Abstract
The resistance of cells to many drugs simultaneously (multidrug resistance) often involves the expression of membrane transporters (Mdrs); each recognizes and expels a broad spectrum of chemically unrelated drugs from the cell. The Escherichia coli Mdr transporter MdfA is able to transport differentially charged substrates in exchange for protons. This includes neutral compounds, namely chloramphenicol and thiamphenicol, and lipophilic cations such as tetraphenylphosphonium and ethidium. Here we show that the chloramphenicol and thiamphenicol transport reactions are electrogenic, whereas the transport of several monovalent cationic substrates is electroneutral. Therefore, unlike with positively charged substrates, the transmembrane electrical potential (negative inside) constitutes a major part of the driving force for the transport of electroneutral substrates by MdfA. These results demonstrate an unprecedented ability of a single secondary transporter to catalyze discrete transport reactions that differ in their electrogenicity and are governed by different components of the proton motive force.
Multidrug resistance (Mdr) transporters that expel drugs from the cytoplasm or cytoplasmic membrane to the external medium constitute one of the major causes of Mdr. Mdr transporters are widely distributed in bacteria, including pathogenic strains (1–4). The prokaryotic Mdr transporters are able to extrude a variety of dissimilar lipophilic compounds, many of which are positively charged under physiological conditions. However, there are some bacterial Mdr proteins that also interact with neutral and zwitterionic drugs, and some transporters export lipophilic anionic drugs (5–8).
We investigated the Mdr phenomenon by using Escherichia coli Mdr transporter MdfA (9) as a model for secondary Mdr transporters. MdfA is a 410-aa-long membrane protein of which close homologues have been identified thus far only in the pathogenic bacteria Salmonella enterica serovar Typhimurium (90% identity) (10) and Yersinia pestis (73% identity) (11). Cells expressing MdfA from a multicopy plasmid exhibit Mdr resulting from active drug extrusion driven by the proton electrochemical gradient. Previous studies have proposed that MdfA is a drug/proton antiporter (12). As predicted from the hydropathy plot of the protein and confirmed by gene-fusion studies, the putative 12 transmembrane regions of MdfA have only one charged amino acid residue, glutamate at position 26, which is embedded in the membrane, in the middle of putative transmembrane segment 1 (13). As demonstrated by mutational analysis, the amino acid residue at this position is important for substrate recognition and binding by MdfA but not for proton translocation (ref. 14 and J.A., O.L., and E.B., unpublished data). As mentioned, MdfA recognizes compounds that are not only structurally unrelated but also dissimilar in charge, and recent work has demonstrated simultaneous binding of such substrates to the transporter (15). This property poses an interesting question regarding the electrogenicity of the substrate/proton exchange with positively charged compounds versus electroneutral ones.
In E. coli, secondary transport is driven mostly by energetically downhill influx of protons. Depending on the proton/substrate stoichiometry of the reaction and the charge on the substrate, exchange of protons with a substrate by antiporters may result in a net movement of electric charges (electrogenic transport) or no net movement of charges (electroneutral transport). Electrogenic transport will be driven by both components (Δψ and ΔpH) of the proton electrochemical gradient (Δμ̄H+), but when transport is electroneutral, ΔpH should be the primary driving force. Importantly, whereas Δμ̄H+ remains relatively constant at external pH values ranging from 5.0 to 8.0, the electrical (Δψ) and chemical (ΔpH) components change. Because internal pH is kept constant at 7.6 as external pH is raised, ΔpH (inside alkaline) drops and even becomes inverted (inside acid) above pH 7.6 (16), whereas Δψ (inside negative) increases. Accordingly, ΔpH-driven electroneutral transport processes should be inhibited at alkaline external pH values, whereas electrogenic transport processes should be less affected. Of the secondary transport proteins described to date, they catalyze either electrogenic or electroneutral translocation reactions. In this regard, Mdr transporters represent a unique subset of secondary transporters. Because of their ability to transport differentially charged substrates, they are theoretically capable of catalyzing transport reactions that are electrically distinct. Most probably, a transport process that involves Δμ̄H+-dependent active extrusion of neutral drugs is electrogenic, but the degree to which Δψ or ΔpH (inside negative and alkaline) contributes to the uptake or extrusion of a given solute may vary (17).
Here we have examined the intriguing possibility that different MdfA-mediated transport reactions exist, each accommodating a distinct group of substrates. The results demonstrate that the MdfA-catalyzed transport of neutral substrates is indeed electrogenic, whereas transport of cationic substrates is electroneutral. Thus, in addition to their promiscuity regarding substrate recognition, Mdr transporters are also versatile regarding the component of Δμ̄H+ used for transport.
Materials and Methods
Materials.
E. coli lipids were from Avanti. [3H]Tetraphenylphosphonium (TPP+) [32 Ci/mmol (1 Ci = 37 GBq)] was purchased from Amersham Pharmacia, and [3H]chloramphenicol (20 Ci/mmol) was purchased from NEN. Nigericin, valinomycin, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), ATP, ethidium bromide (EtBr), chloramphenicol, kanamycin, Hoechst 33342, DNase, phosphatidyl choline, and ampicillin all were purchased from Sigma. Benzalkonium chloride was obtained from Calbiochem, and thiamphenicol was obtained from ICN. Oxonol V and 9-amino-6-chloro-2-methoxyacridine were purchased from Molecular Probes. N-dodecyl-maltoside was purchased from Anatrace (Maumee, OH). His Bind resin was obtained from Novagen and used for purification of MdfA-6His. Cellulose acetate and nitrocellulose filters (0.2 μm) were from Schleicher & Schüll. Polycarbonate filters (0.4 μm) were from Osmonics (Minnetonka, MN). All other materials were reagent-grade and obtained from commercial sources.
Drug-Resistance Assays.
E. coli UTmdfA∷kan (14) was transformed with the indicated plasmid (pT7-5 or pT7-5 mdfA-6His), and after 1 h of recovery at 37°C the transformants were diluted as indicated and plated over LB agar plates. The plates were supplemented with 75 mM bis-Tris-propane at the desired pH and ampicillin (100 μg/ml), kanamycin (30 μg/ml), and the indicated concentrations of the test compounds (chloramphenicol, thiamphenicol, benzalkonium, or EtBr). The ability of the transformants to form single colonies was recorded after 36 h at 37°C.
Transport Assays in Whole Cells.
EtBr-efflux assays (6) and chloramphenicol or TPP+ uptake assays (15) were conducted as described with the following modifications: For EtBr efflux, E. coli UTL2mdfA∷kan cells harboring pT7-5 or pT7-5 mdfA-6His (15) were grown to an OD600 of 0.8 units in LB broth and kept on ice. Two-milliliter aliquots (OD600 of 0.3 units) were pelleted and resuspended in 2 ml of 50 mM potassium phosphate at the desired pH and supplemented with 100 μM CCCP and 5 μM EtBr. Cells were incubated for 5′ at 35°C, pelleted, and resuspended in the same buffer but without CCCP. Transport was initiated by the addition of 0.2% glucose. For chloramphenicol uptake, E. coli UTmdfA∷kan cells harboring pT7-5 or pT7-5 mdfA-6His were grown to an OD600 of 0.5 units in LB broth, washed once in 50 mM potassium phosphate at the desired pH, resuspended in the same buffer, at an OD420 of 30 units, and aliquoted (50 μl). After 15 min recovery at 35°C in the presence of 0.2% glucose, transport was initiated by the addition of an equal volume (50 μl) of the same buffer containing [3H]chloramphenicol. [3H]TPP+ uptake was conducted essentially the same as with chloramphenicol. UTL2mdfA∷kan cells were resuspended to an OD420 of 13.5 units. In experiments with the ionophores valinomycin (5 μM) or nigericin (2 μM), the outer-membrane permeability mutant was made further permeable to the ionophores by a 15-min incubation at 35°C in the presence of 2.5 mM EDTA, 1% ethanol, and the test ionophore.
Preparation of inverted Membrane Vesicles.
E. coli UTL2mdfA∷kan cells overexpressing MdfA were grown and treated as described (15). Inverted membrane vesicles were prepared from these cells as described (18). Cells were washed once in 50 mM potassium phosphate, pH 7.5/5 mM MgSO4. Cells (0.2 g/ml) in 50 mM potassium phosphate, pH 7.5/5 mM MgSO4/30 μg/ml DNase/0.5 mM Pefablock/10 mM 2-mercaptoethanol were disrupted by French Press at 8,000 psi (1 psi = 6.89 kPa) (three passages). Vesicles in 50 mM potassium phosphate, pH 7.5/10% glycerol were frozen in liquid nitrogen and kept at −80°C.
Hoechst 33342 Transport in Inverted Membrane Vesicles.
Transport of Hoechst 33342 was conducted as described (19) with the following modifications: 4 μl of vesicles (30 mg of protein per ml) were diluted into 2 ml of 50 mM Hepes, pH 7.0/50 mM potassium gluconate/10 mM magnesium sulfate/0.2 μM Hoechst 33342. Transport was initiated by the addition of 0.2 mM ATP. Nigericin and valinomycin (2 μM each) were added as indicated.
Membrane Potential Measurements in Inverted Membrane Vesicles.
Forty microliters of vesicles (30 mg of protein per ml) were diluted into 2 ml of 50 mM Hepes, pH 7.0/50 mM potassium gluconate/10 mM magnesium sulfate/0.5 μM oxonol V. A transmembrane potential was generated by the addition of 0.4 mM ATP. CCCP (10 μM) was used to completely abolish the membrane potential. The fluorescence was measured in a Perkin–Elmer SL50 fluorimeter with excitation and emission wavelengths of 599 and 634 nm, respectively, and a slit width of 10 nm.
Purification and Reconstitution of MdfA.
MdfA was purified to near homogeneity as described (15). For reconstitution, a combination of methods was used (20–22). Seventy-five milligrams of 3:1 E. coli lipids (acetone/ether washed) and egg-yolk phosphatidyl choline were dissolved in 2 ml of 20 mM sodium phosphate, pH 7.0/100 mM sodium gluconate/2 mM magnesium sulfate/0.3% glycerol/0.03% N-dodecyl-maltoside. Dithiothreitol (1 mM) was added to the argon-flushed lipids, which then were subjected to two rounds of freezing (in liquid nitrogen) and thawing (undisturbed at room temperature). Next, preformed liposomes were prepared by extrusion (10 times) through a double layer of 0.4-μm polycarbonate filters, and purified MdfA (0.5 ml of 2 mg/ml protein) was added in 20 mM Tris⋅HCl, pH 7.9/0.5 M NaCl/250 mM imidazole/0.1% N-dodecyl-maltoside/1% glycerol/0.2 mg/ml lipids. As a control, 0.5 ml of the same buffer but without protein was added to identically treated lipids. The mixture (2.5 ml) was agitated gently at 4°C for 30 min and then squirted (at room temperature) into vigorously stirred 210 ml of argon-flushed 20 mM sodium phosphate, pH 7.0/100 mM Na-gluconate/2 mM magnesium sulfate. After 20 min of stirring at room temperature, proteoliposomes were collected by 1 h of centrifugation at 185,000 × g, and resuspended in the same buffer at a protein concentration of 0.8 mg/ml. Proteoliposomes were aliquoted, frozen in liquid nitrogen, and stored at −80°C for up to 6 months.
Transport Assays in Proteoliposomes.
For all experiments, proteoliposomes were thawed undisturbed at room temperature and diluted at least 10-fold with the desired loading buffer. After two cycles of freezing and thawing, the proteoliposomes were extruded (7–10 times until clear) through a double layer of 0.4-μm polycarbonate filters. After centrifugation (7 min at 280,000 × g), the proteoliposomes were resuspended in the same buffer at a protein concentration of 8 mg/ml (for TPP+ uptake) or 2 mg/ml (for chloramphenicol uptake). For evaluation of diffusion potential formation, proteoliposomes or liposomes were routinely assayed by fluorescence spectrometry (Perkin–Elmer SL50 fluorimeter) by using oxonol V (0.5 μM; excitation, 599 nm; emission, 634 nm; slit width, 10 nm) for monitoring Δψ and 9-amino-6-chloro-2-methoxyacridine (1 μM; excitation, 409 nm; emission, 474 nm; slit width, 3 nm) for ΔpH. For generation of ΔpH (inside acidic), proteoliposomes were loaded with 150 mM NH4Cl/50 mM potassium gluconate/5 mM Tris⋅HCl, pH 6.5 [and diluted 100-fold into the same buffer (control, no energy gradient)], or 150 mM choline chloride/50 mM potassium gluconate/5 mM Tris⋅HCl, pH 7.5. For generation of Δψ (inside positive), proteoliposomes were loaded with 120 mM sodium gluconate/2 mM magnesium sulfate/5 mM sodium phosphate, pH 7.0 [and diluted 100-fold into the same buffer (control, no energy gradient)], or 120 mM potassium gluconate/2 mM MgSO4/5 mM potassium phosphate, pH 7.0/5 μM valinomycin. For the generation of Δμ̄H+ (inside positive and acidic), proteoliposomes were loaded with 120 mM sodium gluconate/2 mM magnesium sulfate/150 mM NH4Cl/5 mM Tris⋅HCl, pH 6.5 [and diluted 100-fold into the same buffer (control, no energy gradient)], or 120 mM potassium gluconate/2 mM MgSO4/150 mM choline chloride/5 mM Tris⋅HCl, pH 7.5./5 μM valinomycin. Transport was conducted in 50- (TPP+) or 200-μl (chloramphenicol) samples and terminated by the addition of 1.5 ml of ice-cold LiCl (0.1 M) and filtration through 0.2-μm filters (nitrocellulose for chloramphenicol or cellulose acetate for TPP+) followed by an additional 1.5-ml wash. Experiments were repeated three times, each in triplicate.
Results
The MdfA-Mediated Resistance Toward Cationic and Neutral Drugs Is Affected Differently by Changes in the External pH.
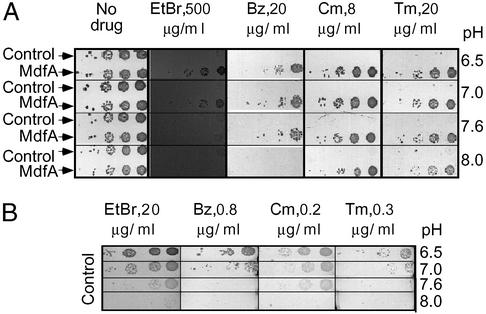
Initial evidence suggesting that the transport reactions of differentially charged substrates may differ originated from the results of drug-resistance assays on solid medium. Drug resistance to positively charged and uncharged compounds was tested under various pH conditions and over a broad range of drug concentrations. The results of a representative experiment are shown in Fig. 1. In the absence of drugs, cells harboring either plasmid, pT7-5 or pT7-5 (mdfA), grew equally well in the pH range of 6.5–8.0. However, when the positively charged substrates EtBr or benzalkonium were added to the medium, only cells expressing MdfA were able to grow, but the growth was attenuated at basic pH. In contrast, the MdfA-mediated resistance to the neutral drugs chloramphenicol and thiamphenicol was maintained also at pH 8.0. Because no growth of control cells (cells that do not express MdfA) was observed at the drug concentrations tested (Fig. 1A), the effect of pH on their intrinsic drug resistance was tested at extremely low drug concentrations (2–4% of the drug concentration used in Fig. 1A). As shown (Fig. 1B), Mdr of control cells was also affected by external pH changes. However, unlike MdfA-expressing cells, control cells are generally less resistant at basic pH toward both cationic and neutral drugs.
Figure 1.
Resistance of E. coli UTmdfA∷kan cells toward differentially charged drugs at various pH values. (A) Cells harboring plasmid pT7-5 (upper line, each panel) or pT7-5 MdfA (lower line, each panel) were tested for their ability to grow in the presence of each of several drugs at various pH values. Ten-fold dilutions of cells were plated from right to left. The drugs tested were EtBr at 500 μg/ml, benzalkonium chloride (Bz) at 20 μg/ml, chloramphenicol (Cm) at 8 μg/ml, and thiamphenicol (Tm) at 20 μg/ml. (B) Mdr of control cells (harboring plasmid pT7-5) was tested at the indicated pH values. Ten-fold dilutions of cells were plated from right to left. The drugs tested were EtBr at 20 μg/ml, benzalkonium chloride at 0.8 μg/ml, chloramphenicol at 0.2 μg/ml, and thiamphenicol at 0.3 μg/ml.
The Transport of Positively Charged Substrates Is Inhibited at a Basic pH, Whereas the Transport of Chloramphenicol Remains Unaffected.
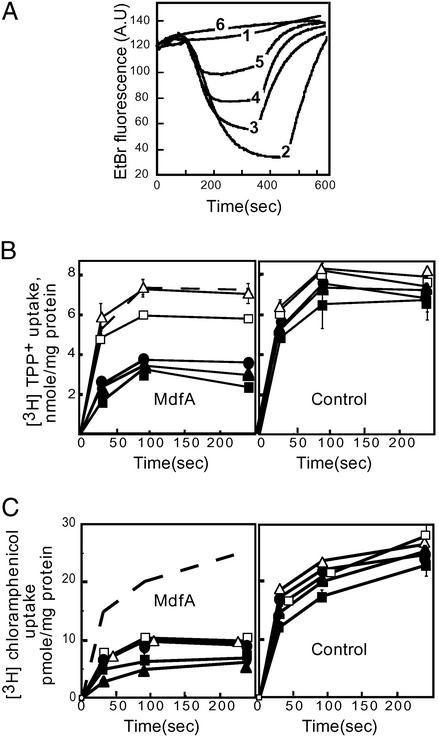
The dissimilar pH-dependent resistance phenotypes described above might reflect a pH-dependent transport phenomenon. The ability of control and MdfA-expressing E. coli cells to extrude drugs against a concentration gradient was tested at a pH range of 6.5–8.5. Transport of each of the positively charged substrates EtBr and TPP+ by MdfA-expressing cells was inhibited gradually by increasing the external pH, with a complete arrest of transport at pH 8.5 (Fig. 2 A and B Left). In contrast, transport of the uncharged chloramphenicol was affected to a much lesser degree by the external pH, and substantial transport of chloramphenicol was still apparent at pH 8.5 (Fig. 2C Left). Importantly, the uptake of all the test drugs by control cells (that do not express MdfA) is only slightly affected by identical pH changes (Fig. 2 B Right and C Right). Therefore, it seems likely that the pH dependence of MdfA-mediated resistance to cationic drugs (Fig. 1) is due to the inhibitory effect of a basic pH on their transport.
Figure 2.
Effect of pH on transport of positively charged and neutral substrates. (A) Efflux of EtBr (5 μM) by preloaded cells. EtBr fluorescence is increased after interaction with DNA and RNA, and efflux of EtBr is represented by a decrease in fluorescence. Trace 1, control cells lacking MdfA at pH 7.0. Traces 2–6, EtBr efflux mediated by MdfA-expressing cells at pH 6.5, 7.0, 7.5, 8.0, and 8.5, respectively. Glucose (0.2%) was added at 100 sec to energize the cells. CCCP (10 μM) was added after 300–500 sec to abolish the Δμ̄H+-driven transport. A.U., arbitrary units. (B Left) MdfA-mediated decreased accumulation of [3H]TPP+ (50 μM) in energized cells. (C Left) MdfA-mediated decreased accumulation of [3H]chloramphenicol (2 μM) in energized cells. (B Right and C Right) Accumulation of [3H]TPP+ (50 μM) or [3H]chloramphenicol (2 μM) by control cells. For direct comparison, the traces representing control cells assayed at pH 7.0 are shown also as dashed traces (B Left and C Left). Filled squares, pH 6.5; filled triangles, pH 7.0; filled circles, pH 7.5; open squares, pH 8.0; and open triangles, pH 8.5. Error bars are indicated unless they are smaller than the icons.
The Dissipation of Δψ or ΔpH Has Distinct Effects on the Transport of Differentially Charged Substrates.
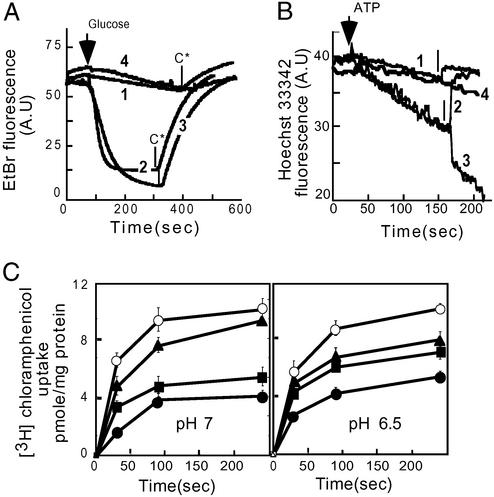
By gradually raising the extracellular pH, the ΔpH (inside alkaline) across the cytoplasmic membrane is diminished progressively, reaching 0 mV at a pH value of ≈7.6, whereas reversing its direction at higher pH (23). Therefore, because both the MdfA-mediated resistance to and transport of cationic substrates require an inwardly directed proton gradient, we speculated that their transport is driven by the ΔpH. Conversely, because resistance and transport with the uncharged drugs were shown to be less dependent on the pH and presumably the proton gradient, we reasoned that for this group of substrates, the transport might be driven also by the Δψ (inside negative). To test this hypothesis, we assayed the transport of various drugs in the presence of ionophores that selectively abolish either component of Δμ̄H+. To facilitate the accessibility of ionophores to the cytoplasmic membrane, we used the outer-membrane permeability mutant, E. coli UTL2 (24), and EDTA/ethanol treatment. Rapid EtBr efflux from cells expressing MdfA was observed compared with control cells (Fig. 3A, traces 2 and 1, respectively). However, EtBr efflux was abolished completely by nigericin, which in the presence of potassium selectively abolishes the ΔpH (Fig. 3A, trace 4). In contrast, the transport of EtBr remained unchanged or even slightly accelerated in the presence of valinomycin, which under these conditions selectively abolishes Δψ (Fig. 3A, trace 3). With the control cells, no effect of the ionophores on the slow efflux was observed (data not shown). Similar results were obtained in transport experiments of the cationic substrate Hoechst 33342 (19), with inverted membrane vesicles prepared from MdfA-expressing cells. The results show substantial Hoechst 33342 transport by MdfA-containing vesicles compared with control vesicles (Fig. 3B, compare the first 150 sec of traces 2 and 3 with trace 1). The MdfA-mediated transport was abolished by nigericin (Fig. 3B, trace 2) yet was stimulated markedly by valinomycin, suggesting a role for ΔpH (Fig. 3B, trace 3). No effect of valinomycin was observed in control vesicles (Fig. 3B, trace 4). Next we tested the effects of the same ionophores on transport of the neutral substrate chloramphenicol at pH 7.0 (Fig. 3C Left) and 6.5 (Fig. 3C Right). In both cases, cells expressing MdfA accumulated less chloramphenicol than control cells, indicating that MdfA expels the antibiotic. However, unlike with cationic substrates, the transport of chloramphenicol at pH 7.0 was inhibited drastically by valinomycin. Under these conditions (pH 7.0) nigericin inhibited transport to a much lesser extent (Fig. 3C Left). At pH 6.5 the inhibitory capacity of both ionophores was almost identical, and substantial transport was observed in both cases. The effects of the ionophores on substrate accumulation by control cells were negligible (data not shown). These results suggest that the transport of cationic substrates is driven by ΔpH and not by Δψ, whereas the transport of neutral compounds depends on both components of Δμ̄H+. The stimulation of the transport of positively charged substrates by valinomycin reflects a compensatory increase in ΔpH after depletion of Δψ.
Figure 3.
Effect of dissipation of Δψ or ΔpH on the transport of differentially charged substrates by MdfA. (A) Efflux of EtBr (5 μM) from preloaded EDTA/ethanol-treated E. coli UTLmdfA∷kan cells was measured at pH 7.0. Trace 1, control cells lacking MdfA; trace 2, MdfA-expressing cells; trace 3, MdfA-expressing cells in the presence of 5 μM valinomycin; trace 4, MdfA-expressing cells in the presence of 2 μM nigericin. Glucose (0.2%) was added at 80 sec to energize the cells. CCCP (C*) (10 μM) was added, as indicated by the arrows, to abolish Δμ̄H+-driven transport. A.U., arbitrary units. (B) Transport of Hoechst 33342 in inverted membrane vesicles prepared from cells overexpressing MdfA or control cells at pH 7.0. Transport was initiated by the addition of 0.2 mM ATP as indicated. Trace 1, control vesicles [nigericin (2 μM) was added as indicated by an arrow]; trace 2, MdfA-containing vesicles [nigericin (2 μM) was added as indicated by an arrow]; trace 3, MdfA vesicles [valinomycin (2 μM) was added as indicated by an arrow]; trace 4, control vesicles [valinomycin (2 μM) was added as indicated by an arrow]. (C) Accumulation of [3H]chloramphenicol (2 μM) by EDTA/ethanol-treated E. coli UTLmdfA∷kan cells at pH 7.0 (Left) and 6.5 (Right). Open circles, control cells; filled circles, MdfA-expressing cells; filled triangles, MdfA-expressing cells in the presence of 5 μM valinomycin; filled squares, MdfA-expressing cells in the presence of 2 μM nigericin. Error bars are indicated unless they are smaller than the icons.
The Membrane Potential Is Dissipated Only by the Transport of Electroneutral Drugs.
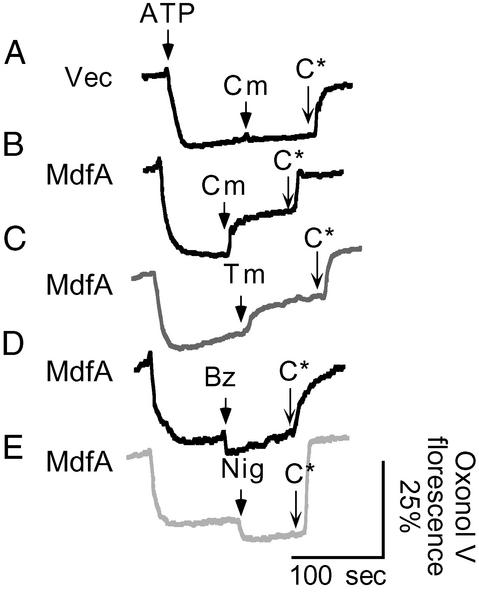
Thus far, the results have suggested that the MdfA-mediated transport of cationic substrates differs from that of uncharged substrates with regard to the driving force. To explore the electrogenicity of these presumably distinct transport reactions, we measured the effect of the transport of various drugs on a preestablished Δψ. Inverted membrane vesicles were energized by the addition of ATP, and the formation of a Δψ (inside positive) was followed by fluorescence spectroscopy using the Δψ-sensitive dye oxonol V. The Δψ generated by vesicles prepared from MdfA-expressing cells or from control cells was indistinguishable (Fig. 4). After the Δψ achieved a steady-state level, various substrates were added in concentrations that had no effect on the oxonol V fluorescence or on the Δψ generated by control vesicles (Fig. 4A, data not shown). The addition of benzalkonium to MdfA-containing vesicles did not dissipate the Δψ but rather caused a small but reproducible increase in its magnitude (Fig. 4D). A similar effect on Δψ was observed by the addition of nigericin (Fig. 4E). In both cases, dissipation of the ΔpH resulted in an increase of Δψ. However, in contrast to the effects of benzalkonium or nigericin, adding chloramphenicol or thiamphenicol to MdfA-containing vesicles resulted in a marked dissipation of Δψ (Fig. 4 B and C). Taken together, these results suggest that the transport of cationic substrates is electroneutral, whereas that of neutral substrates is electrogenic.
Figure 4.
Changes in the transmembrane electrical potential (inside positive) after additions of differentially charged substrates. Membrane vesicles were energized by the addition of 0.4 mM ATP, and the consequent membrane potential was monitored by oxonol V (0.5 μM) fluorescence. CCCP (C*, 5 μM) was added to completely abolish the membrane potential. The upper-most trace represents control vesicles, and all other traces represent MdfA-containing vesicles. Substrates were added where indicated at the following concentrations: 15 μM chloramphenicol (Cm), 15 μM thiamphenicol (Tm), 2 μM benzalkonium chloride (Bz), and 0.2 μM nigericin (Nig). Vec, vector, no MdfA.
Reconstituted MdfA Catalyzes both Electrogenic and Electroneutral Transport.
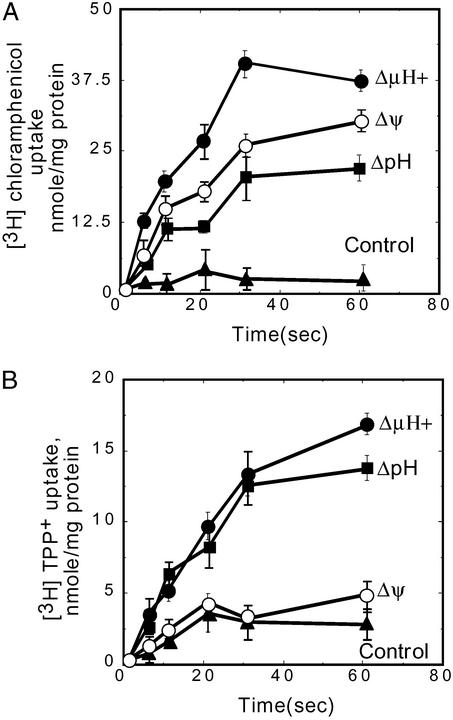
To determine whether the MdfA protein suffices to catalyze both electrogenic and electroneutral transport, we purified and reconstituted the transporter in proteoliposomes. The reconstituted MdfA is functional, as demonstrated by transport assays with chloramphenicol and TPP+ (Fig. 5). Transport assays were driven by artificially imposed diffusion potentials (see Materials and Methods). The established potentials were evaluated routinely by using oxonol V and 9-amino-6-chloro-2-methoxyacridine fluorescence for Δψ and ΔpH, respectively. The uptake of chloramphenicol into proteoliposomes was driven not only by an artificially imposed Δμ̄H+ but also by an imposed Δψ (inside positive), suggesting that, by definition, transport is electrogenic. As expected, ΔpH alone (inside acid) also drove chloramphenicol uptake by MdfA-containing liposomes (Fig. 5A). With the positively charged substrate TPP+, the Δμ̄H+-driven transport was similar to that observed when only a ΔpH (inside acid) was imposed. However, generating only a Δψ (inside positive) failed to drive TPP+ transport into the proteoliposomes (Fig. 5B). These results indicate that unlike with the neutral substrate chloramphenicol, the Δψ is not sufficient for the active transport of TPP+, whereas ΔpΗ suffices for the uptake. Therefore, MdfA catalyzes both the electrogenic and electroneutral transport reactions without the need for accessory components.
Figure 5.
Uptake of [3H]chloramphenicol or [3H]TPP+ into MdfA proteoliposomes. (A) Uptake of 2 μM [3H]chloramphenicol: 2 μl of proteoliposomes (1.5 μg of protein per μl) were diluted 100-fold into the appropriate buffer (as detailed in Materials and Methods) to impose the indicated diffusion potentials. (B) Uptake of 1 μM [3H]TPP+: 0.5 μl of proteoliposomes (8 μg of protein per μl) were diluted 100-fold into the appropriate buffer (as detailed in Materials and Methods) to impose the indicated diffusion potentials. Net values are shown, after subtraction of the amount of substrate accumulated in liposomes devoid of MdfA, under identical conditions. Error bars are indicated.
Discussion
Most secondary transporters are specific regarding their substrate recognition properties and transport driving forces. In contrast, Mdr transporters are remarkably promiscuous in various basic aspects of active transport. Unlike the former transporters that specialize in the transport of a well-defined set of molecules, Mdr transporters are able to extrude a large number of chemically dissimilar compounds. In addition to being dissimilar in their chemical structure, the substrates of many Mdr transporters also differ in their charge. Moreover, as demonstrated here with MdfA, the remarkable versatility of these transporters is exemplified further by their ability to catalyze dissimilar transport reactions.
Theoretically, in extruding a positively charged compound across the cell membrane, the transporter must move the charges against the transmembrane electrical potential, Δψ (inside negative), unlike with uncharged compounds. Accordingly, the transport of uncharged compounds seems more energetically favorable than the transport of charged substrates. Nevertheless, lipophilic cations clearly make up the majority among the substrates of MdfA. From a bioenergetic standpoint, a transporter that has to deal with differentially charged substrates has at least three theoretical modes of action: (i) the transporter may use different drug/proton stoichiometries with neutral versus positively charged, thus balancing the charge difference between the substrates with the appropriate number of counter ions; (ii) the transporter may cotransport an additional charged molecule with some of its substrates to reach the same net charge movement in each of the transport reactions; and (iii) the transporter may use an equal drug/proton stoichiometry with all the substrates, and because the charge of the substrates differs, this must result in different electrogenicities of the different transport reactions. Here we investigated this issue by using MdfA as a model Mdr transporter. The results clearly demonstrate the existence of two types of transport reactions that differ in their electrogenicity. With the monovalent cationic substrates, because their transport is electroneutral, we suggest a proton/substrate stoichiometry of 1 for these substrates. Also for the electroneutral substrates, because their transport is electrogenic, we suggest a proton/substrate stoichiometry of 1, although higher values cannot be ruled out at this stage.
Initial indications of dissimilar transport reactions could already be deduced from experiments examining the in vivo function of MdfA as an Mdr protein. By increasing the external pH, the resistance of cells expressing MdfA to cationic drugs was hampered, yet resistance to uncharged drugs remained unaffected (Fig. 1). The differential effect of pH was shown to be specific for MdfA-expressing cells. Direct evidence for a strong correlation between the export of positively charged drugs and the ΔpH (inside alkaline) came from transport assays with whole cells. In contrast, the transport of an uncharged drug proved to depend less on the ΔpH (Fig. 2), probably because of the MdfA ability to use also Δψ as a driving force for transport of uncharged drugs. Similar conclusions were derived from transport experiments of differentially charged substrates in the presence of valinomycin or nigericin (Fig. 3), which strongly indicated that the transport of positively charged substrates depends solely on a ΔpH and completely independent of the Δψ. In contrast, the transport of uncharged substrates proved to depend on both components of the Δμ̄H+. The experiments described in Fig. 3C clearly demonstrate the versatile ability of MdfA in using either component of Δμ̄H+ for transport of an uncharged substrate. At pH 7.0, Δψ constitutes ≈70% of the proton motive force, whereas ΔpH constitutes ≈30% (23). Accordingly, the addition of valinomycin abolishes a major part of the MdfA-mediated chloramphenicol transport, whereas addition of nigericin exerts a minor effect (Fig. 3C Left). At pH 6.5, the contribution of Δψ and ΔpH to the overall driving force is approximately equal (23), as also reflected by the inhibitory effect of the ionophores on chloramphenicol transport (Fig. 3C Right). Transport experiments with reconstituted MdfA (Fig. 5) suggested that its ability to perform dissimilar transport reactions is independent of accessory components and is an inherent property of the transporter. This phenomenon was established further in inverted membrane vesicles and preformed Δψ (inside positive). The addition of electroneutral drugs consumed the Δψ, whereas the transport of positively charged drugs led to an increase in the magnitude of the Δψ (Fig. 4). Taking into account that Δμ̄H+ is a relatively constant value, the consumption of ΔpH through the electroneutral exchange of a positively charged drug and a proton will bring about a concomitant increase in Δψ. A similar effect on Δψ was exerted by nigericin, which operates as an electroneutral H+/K+ exchanger, hence converting ΔpH to Δψ (Fig. 4).
Because for secondary transport systems described to date transport has been characterized as being either electrogenic or electroneutral, MdfA provides an example of a secondary transporter able to catalyze both types of transport. These results do not only offer an explanation for a basic phenomenon, how Mdr transporters deal with substrates differing in their charge, but also raise clinically oriented considerations in the treatment of multidrug-resistant bacterial strains; e.g., the use of certain positively charged drugs may be advantageous in a basic environment.
Acknowledgments
This research was supported by a grant from the Y. Leon Benoziyo Institute for Molecular Medicine at the Weizmann Institute of Science (to E.B.) and short-term European Molecular Biology Organization Fellowship ASTF 9855 (to O.L.).
Abbreviations
- Mdr
multidrug resistance
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- EtBr
ethidium bromide
- TPP+
tetraphenylphosphonium
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Levy S B. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 3.Nikaido H. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 4.Markham P N, Neyfakh A A. Curr Opin Microbiol. 2001;4:509–514. doi: 10.1016/s1369-5274(00)00243-5. [DOI] [PubMed] [Google Scholar]
- 5.Lewis K, Naroditskaya V, Ferrante A, Fokina I. J Bioenerg Biomembr. 1994;26:639–646. doi: 10.1007/BF00831539. [DOI] [PubMed] [Google Scholar]
- 6.Edgar R, Bibi E. J Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack D L, Storms M L, Tchieu J H, Paulsen I T, Saier M H., Jr J Bacteriol. 2000;182:2311–2313. doi: 10.1128/jb.182.8.2311-2313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zgurskaya H I, Nikaido H. Mol Microbiol. 2000;37:219–225. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- 9.Bibi E, Adler A, Lewinson O, Edgar R. J Mol Microbiol Biotechnol. 2001;3:171–177. [PubMed] [Google Scholar]
- 10.Parkhill J, Wren B W, Thomson N R, Titball R W, Holden M T, Prentice M B, Sebaihia M, James K D, Churcher C, Mungall K L, et al. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 11.Parkhill J, Dougan G, James K D, Thomson N R, Pickard D, Wain J, Churcher C, Mungall K L, Bentley S D, Holden M T, et al. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 12.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. J Biochem (Tokyo) 1998;124:187–193. doi: 10.1093/oxfordjournals.jbchem.a022078. [DOI] [PubMed] [Google Scholar]
- 13.Adler J, Bibi E. J Bacteriol. 2002;184:3313–3320. doi: 10.1128/JB.184.12.3313-3320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar R, Bibi E. EMBO J. 1999;18:822–832. doi: 10.1093/emboj/18.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinson O, Bibi E. Biochemistry. 2001;40:12612–12618. doi: 10.1021/bi011040y. [DOI] [PubMed] [Google Scholar]
- 16.Padan E, Schuldiner S. J Membr Biol. 1987;95:189–198. doi: 10.1007/BF01869481. [DOI] [PubMed] [Google Scholar]
- 17.Ramos H, Kaback H R. Biochemistry. 1977;16:854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- 18.Reenstra W W, Patel L, Rottenberg H, Kaback H R. Biochemistry. 1980;19:1–9. doi: 10.1021/bi00542a001. [DOI] [PubMed] [Google Scholar]
- 19.Putman M, Koole L A, van Veen H W, Konings W N. Biochemistry. 1999;38:13900–13905. doi: 10.1021/bi991262k. [DOI] [PubMed] [Google Scholar]
- 20.Viitanen P, Garcia M L, Kaback H R. Proc Natl Acad Sci USA. 1984;81:1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigaud J L, Pitard B, Levy D. Biochim Biophys Acta. 1995;1231:223–246. doi: 10.1016/0005-2728(95)00091-v. [DOI] [PubMed] [Google Scholar]
- 22.Knol J, Sjollema K, Poolman B. Biochemistry. 1998;37:16410–16415. doi: 10.1021/bi981596u. [DOI] [PubMed] [Google Scholar]
- 23.Zilberstein D, Schuldiner S, Padan E. Biochemistry. 1979;18:669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]
- 24.Beja O, Bibi E. Proc Natl Acad Sci USA. 1996;93:5969–5974. doi: 10.1073/pnas.93.12.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]