Abstract
The carboxy-terminal 150 residues of the focal adhesion kinase (FAK) comprise the focal adhesion-targeting sequence, which is responsible for its subcellular localization. The mechanism of focal adhesion targeting has not been fully elucidated. We describe a mutational analysis of the focal adhesion-targeting sequence of FAK to further examine the mechanism of focal adhesion targeting and explore additional functions encoded by the carboxy-terminus of FAK. The results demonstrate that paxillin binding is dispensable for focal adhesion targeting of FAK. Cell adhesion-dependent tyrosine phosphorylation strictly correlated with the ability of mutants to target to focal adhesions. Focal adhesion targeting was also a requirement for maximal FAK-dependent tyrosine phosphorylation of paxillin and FAK-related nonkinase (FRNK)–dependent inhibition of endogenous FAK function. However, there were additional requirements for these latter functions because we identified mutants that target to focal adhesions, yet are defective for the induction of paxillin phosphorylation or the dominant-negative function of FRNK. Furthermore, the paxillin-binding activity of FRNK mutants did not correlate with their ability to inhibit FAK, suggesting that FRNK has other targets in addition to paxillin.
INTRODUCTION
The focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase (PTK) that is localized to focal adhesions (Hanks et al., 1992; Schaller et al., 1992). FAK colocalizes with transmembrane receptors for the extracellular matrix called integrins. Engagement of integrins with their ligand induces tyrosine phosphorylation and enzymatic activation of FAK (Guan and Shalloway, 1992; Hanks et al., 1992; Kornberg et al., 1992; Lipfert et al., 1992). Thus, FAK is involved in the transmission of cytoplasmic signals after integrin-dependent cell adhesion. FAK has been implicated in controlling several integrin-dependent biological processes, including cell spreading, migration, survival, and regulation of cell cycle (Frisch and Francis, 1994; Ilic et al., 1995; Gilmore and Romer, 1996; Hungerford et al., 1996; Richardson and Parsons, 1996; Zhao et al., 1998).
The catalytic domain of FAK is centrally located and is flanked by large amino- and carboxy-terminal noncatalytic domains. The amino-terminal domain binds peptides, mimicking the carboxy terminus of the β1 integrin subunit in vitro (Schaller et al., 1995), but this putative interaction is dispensable for integrin-dependent tyrosine phosphorylation of FAK in vivo (Tahiliani et al., 1997; Shen and Schaller, 1999). The carboxy terminus contains binding sites for a number of focal adhesion-associated proteins and signaling molecules. The carboxy-terminal 150 amino acids of FAK contain binding sites for both paxillin and talin (Turner and Miller, 1994; Chen et al., 1995; Hildebrand et al., 1995). This region of FAK is called the focal adhesion-targeting (FAT) sequence and is responsible for directing FAK to its focal adhesion location (Hildebrand et al., 1993). Between the catalytic domain and FAT sequence of FAK there are two proline-rich regions that serve as docking sites for the Src homology (SH)3 domains of p130cas and GTPase-activating protein for Rho associated with FAK (GRAF) (Polte and Hanks, 1995; Harte et al., 1996; Hildebrand et al., 1996). In addition to these binding partners, there are two tyrosine residues in FAK that can become phosphorylated to create SH2 domain-binding sites (Schaller et al., 1994; Xing et al., 1994; Calalb et al., 1995). The major site of autophosphorylation of FAK, tyrosine 397, lies to the amino-terminal side of the catalytic domain and serves as a binding site for the SH2 domain of Src (Schaller et al., 1994; Xing et al., 1994). Interestingly, there is a functional Src SH3 domain-binding site in this vicinity that also contributes to the stability of the FAK/Src complex (Thomas et al., 1998). When phosphorylated, tyrosine 397 also functions as a docking site for the SH2 domains of other signaling molecules, including phosphatidylinositol 3′-kinase and phospholipase Cγ (Reiske et al., 1999; Zhang et al., 1999). The SH2 domain of growth factor receptor-binding protein 7 (Grb7) also binds to tyrosine-phosphorylated FAK and may interact with phosphorylated tyrosine 397 (Han and Guan, 1999). A tyrosine residue in the carboxy-terminal domain of FAK, tyrosine 925, serves as a growth factor receptor-binding protein 2 (Grb2)-binding site when phosphorylated and is proposed to function in the activation of the Ras/mitogen-activated protein kinase pathway (Schlaepfer et al., 1994; Schlaepfer and Hunter, 1996, 1997).
Cell adhesion kinase β (CAKβ) (also known as Pyk2, CADTK, RAFTK, and FAK2) is an FAK-related PTK (Avraham et al., 1995; Lev et al., 1995; Sasaki et al., 1995; Herzog et al., 1996; Yu et al., 1996). Like FAK, CAKβ has large amino- and carboxy-terminal domains flanking the central catalytic domain and the two exhibit 45% overall amino acid identity. The regions of greatest homology are the catalytic domain (60% identity) and the FAT sequence of FAK (61% identity). There is some evidence that CAKβ can be regulated by integrin-dependent cell adhesion, but the major stimuli that induce tyrosine phosphorylation of CAKβ are growth factors, neuropeptides, and other stimuli that elevate cytoplasmic calcium (Lev et al., 1995; Dikic et al., 1996; Li et al., 1996; Yu et al., 1996; Hiregowdara et al., 1997; Schaller and Sasaki, 1997; Brinson et al., 1998). Although CAKβ has been reported to localize to focal adhesions, many reports describe a diffuse cytoplasmic localization (Schaller and Sasaki, 1997; Sieg et al., 1998; Zheng et al., 1998). However, the C-terminal noncatalytic domain localizes to focal adhesions when expressed autonomously, demonstrating that CAKβ does contain a functional FAT sequence (Schaller and Sasaki, 1997; Xiong et al., 1998). Interestingly, the carboxy-terminal noncatalytic domains of FAK and CAKβ have been described as naturally occurring, autonomously expressed variants of FAK and CAKβ (Schaller et al., 1993; Xiong et al., 1998). These variants have been called FAK-related nonkinase (FRNK), CAKβ-related nonkinase (CRNK), or Pyk2-related nonkinase (PRNK). The differential expression of FAK and FRNK is apparently regulated at the level of transcription because the mRNAs encoding these two proteins arise from two distinct promoters within the FAK gene (Nolan et al., 1999). Presumably expression of CAKβ and CRNK/PRNK are regulated by a similar mechanism. Although the functions of endogenous FRNK and CRNK/PRNK have not been fully elucidated, both FRNK and CRNK have been used experimentally as dominant-negative mutants to perturb signaling via endogenous, wild-type FAK and CAKβ (Richardson and Parsons, 1996; Li et al., 1999).
The carboxy-terminal FAT sequence of FAK is indispensable for its normal function in signal transduction (Shen and Schaller, 1999). Deletion mutants that ablate targeting to focal adhesions fail to become tyrosine phosphorylated in response to integrin-dependent cell adhesion. Similarly, these mutants fail to induce tyrosine phosphorylation of focal adhesion-associated substrates. Therefore, either localization to focal adhesions per se is a major prerequisite for FAK function, or carboxy-terminal sequences have a second role in addition to focal adhesion targeting that is important for FAK function. Because a chimeric molecule in which the FAT sequence of FAK is replaced by the focal adhesion-targeting sequence of vinculin exhibits very similar properties to wild-type FAK, the former hypothesis is favored (Shen and Schaller, 1999). These findings further underscore the importance of focal adhesion targeting in FAK function, yet the mechanism by which FAK localizes to focal adhesions remains controversial. The carboxy-terminal 150 residues of FAK contains the FAT sequence and a binding site for the focal adhesion-associated protein paxillin (Hildebrand et al., 1993, 1995). Deletion analysis has failed to separate the sequences responsible for focal adhesion targeting and paxillin binding (Hildebrand et al., 1995). A number of mutants of FAK that contain point mutations that disrupt paxillin binding fail to localize to focal adhesions, suggesting that paxillin binding is required for focal adhesion targeting of FAK (Tachibana et al., 1995). This result is in disaccord with reports of FAK variants that are unable to bind paxillin yet correctly localize to focal adhesions (Schaller et al., 1993; Hildebrand et al., 1995; Sieg et al., 1999). Talin binding also has been proposed as the mechanism of focal adhesion targeting because talin was reported to coimmunoprecipitate with FAK, which efficiently targets to focal adhesions, but not with CAKβ, which does not (Zheng et al., 1998). However, talin-binding activity does not strictly correlate with the ability of FAK mutants to target to focal adhesions (Hildebrand et al., 1993; Chen et al., 1995). To further explore the mechanism of focal adhesion targeting of FAK, and to examine whether the FAT sequence may perform other functions in addition to targeting, a further mutational analysis was carried out.
Because the carboxy-terminal domains of FAK and CAKβ exhibit a number of common features, including paxillin binding and a functional FAT sequence (Salgia et al., 1996; Hiregowdara et al., 1997; Li and Earp, 1997; Schaller and Sasaki, 1997; Xiong et al., 1998), conserved residues were targeted for mutagenesis. Seven mutants containing multiple alanine substitutions were engineered and analyzed. The results provide additional evidence that paxillin-binding activity is dispensable for focal adhesion targeting of FAK. Cell adhesion-dependent regulation of tyrosine phosphorylation of FAK strictly correlated with the ability of FAK mutants to localize to focal adhesions. Although focal adhesion targeting was required for efficient FAK-dependent tyrosine phosphorylation of the focal adhesion-associated protein paxillin and the ability of FRNK to function as a dominant-negative mutant of FAK, some mutants were defective for these functions despite correctly localizing to focal adhesions. These results suggest that the carboxy-terminus of FAK has other functions besides targeting FAK to focal adhesions, that the mechanism of the dominant-negative action of FRNK is not simply by displacing FAK from focal adhesions, and that FAK targets to focal adhesions independent of paxillin binding.
MATERIALS AND METHODS
Cells and Viruses
As previously described, chicken embryo (CE) cells were isolated from day 9 embryos and maintained in DMEM + 4% fetal bovine serum + 1% chick serum (Reynolds et al., 1989). NIH 3T3 cells were maintained in DMEM + 10% calf serum and 293 cells in DMEM/F12 + 10% fetal bovine serum. For expression in CE cells, FAK and FRNK variants were subcloned into the replication competent, avian retroviral vector called RCAS and cells transfected as previously described (Reynolds et al., 1989; Schaller et al., 1993). For expression in mammalian cells, FAK variants were engineered as green fluorescent protein (GFP) fusion proteins in the pEGFP-C3 vector (Clontech, Palo Alto, CA). The constructs were introduced into NIH 3T3 and 293 cells by using Lipofectamine (Life Technologies, Gaithersburg, MD) according to the manufacturer's recommended protocol. For cell adhesion experiments, cells were trypsinized, maintained in suspension, and plated onto fibronectin-coated dishes as previously described (Shen and Schaller, 1999). In some experiments cells were treated with 2 μM pervanadate for 30 min prior to lysis to inhibit cellular protein tyrosine phosphatases.
Molecular Biology
Mutations were engineered into the FAK cDNA by site-directed mutagenesis with the Altered Sites kit (Promega, Madison, WI) and mutants were identified by sequencing with a Sequenase kit (Amersham, Piscataway, NJ). A segment of the FAK cDNA containing the point mutation(s) was subcloned into other vectors for further analysis of the properties of these mutants. For expression as a glutathione-S-transferase (GST) fusion protein, the Nru I-EcoRI fragment (nucleotides 2717–3248) or the NheI-EcoRI fragment (nucleotides 2948–3248) was excised from the mutagenesis vector pAlter and substituted for the corresponding fragment from GST-H-FAK, which contains codons 765-1052 of wild-type FAK inserted into the pGex2TK vector (Pharmacia, Piscataway, NJ) (Shen et al., 1998). For expression as full-length FAK in CE cells, the mutations were first rescued into an epitope-tagged, full-length FAK cDNA in the pBluescript vector (Stratagene, La Jolla, CA) (Schaller et al., 1993). This was achieved by amplifying the fragment of FAK containing the point mutation(s) by polymerase chain reaction (PCR), and then substituting either the Nru I/ClaI fragment (nucleotides 2717–3167 of FAK) or the NheI/ClaI fragment (nucleotides 2948–3167 of FAK) of the reaction product for the corresponding fragment of the epitope-tagged FAK. The KT3 epitope tag is fused in-frame with the FAK sequences at the ClaI site. The mutated, tagged FAK insert was then subcloned into the replication competent RCAS A retroviral vector. For expression as FRNK constructs, the sequences encoding FRNK were amplified by PCR with the mutated pAlter-FAK plasmids as template. The two primers, one that annealed 5′ of the FRNK initiation codon and one that annealed in the multiple cloning site of pAlter downstream of the termination codon, were designed to contain SpeI sites. The amplification product was subcloned into pBSX, a pBluescript derivative containing a SalI-XbaI fragment from the RCAS A vector. The FRNK and the RCAS A vector sequences were excised by using SalI and inserted into RCAS A. For expression as a GFP fusion protein, the wild-type FAK cDNA was cloned in-frame with the GFP-encoding sequences of pEGFP-C3 (Clontech). Mutated FAK sequences were introduced into this construct by substitution of the Nru I/SalI fragment of the mutated FAK sequences in pAlter (extending from nucleotide 2717 of FAK into the multiple cloning site of pAlter) with the corresponding Nru I/SalI fragment of pEGFP-FAK (extending from nucleotide 2717 of FAK into the multiple cloning site of pEGFP-C3). All of the amplified sequences were completely sequenced to verify that no unintended mutations were introduced during the procedure. DNA was sequenced at the University of North Carolina-Chapel Hill Automated DNA Sequencing Facility on a model 377 DNA Sequencer (Perkin Elmer-Cetus, Applied Biosystems Division, Norwalk, CT) by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA Polymerase, FS (Perkin Elmer-Cetus, Applied Biosystems Division).
Protein Analysis
Cells were lysed in modified RIPA buffer (Thomas et al., 1999), Tx-RIPA buffer (Thomas et al., 1999), or IGEPAL lysis buffer (20 mM Tris, pH 8.0, 137 mM NaCl, 1% IGEPAL detergent, 10% glycerol) containing protease and phosphatase inhibitors as described (Thomas et al., 1999). The protein concentration of lysates was determined by using the bicinchoninic acid assay (Pierce, Rockford, IL). Immunoprecipitations were typically performed by using 0.2–1 mg of cell lysate and 5–30 μl of polyclonal antiserum or 2 μg of purified monoclonal antibody (mAb). After incubation on ice for 1 h immune complexes were precipitated by using protein A Sepharose (Sigma, St. Louis, MO) or anti-mouse agarose beads (Sigma). Immune complexes were washed twice with lysis buffer, twice with phosphate-buffered saline, and then eluted in sample buffer (Laemmli, 1970). Subsequently, samples were analyzed by Western blotting. Polyclonal antisera BC2 and BC4 and mAb 2A7 (generous gifts of Dr. Tom Parsons, University of Virginia, Charlottesville, VA) were used to recognize FAK. A polyclonal antiserum (Thomas et al., 1999) and a commercial mAb (Transduction Labs, Lexington, KY) were used for the analysis of paxillin. The mAb KT3 was used for recognition of the epitope tag (MacArthur and Walter, 1984) and a polyclonal antibody was used for detection of GST (Molecular Probes, Eugene, OR). RC20 was used to detect phosphotyrosine (Transduction Labs). Western blots were incubated with horseradish peroxidase-conjugated secondary antibodies and processed for enhanced chemiluminescence (Amersham).
In Vitro-Binding Assay
The expression of GST-FAK fusion proteins was induced by using 0.1 mM isopropyl-1-thio-β-d-galactopyranoside. Escherichia coli were harvested, lysed by sonication, and the fusion proteins purified by using glutathione agarose beads (Sigma) as previously described (Thomas et al., 1999). CE cell lysates prepared in IGEPAL lysis buffer were precleared with 5 μg of GST. The supernatant was then incubated with 5 μg of fusion protein immobilized on glutathione agarose beads for 1 h at 4°C. The beads were washed twice with lysis buffer and twice with phosphate-buffered saline. Bound proteins were eluted by boiling in sample buffer (Laemmli, 1970) and analyzed by Western blotting.
Fluorescence
For immunofluorescence, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline for 7 min at room temperature, and then permeabilized with 0.5% triton X-100. As previously described, epitope-tagged proteins were detected with KT3 (Schaller and Sasaki, 1997). Cells were stained with mAb 2A7 to detect exogenously expressed untagged FRNK. Primary mAbs were detected by staining with a rhodamine-conjugated anti-mouse antibody (Jackson Immunoresearch Laboratories, West Grove, PA). For examination of GFP-FAK, cells were plated onto glass coverslips and incubated overnight at 37°C. The cells were then fixed and examined by microscopy. The cells were visualized by using either a Leitz Orthoplan microscope or Zeiss Axiophot microscope. Images were collected with a microMax 5 mHz cooled CCD camera (Princeton Instruments, Monmouth Junction, NJ) and MetaMorph imaging software (Universal Imaging Corp., West Chester, PA).
RESULTS
Targeting Carboxy-Terminal Residues of FAK for Mutagenesis
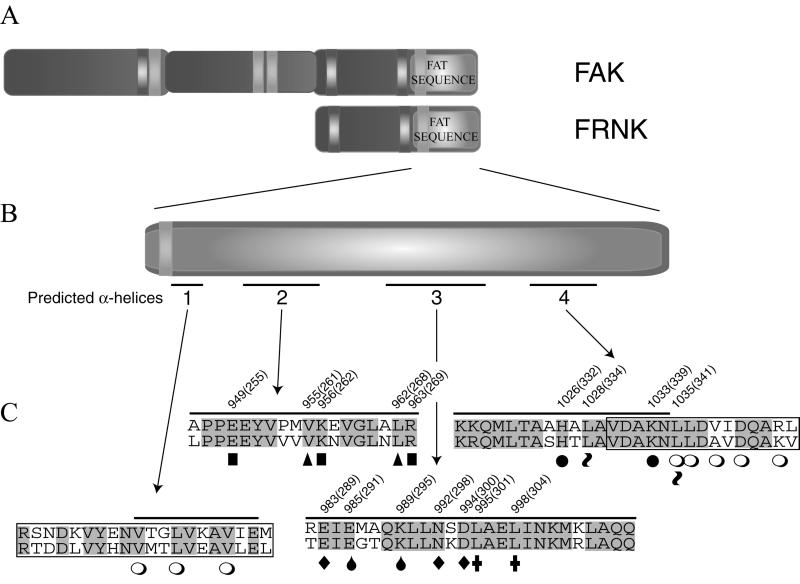
CAKβ is an FAK-related PTK that also exhibits paxillin binding and contains a focal adhesion-targeting sequence at its C terminus (Salgia et al., 1996; Hiregowdara et al., 1997; Li and Earp, 1997; Schaller and Sasaki, 1997; Xiong et al., 1998). Because the C termini of FAK and CAKβ are 61% identical, conserved residues likely mediate these functions. The secondary structure of this region of FAK is predicted to be α-helical and the α-helices are amphipathic in nature. Alanine substitutions were made for a number of residues conserved between FAK and CAKβ (Figure 1). Furthermore, these substitutions were designed to remove either charged residues or hydrophobic residues from the surface of an amphipathic α-helix. Oligonucleotide-directed mutagenesis was used to generate seven mutants, each containing two or three substitutions, and the mutants were identified by nucleotide sequencing. Sequence analysis revealed that one of the mutants contained an aspartic acid substitution for asparagine (at FAK residue 992) in addition to the two targeted mutations. This mutation was introduced during the mutagenesis reaction and not during subsequent amplification of the sequence. The other variants had no alterations other than the engineered alanine substitutions.
Figure 1.
Carboxy-terminal FAK Mutations. (A) Schematic illustration of full-length FAK is shown. (B) Schematic illustration of the FAT sequence is shown. Bars underneath the schematic indicate the positions of four predicted α-helices in the FAT sequence, which are designated 1–4. (C) Amino acid sequences of four regions of the FAT sequence of FAK are aligned with the corresponding sequences of CAKβ. The positions of the predicted α-helices are overlined and designated 1–4. Residues that are identical in FAK (upper sequence) and CAKβ (lower sequence) are shaded. The two boxed sequences in regions 1 and 4 are the vinculin homologous sequences of FAK that have been designated paxillin-binding site 1 (PBS1) and paxillin-binding site 2 (PBS2), respectively. The open circles (○) below the sequences denote point mutations in FAK that have been reported (Tachibana et al., 1995). Alanine residues have been substituted for the indicated FAK residues to create FAKE949/K956/R963 (indicated by ▪), FAKV955/L962 (indicated by ▴), FAKE985/K989 (indicated by drops, ), FAKE983/N992D/D994 (indicated by ♦), FAKL995/L998 (indicated by ✚), FAKH1026/K1033 (indicated by ●), and FAKL1028/L1035 (indicated by tilde signs ). FAKE983/N992D/D994 contains an unintended mutation in addition to the alanine substitutions. Asparagine 992 is mutated to aspartic acid. This mutation also is indicated by a filled diamond (♦). These amino acids also were mutated in FRNK; the residue number in the corresponding FRNK mutants is indicated in parentheses.
FAK Mutants Defective for Paxillin Binding
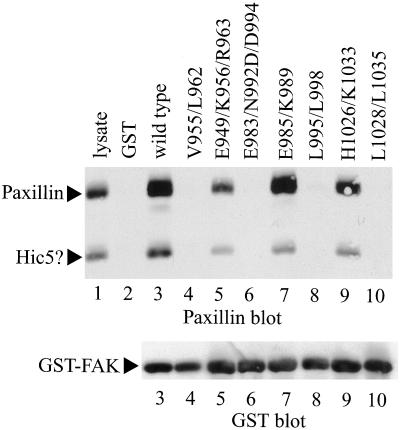
To assess paxillin binding, each mutant was expressed as a GST fusion protein containing FAK codons 765-1052 (Shen et al., 1998). The fusion proteins were immobilized on glutathione agarose beads and incubated with CE cell lysates. Bound paxillin was detected by Western blotting. As previously reported, fusion proteins containing wild-type FAK sequences bound paxillin (Figure 2, lane 3). Binding was specifically mediated by the FAK sequences because GST alone, immobilized to glutathione beads, failed to associate with paxillin (Figure 2, lane 2). In addition, a 50-kDa protein that was recognized by the paxillin antibody also bound GST-FAK. This is presumably the avian homolog of hydrogen peroxide-inducible clone 5 (Hic-5). GST-FAKE985/K989 bound paxillin as well as wild-type GST-FAK (Figure 2, lane 7) (note that the GST-FAK mutants are designated by using the codon numbering of full-length FAK). Two other FAK mutants, GST-FAKE949/K956/R963 and GST-FAKH1026/K1033, also exhibited paxillin binding, although the ability of each to associate with paxillin was slightly reduced relative to wild-type GST-FAK (Figure 2, lanes 5 and 9). The other four FAK mutants, GST-FAKV955/L962, GST-FAKE983/N992D/D994, GST-FAKL995/L998, and GST-FAKL1028/L1035, were all completely defective for paxillin binding in vitro (Figure 2, lanes 4, 6, 8, and 10). The blot was stripped and reprobed with a GST polyclonal antibody to verify that equal amounts of each fusion protein were used in the experiment (Figure 2, bottom). It is noteworthy that the altered residues in three of these mutants lie outside the vinculin homologous sequences of FAK, which have been proposed to comprise the paxillin-binding site (Tachibana et al., 1995).
Figure 2.
Paxillin-binding activity of FAK mutants. Each mutant was expressed as a GST fusion protein and immobilized to glutathione agarose beads. CE cell lysate was precleared by incubation with GST alone immobilized to glutathione agarose beads. The cleared lysates were then incubated with GST (lane 2), GST-Hter (wild type) (lane 3), GST-FAKV955/L962 (lane 4), GST-FAKE949/K956/R963 (lane 5), GST-FAKE983/N992D/D994 (lane 6), GST-FAKE985/K989 (lane 7), GST-FAKL995/L998 (lane 8), GST-FAKH1026/K1033 (lane 9), or GST-FAKL1028/L1035 (lane 10). The beads were washed and bound paxillin was detected by Western blotting (top). Twenty-five micrograms of lysate was run as a control (lane 1). The paxillin band and a second reactive protein that is presumably hydrogen peroxide-inducible clone 5 are indicated by arrows. The blot was stripped and reprobed with a GST antibody as loading control for the GST-FAK fusion proteins (bottom).
Expression of FAK and FRNK Mutants in Avian and Mammalian Cells
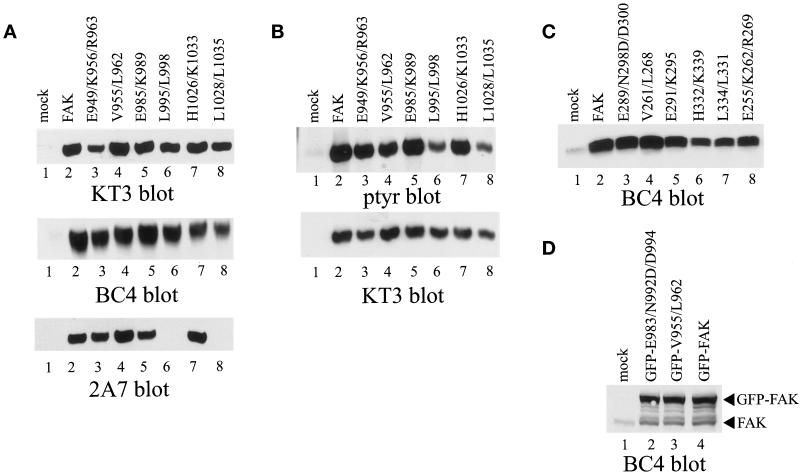
For expression in CE cells, the mutants were rescued into the full-length FAK cDNA containing a carboxy-terminal epitope tag. Although the presence of the tag perturbs the ability of an otherwise wild-type FAK protein to bind paxillin (Hildebrand et al., 1995), tagged wild-type FAK correctly localizes to focal adhesions and its phosphotyrosine content is regulated by cell adhesion (Schaller et al., 1993; Hildebrand et al., 1995; Shen and Schaller, 1999). Each construct was subcloned into the RCAS A avian replication competent retroviral vector and introduced into CE cells by transfection. Expression was initially examined by Western blot analysis of whole cell lysates by using an FAK polyclonal antiserum (BC4) and the KT3 mAb, which recognizes the epitope tag. KT3 did not recognize any proteins in CE cells transfected with the empty RCAS vector (Figure 3A, top, lane 1). In contrast, a 125-kDa reactive species was detected by KT3 in lysates from each of the wild-type and mutant FAK-transfected cells (Figure 3A, top). A similar level of each protein was detected, except for FAKE983/N992D/D994, which was very poorly expressed (our unpublished observations). It is not clear why this construct could not be expressed because FRNK and GFP-FAK constructs containing the same point mutations were expressed to levels similar to wild type. The low level of expression of FAKE983/N992D/D994 precluded its further analysis. Lysates also were probed with BC4, a polyclonal FAK antiserum (Figure 3A, middle). Each of the mutant proteins was recognized by BC4 and was dramatically overexpressed relative to endogenous wild-type FAK, which was undetectable at this exposure. Western blots of each of the mutants also were probed with the 2A7 FAK mAb. Although wild-type FAK and most of the mutants were recognized by 2A7, FAKL995/L998 and FAKL1028/L1035 were not detected in this Western blot (Figure 3A, bottom, lanes 6 and 8). This result represented a specific failure of 2A7 to bind to these two mutants because parallel Western blots demonstrate that these proteins are recognized by both KT3 and BC4 (Figure 3A, top and middle). To examine tyrosine phosphorylation of the mutants, each was immunoprecipitated by using KT3 and analyzed by Western blotting for phosphotyrosine. Both the exogenous wild-type FAK and each of the mutants contained phosphotyrosine (Figure 3B, top). Two of the mutants, FAKL995/L998 and FAKL1028/L1035, exhibited a reduction in the level of tyrosine phosphorylation (Figure 3B, top, lanes 6 and 8). This reduction in tyrosine phosphorylation was not due to differences in recovery of the proteins by immunoprecipitation, as indicated in the control KT3 Western blot (Figure 3B, bottom). Thus, most of the mutants were efficiently expressed as full-length FAK constructs and were tyrosine phosphorylated, although two mutants exhibited reduced tyrosine phosphorylation.
Figure 3.
Expression of FAK, FRNK, and GFP-FAK constructs. (A) Twenty-five micrograms of lysate from control CE cells (lane 1), or CE cells expressing epitope-tagged FAK (lane 2), FAKE949/K956/R963 (lane 3), FAKV955/L962 (lane 4), FAKE985/K989 (lane 5), FAKL995/L998 (lane 6), FAKH1026/K1033 (lane 7), or FAKL1028/L1035 (lane 8) was analyzed by Western blotting with KT3 (top), BC4 (middle), or 2A7 (bottom). (B) FAK was immunoprecipitated from lysates from control CE cells (lane 1), or CE cells expressing epitope-tagged FAK (lane 2), FAKE949/K956/R963 (lane 3), FAKV955/L962 (lane 4), FAKE985/K989 (lane 5), FAKL995/L998 (lane 6), FAKH1026/K1033 (lane 7), or FAKL1028/L1035 (lane 8). The immune complexes were blotted for phosphotyrosine (top). The blot was stripped and reprobed for the exogenous FAK proteins with KT3 (bottom). (C) Twenty-five micrograms of lysate from control CE cells (lane 1), or CE cells expressing FRNK (lane 2), FRNKE289/N298D/D300 (lane 3), FRNKV261/L268 (lane 4), FRNKE291/K295 (lane 5), FRNKH332/K339 (lane 6), FRNKL334/L341 (lane 7), or FRNKE255/K262/R269 (lane 8) was analyzed by Western blotting with BC4. (D) Twenty-five micrograms of lysate from control 293 cells (lane 1) or 293 cells transfected with a plasmid encoding GFP-FAK (lane 4), GFP-FAKE983/N992D/D994 (lane 2), or GFP-FAKV955/L962 (lane 3) was Western blotted by using BC4.
The mutants also were rescued into a full-length FRNK construct (lacking an epitope tag) for expression in CE cells. Each was subcloned into the RCAS A vector and transfected into CE cells. Lysates were analyzed by Western blotting with BC4. The wild-type FRNK protein and each of the mutants were expressed to high levels relative to the level of endogenous FRNK (Figure 3C).
Wild-type FAK and two mutants that were defective for paxillin binding, FAKV955/L962 and FAKE983/N992D/D994, were engineered for expression in mammalian cells as GFP fusion proteins. Using the pEGFP-C3 vector, the FAK sequences were fused in-frame and downstream of the GFP-encoding sequences. The FAK sequences did not contain the carboxy-terminal epitope tag. The resulting plasmids were transiently transfected into 293 cells for expression. Lysates were analyzed by Western blotting with BC4. Control transfected cells, as well as cells transfected with the GFP-FAK constructs, expressed the 125-kDa endogenous wild-type FAK protein (Figure 3D, lane 1). In addition to this protein, cells transfected with the plasmids encoding GFP-FAK fusion proteins contained a protein of 152-kDa that was detected with BC4 (Figure 3D, lanes 2–4). This protein was not expressed in control-transfected cells and migrated with the predicted mobility of the GFP-FAK fusion protein. Although cells transfected with the GFP-FAK constructs appeared to contain higher levels of endogenous FAK, this was likely a degradation product of the full-length GFP-FAK proteins because a number of other minor BC4 reactive species also were present in these lysates. Each of the GFP-FAK constructs was expressed to a similar level.
Subcellular Localization of Mutant Proteins
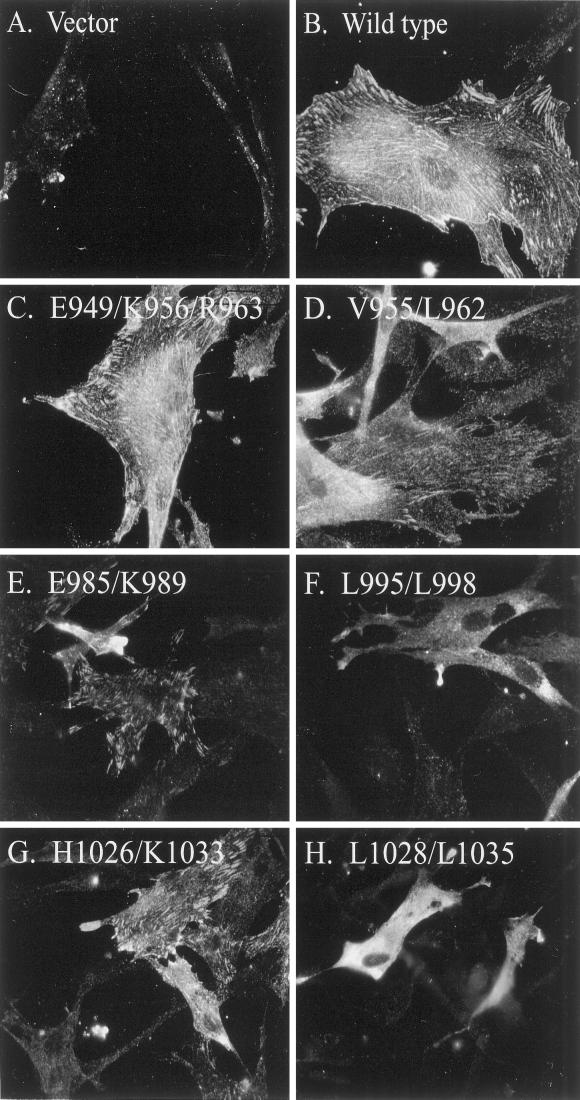
The subcellular localization of the epitope-tagged, FAK mutants in CE cells was determined by immunofluorescence with the KT3 mAb. As previously described, control CE cells that do not express the epitope tag exhibit a very diffuse, pale staining with KT3 (Figure 4A). In contrast, cells expressing epitope-tagged FAK exhibit very prominent focal adhesion staining with the KT3 antibody (Figure 4B). Most of the mutants analyzed targeted to focal adhesions. Like wild-type, epitope-tagged FAK, mutants FAKE949/K956/R963 (Figure 4C), FAKE985/K989 (Figure 4E), and FAKH1026/K1033 (Figure 4G) exhibited prominent focal adhesion localization. FAKV955/L962 also exhibited focal adhesion staining, although the fluorescence intensity was lower (Figure 4D). In contrast, FAKL995/L998 (Figure 4F) and FAKL1028/L1033 (Figure 4H) exclusively exhibited a diffuse cytoplasmic staining pattern with no evidence of specific focal adhesion staining.
Figure 4.
Subcellular localization of epitope-tagged FAK constructs. Control CE cells (A), or CE cells expressing epitope-tagged FAK (B), FAKE949/K956/R963 (C), FAKV955/L962 (D), FAKE985/K989 (E), FAKL995/L998 (F), FAKH1026/K1033 (G), or FAKL1028/L1035 (H) were grown on glass coverslips. The cells were fixed, permeabilized, and stained with mAb KT3. The antibody was detected with rhodamine-conjugated anti-mouse IgG antibody.
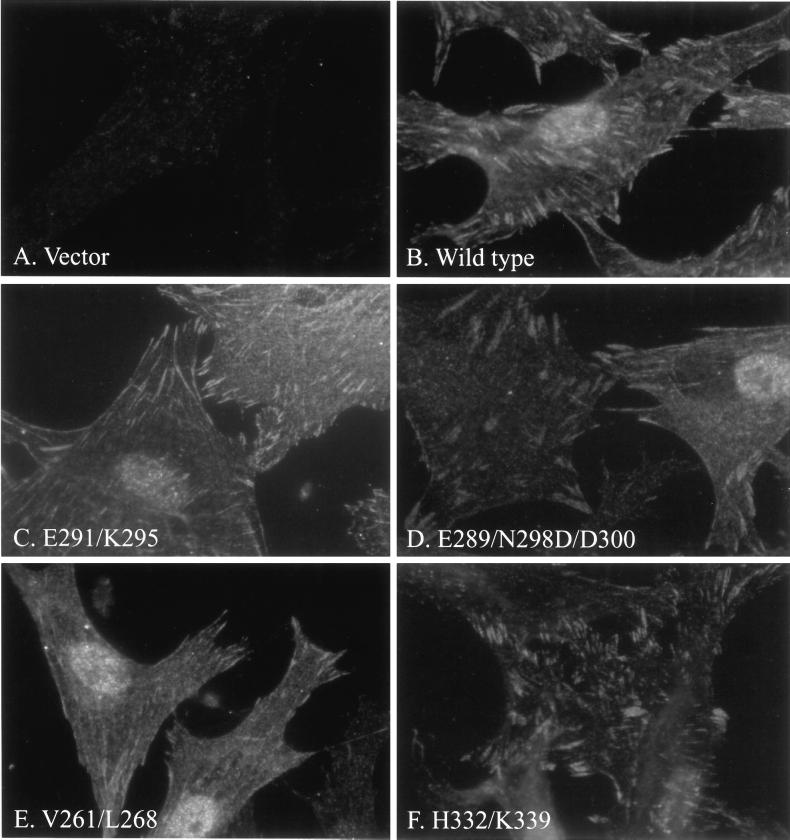
The subcellular localization of untagged FRNK mutants in CE cells was determined by immunofluorescence with mAb 2A7. With the staining conditions used, endogenous wild-type FAK and FRNK exhibited a very pale focal adhesion-staining pattern in control cells transfected with the empty RCAS vector (Figure 5A). Cells expressing exogenous wild-type FRNK were brightly stained with very prominent focal adhesion staining (Figure 5B). Thus, the subcellular localization of the exogenously expressed protein was readily distinguishable from endogenous protein based upon the intensity of the signal. Mutants FRNKE291/K295 (Figure 5C), FRNKE289/N298D/D300 (Figure 5D), FRNKV261/L268 (Figure 5E), and FRNKH332/K339 (Figure 5F) all exhibited a very distinct focal adhesion-staining pattern. FRNKE255/K262/R269 also exhibited focal adhesion staining (our unpublished observations). The localization of FRNKL334/L341 was not determined in this analysis because the mutations eliminated recognition by mAb 2A7.
Figure 5.
Subcellular localization of FRNK mutants. Control CE cells (A), or CE cells expressing FRNK (B), FRNKE291/K295 (C), FRNKE289/N298D/D300 (D), FRNKV261/L268 (E), or FRNKH332/K339 (F) were plated on glass coverslips. The cells were fixed, permeabilized, and stained with mAb 2A7. The mAb was detected with rhodamine-labeled anti-mouse IgG antibody.
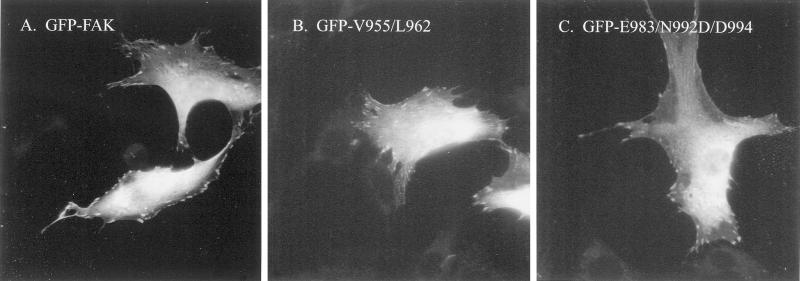
The subcellular localization of GFP-FAK was examined in transiently transfected NIH 3T3 cells. Cells expressing a GFP-wild–type FAK fusion protein exhibited bright cellular/cytoplasmic staining as well as focal adhesion localization (Figure 6A). The subcellular localization of two mutants that were defective for paxillin binding in vitro was determined. Both GFP-FAKV955/L962 (Figure 6B) and GFP-FAKE983/N992/D/D994 (Figure 6C) exhibited the same localization pattern as wild-type FAK. Thus, these mutants can correctly localize to focal adhesions despite their inability to bind paxillin in vitro.
Figure 6.
Subcellular localization of GFP-FAK mutants. NIH 3T3 cells transiently expressing GFP-FAK (A), GFP- FAKV955/L962 (B), or GFP-FAKE983/N992D/D994 (C) were grown on glass coverslips, fixed, and examined by fluorescent microscopy.
GFP-FAK Mutants Fail to Bind Paxillin In Vivo
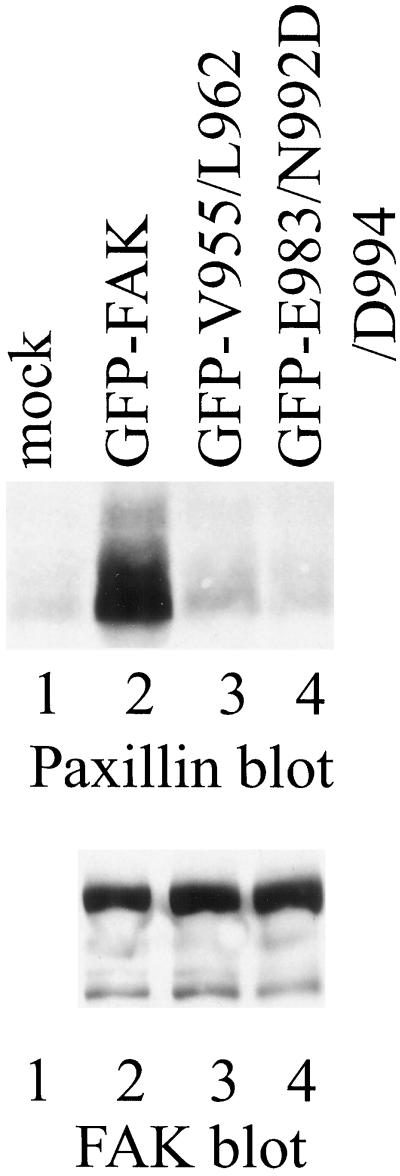
To further examine the ability of FAK mutants to associate with paxillin in vivo, GFP-FAK fusion proteins were expressed transiently in 293 cells. GFP-FAK was immunoprecipitated from cell lysates by using BC4 and the immune complexes were analyzed by Western blotting for paxillin. A small amount of paxillin was coimmunoprecipitated with endogenous wild-type FAK from 293 cells (Figure 7, top, lane 1). However, a significantly increased level of paxillin was detected in immune complexes from cells transfected with GFP-wild–type FAK, demonstrating that the exogenously expressed fusion protein bound paxillin in vivo (Figure 7, top, lane 2). In contrast, the amount of paxillin coimmunoprecipitated from cells transfected with GFP-FAKV955/L962 (Figure 7, top, lane 3) or GFP-FAKE983/N992/D/D994 (Figure 7, top, lane 4) was no greater than the amount of paxillin coimmunoprecipitated with endogenous FAK from control-transfected cells. Thus, these two mutants failed to associate with paxillin in vivo. The blot was stripped and reprobed with BC4 to demonstrate equal immunoprecipitation of each of the GFP fusion proteins (Figure 7, bottom). Endogenous FAK was not detected in the exposure shown in Figure 7. These in vivo-binding data corroborate the results of the in vitro paxillin-binding assay, confirming that these two FAK mutants are defective for paxillin binding.
Figure 7.
Association of paxillin with GFP-FAK. FAK was immunoprecipitated with BC4 from lysates of control 293 cells (lane 1) or 293 cells transiently expressing GFP-FAK (lane 2), GFP-FAKV955/L962 (lane 3), or GFP-FAKE983/N992D/D994 (lane 4). Coimmunoprecipitated paxillin was detected by Western blotting (top). The blot was stripped and reprobed with BC4 to verify equal recovery of the GFP-FAK fusion proteins by immunoprecipitation (bottom).
Cell Adhesion-Dependent Tyrosine Phosphorylation of FAK Mutants
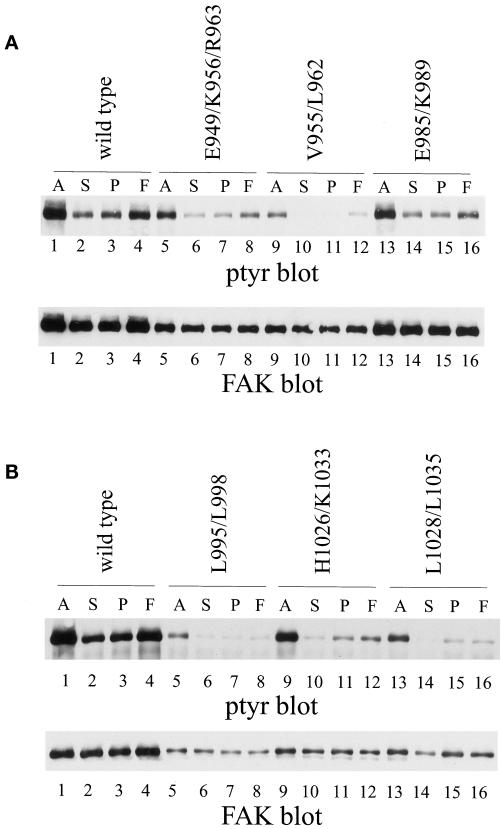
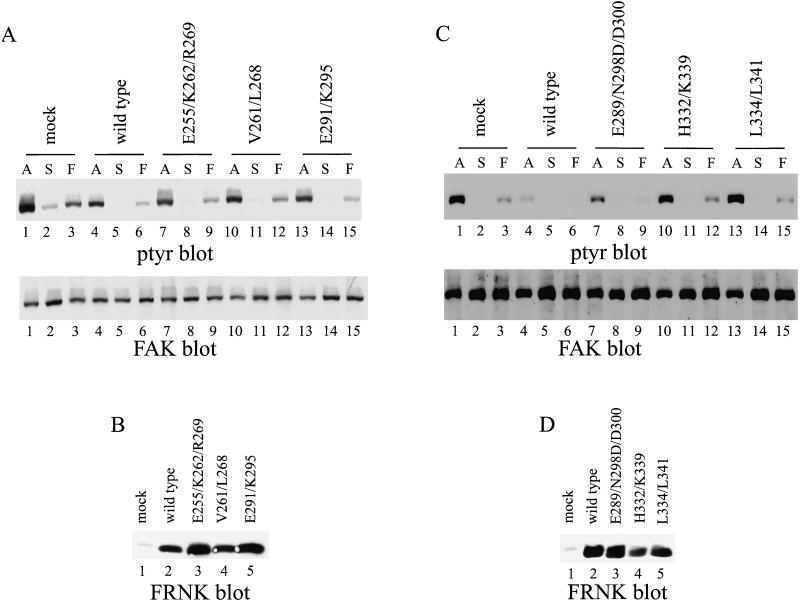
The major stimulus regulating tyrosine phosphorylation of FAK is cell adhesion. To establish whether the FAK mutants were regulated by cell adhesion, CE cells expressing each were held in suspension or plated onto poly-l-lysine– or fibronectin–coated dishes and incubated for 30 min at 37°C before lysis. Exogenously expressed FAK was immunoprecipitated by using mAb KT3 and the immune complexes analyzed by Western blotting for phosphotyrosine. Wild-type tagged FAK was phosphorylated in cells in culture. The phosphotyrosine content was dramatically reduced when cells were held in suspension. It became tyrosine phosphorylated when cells adhered to fibronectin-coated surfaces, but was poorly phosphorylated in response to adhesion to poly-l-lysine (Figure 8A & B, top, lanes 1–4). FAKE985/K989 responded very similarly to wild-type FAK (Figure 8A, top, lanes 13–16). Mutants FAKE949/K956/R963 (Figure 8A, top, lanes 5–8), FAKH1026/K1033 (Figure 8B, top, lanes 9–12), and FAKV955/L962 (Figure 8A, top, lanes 9–12) also exhibited cell adhesion-dependent tyrosine phosphorylation. Differences in the level of expression of the mutants in this experiment make it difficult to compare relative levels of phosphotyrosine between mutants. However, it is apparent that FAKE949/K956/R963, FAKH1026/K1033, and FAKV955/L962 contain elevated levels of phosphotyrosine when cells are plated onto fibronectin relative to the poly-l-lysine–plated controls. Only two mutants, FAKL995/L998 and FAKL1028/L1035, were defective for cell adhesion-dependent tyrosine phosphorylation. FAKL995/L998 (Figure 8B, top, lanes 5–8) and FAKL1028/L1035 (Figure 8B, top, lanes 13–16) were tyrosine phosphorylated in cells growing in culture. The phosphotyrosine content of each mutant was reduced when cells were held in suspension in serum-free medium. However, neither mutant became tyrosine phosphorylated when cells were plated on fibronectin. The phosphotyrosine level of each was similar in cells adhered to fibronectin or to poly-l-lysine–coated dishes for 30 min. These two mutants also exhibit a defect in localization, reinforcing the hypothesis that focal adhesion targeting is essential for cell adhesion-dependent regulation of FAK.
Figure 8.
Cell adhesion-dependent regulation of tyrosine phosphorylation mutants of FAK. CE cells expressing wild-type, epitope-tagged FAK (A and B, lanes 1–4) or FAKE949/K956/R963 (A, lanes 5–8), FAKV955/L962 (A, lanes 9–12), FAKE985/K989 (A, lanes 13–16), FAKL995/L998 (B, lanes 5–8), FAKH1026/K1033 (B, lanes 9–12), or FAKL1028/L1035 (B, lanes 13–16) were analyzed. Adherent cells (A) (lanes 1, 5, 9, and 13) or cells held in suspension (S) (lanes 2, 6, 10, and 14) or plated onto poly(l-lysine) (P) (lanes 3, 7, 11, and 15) or fibronectin (F) (lanes 4, 8, 12, and 16) were lysed. Exogenous FAK was immunoprecipitated with KT3 and Western blotted for phosphotyrosine (tops). The blot was stripped and reprobed with BC4 (bottoms).
Mutants Exhibit Defects in Induction of Paxillin Phosphorylation
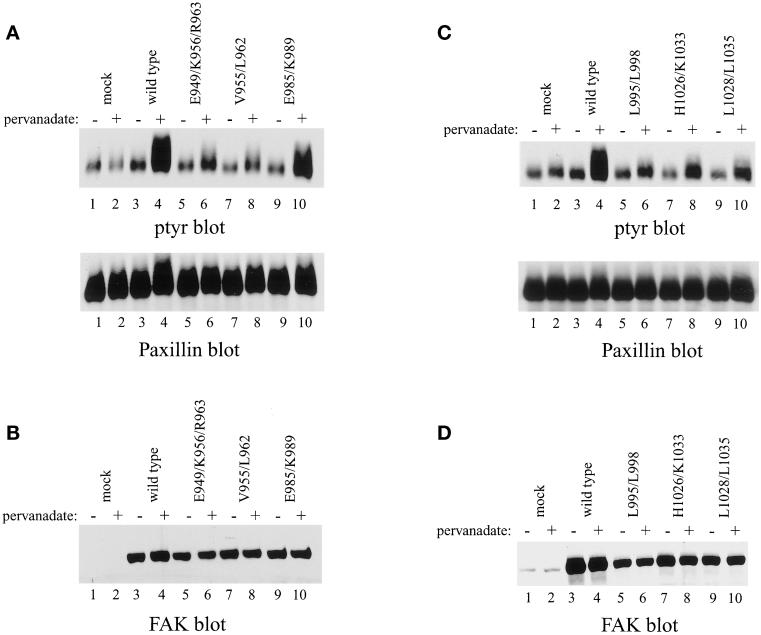
Tyrosine phosphorylation of paxillin can be induced in an FAK-dependent manner. Pharmacological inhibition of protein tyrosine phosphatases in FAK overexpressing CE cells leads to enhanced phosphorylation of paxillin (Schaller and Parsons, 1995; Schaller and Sasaki, 1997; Shen and Schaller, 1999). Each of the FAK mutants was examined to determine whether its ability to induce paxillin phosphorylation was impaired. CE cells were left untreated or treated with 2 μM pervanadate for 30 min before lysis. Paxillin was immunoprecipitated and analyzed by Western blotting for phosphotyrosine. Under these conditions, pervanadate treatment did not alter the phosphotyrosine content of paxillin in CE cells (Figure 9, A and B, top, lanes 1 and 2). In contrast, pervanadate induced a very dramatic increase in tyrosine phosphorylation of paxillin in CE cells overexpressing wild-type, epitope-tagged FAK (Figure 9, A and B, top, lanes 3 and 4). Similarly, paxillin phosphorylation was enhanced upon pervanadate treatment of CE cells expressing FAKE985/K989, although the response was not as robust (Figure 9A, top, lanes 9 and 10). All of the other mutants exhibited a dramatically weaker induction of paxillin phosphorylation upon pervanadate treatment (Figure 9, A and C, top). Two mutants that fail to target to focal adhesions, FAKL995/L998 and FAKL1028/L1035, did induce a small increase in tyrosine phosphorylation of paxillin (Figure 9C, lanes 5, 6, 9, and 10). However, the level of tyrosine phosphorylation of paxillin induced by these mutants was very low relative to the level of phosphorylation induced by wild-type FAK (Figure 9C, lanes 3 and 4). The reduction in tyrosine phosphorylation of paxillin was not due to differential recovery of paxillin by immunoprecipitation (Figure 9, A and C, bottom) or due to differences in level of expression of the mutant FAK proteins (Figure 9, B and D). This result demonstrates that the induction of tyrosine phosphorylation of paxillin by FAK in this system does not strictly correlate with the localization of FAK to focal adhesions. Although focal adhesion targeting is important for efficient induction of paxillin phosphorylation, some of the mutants correctly localize to focal adhesions yet induce paxillin phosphorylation poorly.
Figure 9.
Induction of tyrosine phosphorylation of paxillin by FAK mutants. (A and C) Control CE cells (lanes 1 and 2) or CE cells expressing wild-type, epitope-tagged FAK (lanes 3 and 4) or FAKE949/K956/R963 (A, lanes 5 and 6), FAKV955/L962 (A, lanes 7 and 8), FAKE985/K989 (A, lanes 9 and 10), FAKL995/L998 (C, lanes 5 and 6), FAKH1026/K1033 (C, lanes 7 and 8), or FAKL1028/L1035 (C, lanes 9 and 10) were left untreated (odd lanes) or treated with pervanadate (even lanes). The cells were lysed and paxillin was immunoprecipitated and Western blotted for phosphotyrosine (A and C, top). The blot was striped and reprobed for paxillin (A and C, bottom). (B and D), Twenty-five micrograms of cell lysate was analyzed for FAK expression by Western blotting with BC4.
Characterization of Dominant-Negative Activity of FRNK Mutants
Overexpression of wild-type FRNK in CE cells blocks cell spreading and tyrosine phosphorylation of endogenous wild-type FAK, paxillin, and tensin (Richardson and Parsons, 1996). Each mutant was tested for its ability to inhibit tyrosine phosphorylation of endogenous FAK in cells in culture and after cell adhesion to fibronectin. Cells were lysed and endogenous FAK was immunoprecipitated with BC2, an antiserum recognizing the catalytic domain of FAK and thus unable to recognize the exogenously expressed FRNK (Richardson and Parsons, 1996). The immune complexes were analyzed by Western blotting for phosphotyrosine. As previously described, expression of wild-type FRNK reduced tyrosine phosphorylation on endogenous FAK both in adherent cells growing in culture and in cells plated onto fibronectin (Figure 10, A and C, top, lanes 4 and 6). Mutant FRNKE291/K295 behaved similarly to wild-type FRNK, causing a reduction in tyrosine phosphorylation of endogenous FAK in both adherent cells and in cells plated onto fibronectin (Figure 10A, top, lanes 13 and 15). Three mutants exhibited a partial defect in functioning as a dominant-negative mutant in this assay. Expression of FRNKE255/K262/R269, FRNKV261/268, or FRNKE289/N298D/D300 caused a reduction in the phosphotyrosine content of endogenous FAK, but the level of phosphotyrosine was higher than in cells expressing wild-type FRNK (Figure 10A, top, lanes 7–12 and Figure 10C, top, lanes 7–9). In contrast, two mutants were completely defective for inhibiting tyrosine phosphorylation of endogenous FAK. In cells expressing FRNKH332/K339 or FRNKL334/L341 the level of tyrosine phosphorylation of endogenous FAK was similar to the levels observed in control cells transfected with the empty RCAS vector (Figure 10C, top, lanes 10–15). Differences in the level of phosphotyrosine were not due to differences in recovery of FAK by immunoprecipitation (Figure 10, A and C, bottom). Control blots demonstrated that each of the FRNK mutants was expressed to high levels (Figure 10, B and D). Although these results demonstrate that targeting to focal adhesions was required for FRNK to function as a dominant-negative mutant, some mutants that targeted to focal adhesions were compromised in their ability to act in a dominant-negative manner. This suggests that an additional function is required for the dominant-negative activity of FRNK.
Figure 10.
Inhibition of tyrosine phosphorylation of endogenous FAK by FRNK mutants. (A and C) Control CE cells (lanes 1–3) or CE cells expressing wild-type FRNK (lanes 4–6) or FRNKE255/K262/R269 (A, lanes 7–9), FRNKV261/L268 (A, lanes 10–12), FRNKE291/K295 (A, lanes 13–15), FRNKE289/N298D/D300 (C, lanes 7–9), FRNKH332/K339 (C, lanes 10–12), or FRNKL334/L341 (C, lanes 13–15) were analyzed. Adherent cells (A) (A and C, lanes 1, 4, 7, 10, and 13) or cells held in suspension (S) (lanes 2, 5, 8, 11, and 14) or cells plated onto fibronectin (F) (lanes 3, 6, 9, 12, and 15) were lysed and endogenous FAK was immunoprecipitated by using BC2. The immune complexes were analyzed by Western blotting for phosphotyrosine (A and C, top). The blots were stripped and reprobed for FAK (A and C, bottom). (B and D) Twenty-five micrograms of cell lysate was analyzed for FRNK expression by Western blotting with BC4.
DISCUSSION
In this report, we describe the generation of seven FAT sequence mutants of FAK, each containing multiple alanine substitutions. The results of this analysis are summarized in Table 1. Four of these mutants exhibit severe defects in paxillin binding. Two mutants fail to correctly localize to focal adhesions and are not regulated by cell adhesion. Most of the mutants are unable to induce tyrosine phosphorylation of paxillin to the same levels induced by wild-type FAK in vivo. Several of the mutants are also defective at inhibiting tyrosine phosphorylation of endogenous FAK when expressed as dominant-negative FRNK constructs. The findings support the hypothesis that paxillin binding is dispensable for focal adhesion targeting of FAK. Cell adhesion-dependent regulation of FAK strictly correlated with localization to focal adhesions. However, focal adhesion targeting is not sufficient for the induction of paxillin phosphorylation by FAK or for the inhibition of endogenous FAK signaling by FRNK.
Table 1.
Summary of properties of FAK and FRNK mutants
| FAK mutant | FRNK mutant | Bind PAXa | Target FAs FAK | Target FAs GFP-FAK | Target FAs FRNK | Bind PAXb | Regulation | Pax PTYR | Dominant negative |
|---|---|---|---|---|---|---|---|---|---|
| FAK | FRNK | +++ | + | + | + | + | + | +++ | ++ |
| E949/K956/R963 | E255/K262/R269 | ++ | + | n.d. | + | n.d. | + | + | + |
| V955/L962 | V261/L268 | − | + | + | + | − | + | + | + |
| E985/K989 | E291/K295 | +++ | + | n.d. | + | n.d. | + | +++ | ++ |
| E983/N992D/D994 | E289/N298D/D300 | − | n.d. | + | + | − | n.d. | n.d. | + |
| L995/L998 | − | − | − | n.d. | n.d. | n.d. | − | − | n.d. |
| H1026/K1033 | H332/K339 | ++ | + | n.d. | + | n.d. | + | − | − |
| L1028/L1035 | L334/L341 | − | − | n.d. | n.d. | n.d. | − | − | − |
n.d., not determined.
Paxillin-binding activity of GST-FAK fusion proteins in vitro.
Paxillin-binding activity of GFP-FAK fusion proteins in vivo.
Both FAK and vinculin bind to the amino-terminal half of paxillin (Turner and Miller, 1994). The paxillin-binding site in vinculin has been defined and this region exhibits sequence homology with two discontinuous sequences within the FAT sequence of FAK (Wood et al., 1994; Tachibana et al., 1995). Point mutations introduced into the vinculin homologous sequences in FAK perturbed paxillin binding, reinforcing the hypothesis that these sequences mediate binding to paxillin (Tachibana et al., 1995). However, in this report several other paxillin-binding defective mutants that contain lesions outside of the region of vinculin homology have been identified (FAKV955/L962, FAKE/983/N992/D300, and FAKL995/L998). Therefore, the paxillin-binding region of FAK extends beyond this region of homology with vinculin. This finding is not surprising given the results of a deletion analysis of paxillin. Although both FAK and vinculin were demonstrated to bind to the LD2 motif of paxillin, FAK, but not vinculin, was shown to bind to a second LD motif (LD3) in paxillin (Brown et al., 1996). One simple model of interaction is that the vinculin homologous sequences in FAK bind to LD2 and FAK residues outside of the sequences of vinculin homology interact with LD3. One prediction of this model is that specific point mutations in FAK would disrupt binding to LD3 and leave binding to LD2 unperturbed. However, the mutants described in this report exhibit profound defects in binding to both the LD2 and LD3 motifs of paxillin (Thomas et al., 1999). Therefore, this simple model of interaction is incorrect.
There are two prevailing models for focal adhesion targeting of FAK. First, because a number of FAK mutants that do not bind paxillin fail to target to focal adhesions, paxillin has been proposed as the binding partner that targets FAK to focal adhesions (Tachibana et al., 1995). Second, talin has been proposed as the binding partner that targets FAK to focal adhesions (Zheng et al., 1998). The first model is not strictly correct because a carboxy-terminal, epitope-tagged FAK variant fails to bind paxillin yet still localizes to focal adhesions (Schaller et al., 1993; Hildebrand et al., 1995). Furthermore, two mutants described in this report, FAKV955/L962 and FAKE983/N992D/D994, fail to associate with paxillin in vitro and in vivo but correctly localize to focal adhesions. Recently, an additional analysis of the L1034S FAK mutant (equivalent to our L1035 mutant; the discrepancy in residue number reflects a species difference between avian and murine FAK) has been reported (Sieg et al., 1999). This mutation disrupts paxillin binding, but does not perturb the localization of FAK to focal adhesions. Interestingly, this same mutation disrupts targeting of a GST-FRNK fusion protein and FRNK to focal adhesions, suggesting that focal adhesion targeting of FAK and FRNK might occur via distinct mechanisms (Tachibana et al., 1995; Sieg et al., 1999). It is not obvious why two proteins with identical primary structure would use different mechanisms to achieve the same end, i.e., target to focal adhesions. From our analysis FAKL1028/L1035 did not target to focal adhesions, perhaps suggesting that mutation of leucine 1028 was the critical alteration that ablates focal adhesion-targeting activity.
Clearly, the results of these analyses provide compelling evidence that paxillin binding is dispensable for focal adhesion targeting of FAK. However, examination of all FAK mutants that fail to target to focal adhesions reveals that each is defective for paxillin binding (Hildebrand et al., 1995; Tachibana et al., 1995). Thus, it is possible that there are two targeting mechanisms of FAK, one paxillin dependent and the other paxillin independent. Given this scenario, FAK mutants that fail to target to focal adhesions would be defective for paxillin binding and defective for engagement with the paxillin-independent mechanism of targeting. One candidate-binding partner for this latter mechanism of targeting is obviously talin. We have been unable to evaluate this hypothesis because we have been unable to consistently demonstrate an interaction between wild-type FAK and talin by coimmunoprecipitation or in in vitro-binding assays with GST fusion proteins. Perhaps this is a low-affinity interaction that cannot be detected under the lysis conditions used. Alternatively, FAK may target to focal adhesions via an undiscovered interaction.
Most of the mutants analyzed exhibited cell adhesion-dependent tyrosine phosphorylation. The two exceptions were FAKL995/L998 and FAKL1028/L1035, which also failed to localize to focal adhesions. This finding corroborates previous findings that correct localization is a requirement for cell adhesion-dependent regulation of FAK (Shen and Schaller, 1999). Each of the mutants that targeted to focal adhesions exhibited cell adhesion-dependent tyrosine phosphorylation. Recall that these constructs contain a carboxy-terminal epitope tag that ablates paxillin-binding activity. Therefore, cell adhesion-dependent tyrosine phosphorylation of FAK is independent of paxillin binding.
Two of the mutants that failed to target to focal adhesions induced very low levels of tyrosine phosphorylation of paxillin. There are a number of possible explanations for this observation. Perhaps these mutants induce tyrosine phosphorylation of a pool of paxillin that is not focal adhesion localized. Alternatively, the FAK mutants may stimulate a signaling pathway that indirectly results in tyrosine phosphorylation of paxillin in focal adhesions, e.g., via activation of Src. Regardless of the mechanism of paxillin phosphorylation by these mutants, the induction of tyrosine phosphorylation is very weak relative to the induction of paxillin phosphorylation by wild-type FAK. Thus, focal adhesion targeting was a prerequisite for efficiently inducing tyrosine phosphorylation of paxillin. However, it was not sufficient to induce paxillin phosphorylation because several mutants localized to focal adhesions but only weakly induced paxillin phosphorylation. This observation was surprising because we have described a FAK/vinculin chimera in which the FAT sequence of FAK is replaced with the amino-terminal focal adhesion targeting sequence of vinculin. This construct targets to focal adhesions and can induce tyrosine phosphorylation of paxillin in vanadate-treated CE cells, suggesting that colocalization of the catalytic domain of FAK with paxillin was sufficient to induce tyrosine phosphorylation of paxillin (Shen and Schaller, 1999). One hypothesis that explains these observations is that FAK and paxillin may need to colocalize in focal adhesions in a specific spatial arrangement for FAK-dependent tyrosine phosphorylation of paxillin. Presumably, the FAK/vinculin chimera can mimic the required organization between wild-type FAK and paxillin within focal adhesions, whereas some FAK mutants cannot. It may be noteworthy that the vinculin sequences in the FAK/vinculin chimera contain a binding site for talin (Gilmore et al., 1992). Our results also demonstrate that tyrosine phosphorylation of paxillin by exogenously expressed FAK was not dependent upon physical association with paxillin because the FAK constructs were all carboxy-terminally epitope tagged and unable to bind paxillin. This result is corroborated by analysis of a paxillin mutant that is defective for FAK binding. This mutant shows a partial reduction in tyrosine phosphorylation relative to wild-type paxillin, suggesting that tyrosine phosphorylation is partially dependent and partially independent of FAK binding (Thomas et al., 1999). Although paxillin binding is dispensable for the induction of paxillin phosphorylation by FAK, these findings suggest that there is a second requirement in addition to focal adhesion targeting of FAK.
FRNK has been used as a dominant-negative mutant to block biochemical signaling via FAK and to inhibit cell spreading and motility, two biological processes controlled by FAK (Gilmore and Romer, 1996; Richardson and Parsons, 1996). To function as a dominant-negative mutant, FRNK must localize to focal adhesions because FRNKL334/L341 does not inhibit tyrosine phosphorylation of endogenous FAK when expressed in CE cells. This finding is consistent with the observation that a FRNK mutant (L1034S) that fails to target to focal adhesions cannot impair cell motility (Sieg et al., 1999). However, targeting to focal adhesions is not sufficient for FRNK to block FAK signaling because FRNKH332/K339 correctly localizes to focal adhesions, yet does not cause a reduction in tyrosine phosphorylation of endogenous FAK. Similarly, an epitope-tagged variant of FRNK that localizes to focal adhesions is also ineffective as a dominant-negative mutant (Richardson et al., 1997). Because this variant fails to bind paxillin, it was suggested that paxillin binding was a prerequisite for the dominant-negative activity of FRNK. However, the dominant-negative activity of the mutants reported herein does not correlate with paxillin-binding activity. These observations suggest that, in addition to focal adhesion targeting and paxillin binding, there are other requirements for the dominant-negative activity of FRNK.
Paxillin binding was previously proposed as the mechanism for focal adhesion targeting of FAK and as an essential feature for the dominant-negative function of FRNK. Although our results do not strictly exclude a role for paxillin binding in these functions, they clearly demonstrate a paxillin-independent mechanism of focal adhesion targeting for FAK and suggest that paxillin is not the sole target for FRNK-dependent inhibition of FAK. Elucidation of FAK targeting and signaling clearly requires the identification of the FAT sequence-binding partners mediating these functions.
ACKNOWLEDGMENTS
We thank Drs. Keith Burridge and Ben Peng for the use of their microscope facilities. We also thank the members of the Schaller and Burridge laboratories, especially Veronica Gabarra, Patrick Lyons, Sarita Sastry, Simone Schoenwaelder, and Betty Liu, for helpful comments during the course of this study. This study was supported by grant RPG-96-021-04-CSM from the American Cancer Society (to M.D.S.).
Abbreviations used:
- CAKβ
cell adhesion kinase β
- cas
crk-associated substrate
- CE
chicken embryo
- CRNK
CAKβ-related nonkinase
- FAK
focal adhesion kinase
- FAT
focal adhesion targeting
- FRNK
FAK-related nonkinase
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- PCR
polymerase chain reaction
- PRNK
Pyk2-related nonkinase
- PTK
protein tyrosine kinase
- Pyk
proline-rich tyrosine kinase
- RAFTK
related adhesion focal tyrosine kinase
- SH
Src homology
REFERENCES
- Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- Brinson AE, Harding T, Diliberto PA, He Y, Li X, Hunter D, Herman B, Earp HS, Graves LM. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor. Dependence on calcium and the actin cytoskeleton. J Biol Chem. 1998;273:1711–1718. doi: 10.1074/jbc.273.3.1711. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Jackson P, Waites GT, Critchley DR. Further characterization of the talin-binding site in the cytoskeletal protein vinculin. J Cell Sci. 1992;103:719–731. doi: 10.1242/jcs.103.3.719. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Han DC, Guan JL. Association of focal adhesion kinase with Grb7 and its role in cell migration. J Biol Chem. 1999;274:24425–24430. doi: 10.1074/jbc.274.34.24425. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Herzog H, Nicholl J, Hort YJ, Sutherland GR, Shine J. Molecular cloning and assignment of FAK2, a novel human focal adhesion kinase, to 8p11.2-p22 by nonisotopic in situ hybridization. Genomics. 1996;32:484–486. doi: 10.1006/geno.1996.0149. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Taylor JM, Parsons JT. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol Cell Biol. 1996;16:3169–3178. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiregowdara D, Avraham H, Fu Y, London R, Avraham S. Tyrosine phosphorylation of the related adhesion focal tyrosine kinase in megakaryocytes upon stem cell factor and phorbol myristate acetate stimulation and its association with paxillin. J Biol Chem. 1997;272:10804–10810. doi: 10.1074/jbc.272.16.10804. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions [see comments] Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Li J, Avraham H, Rogers RA, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–8924. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur H, Walter G. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J Virol. 1984;52:483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK [published erratum appears in Nature 1996 Jun 27;381:810] Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Salgia R, Avraham S, Pisick E, Li JL, Raja S, Greenfield EA, Sattler M, Avraham H, Griffin JD. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271:31222–31226. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Sasaki T. Differential signaling by the focal adhesion kinase and cell adhesion kinase beta. J Biol Chem. 1997;272:25319–25325. doi: 10.1074/jbc.272.40.25319. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer GP. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases [published erratum appears in Mol. Cell. Biol. 1996 Dec; 16:7182–7184] Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Shen Y, Schaller MD. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol Biol Cell. 1999;10:2507–2518. doi: 10.1091/mbc.10.8.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Schneider G, Cloutier JF, Veillette A, Schaller MD. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J Biol Chem. 1998;273:6474–6481. doi: 10.1074/jbc.273.11.6474. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Sato T, D'Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1099. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani PD, Singh L, Auer KL, LaFlamme SE. The role of conserved amino acid motifs within the integrin beta3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J Biol Chem. 1997;272:7892–7898. doi: 10.1074/jbc.272.12.7892. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Cooley MA, Broome JM, Salgia R, Griffin JD, Lombardo CR, Schaller MD. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J Biol Chem. 1999;274:36684–36692. doi: 10.1074/jbc.274.51.36684. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Wood CK, Turner CE, Jackson P, Critchley DR. Characterization of the paxillin-binding site and the C-terminal focal adhesion targeting sequence in vinculin. J Cell Sci. 1994;107:709–717. [PubMed] [Google Scholar]
- Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WC, Macklem M, Parsons JT. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci. 1998;111:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]
- Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G, Hanks SK. Focal adhesion kinase promotes phospholipase C-gamma1 activity. Proc Natl Acad Sci USA. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Xing Z, Bian ZC, Guo C, Akbay A, Warner L, Guan JL. Differential regulation of Pyk2 and focal adhesion kinase (FAK). The C-terminal domain of FAK confers response to cell adhesion. J Biol Chem. 1998;273:2384–2389. doi: 10.1074/jbc.273.4.2384. [DOI] [PubMed] [Google Scholar]