Abstract
Gene delivery has shown potential in a wide variety of applications, including basic research, therapies for genetic and acquired diseases, and vaccination. Most available nonviral systems have serious drawbacks such as the inability to control and scale the production process in a reproducible manner. Here, we demonstrate a biotechnologically feasible approach for gene delivery, using synthetic cationic amphipathic peptides containing a variable number of histidine residues. Gene transfer to different cell lines in vitro was achieved with an efficiency comparable to commercially available reagents. We provide evidence that the transfection efficiency depends on the number and positioning of histidine residues in the peptide as well as on the pH at which the in-plane to transmembrane transition takes place. Endosomal acidification is also required. Interestingly, even when complexed to DNA these peptides maintain a high level of antibacterial activity, opening the possibility of treating the genetic defect and the bacterial infections associated with cystic fibrosis with a single compound. Thus, this family of peptides represents a new class of agents that may have broad utility for gene transfer and gene therapy applications.
In contrast to most polymers, key parameters such as product identification and quality control are possible for synthetic peptides. Although these compounds offer in addition the possibility of a reproducible and scaleable production process, synthetic peptide-based gene delivery systems are among the least explored.
Until recently, peptides were mostly used as auxiliary agents in combination with other systems (1), providing either a cell targeting moiety (2), a membrane destabilization activity (3), or a nuclear localization function (4). A strategy that is beginning to emerge is the use of multifunctional peptides. Peptides such as KALA (5), ppTG20 (6), and Vpr52–96 (7) at the same time bind DNA and destabilize membranes, two properties that are indispensable for efficient gene transfer in dividing cells. Of note, the two former peptides combine a positively charged lysine or arginine stretch required for DNA binding and an amphipathic membrane-destabilizing domain deriving from the fusogenic peptides GALA (5) and JTS-1 (8). These transfecting peptides have a strong propensity for an α-helical conformation that positions the lysines or arginines on one face of the helix.
Another strategy to achieve efficient release of the delivered material from endosomes consists in using compounds such as polyethyleneimines (PEIs) that are able to capture protons entering the endosome. This induces swelling of the endosomes that leads to membrane disruption (9, 10).
Here we present peptides that exhibit efficient gene transfer activity by mimicking partially the proton sponge activity of PEIs. Amphipathic peptides that are rich in alanine and leucine residues were used as a template. Various numbers of lysine and histidine residues were included in the peptide sequence (Tables 1 and 2). Whereas the lysines at both ends of the peptides might serve for DNA condensation, the histidine residues might favor endosomal escape of the DNA (11).
Table 1.
Sequence of histidine-containing cationic peptides
| Peptide | Sequence | DNA retardation, μg of peptide* |
|---|---|---|
| LAH1 | KKLALALALALHALALALALKKA | 2.5 |
| LAH2 | KKLAHLALALALGLALAHLAKKA | 2.5 |
| LAH3 | KKALALGLHLAHLALHLALALKKA | 2.5 |
| LAH4 | KKALLALALHHLAHLALHLALALKKA | 2.5 |
| LAH5 | KKALLALALHHLAHLAHHLALALKKA | 2.5 |
Amount of peptide (μg) needed for complete retardation of 1 μg of DNA.
Table 2.
LAH4 derivatives and histatin-5
| Peptide | Sequence | Retardation assay, μg of peptide* | Transfection efficiency† | Optimal ratio, μg of peptide/μg of DNA‡ |
|---|---|---|---|---|
| LAH4 | KKALLALALHHLAHLALHLALALKKA | 2.5 | 1,000 | 6 |
| LAK4 | KKLAKALAKALAKALKLALALAKK | 2.5 | ≤1 | 2.5 |
| Histatin-5 | DSHAKRHHGYKRKFHEKHHSHRGY | >50§ | <1 | 7.5 |
| LAH4-L3 | KKALLALALHHLALLAHHLALALKKA-NH2 | <2.5 | 1,150 | 6 |
| LAH4-L4 | KKALLALALHHLALLAHLLALHLKKA-NH2 | 1 | 510 | 4 |
| LAH4-A6 | KKKKALAHLHALAAHLHALAAAALKKK | 2.5 | 140 | 15 |
| LAH4-L6 | KKKKALLHLHLLALHLHLLALLALKKK | 2.5 | 10 | 12 |
| LAH4-G6 | KKKKALGHLHGLAGHLHGLAGGALKK | >50§ | ≤1 | 7.5 |
| H4-LAK4 | HHALLALALKKLAKLALKLALALHHA | 1 | 20 | 5 |
Italics in first row indicate the peptide that was used as reference.
The indicated amount of peptide is the minimal amount required to completely retard 1 μg of plasmid DNA.
We gave the value 1,000 to the luciferase activity obtained with LAH4. Transfection experiments were performed on HepG2 cells.
Amount of peptide per μg of DNA that gives the highest reporter gene expression.
Some of the DNA was retarded, but not all.
Our results demonstrate that the presence of histidine and lysine residues is not sufficient to obtain an efficient transfection agent. Of crucial importance are the number of histidine residues, their position in the sequence, and the pH at which the peptides change from an in-plane to a transmembrane alignment. Because some of the peptides possess antimicrobial activities (12), we propose that antibacterial peptides with high transfection efficiency may have potential for the treatment of cystic fibrosis (CF) as the airways of CF patients are chronically infected with a variety of bacteria.
Materials and Methods
Plasmids.
SMD2-LucΔITR (7.6 kb) and eGFP-C1 (4.7 kb) are expression plasmids encoding the firefly luciferase gene and the green fluorescent protein gene (GFP), respectively, under the control of the human cytomegalovirus (CMV) immediate-early promoter.
Peptides.
Histatin-5 (purity >99%) was purchased from Bachem. H4-LAK4 (purity >98%) was from Synt:em (Nimes, France). LAH4 was synthesized by the authors and by Neosystem [Strasbourg, France; theoretical molecular weight = 2,779.5, molecular weight determined by ES+ (electrospray) = 2,780, purity = 98%]. The different batches of LAH4 had indistinguishable transfection properties. All other peptides were prepared by automated solid-phase synthesis on Millipore 9030 and Applied Biosystems 431 synthesizers, using fluorenylmethoxycarbonyl (Fmoc) chemistry. In crude peptide preparations a predominant peak was observed when analyzed by HPLC with acetonitrile/water gradients. During HPLC purification the main peak was collected and the identity of the product confirmed by matrix-assisted laser desorption ionization (MALDI) MS.
Cell Culture.
DMEM (GIBCO/BRL) was supplemented with 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. Human hepatocarcinoma cells (HepG2 cells; American Type Culture Collection), rabbit aorta smooth muscle cells (Rb1 cells; kindly given by M. Nachtigal, University of South Carolina, Columbia), transformed HEK 293 cells (American Type Culture Collection), and mouse fibroblasts (NIH 3T3, American Type Culture Collection) were used.
Preparation of Polycation/DNA Complexes.
Four micrograms of DNA and the desired amount of 25-kDa PEI, poly l-lysine with a degree of polymerization of 180 (pLys180; Sigma), 1,2-dioleoyl-3-trimethylammonium propane (DOTAP, Avanti Polar Lipids), or peptide were each diluted in 100 μl of 150 mM NaCl. Transfection reagent and DNA solution were mixed and incubated for 20 min at room temperature. Before adding the complexes to the cells, the mixtures were diluted with serum-free medium to a final volume of 1 ml. For some peptides, the complexes were prepared in citrate buffer (pH 5.3).
DNA Retardation Assay.
DNA binding was studied by agarose gel retardation assays. DNA (1 μg) and increasing amounts of peptide were each diluted in 25 μl of 150 mM NaCl or citrate buffer and mixed. After 20 min, samples were electrophoresed through a 1% agarose gel by using TBE buffer (89 mM Tris/89 mM boric acid/2 mM EDTA, pH 8.3). DNA was visualized after ethidium bromide staining.
Transfection Experiments.
Cells were plated in 24-well plates and transfected at 50–80% confluency. For transfection, 0.5 ml of serum-free medium containing the DNA complexes was transferred into each well. After incubation for 2.5–4 h at 37°C, the medium was replaced with DMEM containing 10% FCS. Luciferase activity was measured 24–48 h posttransfection. For transfection experiments with bafilomycin A1 (Sigma, 175 nM) or chloroquine (100 μM), the drugs were added to the DNA complexes after dilution with medium. The luciferase assay was performed as described (13). Luciferase background was subtracted from each value and the transfection efficiency was expressed as total light units per 10 s per well (1 light unit = 10 counts) and are the means of duplicates. The protein content of transfected cells was measured by using the Bio-Rad protein assay. Cytotoxicity was evaluated by performing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, Sigma) assay. Briefly, 1 day after transfection, the cell culture medium was removed and replaced by serum-free DMEM containing 0.5 mg/ml MTT. After incubation at 37°C for 4 h, the medium was removed and 200 μl of DMSO was added to each well to dissolve the formazan crystals produced from the reduction of MTT by viable cells. Absorbance was then measured at 570 nm. Untreated cells were used as control.
Antibacterial Activity.
Escherichia coli (DH5α) was grown in LB until the mid-logarithmic phase. Cells were diluted with LB to OD600 = 0.15, and 200-μl aliquots were added to a 96-well microtiter plate containing 50 μl of a serial dilution of LAH4 with or without DNA. The pH of the culture was adjusted either to 7.4 or 5.5. The OD600 was measured after an incubation of 6 h at 37°C with constant shaking. One hundred percent of relative growth was determined with bacteria cultured in LB medium.
Southern Blot Analysis.
HepG2 cells were transfected with 3 μg of plasmid SMD2-LucΔITR in six-well plates, washed once with PBS, trypsinized, centrifuged, and washed again with PBS. After selective low-molecular-weight DNA extraction (14), the Southern blot analysis was performed as described (7).
Results
Gel-Mobility Shift Assay.
The peptides encompassing the series LAH1–LAH5 were first tested for their capacity to interact with DNA. Therefore, increasing amounts of peptide were mixed with a constant amount of plasmid and the complexes were electrophoresed through an agarose gel. As shown in Table 1, with 2.5 μg of peptide the retardation of DNA was complete. With higher amounts of peptide, the DNA was no more visible, showing that its condensation was sufficiently strong to prevent ethidium bromide intercalation.
Determination of the Transfection Activity.
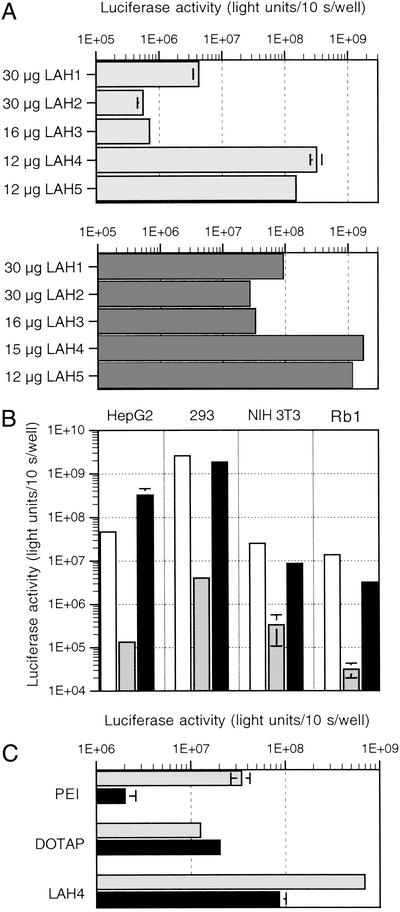
The transfection activity of the LAH series was evaluated on HEK 293 and HepG2 cells. The results presented in Fig. 1A show that the most efficient peptides were those containing four and five histidine residues. LAH1–LAH3 are at least 10 times less efficient than LAH4 or LAH5. In light of these results, we chose LAH4 to continue our study.
Figure 1.
Evaluation of the transfection efficiency of LAH peptides. Increasing amounts of transfection reagent were mixed with a constant amount of reporter plasmid [2 μg of cytomegalovirus (CMV)-Luc per duplicate]. The complexes were incubated for 3 h in serum-free medium. Luciferase activity was measured 24–43 h posttransfection. The transfection efficiency is expressed as total light units per 10 s per well, and the average of duplicates is shown. (A) Transfection efficiency of LAH peptides on HepG2 (Upper) and 293 (Lower) cells. (B) Comparison of the transfection efficiency of LAH4 vs. pLys180 (poly l-lysine with a degree of polymerization of 180) and 25-kDa PEI on four different cell lines. Only the best condition for each reagent is shown (open bars, PEI; gray bars, pLys; black bars, LAH4). (C) HepG2 cells were transfected with PEI [N/P = 13; N/P is the molar ratio of amino nitrogen (present in the transfection agent) to DNA phosphate], DOTAP (N/P = 4), or LAH4 in the presence (black bars) or absence (gray bars) of 175 nM bafilomycin A1.
By using GFP as a reporter gene, we determined that ≈50% of the HEK 293 cells were transfected with LAH4. Toxicity of LAH4/DNA complexes was evaluated after transfection by comparing the total amount of protein per well and the cell number of transfected and nontransfected cells. Thirty hours after transfection the protein content was 88% when compared with nontreated cells. The number of cells remained unchanged when transfection experiments were compared with controls. Thus, the treatment with this peptide was well tolerated by the cells.
Next, the transfection efficiency of LAH4 was compared with that of pLys180 and 25-kDa PEI. The optimal transfection conditions were determined by using increasing concentrations of reagents. The results show that LAH4 is significantly more efficient than polylysine on four different cell lines and that it exhibits an efficiency comparable to PEI (Fig. 1B). In comparison to the cationic peptide KALA, LAH4 mediates 200- and 40-times-higher luciferase expression in HepG2 and HEK 293 cells, respectively (not shown).
Inhibition of the Acidification of Endosomes.
It has recently been shown that inhibition of endosome acidification by H+-ATPase inhibitors such as bafilomycin A1 diminishes significantly the transfection efficiency of PEI. Because this polymer is protonated under physiological conditions, it is assumed that buffering of endosomes by PEI leads to their destabilization (13). We asked whether LAH4, which contains imidazole groups, exhibits similar properties. In the presence of bafilomycin A1, the transfection efficiency of PEI and LAH4 was diminished by 17- and 8-fold, respectively (Fig. 1C). In contrast, the efficiency of the monocationic lipid DOTAP, which is already completely protonated at pH 7, was not altered.
The neutralizing effect of chloroquine, a weak base that accumulates in acidic endocytic vesicles (15), did not influence luciferase expression (data not shown).
Evaluation of the Transfection Activity of LAH4 Variants and Histatin-5.
To obtain better insight into the mechanism of action of LAH4, different modifications were introduced into the peptide sequence. First, we tested whether the presence of histidine residues is required for efficient reporter gene expression. A peptide having only leucine, alanine, and lysine residues was synthesized and its ability to complex DNA and to transfect cells was determined. As shown in Table 2, although LAK4 retarded well the migration of plasmid DNA, it exhibited only poor gene transfer activity.
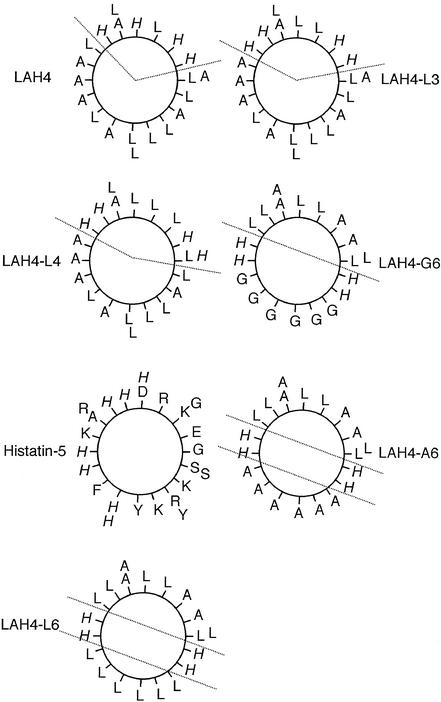
Second, we analyzed whether the presence of a well defined hydrophobic face is necessary for efficient DNA delivery (Fig. 2). Therefore, we included the salivary antimicrobial peptide histatin-5, which presents an equal distribution of hydrophilic residues along its surface (Fig. 2), in our assays (Table 2). Surprisingly, histatin-5 did not fully retard DNA migration, even though high amounts of peptide were used. This was still the case when the complexes were prepared at pH ≤ 5.3, which should allow the protonation of the imidazole groups. The transfection experiments confirmed that histatin-5 is an inefficient DNA carrier.
Figure 2.
Edmundson helical wheel diagrams. The helical wheel projections allow the visualization of the distribution of hydrophobic and polar residues with respect to the helical axis. The histidine residues are in italics.
Third, we studied whether the position of the four histidine residues in the hydrophilic face is of importance. The peptides LAH4, LAH4-L3, and LAH4-L4 (Table 2, Fig. 2) were used to transfect HepG2 cells. Although all three peptides mediated high reporter gene expression, some differences are observed: LAH4-L4 gives reproducibly slightly lower luciferase levels than LAH4 and LAH4-L3, suggesting that optimal transfection efficiency requires that the placement of four histidines in an Edmundson helical wheel diagram leave a high hydrophobic angle (Fig. 2).
Fourth, it was shown that LAH4 completely reorients from parallel to the surface into a transmembrane configuration when the pH is increased from 5 to 7 (16). The midpoint of this transition is pH50 = 6.1. To test whether this behavior is important for the mechanism of transfection, derivatives of LAH4 differing in the midpoint of their in-plane-to-transmembrane transition were tested: pH50 = 4.8 for LAH4-L6, 6.0 for LAH4-A6, and >8.5 for LAH4-G6 (17). Table 2 shows that LAH4-L6 and -A6 were able to complex DNA efficiently and to retard its electrophoretic migration, whereas LAH4-G6, which is considerably more hydrophilic, is unable to fully retard the migration of DNA, even when high doses of peptide are used. In transfection experiments, the three mutants are significantly less efficient than LAH4 (Table 2). Interestingly, LAH4-A6, the peptide having the closest transition midpoint to LAH4, was also the most efficient when compared with LAH4-L6 or -G6.
Finally, we tested whether inversion between lysine and histidine residues (H4-LAK4) affects the transfection efficiency. The results presented in Table 2 show that H4-LAK4 is significantly less active in gene transfer than LAH4.
The toxicity of the peptide/DNA complexes on HepG2 cells after transfection was monitored by measuring the total amount of protein per well and by estimating cell proliferation by the MTT colorimetric assay. The results indicate that the lower transfection efficiency of certain LAH4 mutants when compared with LAH4 is not due to an increased cytotoxicity (data not shown).
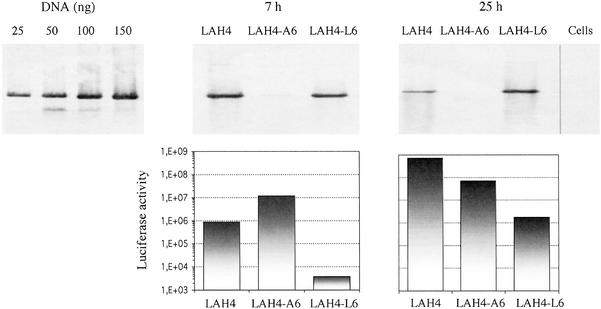
To determine whether the observed differences in reporter gene expression are due to varying efficiencies of DNA delivery, we transfected cells by using LAH4, LAH4-A6, or LAH4-L6. The cells were harvested 7 and 25 h posttransfection and the DNA of low molecular weight was isolated and quantified by Southern blot analysis. At the same time, expression of the reporter gene was monitored. The results demonstrate that LAH4-L6 delivers almost as much DNA as LAH4, although this peptide is significantly less efficient in mediating reporter gene expression (Fig. 3). Surprisingly, LAH4-A6 delivers 80 times less DNA into cells than LAH4, although 1 day posttransfection the difference in luciferase activity is only one log unit.
Figure 3.
Efficiency of plasmid DNA delivery. HepG2 cells were transfected with peptide/cytomegalovirus (CMV)-Luc complexes. At 7 and 25 h after transfection, cells were harvested and lysed and the low-molecular-weight DNA was recovered. At the same time, the luciferase activity was measured. The blot at Left serves as a standard curve and corresponds to the results obtained after hybridization of 25, 50, 100, and 150 ng of CMV-Luc. Graphs below the blots show the corresponding luciferase activity.
These results show that minor modifications of the sequence of LAH4 can result in significant changes in the capacity to mediate gene transfer. Moreover, our data show that there is not a simple correlation between the amount of delivered DNA and the level of transgene expression.
Discussion
We have evaluated the transfection efficiency of a series of peptides including LAH4 and its derivatives. Our results show that although DNA retardation is observed for peptides encompassing four lysines and one to five histidines (Table 1), only those with four to five histidine residues in the central region of the sequence exhibit high transfection efficiencies (Table 2). In fact, LAH4 allows for gene transfer levels on several cell lines that are comparable to those observed with 25-kDa PEI, which is one of the most efficient transfection reagents (9, 18).
The pK values of the four histidine imidazole groups of LAH4, determined in the presence of dodecylphosphocholine micelles, are 5.4, 5.8, 5.9, and 6.0 (16). The importance of protonation of the imidazole groups was shown with transfections performed in the presence of the proton pump inhibitor bafilomycin A1. Luciferase expression was significantly diminished, but not abolished, in the presence of the drug. Interestingly, the mere presence of four or more histidine residues is not sufficient for efficient gene transfer. This indicates that the mechanism of action of these peptides is different from that of histidylated polylysines (11).
From the experiments performed with LAH4 mutants, the following conclusions can be drawn: (i) the ability of the peptides to complex DNA seems not to be solely linked to the global charge of the peptide, suggesting that structural requirements need to be fulfilled (LAH4-G6 and histatin-5 for example do not retard DNA migration well); (ii) comparison of results obtained with LAH4 and H4-LAK4 shows that the histidine residues have to be positioned in the core of the peptide; and (iii) the peptides LAH4-L3, LAH4-L4, and LAH4 all show pronounced transfection efficiencies, indicating that the position of the histidine residues within the polar face is important, even though minor modifications are tolerated.
Structural investigations using circular dichroism (CD)-fourier transform infrared (FTIR) and attenuated total reflection (ATR)-FTIR spectroscopy indicate that in the presence of membranes the LAH4 family exhibits a high propensity for α-helical conformations with all histidine residues located on one face of the helix (12, 16, 19). Furthermore, when the peptides are reconstituted into bilayers aligned with the normal parallel to the magnetic direction, 15N solid-state NMR spectroscopy indicates that the interactions of the LAH4 peptides with membranes are strongly modulated by the pH-dependent chemical potential of the histidine side chains. As a result, the LAH4 helix acts as a pH-dependent molecular switch, having a transmembrane orientation at neutral pH (15N chemical shift close to 200 ppm) and an in-plane orientation at pH <6 (15N chemical shift <90 ppm) (16). The transition midpoint around pH 6.2 was confirmed by following the dichroic ratio measured in oriented ATR-FTIR spectra. Experiments with this latter technique also indicated that the in-plane–transmembrane transition is reversible (19). Interestingly, in this study all peptides exhibiting high transfection efficiencies also show pH-dependent membrane alignments with transition midpoints around pH 6 (16, 17). This suggests that pH-dependent membrane insertion of the peptides constitutes an important part of the DNA import mechanism.
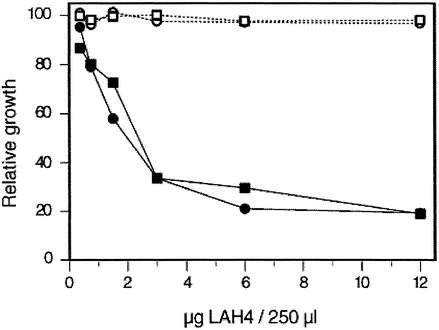
In patients with CF the airways are colonized and chronically infected with a variety of bacteria (20), despite the presence of many antibacterial proteins and peptides. It has been proposed that in CF an increase in the airway surface liquid salt concentration inhibits the activity of antibacterial factors (21). However, increased amounts of antibiotics can partially compensate for this. Thus, addition of exogenous antibacterial peptides might have therapeutic value (22). LAH4 was shown to possess significant antibiotic activity, although it does not lyse human erythrocytes at the same concentrations (12). We tested whether LAH4 still possesses its antibiotic activity once complexed to DNA. Our results, obtained with E. coli, show that the antibacterial activity is independent of its association with DNA (Fig. 4). As previously reported, this activity was found to be pH dependent (Fig. 4; ref. 12). Interestingly, a similar pH-dependent antibacterial activity was recently characterized for calcitermin, a histidine-rich peptide found in human airways (23).
Figure 4.
Antibacterial activity of LAH4. Growth of E. coli was determined at pH 7.4 (open symbols) and 5.5 (filled symbols) as a function of peptide concentration for LAH4 in the absence (circles) or presence (squares) of DNA (LAH4/DNA with a wt/wt ratio of 6/1).
Although more experiments have to be performed, including on bacterial strains commonly found in CF patients (e.g., Pseudomonas aeruginosa and Staphylococcus aureus), administration of peptide antibiotic/DNA complexes has potential in the treatment of the genetic defect and bacterial infections associated with CF.
Acknowledgments
This work was performed with the financial support of the Association Française contre les Myopathies and Vaincre la Mucoviscidose.
Abbreviations
- CF
cystic fibrosis
- PEI
polyethyleneimine
References
- 1.Mahato R I, Monera O D, Smith L C, Rolland A. Curr Opin Mol Ther. 1999;1:226–243. [PubMed] [Google Scholar]
- 2.Harbottle R P, Cooper R G, Hart S L, Ladhoff A, McKay T, Knight A M, Wagner E, Miller A D, Coutelle C. Hum Gene Ther. 1998;9:1037–1047. doi: 10.1089/hum.1998.9.7-1037. [DOI] [PubMed] [Google Scholar]
- 3.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. J Biol Chem. 1994;269:12918–12924. [PubMed] [Google Scholar]
- 4.Zanta M A, Belguise-Valladier P, Behr J P. Proc Natl Acad Sci USA. 1999;96:91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyman T B, Nicol F, Zelphati O, Scaria P V, Plank C, Szoka F C., Jr Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 6.Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, Jacobs E. Mol Ther. 2002;5:104–114. doi: 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- 7.Kichler A, Pages J C, Leborgne C, Druillennec S, Lenoir C, Coulaud D, Delain E, Le Cam E, Roques B P, Danos O. J Virol. 2000;74:5424–5431. doi: 10.1128/jvi.74.12.5424-5431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk S, Sparrow J T, Hauer J, Mims M P, Leland F E, Woo S L, Smith L C. Gene Ther. 1996;3:48–57. [PubMed] [Google Scholar]
- 9.Boussif O, Lezoualc'h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas M, Klibanov A M. Proc Natl Acad Sci USA. 2002;99:14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Midoux P, Monsigny M. Bioconjugate Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 12.Vogt T C, Bechinger B. J Biol Chem. 1999;274:29115–29121. doi: 10.1074/jbc.274.41.29115. [DOI] [PubMed] [Google Scholar]
- 13.Kichler A, Leborgne C, Coeytaux E, Danos O. J Gene Med. 2001;3:135–144. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- 14.Hirt B. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 15.Erbacher P, Roche A C, Monsigny M, Midoux P. Exp Cell Res. 1996;225:186–194. doi: 10.1006/excr.1996.0169. [DOI] [PubMed] [Google Scholar]
- 16.Bechinger B. J Mol Biol. 1996;263:768–775. doi: 10.1006/jmbi.1996.0614. [DOI] [PubMed] [Google Scholar]
- 17.Bechinger B. FEBS Lett. 2001;504:161–165. doi: 10.1016/s0014-5793(01)02741-7. [DOI] [PubMed] [Google Scholar]
- 18.Kichler A, Behr J P, Erbacher P. In: Non-viral Vectors for Gene Therapy. Huang L, Hung M-C, Wagner E, editors. San Diego: Academic; 1999. pp. 191–206. [Google Scholar]
- 19.Bechinger B, Ruysschaert J M, Goormaghtigh E. Biophys J. 1999;76:552–563. doi: 10.1016/S0006-3495(99)77223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilligan P H. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 22.Travis S M, Conway B A, Zabner J, Smith J J, Anderson N N, Singh P K, Greenberg E P, Welsh M J. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 23.Cole A M, Kim Y H, Tahk S, Hong T, Weis P, Waring A J, Ganz T. FEBS Lett. 2001;504:5–10. doi: 10.1016/s0014-5793(01)02731-4. [DOI] [PubMed] [Google Scholar]