tank transfer system (Minigel transfer unit, Bio-Rad) at 90 mA for 15 h. Membranes were blocked (1 h in PBS, pH 7.2, containing 5% calf serum and 0.1% Tween-20), incubated for 2 h at room temperature with primary antibodies, followed by HRP-conjugated goat anti-rabbit IgG (1 h incubation, 1:3000 dilution) and detection by ECL. Quantification of protein bands was done by densitometry with the use of ScanAnalysis software (Biosoft, Cambridge, United Kingdom).
Transfection Procedure and Selection of Cell Lines Stably Overexpressing PC
CHO-K1 or MDCK cells were plated at 1–2 × 105 cells per six-well plate, cultured overnight, washed twice with serum and antibiotic-free medium, and transfected with 2 μg of pIRES-PC using 10 μl of Lipofectamine (Life Technologies/BRL). After 5 h of incubation, 1 ml of medium containing 20% FCS was added. Cells were then cultured for 1 d and split into 96-well plates by serial dilution, 0.5 cells/well (CHO-K1 cells) or into two 100-mm dishes (MDCK cells), followed by selection for G418 resistance (0.5 mg/ml; Life Technologies/BRL). G418-resistant colonies were isolated after 10–14 d. Three CHO-K1 cell clones (CHO-PC5, CHO-PC13, and CHO-PC14 cells) and two MDCK cell clones (MDCK-PC6 and MDCK-PC8 cells) overexpressing PC were obtained.
For establishment of a PC-inducible cell line, EcR-CHO cells (Invitrogen) stably expressing the ecdysone receptor (RXR and VgEcR) were transfected with the pIND-PC plasmid using Lipofectamine as described above, followed by selection for G418 resistance (0.5 mg/ml) for 10 d. One clone overexpressing PC (EcR-CHO-PC) after induction with ponasterone A (ecdysone analog; Invitrogen) was obtained.
Cell Aggregation Assay
The assay used is a modification of a previously described method (Takeichi, 1977). To improve detachment efficiency, MDCK cells were cultured on 100-mm dishes overnight in MEM containing 0.1 mM Ca2+ (low-calcium medium) supplemented with 5% dialyzed FCS. Subconfluent cell layers were rinsed twice with Ca2+- and Mg2+-free PBS and detached by incubation in HBSS containing 1 mM EDTA at 37°C for 5 or 20 min. After washing twice in HBSS containing 1% BSA (HBSS/BSA), cells were resuspended (5 × 105 cells/ml) in HBSS/BSA by three passages through an 18-gauge needle. The cell suspension (0.5 ml/well) was seeded in a 24-well plate previously coated with 2% BSA in HBSS and allowed to aggregate for 0–180 min in the presence of 1 mM CaCl2 or 1 mM EGTA at 37°C on a rotating shaker (80 rpm). The reaction was stopped by the addition of 0.5 ml of 25% glutaraldehyde per well (Electron Microscopy Sciences, Fort Washington, PA). Aggregation was quantified by counting representative aliquots from each sample on a hematocytometer using phase-contrast optics. The number of cells in aggregates of more than three cells as well as the total number of cells were counted from four 1-mm squares of the hematocytometer grid. At least 600 cells were counted from each sample. Quantification of aggregation was estimated by the following formula: % aggregation = (N0 − Nt)/N0 × 100, where Nt is the total number of particles at the incubation time t, and N0 is the total number of cells.
Sialidase Treatment
Cells detached as described above were resuspended (5 × 106 cells/ml) in RPMI-1640 containing 50 mM HEPES (pH 6.9) and incubated for 30 min at room temperature with Arthrobacter ureafaciens sialidase (20 mU/ml; Sigma). After washing twice in HBSS/BSA, cells were divided into three aliquots: one was used for the cell aggregation assay, one was lysed immediately, and the remaining portion was lysed after incubation for 180 min at 37°C.
Pulse-Chase Experiments
Cells were incubated for 30 min in methionine- and cysteine-free DMEM, pulsed for 15 min with DMEM containing 0.2 mCi/ml [35S]EasyTag Express protein-labeling mix, followed by washing with MEM/10% FCS and incubation in the same medium with excess unlabeled methionine and cysteine. At selected intervals, cells were washed three times with PBS, lysed in 800 μl of RIPA buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% deoxycholic acid, 1% BSA) containing protease inhibitors (10 μg/ml leupeptin and 1 mM PMSF), and centrifuged. Immunoprecipitation was performed with 2 μg of affinity-purified, anti-PC serum (0601) or 10 μl of preimmune serum at 4°C overnight. Immune complexes were precipitated with protein A–Sepharose (Bio-Rad), and pellets were washed three times in RIPA buffer and three times in PBS. Immunoprecipitates were divided into three portions and resuspended in 15 μl of 100 mM citrate phosphate buffer (pH 5.5). Three milliunits of endoglycosidase H (endo H) (Streptomyces plicatus; Boehringer Mannheim, Indianapolis, IN) was added to one aliquot and 5 mU of sialidase (Arthrobacter ureafaciens) was added to another. Samples were incubated at 37°C for 3 h, after which 15 μl of 2× SDS sample buffer was added, and the samples were boiled for 5 min and electrophoresed on 6% SDS-PAGE gels. Gels were stained with Coomassie brilliant blue, soaked for 30 min in Amplify (Amersham, Arlington Heights, IL), and exposed to Biomax MR x-ray film (Eastman Kodak, Rochester, NY) with an intensifying screen for 8 d.
Measurement of TER
Parental or transfected MDCK cells were seeded on polycarbonate Transwell filters (Costar, Cambridge, MA) at a high density (∼105 cells/cm2) and allowed to grow to confluence for a 3-d period. TER was measured with a Millipore ERS electrical resistance system, calculated from the measured voltage, and normalized by the area of the monolayer according to the manufacturer's instructions. To investigate the effect of sialidase treatment on TER, cells were washed with serum-free MEM and incubated at 37°C for 60 min in the same medium with or without sialidase (20 mU/ml). TER was measured after 30 and 60 min of sialidase treatment. Values are means ± SE of multiple determinations on four different cell layers. All resistance values are in Ω/cm2.
RESULTS
Cloning and Sequencing of Rat PC cDNA
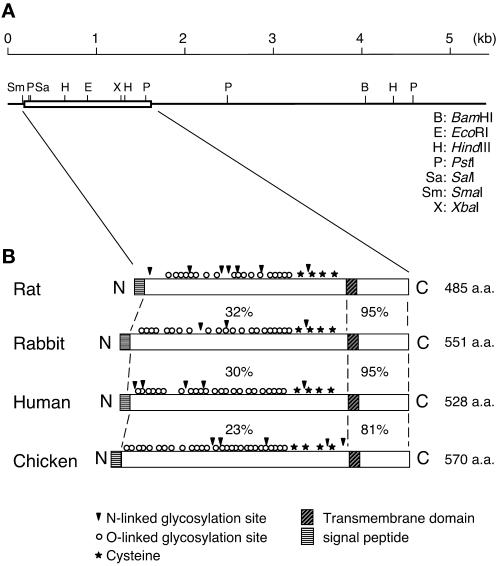
To obtain the complete rat PC cDNA, we carried out 5′ and 3′ RACE on a Marathon Ready rat kidney cDNA library and obtained two products (∼1.5 and ∼4 kilobase [kb]) that covered the entire PC cDNA. The cDNA for rat PC encompasses 5.5 kb, with an ORF encoding 485 aa (Figure 1). By Northern blotting, the cDNA probes hybridized with a 5.5-kb transcript of rat glomerular mRNA. The primary structure of rat PC predicts an ∼60 kDa type 1 transmembrane protein with a 24-aa putative N-terminal signal peptide, a 360-aa ectodomain, a 26-aa transmembrane domain, and a 75-aa C-terminal cytoplasmic tail (Figure 2). The ectodomain of rat PC is shorter than that of PC from other species and has several gaps, possibly resulting from alternative splicing. Comparison of the amino acid sequence of rat PC with that from rabbit, human, and chicken PC showed an overall amino acid homology of 40, 41, and 33%, respectively (Figure 1). As reported for other species, the homologies of the transmembrane and cytoplasmic domains are very high (95% in the case of rabbit and human PC, and 81% for chicken PC), whereas those of the ectodomain are much lower (32, 30, and 23%, respectively) (Figure 2). The overall structure of PC among these species is very similar (Figure 2): the N-terminal portion of the ectodomain is rich in serine, threonine, and proline residues and has seven potential sites for N-linked and numerous putative sites for O-linked glycosylation.
Figure 1.
Amino acid sequence alignment of rat, rabbit, human, and chicken PC. Note the low homology and variable length of the ectodomains and the high homology of the putative transmembrane domains (box) and cytoplasmic domains of all four proteins. The location of the cysteine residues (asterisks) is highly conserved. Potential PKC (●) and casein kinase II (○) phosphorylation sites are indicated. Black, amino acid identity; gray, amino acid similarity. Data are from Kershaw et al. (1995, 1997) and McNagny et al. (1997).
Figure 2.
Scheme of rat PC cDNA and predicted protein structure. (A) Restriction enzyme map of rat PC cDNA showing the 5′ and 3′ untranslated regions and coding region (box). (B) Scheme of the comparative protein structure of rat, rabbit, human, and chicken PC. The rat PC cDNA is ∼5.5 kb with an ORF encoding 485 aa. The primary structure of rat PC predicts a type 1 transmembrane protein consisting of a putative 24-aa N-terminal signal peptide sequence (striped box), a 360-aa ectodomain, a 26-aa single transmembrane-spanning domain (hatched box), and a 75-aa C-terminal cytoplasmic tail. The ectodomain of rat PC is shorter than that of PC from other species. It has seven potential sites for N-linked glycosylation (arrowheads) and numerous potential sites for O-linked glycosylation (○). Four cysteines (★) for potential disulfide linkage believed to form two loop structures are conserved in all species. The percentages of amino acid identity between the ectodomains or transmembrane and cytoplasmic domains of rat PC and those of PC from other species are shown.
Expression of PC in CHO-K1 and MDCK Cells
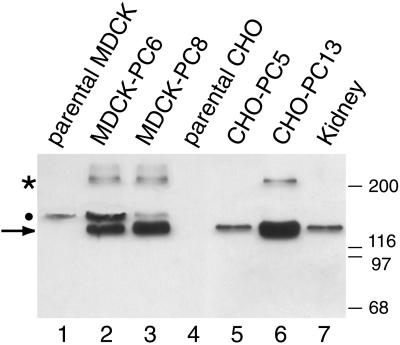
To investigate the effect of overexpression of PC on cell behavior, we transfected rat PC cDNA into CHO-K1 and MDCK cells. We obtained several cell lines stably expressing PC (CHO-PC5, lowest expression; CHO-PC13, highest expression; and CHO-PC14, moderate expression; MDCK-PC6, moderate expression; and MDCK-PC8, high expression) (Figure 3). By SDS-PAGE, PC expressed in transfected CHO-K1 or MDCK cells appears as two bands, ∼140 kDa (major) and 250 kDa (minor), comparable to those seen for PC in rat kidney. The high apparent molecular mass compared with its predicted molecular weight is characteristic of heavily O-glycosylated glycoproteins. The 250-kDa band most likely represents a dimer of PC and is recognized by both monoclonal and polyclonal anti-PC (Dekan et al., 1991).
Figure 3.
Immunoblotting analysis of PC expressed in CHO-K1 and MDCK cells. PC (arrow) is expressed at high levels in stably transfected MDCK-PC8 (lane 3) and CHO-PC13 (lane 6) cells and at lower levels in MDCK-PC6 (lane 2) and CHO-PC5 (lane 5) cells. PC is seen as a 140-kDa band comparable to that found in rat kidney lysates (lane 7). PC is not expressed in parental CHO (lane 4) or MDCK (lane 1) cells. The 250-kDa band (asterisk), previously described in rat kidney (Dekan et al., 1991), most likely represents a dimer of PC. A nonspecific, 160-kDa band (dot) that cross-reacts with anti-PC serum (0601) is seen in nontransfected and transfected MDCK cells. Cells stably transfected with the pIRES-PC expression vector were lysed in TDU buffer, as described in MATERIALS AND METHODS. A total of 100 μg of cell lysate (lanes 1–6) or kidney lysate (lane 7) was separated by 7.5% SDS-PAGE and immunoblotted with anti-PC serum (0601). Markers indicate molecular mass in kilodaltons.
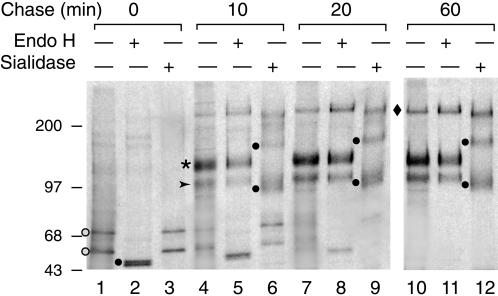
To examine the biosynthesis and intracellular processing of PC, we performed pulse-chase experiments on transfected MDCK cells (Figure 4). At 0 min (end of a 15-min pulse), two precursors, ∼70 and ∼55 kDa, were found (Figure 4, lane 1). Both species were susceptible to endo H digestion, yielding a doublet of ∼50 kDa (Figure 4, lane 2). At 10 and 20 min of chase, two broader bands, ∼140 and ∼100 kDa, appeared (Figure 4, lanes 4 and 7). Both were insensitive to endo H but were sensitive to sialidase digestion, yielding ∼170- and ∼95-kDa bands. This anomalous migration behavior resulting from loss of negative charge is characteristic of PC from rat kidney (Dekan et al., 1991) and is not unusual for heavily sialylated glycoproteins. After 20 min of chase, a 250-kDa band accumulated that most likely represents a dimer of PC. At 60 min of chase, the ∼70- and ∼55-kDa precursors were no longer present (Figure 4, lane 10). This indicates that most of the PC is converted to an endo H–resistant, sialidase-sensitive form by 60 min of chase.
Figure 4.
Pulse-chase analysis of PC processing. At the end of the pulse (0 min, lane 1), two precursors of ∼70 and ∼55 kDa (open circles) are seen that are sensitive to endo H digestion (lane 2), yielding an ∼50-kDa doublet (closed circle). At 10 min of chase (lane 4), two bands, a mature glycosylated ∼140-kDa band (asterisk) and an immature glycosylated ∼100-kDa band (arrowhead), are seen. Both are insensitive to endo H but sensitive to sialidase digestion, yielding ∼170- and ∼95-kDa bands (closed circles). After 20 min of chase (lanes 7–9), a 250-kDa band gradually accumulates that most likely represents a dimer of PC (diamond). At 60 min of chase (lanes 10–12), the two PC precursors (∼70 and ∼55 kDa) are no longer present, but the two sialidase-sensitive bands persist. Size markers indicate molecular mass in kilodaltons. MDCK-PC8 cells, stably expressing PC, were pulsed for 15 min with [35S]EasyTag Express protein-labeling mix and chased in medium containing excess unlabeled methionine and cysteine as described in MATERIALS AND METHODS. Lysates were collected at 0–60 min after pulse, and PC was immunoprecipitated with affinity-purified anti-PC IgG (0601). Immunoprecipitates were incubated with or without endo H (Streptomyces plicatus) or sialidase (Arthrobacter ureafaciens), separated by SDS-PAGE, and detected by fluorography.
By immunofluorescence, PC was distributed diffusely over the entire cell surface (basal as well as apical) in transfected, nonpolarized CHO-K1 cells (Figure 5a), whereas in polarized MDCK cells, PC was expressed in a punctate pattern only on the apical surface (Figure 5, b and c). This indicates that overexpressed PC is correctly targeted to the apical plasma membrane in MDCK cells, which is equivalent to the location of endogenous PC in the glomerular epithelium and vascular endothelium (Kerjaschki et al., 1984; Horvat et al., 1986; Schnabel et al., 1989). No staining was detected in nontransfected cells (see Figure 9a).
Figure 5.
Immunofluorescence staining of PC in transfected CHO-K1 and MDCK cells. (a) In nonpolarized CHO-PC13 cells stably expressing PC, PC is distributed diffusely over the entire cell surface. (b and c) In MDCK-PC8 cells, PC is expressed in a punctate pattern on the apical surface (above the level of the TJ) as seen in both en face (b) and in X-Z cross-sections (c) taken in 0.1-μm steps through the cells. Thus, PC is correctly targeted to the apical plasma membrane in MDCK cells, which is equivalent to its location in the glomerular epithelium. Cells were grown on glass coverslips, fixed in paraformaldehyde, permeabilized, and immunostained with anti-PC mAb 5A as described in MATERIALS AND METHODS. Bars, 10 μm.
Figure 9.
Effect of PC overexpression on distribution of occludin, ZO-1, and pan-cadherin in MDCK cells. Localization of PC, occludin, ZO-1, and cadherin in parental MDCK cells (a, c, e, and g) and MDCK-PC8 cells stably overexpressing PC (b, d, f, and h). A honeycomb-like staining pattern is observed for both occludin (c) and ZO-1 (e) in parental cells when the focus is at the TJ level. In transfected MDCK cells, staining for both occludin (d) and ZO-1 (f) is more concentrated at the intersection (arrowheads) of cell–cell boundaries where three cells meet. In parental cells, staining for cadherin is uniform along the entire lateral membrane (g), whereas in transfected MDCK-PC8 cells (h), cadherin staining is less uniform and is more concentrated between the intersections (asterisks), rather than at the intersections (arrows), when the focus is at the level of the junctions. Cells were fixed in 2% paraformaldehyde and processed for double immunofluorescence as described in MATERIALS AND METHODS. Bars, 10 μm.
Expression of PC Decreases Cell–Cell Adhesion in CHO-K1 and MDCK Cells
To investigate the effects of PC on cell adhesion, the ability of the transfectants to aggregate was assessed using a well-established cell aggregation assay (Takeichi, 1977). CHO-PC13 cells, the highest PC expression clone, showed complete inhibition of aggregation in both the presence and absence of Ca2+, whereas CHO-PC5 cells, the lowest expression clone, showed ∼35% reduction in aggregation in both the presence and absence of Ca2+ (Figure 6a). In addition, aggregation was slower in the absence of Ca2+. Similarly, in MDCK-PC6 and MDCK-PC8 cell lines, aggregation was reduced by 60 and 80% (Figure 6b). These results indicate that the cells expressing PC do not aggregate as efficiently as parental cells, that the expression of PC has a marked antiadhesion effect on both cell types, and that this effect directly correlates with the level of PC expression.
Figure 6.
Antiadhesion effect of PC expression on cell aggregation. (A) Aggregation assay of parental CHO-K1 cells and CHO-PC5 and CHO-PC13 cells stably expressing PC. CHO-PC13 cells, the highest PC expression clone, showed complete inhibition of aggregation in both the presence and absence of Ca2+. CHO-PC5 cells, expressing the lowest level of PC, showed a 35% reduction in aggregation after 180 min of incubation in both the presence and absence of Ca2+. However, aggregation was slower in the absence of Ca2+, because it was reduced at 60 and 120 min compared with parental CHO cells. (B) Aggregation assay of parental MDCK cells and MDCK-PC6 and MDCK-PC8 cells stably overexpressing PC. Aggregation in MDCK-PC6 and MDCK-PC8 cells was reduced 60 and 80%, respectively, compared with parental cells. Cell suspensions were seeded and allowed to aggregate for 0 to 180 min in the presence of 1 mM CaCl2 or 1 mM EGTA (in the case of CHO cells) at 37°C as described in MATERIALS AND METHODS. Aggregation was estimated by the following formula: % aggregation = (N0 − Nt)/N0 × 100, where Nt is the total number of particles at the incubation time t and N0 is the total number of cells. Results (means ± SD) represent the average of values obtained in three independent experiments.
Correlation between PC Expression and Antiadhesion Effects on PC-inducible EcR-CHO Cells
The contributions of PC to cell surface properties can be more precisely quantitated by altering its expression level with an inducible system. In EcR-CHO-PC cells induced with ponasterone A (0.1–30 μM) for 24 h, we found a linear increase in the expression of PC with increasing amounts of added hormone up to 10 μM (Figure 7a). By immunofluorescence, PC was distributed over the entire cell surface, as in CHO-K1 cells stably expressing PC. The expression level of PC induced by increasing levels of ponasterone A correlated closely with the antiadhesion effects, i.e., cells induced with 1, 3, or 10 μM ponasterone A showed slight (10%), moderate (40%), or nearly complete (90%) inhibition of aggregation (Figure 7b), respectively.
Figure 7.
Correlation between PC expression levels and the antiadhesion effect of PC-inducible EcR-CHO cells. (A) Expression of PC in inducible EcR-CHO-PC cells stably expressing PC. By immunoblotting, increasing amounts of PC are detected in cells induced with increasing amounts (0.1–10 μM) of ponasterone A. Both the 140- and 250-kDa PC bands are expressed. Cells stably transfected with the inducible expression vector pIND-PC were treated with 0.1–10 μM ponasterone A for 24 h and lysed in TDU buffer, after which 100 μg of cell lysate or kidney lysate (right lane) was separated by 7.5% SDS-PAGE and immunoblotted with anti-PC serum (0601). (B) Aggregation assay of noninduced and induced EcR-CHO-PC cells. The antiadhesion effect correlates closely with the expression level of PC induced by increasing amounts of ponasterone A. Cells induced with 1, 3, or 10 μM ponasterone A showed slight (10%), moderate (40%), or nearly complete (90%) inhibition of aggregation, respectively. At 24 h before their detachment, cells were treated with the indicated amounts of ponasterone A, followed by quantitation of cell aggregation, as in Figure 6. Results (means ± SD) represent the average of values obtained in three independent experiments.
The Antiadhesion Effect of PC Is Reversed by Sialidase Treatment
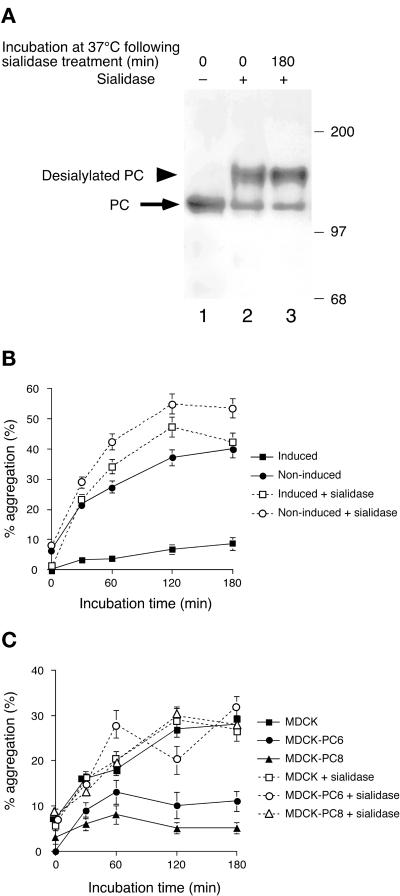
PC is heavily sialylated, which contributes significantly to its high net negative charge (isoelectric point = 4.8). To examine whether the antiadhesion effect of PC is due to sialic acid, we removed the majority of the sialic acid residues by treating both parental and transfected cells with sialidase (A. ureafaciens) before carrying out the cell aggregation assay. The mobility of the majority (∼80%) of the mature PC was markedly decreased after sialidase treatment (Figure 8a, compare lanes 1 and 2). Subsequent incubation of the cells at 37°C for 180 min, the time necessary for the cell aggregation assay, did not significantly change the mobility of PC (Figure 8a, lane 3), indicating that desialylated PC did not acquire new sialic acid residues during this time.
Figure 8.
Effects of sialidase treatment of EcR-CHO-PC cells on cell aggregation. (A) Immunoblotting of PC (arrow) in EcR-CHO-PC cells before (lane 1) and after (lane 2) sialidase treatment. Most of the sialic acid residues have been removed by sialidase treatment, as indicated by the fact that the mobility of PC is markedly decreased (arrowhead). The PC that is not affected by the treatment (lanes 2 and 3) may represent an intracellular pool. (B) Aggregation assay of noninduced or induced EcR-CHO-PC cells after sialidase treatment. The degree of aggregation of the induced (10 μM ponasterone A for 24 h) EcR-CHO-PC cells is increased approximately fivefold after sialidase treatment. (C) Similar aggregation assay of MDCK, MDCK-PC6, and MDCK-PC8 cells after sialidase treatment. MDCK-PC6and MDCK-PC8 cells also show a significant increase in their degree of aggregation (approximately threefold and fivefold, respectively) after sialidase treatment, whereas parental cells are not affected by this treatment. Results (means ± SD) represent the average of values obtained in three independent experiments. Cells were induced with 10 μM ponasterone A for 24 h, detached and resuspended as in Figure 6, and treated for 30 min with or without sialidase (Arthrobacter ureafaciens) at room temperature, and aggregation was quantitated as in Figure 6 (b and c). In some cases (a), cells were lysed (lanes 1 and 2) or first incubated for 180 min at 37°C, as in the cell aggregation assay (lane 3). Lysates (100 μg) were separated by 6% SDS-PAGE and immunoblotted with anti-PC serum (0601). Size markers indicate molecular mass in kilodaltons.
When cell aggregation assays were performed after sialidase treatment, the degree of aggregation of the induced (10 μM ponasterone A for 24 h) EcR-CHO-PC cells was increased (approximately fivefold) after sialidase treatment (Figure 8b). The uninduced EcR-CHO-PC cells also showed a significant increase in aggregation (∼40%) after sialidase treatment, indicating that CHO cells express endogenous, antiadhesion sialoproteins on their cell surface.
MDCK-PC6 and MDCK-PC8 cells also showed significant increases (approximately threefold and fivefold, respectively) in aggregation, whereas parental cells were not affected by sialidase treatment (Figure 8c). These results indicate that sialic acid is responsible for the antiadhesion effect of overexpressed PC.
PC Expression Affects the Distribution of AJ and TJ Proteins and TER
Because the expression of PC during glomerular development is closely coupled to the appearance of open intercellular spaces and the disappearance of TJ and AJ (Schnabel et al., 1989), we examined if PC overexpression affects TJ or AJ morphology or function in cultured cells. No obvious changes in the location of TJ or AJ in transfected versus parental cells was detected by routine transmission electron microscopy.
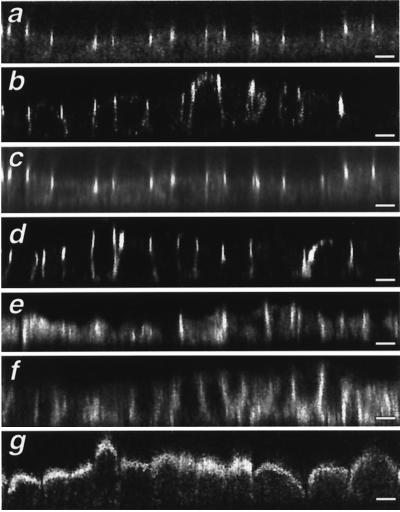
Next, we analyzed the localization of several junctional proteins by immunofluorescence. Parental MDCK cells showed a “honeycomb-like” staining pattern for both occludin (Figure 9c) and ZO-1 (Figure 9e) when the focus was at the TJ level. In MDCK cells stably expressing PC, immunostaining for both TJ proteins was more concentrated at the cell–cell boundaries where three cells intersect (Figure 9, d and f, arrowheads). Differences in cadherin staining were also seen in PC-transfected cells. In parental MDCK cells, E-cadherin was distributed uniformly around the cells when the focus was at the middle of the cells (Figure 9g), whereas in transfected cells, the pattern of staining was less uniform (Figure 9h). The distribution of junctional proteins and overexpressed PC was examined further in X-Z cross-sections by confocal laser scanning microscopy (Figure 10). In parental cells, occludin and ZO-1 colocalized and were found in the usual location of TJ, i.e., along the apical cell surface where the apical and lateral cell membranes meet (Figure 10, a and c). In transfected cells, both TJ proteins were also restricted to junctions between the apical and lateral membranes; however, the staining intensity was more variable compared with that in parental cells. (Figure 10, b and d). The distribution of overexpressed PC (Figure 10g) did not overlap with that of occludin or ZO-1. The most striking change was in cadherin staining, which was found to shift toward the basal cell surface (Figure 10f) in transfected cells (Figure 10e). By immunoblotting, no effect of PC overexpression on the expression levels of junctional proteins (E-cadherin, occludin, or ZO-1) was seen in either CHO-K1 or MDCK cells.
Figure 10.
Confocal microscopic analysis of the distribution of occludin, ZO-1, and PC in MDCK cells. X-Z sections (0.1-μm motor step) through parental MDCK cells (a, c, and e) and MDCK-PC8 cells stably expressing PC (b, d, f, and g). In both cases, occludin (a and b) and ZO-1 are restricted to junctions between adjoining cells; however, in cells expressing PC, the staining intensity of these TJ proteins between pairs of cells is more variable (b and d). The distribution of overexpressed PC (g) did not overlap that of occludin or ZO-1. Cadherin staining is more broadly distributed along the lateral cell surface extending more basally in MDCK-PC8 (f) than in parental cells (e). Bars, 10 μ m.
Because we observed a change in the distribution of AJ and TJ proteins, it was of particular interest to determine whether these cells would still form an electrically tight epithelial monolayer. Therefore, we next measured TER of monolayers formed by either parental or transfected MDCK cells on a permeable support. Because expression of PC did not affect the number of cells per filter, TER from different cell lines could be compared directly. As shown in Table 1, MDCK-PC6 and MDCK-PC8 cells exhibited ∼20 and 40% reductions in TER, respectively, compared with parental cells. To determine whether sialic acid is important for the effect of PC expression on TER, we measured TER after sialidase treatment of MDCK-PC8 cells. We found that the effect of PC expression on TER is completely reversed by sialidase treatment but does not affect the TER of parental MDCK cells (Table 2). We conclude that PC overexpression affects the electrical tightness of the MDCK monolayer, that the decrease in TER correlates inversely with the amount of PC expressed (Figure 3 and Table 1), and that the effect is due to sialic acid present on PC.
Table 1.
TER in parental MDCK cells and MDCK cells stably overexpressing PC
 |
Values are means ± SE of multiple determinations on four different cell layers. MDCK-PC6 and MDCK-PC8 cells had significantly lower resistances than wild-type MDCK cells (p < 0.02 and 0.001 in t test, respectively.)
Table 2.
The effect of sialidase treatment on TER in parental MDCK and MDCK-PC8 cells
 |
Values are means ± SE of multiple determinations on four different cell layers. Sialidase treatment significantly increased the TER of MDCK-PC8 cell monolayers but did not affect the TER of parental MDCK cells (p < 0.001 and p = 0.56 in t test, respectively). After sialidase treatment, MDCK-PC8 cells showed virtually the same resistance as parental MDCK cells.
DISCUSSION
This is the first report to demonstrate the antiadhesion function of PC and to obtain the complete amino acid sequence of rat PC. The data clearly demonstrate that the rat protein is a homologue of the previously cloned rabbit (Kershaw et al., 1995) and human PC-like proteins (Kershaw et al., 1997) and chicken thrombomucin (McNagny et al., 1997).
Although PC is expressed on the endothelium and podocyte in situ, its expression is rapidly lost from the cell surface of podocytes as they grow out from glomeruli in culture (Holthofer et al., 1991; Yaoita et al., 1995) and from podocytes or endothelial cell lines (Delarue et al., 1991; Ardaillou et al., 1992). Therefore, until now it has been impossible to study the functions of PC in cultured cells. Using stably transfected CHO-K1, MDCK, and inducible EcR-CHO cells, we have shown that overexpression of PC inhibits cell–cell adhesion in an expression level–dependent manner and that the antiadhesion effect is due to sialic acid residues present on PC. We further report the surprising finding that overexpression of PC affects the distribution of AJ and TJ proteins and TER. We verified that its targeting properties resemble those in vivo, because PC is localized exclusively on the apical membrane of MDCK cells. Moreover, the abundance of mature, glycosylated protein in these cells indicates that it is for the most part processed in a physiological manner. The availability of cell lines stably expressing PC will be quite valuable for understanding the functions of PC and its effects on cells.
Antiadhesion effects have also been reported for other so-called membrane-associated mucins. For example, epiglycanin, which is highly expressed on the TA3/Ha mouse mammary tumor cell line, inhibits E-cadherin and integrin-mediated adhesion (Kemperman et al., 1994). Similarly, ectopic expression of episialin in adherent cell lines also inhibits E-cadherin and integrin-mediated adhesion (Wesseling et al., 1995, 1996). It has been postulated that a high density of mucin-like oligosaccharides at the cell surface can prevent cell–cell adhesion either through steric hindrance by masking adhesion molecules or by charge repulsion as a result of the presence of abundant sialic acid residues, which give these molecules a high negative charge. In the case of PC, we demonstrated that charge repulsion is the main mechanism involved, because removal of sialic acid from cells overexpressing PC restores normal adhesion properties. The neuronal cell adhesion molecule N-CAM has also been shown to reduce cell–cell and cell–matrix adhesion by charge repulsion when the molecule is polysialylated (Ardman et al., 1992; Yang et al., 1992; Rutishauser and Landmesser, 1996).
So far, a small number of podocyte membrane proteins have been defined, including megalin, an endocytic receptor located in clathrin-coated vesicles that mediates uptake of a number of ligands and serves as antigenic target in rat Heymann nephritis (Saito et al., 1994; Farquhar et al., 1995); glomerular epithelial protein 1, a transmembrane protein tyrosine phosphatase located on the apical surface of foot processes (Thomas et al., 1994); and podoplanin, a 43-kDa membrane glycoprotein also localized mainly (90%) on the apical surface of the glomerular epithelium (Breiteneder-Geleff et al., 1997). Podoplanin mediates collapse of the characteristic podocyte architecture when complexed by antibodies in situ (Matsui et al., 1998). It is expressed not only in the glomerular epithelium but also in extrarenal tissues, including nonpolarized cells, and its function remains to be determined. α3β1 integrin, found on the basolateral surface of podocytes, also appears to be required for the formation of mature foot processes, because α3β1-deficient mice failed to form normal foot processes (Kreidberg et al., 1996; Wang et al., 1999).
Several junctional proteins have also been localized in glomeruli, including a special isoform of ZO-1, ZO-1α, that is present only in labile TJ (Kurihara et al., 1992a; Balda and Anderson, 1993) and is concentrated in the cytoplasm along the slit diaphragms, P-cadherin (Reiser et al., 2000), and nephrin, a putative transmembrane protein and a member of the immunoglobulin family of cell adhesion molecules. Nephrin is expressed exclusively in the glomerulus, where it is located in the slit diaphragm region (Holzman et al., 1999; Ruotsalainen et al., 1999). The nephrin gene is mutated in the congenital nephrotic syndrome of the Finnish type, leading to loss of the foot process organization in glomeruli (Kestila et al., 1998; Lenkkeri et al., 1999). Nephrin and P-cadherin are believed to contribute to the formation of the slit diaphragm, which is proposed to represent a modified AJ (Reiser et al., 2000).
In the developing kidney, PC appears on the glomerular epithelium at the early S-shaped body stage, when the epithelium begins to differentiate, and its expression is closely coupled to the appearance and interdigitation of foot processes, the appearance of open intercellular spaces, junctional migration, the disappearance of typical TJs and AJ, and the appearance of slit diaphragms (Schnabel et al., 1989). As the glomerular epithelium matures, PC is restricted to the membranes above the level of the slit diaphragms (Schnabel et al., 1989). It has also been demonstrated that neutralization of the glomerular surface charge by perfusion of polycations or removal of sialic acid residues by sialidase digestion in vivo causes a narrowing or collapse of the filtration slits, spreading and simplification of the foot processes (Seiler et al., 1977), and tyrosine phosphorylation of ZO-1 (Kurihara et al., 1995). These findings emphasize the lability of the slit structure and its dependence on the negative surface charge of the podocyte, which is carried for the most part by PC. The present study supports these findings and provides direct evidence that PC functions as an antiadhesin that maintains an open filtration pathway between neighboring foot processes. By immunofluorescence, we showed the relocalization of cadherin, occludin, and ZO-1 in MDCK cells overexpressing PC. In particular, cadherin expression seems to be more affected than does the expression of occludin or ZO-1.
How does PC affect the localization of AJ and TJ proteins? We postulate that the mechanism involves interaction of both AJ and TJ proteins and PC with the actin cytoskeleton. The AJ consists of integral membrane proteins (cadherins) and cytoplasmic proteins (α-, β-, and γ-catenins) that link cadherins to the actin cytoskeleton (Yap et al., 1997; Steinberg and McNutt, 1999). The TJ is also composed of both integral (occludin, claudin) and peripheral (ZO-1, -2, and -3, cingulin) membrane proteins linked to the actin cytoskeleton (Mitic and Anderson, 1998; Stevenson and Keon, 1998; Fanning et al., 1999; Tsukita and Furuse, 1999). TJs function as seals to restrict the passage of proteins through the intercellular spaces, as fences to prevent the mixing of apical and basolateral plasmalemmal domains, and as channels to regulate the passage of ions and water through the intercellular spaces (Mitic and Anderson, 1998; Stevenson and Keon, 1998; Fanning et al., 1999). Many lines of evidence suggest that paracellular permeability through TJ is influenced by the state of perijunctional actin (Madara et al., 1992). Many signaling molecules are concentrated along the AJ and TJ, including tyrosine kinases, Ca2+, PKC, heterotrimeric G proteins, calmodulin, cAMP, lipid second messengers, and phospholipase C (Mitic and Anderson, 1998), and these have been reported to affect TJ permeability, presumably through changes in actin organization.
The organization of the epithelial foot processes and glomerular filtration slits is also known to be influenced by the state of the actin cytoskeleton, as originally suggested by the finding that the change in the shape of podocytes and the loss of foot processes and filtration slits that occur when glomeruli are placed in culture are prevented when glomeruli are incubated in the presence of cytochalasin, a potent inhibitor of actin polymerization (Andrews and Stauver, 1979; Andrews, 1981). Immunoelectron microscopic studies have shown a high concentration of actin, α-actinin, and myosin in the foot processes of podocytes (Drenckhahn and Franke, 1988). Moreover, we (Kurihara et al., 1995) and others (Hugo et al., 1998) have shown that the cytoskeletal linker protein ezrin is concentrated in the foot processes. Ezrin is a member of the ERM protein family that serves to link membrane proteins to the actin cytoskeleton and to regulate cell adhesion and morphogenesis (Mangeat et al., 1999). The distribution of ezrin overlaps that of PC in the glomerular epithelium, in that it is found in a band underlying the apical membrane of podocytes and is excluded from the area of the slit membrane (Kurihara et al., 1995). This leads us to propose that PC may interact with ezrin or a similar linker protein that connects PC with the actin cytoskeleton and appropriate signaling networks to promote PC-induced distribution of junctional proteins and the decrease in TER.
Our present data, together with earlier observations, demonstrate that the main function of PC is to maintain open slits between neighboring foot processes in the glomerular epithelium. PC on endothelia may also help to keep the vascular lumen open and repel circulating leukocytes. Because PC has recently been found to be present in thrombocytes (McNagny et al., 1997; Miettinen et al., 1999), it may also prevent thrombocytes from inappropriately adhering to endothelia.
Several remaining intriguing questions require further elucidation. In particular, information on the interactions of the highly conserved cytoplasmic domain of PC is needed and should shed light on its biological functions.
ACKNOWLEDGMENTS
We thank Matthew Hickman for help in the preparation of antibodies and Dr. Larry Goldstein (Department of Cellular and Molecular Medicine and Howard Hughes Medical Research Institute, University of California San Diego) for the use of his Bio-Rad MRC 1024 confocal microscope. W.Y.G is a member of the Medical Scientist (MD/PhD) Training Program at the University of California San Diego. This research was supported by grant DK17724 to M.G.F. from the National Institutes of Health. T.T. was supported in part by a fellowship from the Nakatomi Foundation (Tokyo, Japan).
Abbreviations used:
- AJ
adherens junction
- CHO
Chinese hamster ovary
- EcR
ecdysone receptor–expressing
- endo H
endoglycosidase H
- MDCK
Madin-Darby canine kidney
- PC
podocalyxin
- RACE
rapid amplification of cDNA ends
- TER
transepithelial electrical resistance
- TJ
tight junction
Footnotes
The rat podocalyxin cDNA sequence reported in this paper has been deposited in the GenBank database (accession number AF109393).
REFERENCES
- Andrews PM. Investigations of cytoplasmic contractile and cytoskeletal elements in the kidney glomerulus. Kidney Int. 1981;20:549–562. doi: 10.1038/ki.1981.176. [DOI] [PubMed] [Google Scholar]
- Andrews PM, Stauver M. In vitro incubation and study of kidney glomerular epithelial cells. Kidney Int. 1979;15:80–87. doi: 10.1038/ki.1979.11. [DOI] [PubMed] [Google Scholar]
- Ardaillou N, Lelongt B, Turner N, Piedagnel R, Baudouin B, Estrade S, Cassingena R, Ronco PM. Characterization of a simian virus 40-transformed human podocyte cell line producing type IV collagen and exhibiting polarized response to atrial natriuretic peptide. J Cell Physiol. 1992;152:599–616. doi: 10.1002/jcp.1041520320. [DOI] [PubMed] [Google Scholar]
- Ardman B, Sikorski MA, Staunton DE. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci USA. 1992;89:5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–C924. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- Dekan G, Gabel C, Farquhar MG. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci USA. 1991;88:5398–5402. doi: 10.1073/pnas.88.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue F, Virone A, Hagege J, Lacave R, Peraldi MN, Adida C, Rondeau E, Feunteun J, Sraer JD. Stable cell line of T-SV40 immortalized human glomerular visceral epithelial cells. Kidney Int. 1991;40:906–912. doi: 10.1038/ki.1991.292. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Saito A, Kerjaschki D, Orlando RA. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol. 1995;6:35–47. doi: 10.1681/ASN.V6135. [DOI] [PubMed] [Google Scholar]
- Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- Holthofer H, Sainio K, Miettinen A. Rat glomerular cells do not express podocytic markers when cultured in vitro. Lab Invest. 1991;65:548–557. [PubMed] [Google Scholar]
- Holzman LB, John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D. Endothelial cell membranes contain podocalyxin—the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102:484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo C, Nangaku M, Shankland SJ, Pichler R, Gordon K, Amieva MR, Couser WG, Furthmayr H, Johnson RJ. The plasma membrane-actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int. 1998;54:1934–1944. doi: 10.1046/j.1523-1755.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- Kemperman H, Wijnands Y, Wesseling J, Niessen CM, Sonnenberg A, Roos E. The mucin epiglycanin on TA3/Ha carcinoma cells prevents alpha 6 beta 4-mediated adhesion to laminin and kalinin and E-cadherin-mediated cell-cell interaction. J Cell Biol. 1994;127:2071–2080. doi: 10.1083/jcb.127.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Vernillo AT, Farquhar MG. Reduced sialylation of podocalyxin—the major sialoprotein of the rat kidney glomerulus—in aminonucleoside nephrosis. Am J Pathol. 1985;118:343–349. [PMC free article] [PubMed] [Google Scholar]
- Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC. Molecular cloning and characterization of human podocalyxin-like protein: orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem. 1997;272:15708–15714. doi: 10.1074/jbc.272.25.15708. [DOI] [PubMed] [Google Scholar]
- Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC. Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem. 1995;270:29439–29446. doi: 10.1074/jbc.270.49.29439. [DOI] [PubMed] [Google Scholar]
- Kestila M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1992a;89:7075–7079. doi: 10.1073/pnas.89.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol. 1995;268:F514–F524. doi: 10.1152/ajprenal.1995.268.3.F514. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992b;141:805–816. [PMC free article] [PubMed] [Google Scholar]
- Lenkkeri U, et al. Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999;64:51–61. doi: 10.1086/302182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Parkos C, Colgan S, Nusrat A, Atisook K, Kaoutzani P. The movement of solutes and cells across tight junctions. Ann NY Acad Sci. 1992;664:47–60. doi: 10.1111/j.1749-6632.1992.tb39748.x. [DOI] [PubMed] [Google Scholar]
- Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K+-ATPase distributions in polarized epithelia. J Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Breiteneder-Geleff S, Kerjaschki D. Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am Soc Nephrol. 1998;9:2013–2026. doi: 10.1681/ASN.V9112013. [DOI] [PubMed] [Google Scholar]
- McNagny KM, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol. 1997;138:1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AF, Blau E, Vernier RL. Glomerular polyanion: alteration in aminonucleoside nephrosis. Lab Invest. 1970;23:649–657. [PubMed] [Google Scholar]
- Miettinen A, Dekan G, Farquhar MG. Monoclonal antibodies against membrane proteins of the rat glomerulus: immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990;137:929–944. [PMC free article] [PubMed] [Google Scholar]
- Miettinen A, Solin ML, Reivinen J, Juvonen E, Vaisanen R, Holthofer H. Podocalyxin in rat platelets and megakaryocytes. Am J Pathol. 1999;154:813–822. doi: 10.1016/S0002-9440(10)65328-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family Proc. Natl Acad Sci USA. 1994;91:9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E, Dekan G, Miettinen A, Farquhar MG. Biogenesis of podocalyxin—the major glomerular sialoglycoprotein—in the newborn rat kidney. Eur J Cell Biol. 1989;48:313–326. [PubMed] [Google Scholar]
- Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36:48–61. [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC. GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase: identification, molecular cloning, and characterization in rabbit. J Biol Chem. 1994;269:19953–19962. [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Symons JM, Goldstein SL, McDonald A, Miner JH, Kreidberg JA. α3β1 integrin regulates epithelial cytoskeletal organization. J Cell Sci. 1999;112:2925–2935. doi: 10.1242/jcs.112.17.2925. [DOI] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Yin X, Rutishauser U. Intercellular space is affected by the polysialic acid content of NCAM. J Cell Biol. 1992;116:1487–1496. doi: 10.1083/jcb.116.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita E, Yamamoto T, Takashima N, Kawasaki K, Kawachi H, Shimizu F, Kihara I. Visceral epithelial cells in rat glomerular cell culture. Eur J Cell Biol. 1995;67:136–144. [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]