Abstract
The herpes simplex virus 1 (HSV-1) replicative cycle begins by binding of the viral activator, VP16, to a set of sequences in the immediate-early (IE) gene promoters. With the aim of inhibiting this cycle, we have constructed a number of synthetic zinc-finger DNA-binding peptides by using recently reported methods. Peptides containing either three or six fingers, targeted to a viral promoter, were engineered as fusions with a KOX-1 transcription repression domain. These proteins bound to the HSV-1 IE175k (ICP4) promoter, in vitro, with nanomolar or subnanomolar binding affinity. However, in a chloramphenicol acetyltransferase reporter system, only the six-finger protein was found to repress VP16-activated transcription significantly. Thus the longer array of zinc fingers is required to compete successfully against VP16, one of the most powerful natural activators known. We found that the HSV-1 replication cycle can be partially repressed by the six-finger peptide with the viral titer reduced by 90%.
The herpes simplex virus 1 (HSV-1) is a human pathogen most commonly associated with mild epithelial lesions and capable of becoming latent in nerve cells (reviewed in ref. 1). Most HSV-1 infections can be successfully treated with acyclovir and its derivatives; however, a large research effort is currently devoted to developing alternative treatment strategies to tackle drug-resistant strains of HSV-1. Among the new approaches to antiviral treatment, conventional antisense reagents (2, 3) and ribozyme technology (4, 5) are particularly promising. We decided to evaluate the potential for specific antiviral intervention at an alternative level: repression of transcription by customized zinc-finger transcription factors, as shown to be possible by Choo et al. (6).
Over the last decade, Cys2-His2 classical zinc fingers, such as Zif268 (7), have emerged as versatile scaffolds for engineering novel sequence-specific DNA-binding domains (reviewed in refs. 8–10). Protein engineering has demonstrated that zinc fingers are capable of binding to a wide range of DNA sequences (11–13), and furthermore, arrays of finger subunits may be linked to bind longer DNA sequences (14–17). Three- and six-finger domains have been constructed by a number of groups and have successfully activated or repressed single gene targets in a wide variety of systems (7–9). Although zinc fingers have been previously targeted to viral gene sequences, such as the HIV promoter (12, 18, 19), the potential of zinc fingers to selectively inhibit a live viral infection has not yet been addressed (but see the preceding companion paper, ref. 20).
Because viral replication is a complex process, involving multiple and often host-derived transcription factors, it is necessary to examine the biology of HSV-1 in detail, so as to find a “therapeutic window” to allow the specific targeting of the virus, without affecting cellular processes. HSV-1 gene expression proceeds in a sequential and strictly regulated manner and can be divided into at least three phases, termed immediate-early (IE or α), early (β), and late (γ) (21). The IE proteins regulate the expression of later classes of genes as well as their own expression, and the product of the IE3 gene, IE175k (ICP4) is critical for HSV-1 gene regulation (22–24).
Each of the five IE gene promoters of HSV-1 has at least two copies of a conserved enhancer sequence, TAATGARAT (where R represents the nucleotide A or G), which is recognized by a multiprotein transactivation complex (25–27). This complex consists of the virus-encoded activator, VP16, and two cellular factors, namely Oct-1 and HCF-1 (for reviews see refs. 28–31). Because this complex specifically activates only HSV-1 IE genes in a TAATGARAT-dependent manner, it is clear that this process is specific to the virus and provides an opportunity for antiviral therapy. Therefore, we engineered several zinc-finger repressor proteins that would bind to the TAATGARAT region of the IE IE175k gene, and thus allow us to investigate whether it is possible to intervene in HSV-1 viral replicative cycle at the level of transcription. An IE gene was chosen because, if repression is to take place by means of a direct protein–DNA interaction, it is important to target a gene that is expressed at the beginning of the viral cycle, thereby inhibiting infection at the earliest possible stage before the number of viral genomes increases. Additionally, the promoter region of an IE gene is readily accessible to transcription factors, which makes it a seemingly ideal target for zinc fingers. This model also gives a unique opportunity to study competition between a natural and highly potent activator, VP16, and an artificially generated repressor for the same DNA-binding site. This competition, to some extent, reflects natural gene regulatory processes in which different factors interact and compete at a gene promoter to control the level of transcription.
Materials and Methods
Library Selection of Zinc Fingers by Using a Phage Display System.
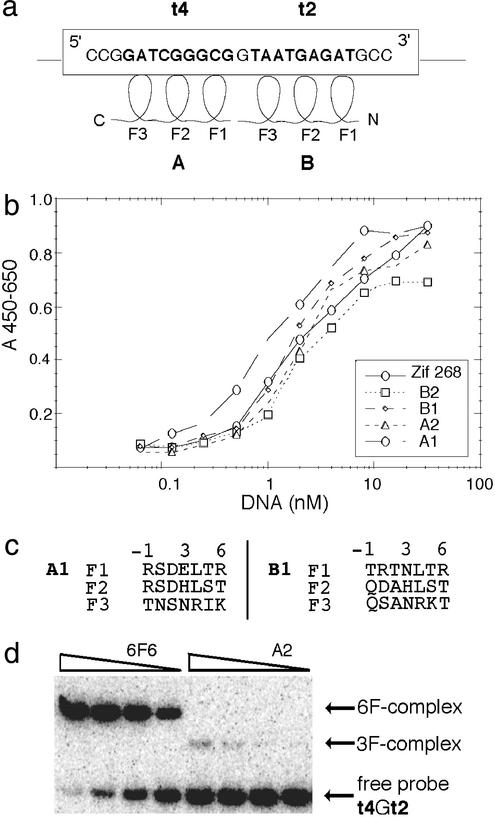
Several 9-bp sequences (including targets t4 and t2, Fig. 1) spanning the transactivation complex binding region of the HSV-1 IE175k promoter were chosen as targets for zinc fingers. These target sequences were used to screen libraries of randomized three-zinc finger peptides, in a Fd-Tet-SN phage display system. Two bipartite GCGG-anchored libraries, Lib12 and Lib23, which had been previously constructed for rapid engineering of three-finger peptides (12, 32), were used for screening. Zinc-finger proteins (designated A) to bind t4 (GATCGGGCG) were selected directly from Lib23 by using the method described by Isalan and colleagues (12, 32).
Figure 1.
Binding of zinc-finger peptides to their target sites. (a) Three-finger proteins (denoted A and B) were selected to bind 9-bp DNA sequences t4 and t2, respectively, within the promoter of the HSV-1 ICP4 (IE175k) gene. These sequences (in bold) are contained in the sequence with the coordinates from −276 to −252 relative to the start of transcription. Note that 5′–3′ direction of DNA runs antiparallel to the N–C direction of the bound zinc-finger protein (fingers marked F1, F2, and F3). (b) Plot of the phage-ELISA results for the four selected three-finger proteins, A1, A2, B1, and B2 (measured by A450 − A650), against serial dilutions of their target sites (t4 or t2 shown in nM). The three-finger Zif268 peptide displayed on phage was used as a control. Apparent Kd values calculated from the graph in b are as follows: Zif268, Kd = 2.0 nM; B2, Kd = 2.0 nM; B1, Kd = 1.9 nM; A2, Kd = 2.2 nM; and A1, Kd = 1.0 nM. (c) Amino acid sequence of the recognition helices (positions from −1 to 6) of the selected best binding peptides. (d) Comparison of binding affinities of three-finger (A2) and six-finger (6F6) peptides in a gel retardation assay in 7% acrylamide. Relevant peptides were synthesized in vitro and incubated with radioactively labeled 40-bp double-stranded DNA probe containing t4 and t2 sequences linked together (t4Gt2). A constant concentration of 0.16 nM DNA was used against 5-fold dilutions of proteins.
Proteins (designated B) to bind the t2 sequence (TAATGAGAT) were generated by a combination of selection and rational design (Fig. 1). Initially, a series of clones that bound the t2 “half-site,” 5′-TAA TGg gcg-3′, were selected from Lib23. Amino acids at positions −1 (contacting A), 1, and 2 of finger 2 (F2) were then engineered by rational design as QDA. The sequence of F1 was also engineered by a combination of rational design and selection. Positions −1, 1, and 2 were randomized, whereas the rest of F1 (positions 3–6) was constructed by rational design as NLTR by using Tramtrack (33) F1 (which binds the same TAG sequence) as a template. This library of clones was screened against the full-length t2 target sequence.
Construction of the Zinc-Finger Expression Plasmids.
The sequences encoding the three-finger peptides were amplified by PCR directly from the selected phage clones, A1, A2, B1, and B2 (Fig. 1), and cloned into pcDNA3.1(−) (Invitrogen), which facilitates expression both in mammalian cells and in vitro. To create a six-finger protein, 6F6, the three-finger peptides B1 and A1, selected to bind the sequences TAATGAGAT (t2) and GATCGGGCG (t4), respectively, were joined by using the linker LRQKDGERP to bridge the 1-bp gap between t2 and t4.
To engineer zinc-finger and repression domain fusions, DNA fragments corresponding to the relevant three-finger or six-finger domains were amplified by PCR and joined with the PCR-amplified regions coding for the nuclear localization signal (34), the Krüppel-associated box (KRAB) repression domain from KOX-1 (35–39), and the c-myc epitope (40). All fusion proteins, designated A1KOX, A2KOX, B1KOX, and 6F6KOX, were expressed by using pcDNA3.1(−), and in all of the cases the N-terminal zinc-finger domain was separated from the KRAB domain by a nuclear localization signal and the epitope tag was at the C terminus. Additionally, the three-finger construct pHIVCKOX, used as a negative control in HSV-1 infections, was generated by exchanging the A1 domain in A1KOX with a three-finger HIVC domain (selected to bind GATGCTGCA). To facilitate fluorescence-activated cell sorting (FACS), 6F6KOX- and B1KOX-coding sequences were subsequently subcloned into pTRACER-CMV/Bsd (Invitrogen) to create p6F6KOX-TR and pB1KOX-TR, respectively. These plasmids express relevant zinc fingers-KOX fusions from the cytomegalovirus (CMV) promoter and Cycle3 GFP-blasticidin from the EF-1 promoter.
Evaluation of Zinc-Finger Binding Affinity and Specificity.
The phage-ELISA method was used to evaluate the Kd and binding specificity of the selected three-finger proteins (41). To test the specificity of the phage-displayed zinc-finger peptides, ELISAs were carried out against the correct target sequence and against closely related sequences. For measurements of Kd, the phage-displayed peptides were tested in ELISAs against serial dilutions of its correct 9-bp target site (ranging from 0.125 to 32 nM). WT Zif268 (fingers 1–3) displayed on phage was used as a control in these experiments.
Gel Retardation Assays.
Zinc-finger peptides were synthesized in vitro by using the TNT T7 Quick coupled transcription/translation system (Promega) and subjected to gel retardation assay by using previously described methods (16). Binding of zinc-finger proteins to the appropriate DNA sequences was tested in the presence and absence of the C-terminal regulatory domain. The six-finger protein, 6F6, was tested against its full-length target and related sites from the promoters of the IE68k gene, the IE110k gene, and the human H2B gene.
Transfection of Mammalian Cell Lines.
Transient transfections of COS-1 and HeLa cells were performed by using the chemical compound FuGene (Roche) and CsCl-purified DNA. We routinely used 1–2 μg of total DNA, equalized in all cases by addition of pUC19 carrier DNA. For chloramphenicol acetyltransferase (CAT) assays, pcDNA3.1(−) was added as required to equalize total levels of CMV promoter input. On average the efficiency of transfection varied between 3% and 33%, as assessed by a standard pLacZ transfection and β-galactosidase assay.
CAT Assays.
Different amounts of zinc-finger constructs were transfected into mammalian cells along with the pPO13 (p175CATΔ380) reporter, which contains the entire HSV-1 IE175k promoter region (positions from −380 to +30) fused to the CAT gene (ref. 42; kindly donated by P. O'Hare). Transcription of the CAT gene was additionally activated by VP16, expressed by pCMV-VP16 (RG50) plasmid (kindly donated by P. O'Hare) added to the transfection mixture in amounts of 2 ng/10 ng of pPO13. Cells were harvested at 40–48 h after transfection and assayed for CAT enzyme by using the CAT ELISA kit (Roche), according to the manufacturer's instructions.
HSV-1 Infection of Transiently Transfected Cells.
Transiently transfected cells (provided that the efficiency of transfection was at least 30%) were infected with various amounts of HSV-1 (strain 17), ranging from 0.01 to 0.5 plaque-forming units (pfu) per cell at 40 h after transfection. Infection was performed in cluster dishes in the presence of 2% FCS at 37°C for 1 h, followed by changing of medium and further incubation at 37°C.
HSV-1 Infection of FACS-Sorted Cells.
To enrich for cells expressing 6F6KOX, before HSV-1 infection, COS-1 cells were transfected with p6F6KOX-TR and at 24 h after transfection were subjected to FACS sorting using GFP as a tracer. Before FACS, cells were resuspended in PBS with propidium iodide (0.005 mg/ml) and strained through a 70-μm cell strainer (Falcon) to remove aggregates. Only cells positive for GFP and negative for propidium iodide were selected and seeded at the desired density and infected, as above, with HSV-1 at 16–24 h after plating. Cells and medium containing viral progeny were harvested at various time points after infection. Cells transfected with pB1KOX-TR or an empty vector (pTRACER-CMV/Bsd) were used as controls.
Immunofluorescence and Immunodetection.
Cells intended for immunofluorescence studies were grown on coverslips placed inside the wells of cluster dishes and transfected and infected, as described above. Cells were fixed on the coverslips by using methanol, and zinc-finger proteins were visualized by using anti-c-myc mAb, 9E10 (Santa Cruz Biotechnology). The IE175k protein was detected with rabbit polyclonal Ab r74 (kindly donated by R. Everett), and immunofluorescence was viewed by using a Bio-Rad confocal microscope.
Total cellular lysates from transfected and infected cells were used for SDS/PAGE and Western blotting. The c-myc epitope-tagged proteins were detected with mAb 9E10, whereas HSV-1 proteins were detected by using anti-VP16 mAb LP1 (kindly donated by A. Minson), polyclonal Ab r191 against IE110k (kindly donated by R. Everett), or the mAb 10176 against IE175k (kindly donated by R. Everett). The signal was visualized by using the ECL detection system (Amersham Pharmacia). The same membrane was stripped and reblotted up to five times.
RNA Analysis.
For quantitative analysis of viral mRNA, infected cells (previously transfected and FACS sorted as described above) were lysed, and total RNA was extracted by using the RNeasy total RNA isolation kit (Qiagen) with RNase-free DNase treatment. RNA samples (500 ng to 5 μg) were reverse transcribed by using the SuperScript First-Strand Synthesis system for RT-PCR (GIBCO/BRL), with random hexamers as primers. Samples of cDNA (20–100 ng) were serially diluted and analyzed by real-time quantitative PCR using TaqMan chemistry. The 18S rRNA was used as an internal standard for each sample. All primers were HPLC-purified, and all TaqMan Quantitation probes were labeled with FAM reporter dye (Applied Biosystems).
Results
Analysis of the Three-Finger Proteins Selected to Bind to the t4 (GATCGGGCG) and t2 (TAATGAGAT) DNA Sequences.
A phage display method (12, 32) was used to select three-finger proteins capable of binding to two adjacent DNA target sites (named t2 and t4) in the HSV-1 IE175k promoter. Selection A was carried out against the t4 DNA site (GATCGGGCG) and selection B against the t2 site (TAATGAGAT) (Fig. 1a). A large number of selected clones were initially screened by phage ELISA to determine which zinc-finger peptides bound with the highest affinity and specificity. The strongest binders, A1, A2, B1, and B2, were then taken for further analysis. These four proteins were tested for binding against serial dilutions of their appropriate target site (Fig. 1b) and were found to exhibit apparent Kd comparable to Zif268 displayed on phage, which was used as a control (Fig. 1b). The relative affinities of A1, A2, B1, and B2 for their target DNA sites were also analyzed in gel retardation assays using in vitro expressed zinc-finger peptides, as exemplified for A2 in Fig. 1d, with results comparable to the ELISA data described above.
For the initial cell culture experiments, the three-finger domains of A1 and A2 were fused to the KRAB repression domain and tested in transient transfections by using the entire IE175k promoter linked to a CAT gene as a reporter. Experiments were conducted in the presence of full-length VP16, expressed from the separate plasmid, to mimic gene activation during HSV-1 infection. Despite high levels of activation by VP16 alone (≈30-fold), suggesting that the IE175k promoter is active and responsive, no significant repression was observed in the presence of either A1KOX or A2KOX (data not shown). This lack of repression was not due to poor expression of the recombinant zinc-finger proteins in the cells because their presence was detected by both immunofluorescence and Western blotting.
Because the IE175k promoter contains only a single t4 site, adjacent to TAATGAGAT bound by VP16, it appeared likely that the three-finger proteins did not bind with high enough affinity to overcome VP16 activation. This conclusion is supported by the observation that the three-finger peptide B1KOX, which binds TAATGAGAT itself, also failed to inhibit viral replication in the HSV-1 titer assay, as described later. Given the above results, we decided to construct and test a six-finger protein, which we hoped would have higher DNA-binding affinity and, therefore, a greater chance of inhibiting the virus.
Analysis of the Six-Finger Protein Binding to the t4Gt2 19-bp (GATCGGGCGGTAATGAGAT) DNA Sequence.
The A1 and B1 three-finger proteins bind to 9-bp target sites in the HSV-1 promoter with a separation of 1 bp. This is convenient because by fusing A1 and B1 a six-finger protein, 6F6, is created that binds over 19 bp of the IE175k promoter, covering the TAATGAGAT sequence and the preceding 5′-region (Fig. 1a).
In gel retardation assays, 6F6 showed, of the order of several hundredfold, greater affinity for the 19-bp t4Gt2 site than any of the three-finger peptides (Fig. 1d). Moreover, despite the high affinity of 6F6 for its full-length DNA site, the protein exhibits very low affinity for related DNA sequences. Such sequences include the IE68k promoter region, which contains only three mismatches over a 19-bp region, and the HSV-1 IE110k and human H2B promoter regions. However, under the same conditions the three-finger peptide, B1, binds strongly to the same IE68k promoter region. In fact, the 6F6 protein (which contains B1) binds with lower affinity to the IE68k promoter (which contains the full t2 site targeted by B1) than the three-finger B1 peptide. This observation suggests that the A1 portion of 6F6 in some way disrupts its binding to the nonideal IE68k sequence, and so in this case, the six-finger protein demonstrates both higher affinity and greater specificity for the IE175k promoter than the three-finger peptide, B1. Furthermore additional domains fused to the C terminus of zinc fingers, e.g., nuclear localization signal and KRAB, do not alter binding affinities of the peptides tested.
In the repression studies, similar to those described for three-finger proteins, different quantities of p6F6KOX were transfected into cells along with a CAT reporter gene under the control of the IE175k promoter and a plasmid encoding VP16. Our results demonstrated that the 6F6KOX protein had a clear inhibitory effect on expression from the VP16-activated reporter gene. Moreover, repression was found to be dose dependent, with 96% repression achieved with the highest dose of the 6F6KOX plasmid in COS cells (Fig. 2). A similar, but slightly lesser, effect was observed in HeLa cells. Even in the absence of the KRAB repression domain, 6F6 was found to partly inhibit CAT expression (Fig. 2), which suggests that the 6F6-zinc-finger protein competes with VP16 for binding to the TAATGAGAT sequence. It is therefore likely that repression of the IE175k promoter by 6F6KOX is due to a combination of binding competition with VP16 and the direct action of KRAB.
Figure 2.
Repression of VP16-activated transcription by the 6F6KOX and 6F6 proteins in the CAT reporter system. COS-1 cells grown in six-well cluster dishes were transiently transfected with a three-vector mixture comprising CAT reporter pPO13, activator pCMV-VP16, and various amounts (5, 50, or 500 ng) of the p6F6KOX- or p6F6-zinc-finger-expressing plasmid. Cells were harvested 40 h after transfection and assayed by using the CAT ELISA kit (Roche), and the total amount of CAT (ng/ml) was plotted on the graph. Activation of the pPO13 reporter by VP16 (pPO13 + VP16) was ≈30-fold over background (pPO13 alone), and repression with 500 ng of p6F6KOX was 2-fold over background, which corresponds to 96% inhibition.
Inhibition of HSV-1 Infection by 6F6KOX.
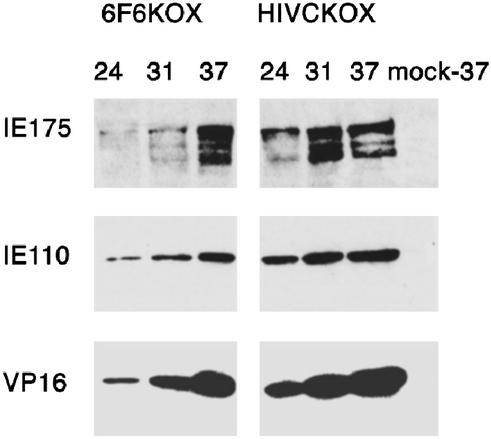
To investigate the effects of the 6F6KOX repressor protein on viral infection, COS-1 cells were transiently transfected either with the p6F6KOX or with a control-zinc finger construct (pHIVCKOX). These cells were then infected with HSV-1 and the time course of viral gene expression was monitored. The results showed a delay in appearance of all classes of HSV-1 proteins (including IE and late) in the cells transfected with p6F6KOX, compared with those transfected with pHIVCKOX (Fig. 3). Taking into account the efficiency of transient transfection (30–35% of cells subsequently infected with HSV-1), the observed inhibitory effect of 6F6KOX on HSV-1 infection can be considered significant.
Figure 3.
Expression of HSV-1 proteins during the course of infection in cells producing 6F6KOX. The course of HSV-1 infection in cells that express either 6F6KOX or a control protein, HIVCKOX, was monitored by using Western blotting. We were unable to detect IE proteins at time points earlier than 24 h after infection, probably because of the low multiplicity of infection used at the start of the assay. COS-1 cells grown in six-well plate cluster dishes were transfected with either p6F6KOX or pHIVCKOX and 40 h later infected with HSV-1 at a multiplicity of 0.05 pfu per cell or mock infected (mock). At various times after infection (as indicated in hours: 24, 31, or 37), cells were lysed directly on the plate by using 300 μl of hot SDS loading buffer. The 50-μl protein samples were separated by SDS/PAGE, transferred onto nitrocellulose, and sequentially probed for HSV-1 proteins. HSV-1 VP16 was detected with mAb LP1 at a 1:1,000 dilution (VP16). HSV-1 IE110k was detected with rabbit polyclonal Ab r191 at a 1:1,000 dilution (IE110), and HSV-1 IE175k was detected with mAb 10176 used at a 1:5,000 dilution (IE175). Membranes were stripped before the addition of each new Ab.
The inhibitory effect of 6F6KOX on viral infection also was observed in an immunofluorescence study (Fig. 4). This experiment further revealed that in the later stages of infection, cells that expressed 6F6KOX showed fewer of the morphological aberrations associated with HSV-1 infection than did neighboring cells that had not been transfected (Fig. 4). Although these results suggest that the 6F6KOX protein slows the infection, the repressor was unable to abolish expression of IE175k, as demonstrated by the occasional presence of both 6F6KOX and the IE175k in the same cell, at this late stage of infection. Nevertheless, in most cases, IE175k was present at lower levels in 6F6KOX-positive cells than in control cells in which HSV-1 infection seemed to progress normally (Fig. 4).
Figure 4.

HSV-1 infection in cells expressing 6F6KOX. COS-1 cells were transfected with p6F6KOX and after 40 h infected with HSV-1 at 0.5 pfu per cell. Cells were harvested at 26 h after infection and probed with Abs. The IE175k protein (anti-IE175, green) was detected with rabbit polyclonal Ab r74 used at a 1:200 dilution and followed by FITC-conjugated anti-rabbit Ab. The c-myc epitope present in the 6F6KOX (anti-c-myc, red) was detected with 9E10 used at a 1:200 dilution and followed by Texas red anti-mouse Ab. At this late stage of infection, typical changes in cell morphology can be observed. These include ballooning and rounding up of infected cells, distortion of the nucleus, apparent thickening of the nuclear membrane, and condensation of chromatin. IE175k in late infection can be found in all cellular compartments, being mainly associated with the nuclear rim and the cytoplasm. Typically, 6F6KOX-positive cells (red) exhibit fewer aberrations, and 6F6KOX is associated with the apparently unchanged nucleus (note intact nucleolus, arrow). (×800.)
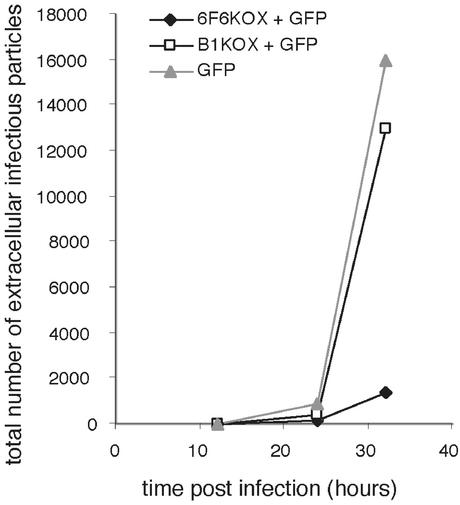
Owing to the limitations of the transient transfection system used, such as variations in the initial levels of protein expression, it is difficult to obtain a precise assessment of the IE175k to 6F6KOX ratio in the infected cells, which could indicate the degree of IE175k inhibition. It would therefore be desirable to study HSV-1 infection within a homogeneous population of 6F6KOX-expressing cells. Unfortunately (as experienced by other researchers, ref. 43), we found great difficulty in generating cell lines that stably expressed this artificial zinc-finger transcription factor. Therefore, to generate a more homogeneous population of cells, we used cell sorting to enrich for 6F6KOX-positive cells within the transiently transfected pool. Cells were transfected with p6F6KOX-TR or control plasmids, which express GFP, and sorted by using GFP as a marker. The selected cells were infected with HSV-1 at the low multiplicity of infection, and the total number of infectious viral particles released into the medium during the course of infection was determined. The results of infection with 0.05 pfu per cell demonstrate a 10-fold reduction in viral titer in cells transfected with p6F6KOX-TR in comparison with cells transfected with pTRACER-CMV/Bsd (Fig. 5). To eliminate the possibility that the observed reduction in HSV-1 titer could be attributed to cell loss caused by a general cytotoxic effect exerted by 6F6KOX, a sample of uninfected cells was analyzed by FACS at the beginning and at the end of the time course experiment. Significantly, in this experiment, no increased cell death was found to be associated with expression of this particular protein.
Figure 5.
Inhibition of HSV-1 productive cycle by 6F6KOX. COS-1 cells were transiently transfected with either p6F6KOX-TR (6F6KOX + GFP) or control plasmids pTRACER-CMV/Bsd (GFP) or pB1KOX-TR (B1KOX + GFP). At 24 h after transfection, cells were FACS sorted and seeded at 0.9 × 106 cells per well in 24-well cluster dishes, and 24 h later the cells were infected with HSV-1 at 0.05 pfu per cell. Culture medium samples (300 μl total) were harvested 12, 24, and 32 h after infection and used for plaque assays on confluent monolayers of COS-1 cells, in 10-fold serial dilutions of virus (performed in duplicate). The graph shows the total number of infectious particles released into the medium at the indicated times (in hours).
Despite the lack of detectable cytotoxicity over the course of the previous experiment, it was still important to demonstrate that the mechanism of HSV-1 inhibition was, as intended, a direct consequence of the repression of the IE175k promoter by 6F6KOX. To address this problem, the production of viral transcripts was analyzed. Owing to the complex effects that the IE175k activator/repressor has on other genes (including other IE genes) at every stage of infection, it was considered vital to assay HSV-1 transcription at the earliest possible time points during the progress of infection. RNA samples were collected between 3 and 6 h after infection with 0.2 pfu per cell and analyzed by real-time PCR. In cells that expressed 6F6KOX, we found that IE175k mRNA levels were reduced by 35–50%, in comparison with cells that expressed either B1KOX or GFP only. Furthermore, in 6F6KOX-expressing cells the reduction in IE175k mRNA correlated with a decrease in the levels of later viral transcripts such as thymidine kinase mRNA. A further control showed, as expected, that the IE63k gene was not inhibited by the zinc-finger repressor. In conclusion, these results are consistent with the specific inhibition of IE175k expression as a direct result of the action of the engineered 6F6KOX transcription factor.
Discussion
In this work we have investigated the use of “designer” zinc-finger transcription factors in regulating the replicative cycle of a live virus, HSV-1. The engineered three- and six-finger proteins were targeted to a 19-bp region of the promoter for the IE gene IE175k, which encodes for a critical viral regulatory protein. This gene was also used as a target by Trang et al. (4, 5), who successfully inhibited HSV-1 replication by using a ribozyme constructed against IE175k mRNA.
The experiments described demonstrate that the most potent zinc finger protein, a six-finger construct, 6F6KOX, was able to significantly repress transcription of the viral DNA. 6F6KOX was designed to bind across one of two conserved TAATGARAT enhancer sequences (the natural binding site of the viral VP16 activator) in the promoter of the IE175k gene. The presence of a second, proximal, TAATGARAT-like sequence (at position −115 to −106 relative to the transcription start site, ref. 42) was an added complication because it was anticipated that VP16 would still bind at this sequence and activate the IE175k gene from this site. In contrast, it was not expected that the 6F6KOX protein would bind over the proximal TAATGARAT site because of 10 differing bases over the equivalent 19-bp region. Given these factors, we feel that the 96% inhibition of IE175k gene transcription achieved by the 6F6KOX protein in the CAT assay system is very significant. Perhaps more significant, however, in the context of our aim, was the 10-fold (90%) reduction in viral titer observed in HSV-1-infected cells, in comparison to control samples. To achieve further repression of HSV-1, other immediate early genes would have to be targeted in parallel with IE175k. Results similar to these presented here, namely levels of HSV-1 inhibition of 80–90%, have been reported for other experimental antiviral approaches, such as antisense oligonucleoside methylphosphonates complementary to IE mRNAs 4 and 5 (2) and decoy oligonucleotides capable of sequestering the IE175k protein (43).
In contrast to the results obtained with 6F6KOX, the three-finger transcription factors lacked any significant activity in the reporter system. This was despite their displaying good affinities for their respective target sites in vitro, which were comparable to the natural three-finger protein, Zif268. It is likely that the success of the six-finger protein was due to a combination of increased binding affinity, a longer half-life, and a slower off-rate when bound to its full-length target site (14), which allow more effective competition with the natural VP16 transcription factor. Similarly, Beerli et al. (45) have presented data that demonstrated the activation of the endogenous erbB gene by using a designer six-finger protein, whereas its three-finger constituents failed to activate the promoter. In contrast, Liu et al. (15) have shown that activation of the VEGF-A gene can be achieved by using three-finger activators that target DNase I-hypersensitive regions in the promoter. We believe that engineered three-zinc finger proteins can play a significant role in customized gene regulation, and we have shown in our own studies that combinations of three-finger proteins, which target different regions of the same promoter, can have greatly enhanced regulatory properties (see also the companion paper, ref. 20). That said, we feel that six-finger arrays will generally exhibit stronger effects than their component three-finger subunits, either individually or in combination.
Zinc-finger technology is the subject of intense research to investigate its possible applications in gene therapy and transgenic organisms. These efforts are in part fuelled by the clear advantages the technology has over other competing systems, such as antisense, ribozymes, or RNAi (RNA interference), in that these do not allow activation of target genes or genomic targeting of useful effector domains. Nonetheless, to date, only a limited number of endogenous cellular genes have been successfully regulated by engineered zinc-finger proteins (6, 14, 46, 47), despite the ease with which zinc fingers can be generated to bind target DNA sites in vitro, and shown to regulate reporter constructs in cell culture systems (reviewed in refs. 7–9).
In the work presented here and in the companion paper (20), we have demonstrated that a designer zinc-finger transcription factor can successfully inhibit the replication of a live virus. Furthermore, we expect that the rapid advances in the fields of zinc-finger engineering and biotherapeutics will make the wide practical applications of this technology ever more feasible, especially in the view of recently reported use of zinc fingers in an in vivo mouse model (47) and in plants (48).
Acknowledgments
We thank P. O'Hare and R. Everett for their scientific advice and for generously donated materials. We also thank H.-J. Thiesen for CMV-tetR-KRAB and A. Minson for LP1. The technical assistance of L. Reynolds with TaqMan analysis is also gratefully acknowledged. This research was funded by the Medical Research Council, United Kingdom. Part of this work was also supported by the National Foundation for Cancer Research, Washington, DC (to M.P.), EC Biomed 2 Grant BMH4-CT96-0375 (to M. Moore), and Grant 6 P04A 018 18 from the Polish State Committee for Scientific Research (to M. Minczuk). M. Minczuk was also sponsored by European Molecular Biology Organization Short-Term Fellowship ASTF9882.
Abbreviations
- HSV-1
herpes simplex virus 1
- CAT
chloramphenicol acetyltransferase
- FACS
fluorescence-activated cell sorter
- CMV
cytomegalovirus
- IE
immediate early
- pfu
plaque-forming unit
- KRAB
Krüppel-associated box
References
- 1.Whitley R J, Roizman B. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 2.Kean J M, Kipp S A, Miller P S, Kulka M, Aurelian L. Biochemistry. 1995;34:14617–14620. doi: 10.1021/bi00045a001. [DOI] [PubMed] [Google Scholar]
- 3.Feng C P, Kulka M, Smith C, Aurelian L. Antisense Nucleic Acid Drug Dev. 1996;6:25–35. doi: 10.1089/oli.1.1996.6.25. [DOI] [PubMed] [Google Scholar]
- 4.Trang P, Kilani A, Kim J, Liu F. J Mol Biol. 2000;301:817–826. doi: 10.1006/jmbi.2000.4022. [DOI] [PubMed] [Google Scholar]
- 5.Trang P, Lee J, Kilani A F, Kim J, Liu F. Nucleic Acids Res. 2001;29:5071–5078. doi: 10.1093/nar/29.24.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choo Y, Sanchez-Garcia I, Klug A. Nature. 1994;372:642–645. doi: 10.1038/372642a0. [DOI] [PubMed] [Google Scholar]
- 7.Choo Y, Isalan M. Curr Opin Struct Biol. 2000;10:411–416. doi: 10.1016/s0959-440x(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 8.Pabo C O, Peisach E, Grant R A. Annu Rev Biochem. 2001;70:313–340. doi: 10.1146/annurev.biochem.70.1.313. [DOI] [PubMed] [Google Scholar]
- 9.Beerli R R, Barbas C F., III Nat Biotechnol. 2002;20:135–141. doi: 10.1038/nbt0202-135. [DOI] [PubMed] [Google Scholar]
- 10.Pavletich N P, Pabo C O. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 11.Greisman H A, Pabo C O. Science. 1997;275:657–661. doi: 10.1126/science.275.5300.657. [DOI] [PubMed] [Google Scholar]
- 12.Isalan M, Klug A, Choo Y. Nat Biotechnol. 2001;19:656–660. doi: 10.1038/90264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreier B, Beerli R R, Segal D J, Flippin J D, Barbas C F., III J Biol Chem. 2001;276:29466–29478. doi: 10.1074/jbc.M102604200. [DOI] [PubMed] [Google Scholar]
- 14.Kim J S, Pabo C O. Proc Natl Acad Sci USA. 1998;95:2812–2817. doi: 10.1073/pnas.95.6.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P-Q, Rebar E J, Zhang L, Liu Q, Jamieson A C, Liang Y, Qi H, Li P X, Chen B, Mendel M C, et al. J Biol Chem. 2001;276:11323–11334. doi: 10.1074/jbc.M011172200. [DOI] [PubMed] [Google Scholar]
- 16.Moore M, Choo Y, Klug A. Proc Natl Acad Sci USA. 2001;98:1432–1436. doi: 10.1073/pnas.98.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore M, Klug A, Choo Y. Proc Natl Acad Sci USA. 2001;98:1437–1441. doi: 10.1073/pnas.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiesen H J. Gene Expr. 1996;5:229–243. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Yang W P, Barbas C F., III Proc Natl Acad Sci USA. 1995;92:344–348. doi: 10.1073/pnas.92.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds L, Ullman C, Moore M, Isalan M, West M J, Clapham P, Klug A, Choo Y. Proc Natl Acad Sci USA. 2003;100:1615–1620. doi: 10.1073/pnas.252770699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honess R W, Roizman B. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLuca N A, Schaffer P A. Mol Cell Biol. 1985;5:1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLuca N A, Courtney M A, Schaffer P A. J Virol. 1984;52:767–776. doi: 10.1128/jvi.52.3.767-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett R D. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Post L E, Mackem S, Roizman B. Cell. 1981;24:555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 26.Campbell M E, Palfreyman J W, Preston C M. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 27.ApRhys C M, Ciufo D M, O'Neill E A, Kelly T J, Hayward G S. J Virol. 1989;63:2798–2812. doi: 10.1128/jvi.63.6.2798-2812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Hare P. Semin Virol. 1993;4:145–155. [Google Scholar]
- 29.Wegner M, Drolet D W, Rosenfeld M G. Curr Opin Cell Biol. 1993;5:488–498. doi: 10.1016/0955-0674(93)90015-i. [DOI] [PubMed] [Google Scholar]
- 30.Herr W. Cold Spring Harbor Symp Quant Biol. 1998;63:599–607. doi: 10.1101/sqb.1998.63.599. [DOI] [PubMed] [Google Scholar]
- 31.Phillips K, Luisi B. J Mol Biol. 2000;302:1023–1039. doi: 10.1006/jmbi.2000.4107. [DOI] [PubMed] [Google Scholar]
- 32.Isalan M, Choo Y. Methods Enzymol. 2001;340:593–609. doi: 10.1016/s0076-6879(01)40444-7. [DOI] [PubMed] [Google Scholar]
- 33.Fairall L, Schwabe J W, Chapman L, Finch J T, Rhodes D. Nature. 1993;366:483–487. doi: 10.1038/366483a0. [DOI] [PubMed] [Google Scholar]
- 34.Kalderon D, Roberts B L, Richardson W D, Smith A E. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 35.Witzgall R, O'Leary E, Leaf A, Onaldi D, Bonventre J V. Proc Natl Acad Sci USA. 1994;91:4514–4518. doi: 10.1073/pnas.91.10.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolin J F, Friedman J R, Meyer W K-H, Vissing H, Thiesen H-J, Rauscher F J., III Proc Natl Acad Sci USA. 1994;91:4509–4513. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellefroid E J, Marine J-C, Ried T, Lecocq P, Riviere M, Amemiya C, Poncelet D A, Coulie P G, de Jong P, Szpirer C, et al. EMBO J. 1993;12:1363–1374. doi: 10.1002/j.1460-2075.1993.tb05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vissing H, Meyer W K-H, Aagaard L, Tommerup N, Thiesen H-J. FEBS Lett. 1995;369:153–157. doi: 10.1016/0014-5793(95)00728-r. [DOI] [PubMed] [Google Scholar]
- 39.Deuschle U, Meyer W K-H, Thiesen H-J. Mol Cell Biol. 1995;15:1907–1914. doi: 10.1128/mcb.15.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evan G I, Lewis G K, Ramsay G, Bishop J M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choo Y, Klug A. Proc Natl Acad Sci USA. 1994;91:11168–11172. doi: 10.1073/pnas.91.23.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Hare P, Hayward G. J Virol. 1987;61:190–199. doi: 10.1128/jvi.61.1.190-199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock R, Giel M, Linher K, Clackson T. Nat Biotechnol. 2002;20:729–733. doi: 10.1038/nbt0702-729. [DOI] [PubMed] [Google Scholar]
- 44.Clusel C, Meguenni S, Elias I, Vasseur M, Blumenfeld M. Gene Expr. 1995;4:301–309. [PMC free article] [PubMed] [Google Scholar]
- 45.Beerli R R, Segal D J, Dreier B, Barbas C F., III Proc Natl Acad Sci USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beerli R R, Dreier B, Barbas C F., III Proc Natl Acad Sci USA. 2000;97:1495–1500. doi: 10.1073/pnas.040552697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebar E J, Huang Y, Hickey R, Nath A K, Meoli D, Nath S, Chen B, Xu L, Liang Y, Jamieson A C, et al. Nat Med. 2002;8:1427–1432. doi: 10.1038/nm1202-795. [DOI] [PubMed] [Google Scholar]
- 48.Oroliz M I, Barbas C F, III, Beachy R N. Proc Natl Acad Sci USA. 2002;99:13290–13295. doi: 10.1073/pnas.202471899. [DOI] [PMC free article] [PubMed] [Google Scholar]