Abstract
Most of the genes that are central to the process of skeletal muscle differentiation remain in a transcriptionally silent or “off” state until muscle cells (myoblasts) are induced to differentiate. Although the mechanisms that contribute to this phenomenon are still unclear, it is likely that histone deacetylases (HDACs), which play an important role in the repression of genes, are principally involved. Recent studies indicate that the initiator of the myogenic program, namely MyoD, can associate with the deacetylase HDAC1 in vivo, and because HDACs are usually recruited to promoters by specific proteins, we considered the possibility that these two proteins might be acting together at the promoters of muscle-specific genes to repress their transcription in myoblasts. In this work, we show by chromatin immunoprecipitation (ChIP) assays that MyoD and HDAC1 are both occupying the promoter of myogenin and that this gene is in a region of repressed chromatin, as revealed by enrichment in histone H3 lysine 9 (Lys-9) methylation and the underacetylation of histones. Surprisingly, after the myoblasts are induced to differentiate, the promoter becomes absent of HDAC1, and eventually the acetyltransferase P/CAF takes it place alongside MyoD. In addition, enrichment of histone H3 acetylation (Lys-9/14) and phosphorylation of Ser-10 can now be observed at the myogenin promoter. These data strongly suggest that in addition to its widely accepted role as an activator of differentiation-specific genes, MyoD also can perform as a transcriptional repressor in proliferating myoblasts while in partnership with a HDAC.
The differentiation of skeletal myoblasts (myogenesis) depends on either one of two members of the myogenic basic helix–loop–helix (bHLH) family of transcription factors: MyoD or Myf5 (1). The prototype, MyoD, has been shown to establish the myogenic lineage during embryogenesis and to regulate the myogenic program in satellite cells, the stem cells of adult skeletal muscles (2). In initiating the differentiation program, MyoD forms a heterodimer with ubiquitously expressed E proteins (E12/E47), and, together, they bind to a consensus DNA sequence termed an E-box (CANNTG), present in the regulatory regions of both muscle and nonmuscle genes (1). In addition, MyoD also cooperates with members of the myocyte enhancer factor-2 (MEF2) family of proteins to activate the expression of muscle-specific genes (1). The MEF2 proteins recently have been found to associate with the class 11 histone deacetylases (HDACs) HDAC4 and HDAC5 (3).
It is well documented that MyoD is expressed in myoblasts before the activation of the myogenic program, and this enigma is common to muscle cells in both developmental myogenesis and tissue culture systems (4, 5). Under these circumstances, MyoD is unable to activate myogenic transcription, and therefore the requirement for MyoD to be continuously expressed in undifferentiated muscle cells is still unclear. However, numerous studies, including our own, have provided considerable information regarding the way in which MyoD may be negatively controlled in proliferating myoblasts. For example, evidence suggests that MyoD might be regulated by posttranscriptional events, which include either direct association with proteins (e.g., Id, Twist, cdk4, Mist1, and MEK1) and/or phosphorylation (1, 6). More recently, our laboratory has shown that a class 1 HDAC (HDAC1) can block the function of MyoD in initiating the myogenic program under conditions of differentiation (7). Others have now extended our results by showing that the retinoblastoma protein (pRb) can displace HDAC1 from MyoD upon differentiation (8), thereby reducing HDAC1's effect on MyoD. The ability of pRb to remove HDAC1 from MyoD also may be viewed as a necessary step for MyoD's association with the histone acetyltransferase P/CAF, which occurs almost immediately after myogenic differentiation begins (7). This interaction might very well explain the acetylation of endogenous MyoD, which is observed thereafter, and only during the course of myogenesis (7, 9), although there is some indication that MyoD also may be acetylated in undifferentiated myoblasts as well (10). Finally, P/CAF, as well as the histone acetyltransferase p300, has the ability to acetylate MyoD in vitro (10, 11), and these acetyl markings seem to mediate an interaction with the bromodomain of the cAMP response element-binding protein (CREB)-binding protein (CBP), at least in vitro (9).
Apart from acetylating nonhistone proteins, P/CAF and p300/CBP [histone acetylases (HATs)] are both able to acetylate lysine residues located in the N-terminal tails of nucleosomal core histones (12). Indeed, markings of acetylation, especially on histone H3 at Lys-9 and/or Lys-14, recently have been shown to be important for transcriptional activation in both yeast and mammalian cells (13). Furthermore, the phosphorylation of Ser-10 on histone H3 also plays a role in the induction of transcription in these systems as well (13), and evidence suggests that this marking facilitates the acetylation of H3, leaving it therefore doubly modified (14, 15). Conversely, the methylation of Lys-9 on histone H3 along with the association of the heterochromatin protein 1 (HP1) has been shown to be a critical marker for the repression of transcription in both heterochromatin and euchromatin regions (16–18). The methylation of this residue is catalyzed by the SUV39 family of proteins, which includes SUV39H1, G9A, and ESET (19–21). The enzymes that provide the switch to repressive chromatin are the HDACs. By decreasing acetylation, HDACs, which are targeted to specific sites and/or promoters by repressor proteins, create a favorable environment for the subsequent methylation of histones, which mostly occurs on H3 and H4 (13).
After our initial observations on MyoD's ability to form a functional complex with either HDAC1 or P/CAF in muscle cells (7), we now demonstrate by chromatin immunoprecipitation (ChIP) assays that MyoD can associate with the myogenin promoter along with HDAC1 or P/CAF in undifferentiated and differentiated myoblasts, respectively. Moreover, the replacement of HDAC1 by P/CAF within this promoter helps MyoD to drive differentiation, as evidenced by the expression of myogenin, which occurs shortly thereafter. We also show that methylated H3 histones are associated with this promoter in undifferentiated myoblasts and that these histones become phosphorylated (Ser-10) and acetylated (Lys-9/14) after myoblasts are induced to differentiate. Together, these data show that MyoD is actively involved in both the repression and activation of the myogenin gene in live muscle cells.
Materials and Methods
Cell Culture, Nuclear Extracts, and Antibodies.
C2C12 skeletal myoblasts (kindly provided by N. Rosenthal, Massachusetts General Hospital, Boston) were maintained in growth medium (GM) or differentiation medium (DM) for a period of 36 h, as described (7, 22). When cultured in DM, these cells begin to form morphological and biochemically differentiated myotubes within 24 h, as reported (23). The preparation of nuclear extract from C3H 10T1/2 cells, myoblasts, and differentiated cells was carried out as described (22). Antibodies recognizing E2F1 (sc-193x), MyoD-a (sc-760), MyoD-b (sc-304), HDAC1-a (sc-7872), HDAC1-b (sc-6298), and normal rabbit IgG (sc-2027) were obtained from Santa Cruz Biotechnology. Antibodies specific for HDAC1-c (2062), and acetyl (Lys-9)-phospho (Ser-10)-histone H3 (9711) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against acetylated histone H3, acetylated histone H3 (Lys-9), dimethyl histone H3 (Lys-9), and phospho (Ser-10)-acetyl (Lys-14)-histone H3 were obtained from Upstate Biotechnology (Lake Placid, NY). Antibody specific for myogenin (F5D) was from PharMingen. Anti-P/CAF was kindly provided by Y. Nakatani (Harvard Medical School, Boston; ref. 24).
ChIP and ChIP Reimmunoprecipitations.
Chromatin preparation and the immunoprecipitation of such was performed as described (25). Briefly, C2 cells in GM or DM for 36 h were cross-linked with formaldehyde (to 1% final concentration) for 10 min at room temperature. Nuclei from these cells were then sonicated under conditions of standardization and to an average length of 500–800 bp. The sonicated nuclei were then purified by a CsCl step gradient and, afterward, dialyzed against TE buffer (10 mM Tris⋅HCl, pH 8.0/1 mM EDTA/0.5 mM EGTA/10% glycerol). One hundred micrograms of purified chromatin was precleared with a mixture of protein A and protein G Sepharose (blocked previously with 1 mg/ml salmon sperm DNA and 1 mg/ml BSA). Twenty-five percent of the precleared chromatin was set aside (input control), and the rest then was immunoprecipitated with 2.0 μg of antibody or 10 μl of antibody solution. The procedures for immunoprecipitations, followed by RNase A and proteinase K treatment, the reversal of formaldehyde cross-linking, and the extraction of samples, were identical to those described (25), except the DNAs were ethanol precipitated in the presence of Pellet Paint Co-Precipitant (Novagen). Pellets were resuspended in 25 μl of H20, and 1.5 μl was used for semiquantitative PCR.
For ChIP reimmunoprecipitations, 100 μg of CsCl-purified chromatin was initially immunoprecipitated in parallel with an antibody specific for HDAC1, P/CAF, or E2F1. The immunoselective DNA fragments then were released from the primary immunoprecipitation by incubation with 10 mM DTT at 37°C for 30 min in TE buffer (26). Afterward, the samples were centrifuged, and the supernatant was transferred into a fresh tube. The supernatants were then diluted to 1 ml in buffer (1% Triton X-100/2 mM EDTA/150 mM NaCl/20 mM Tris⋅HCl, pH 8.1), reimmunoprecipitated with the second antibody specific for E2F1 or MyoD, and processed as described above. In the end, 5.0 or 7.5 μl of sample was used for PCR analysis.
Thirty cycles of PCR were performed in a reaction volume of 25 μl with 1.5 or 5.0 μl of immunoprecipitated material, 10 pmol of each primer set, 0.25 units of Taq DNA polymerase (Roche Molecular Biochemicals), and 0.1 μCi (1 Ci = 37 GBq) of [α-32P]dCTP in buffer. All PCRs were performed with an MJ Research (Cambridge, MA) PTC-100 thermal cycler, and optimal conditions, as well as the linear range for all primer pairs, were determined empirically. PCR products were fractionated on 8% native polyacrylamide gels and visualized by autoradiography. To amplify the mouse myogenin promoter regions, the following primer sets were used: E2/E1 (+) 5′-GAATCACATGTAATCCACTGGA-3′ and (−) 5′-ACGCCAACTGCTGGGTGCCA-3′. Primer sets for the mouse E2F1 promoter were: (+) 5′-CGGAGCCTCCGTCGTCACAGCCGC-3′ and (−) 5′-CGGCCGCCGCTGCCTGCAAAGTCC-3′; and for the GAPDH fragment: (+) 5′-AAGCCAAACTAGCAGCTAGG-3′ and (−) 5′-GGGCTAGTCTATCATTGCAG-3′.
For input control, 25 μg of cross-linked chromatin was incubated at 65°C for 12 h to reverse the cross-links. After processing as described above, the DNA was dissolved in H20, and 0.04% of the total was assayed for PCR by using the GAPDH primer sets.
Western Blotting.
To detect MyoD, cross-linked chromatin was boiled for 30 min in 1× Laemmli buffer to reverse formaldehyde cross-links (25). Afterward, the boiled samples were electrophoresed on an SDS/10% polyacrylamide gel and transferred to a membrane, and an anti-MyoD antibody (sc-760) was used as a probe. Detecting MyoD or myogenin in nuclear extracts of either C2 or C3H 10T1/2 cells by Western analysis using antibodies specific for either MyoD or myogenin was as described (7).
Results and Discussion
MyoD Occupies the Myogenin Promoter in Undifferentiated Myoblasts.
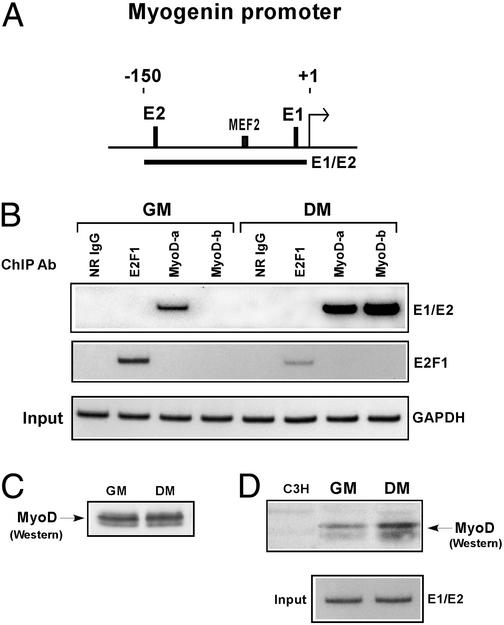
As discussed above, studies indicate that MyoD can associate with HDAC1 in undifferentiated myoblasts but not in differentiated myotubes (7, 8). Because deacetylases are recruited by repressor proteins to specific promoters for transcriptional silencing (27), we considered the possibility that MyoD, despite its role as a transcriptional activator, might be involved in maintaining the repression of muscle-specific genes by targeting HDACs to their promoters. To test this idea, we used ChIPs to determine whether MyoD might in fact be actively engaged at the myogenin promoter, which is known to be repressed in undifferentiated myoblasts (2). As a comparison, we also performed this assay on myoblasts undergoing differentiation, because this gene is one of the first to be rapidly induced by MyoD upon myogenesis (2). Therefore, equivalent amounts of cross-linked chromatin from myoblasts cultured under conditions of GM or DM was immunoprecipitated in parallel with two antibodies that recognize distinct epitopes of MyoD. The precipitated DNA then was subject to PCR amplification with the use of a pair of primers, spanning two of the E-box sites (E1 and E2) located in the proximal region of myogenin promoter (Fig. 1A). Fig. 1B shows that MyoD can unequivocally bind to this promoter in undifferentiated myoblasts and, not surprisingly, in cells undergoing differentiation as well, although in this case the association was more pronounced. The specificity of this assay is shown by the fact that the binding of MyoD was not observed when a normal rabbit IgG or an antibody against the E2F1 transcription factor was used in the analysis. In addition, E2F1 was not associated with the myogenin promoter, but rather with a promoter containing its own DNA binding site (Fig. 1B). That E2F1's expression becomes down-regulated as myoblasts undergo differentiation (28) perhaps explains why its interaction with the promoter was less evident in differentiated myoblasts.
Figure 1.
MyoD binds to the endogenous myogenin promoter in undifferentiated and differentiated myoblasts. (A) Schematic representation of the myogenin promoter. E-boxes (E1 and E2), as well as the myocyte enhancer factor-2 (MEF2) binding site, are depicted relative to the +1 transcriptional start site. Solid bar represents the region amplified by the indicated primer sets, described in Materials and Methods and used in the PCR. (B) For ChIP analysis, equivalent amounts of chromatin from myoblasts (C2C12) cultured in either GM or DM were immunoprecipitated in parallel with normal rabbit IgG (NR IgG), antibodies specific for MyoD (MyoD-a and MyoD-b), and an anti-E2F1 antibody. The purified myogenin and E2F1-dependent promoters then were analyzed by semiquantitative PCR and detected by autoradiography. Bottom (GAPDH) shows the DNA input (0.04%) and serves as a control for confirming that equivalent amounts of chromatin were used in each of the ChIP assays. (C) Nuclear extracts from myoblasts cultured in GM or DM were separated by SDS/PAGE, and the relative levels of MyoD were determined by Western blotting using anti-MyoD-a. (D) Equivalent amounts of cross-linked chromatin from myoblasts cultured in GM or DM were resolved by SDS/PAGE alongside a nuclear extract from C3H 10T1/2 cells, which are absent of MyoD. The relative levels of MyoD were detected by Western blotting, using anti-MyoD-a. Lower indicates by PCR that equivalent amounts of cross-linked chromatin were used in this experiment.
There are two likely reasons why both of the anti-MyoD antibodies were more efficient in immunoprecipitating MyoD-promoter DNA fragments from differentiated versus undifferentiated myoblasts (Fig. 1B). The first relates to the unmasking of epitopes, only because MyoD has been shown to change in physical property and conformational form once differentiation occurs (5, 11). The second reason considers the possibility that MyoD may be transiently occupying the myogenin promoter in myoblasts before differentiation, providing, of course, that the MyoD-a antibody is equally effective in recognizing MyoD whether it is expressed in undifferentiated or differentiated myoblasts. Whichever the reason, they are both supported by the fact that the levels of MyoD protein in nuclear extracts prepared from myoblasts cultured in either GM or DM seem to be comparatively similar (Fig. 1C).
The observations shown in Fig. 1B prompted us to investigate by Western analysis whether the relative amounts of MyoD on cross-linked chromatin from undifferentiated versus differentiated myoblasts were equivalent. In this experiment, we found the quantity of MyoD to be slightly more on chromatin from cells cultured in DM than in GM (Fig. 1D). Although this result supports the possibility that MyoD is transiently associating with myogenin and perhaps other MyoD-regulated promoters in undifferentiated cells, it also suggests that MyoD might be restricted with respect to the number of MyoD-mediated promoters it can permanently occupy in these cells.
MyoD Occupies the Myogenin Promoter with HDAC1 in Undifferentiated Myoblasts and with P/CAF After Differentiation.
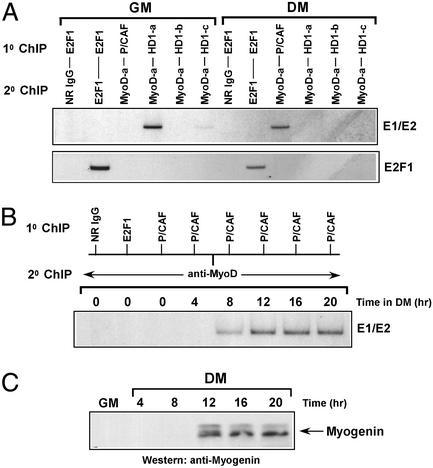
Because the initiation of myogenesis results in a switch from MyoD–HDAC1 to MyoD–P/CAF association in vivo (7), we performed serial ChIP assays to determine whether MyoD was linked with HDAC1 at the myogenin promoter in undifferentiated myoblasts and, conversely, with P/CAF in differentiated cells. The strategy for this experiment was as follows. Equivalent amounts of cross-linked chromatin, in parallel, were initially immunoprecipitated with antibodies specific for HDAC1, P/CAF, or E2F1. Afterward, the immunoselective DNA fragments were released from each of these antibodies (26) and then reimmunoprecipitated with antibodies directed against MyoD and E2F1. As before, PCR primers spanning both of the E-box sites (E1 and E2) within the promoter were used for amplification. In undifferentiated myoblasts, MyoD and HDAC1 both were assembled on the myogenin promoter, whereas P/CAF was not (Fig. 2A). However, after myoblasts were induced to differentiate, we found that although MyoD was still occupying this promoter it was no longer with HDAC1, but instead with P/CAF (Fig. 2A). The switch between HDAC1 and P/CAF association with the myogenin promoter may be explained by the observation that HDAC1 levels decrease during myogenesis, whereas P/CAF levels do not (7, 8). Finally, the specificity of this assay is exemplified by the fact that E2F1 was bound to its own promoter in proliferating myoblasts, whereas in the differentiated cells there was very little binding (Fig. 2A), and this observation is consistent with the results shown in Fig. 1B.
Figure 2.
(A) The myogenin promoter is occupied by both MyoD and HDAC1 or MyoD and P/CAF in undifferentiated and differentiated myoblasts, respectively. Equivalent amounts of cross-linked chromatin from C2 cells cultured in GM or DM initially were immunoprecipitated in parallel with antibodies specific for E2F1, P/CAF, and three different antibodies against HDAC1. The captured protein DNA fragments then were eluted from the immunoprecipitates (see Materials and Methods) and, afterward, reimmunoprecipitated with anti-MyoD (MyoD-a). In parallel, complexes of E2F1 also were eluted in the same manner and then reimmunoprecipitated with either normal rabbit IgG or anti-E2F1. Reimmunoprecipitated products were analyzed by PCR using primers spanning both the E1 and E2 sites of the myogenin promoter or spanning the promoter of the E2F1 gene. (B) P/CAF is recruited to the myogenin promoter shortly after myoblasts undergo differentiation. Cross-linked chromatin from C2 cells cultured in GM or DM for the indicated times was serially immunoprecipitated and then analyzed by PCR in a manner as described in A. (C) The myogenin gene is activated in myoblasts only after its promoter is occupied by MyoD and P/CAF. Myoblasts were cultured in GM or DM for the indicated times, and myogenin was detected by Western blotting using anti-myogenin antibody.
To reiterate, MyoD associates with P/CAF only after myoblasts have been induced to differentiate, and this interaction does not seem to be initiated because of an increase in the levels of P/CAF (7). Perhaps more important is that this interaction, which has been shown to occur within 8 h, coincides with the appearance of an acetylated form of MyoD (7). Because it has been speculated that the acetylation of a DNA-bound MyoD may be necessary for activating transcription from a MyoD-regulated promoter (11), we first performed serial ChIP assays to assess the time in which P/CAF begins to occupy the myogenin promoter in differentiated myoblasts along with MyoD, and then we determined by Western analysis whether this event might correspond to the induction of myogenin expression. As shown in Fig. 2B, P/CAF was found to be associated with the myogenin promoter ≈8 h after differentiation, and, together, P/CAF and MyoD continued their occupancy until the end of this experiment (20 h). Perhaps more important is that this finding was nearly in agreement with the kinetics of myogenin transcriptional activation, with levels of myogenin accumulating by 12 h and reaching a maximum well within 20 h of differentiation (Fig. 2C). Together, these results indicate that MyoD and P/CAF are both required for switching “on” the early myogenic marker myogenin, whose expression is critical to the process of myogenesis.
MyoD and HDAC1 Are Functionally Connected in Affecting Chromatin Structure.
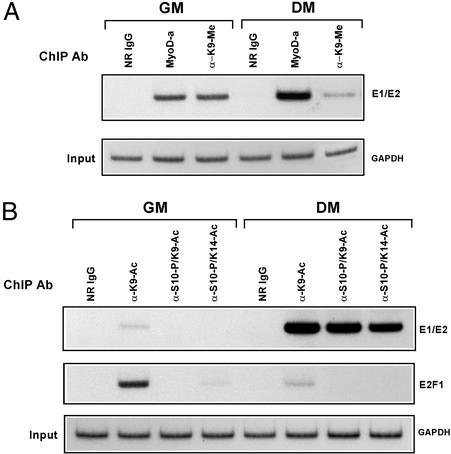
Given that HDACs act locally at promoters to silence gene activity (27), we performed ChIP analysis on undifferentiated myoblasts to determine whether the histones at the MyoD-binding sites of the myogenin promoter were absent of acetylation. Thus, we focused our efforts on whether histone H3 was methylated at Lys-9, only because of the likelihood that this residue would not be methylated if it were covalently attached to an acetyl group (19), therefore indicating the necessity of a deacetylase activity. As shown in Fig. 3A, the H3 histones surrounding the myogenin promoter in undifferentiated myoblasts were at least dimethylated, specifically at Lys-9. Most gratifying, however, is that this marking was dramatically reduced in myoblasts that had undergone differentiation and, in addition, that the reversal of this modification correlated with the expression of myogenin (Fig. 2C).
Figure 3.
Analysis of histone H3 methylation, phosphorylation, and acetylation at the myogenin promoter in undifferentiated versus differentiated cells. (A) Histone methylation at the myogenin promoter changes after differentiation. Cross-linked chromatin from myoblasts or differentiated cells was immunoprecipitated in parallel with anti-MyoD (MyoD-a), anti-H3-methyl-Lys-9, or normal rabbit IgG and then analyzed by semiquantitative PCR using primers spanning both the E1 and E2 sites. PCR products were run on 8% polyacrylamide gels and visualized by autoradiography. Lower represents the input control (GAPDH) and confirms the use of equivalent amounts of chromatin in each of the ChIP reactions. (B) Histone phosphorylation and acetylation occur at the myogenin promoter after differentiation. Equivalent amounts of cross-linked chromatin from cells cultured in GM or DM were immunoprecipitated in parallel with normal rabbit IgG and the following antibodies: anti-H3-K9-Ac, anti-H3-Ser-10-P/K9-Ac, anti-H3-Ser-10-P/K14-Ac, and E2F1. Precipitated products were analyzed by PCR using primers spanning E1/E2 of myogenin and the E2F1 gene. Input control (GAPDH) was analyzed as described in A.
Our finding that the myogenin promoter in differentiated myoblasts was undermethylated prompted us to investigate whether it was now displaying patterns of H3 phosphorylation and acetylation, specifically at Ser-10 and at Lys-9 and/or Lys-14. These particular markings recently have been correlated to the activation of genes in both yeast and mammalian cells (14, 15, 29). As shown in Fig. 3B, ChIP assays with the use of dual-specificity antibodies demonstrated that the myogenin promoter was indeed surrounded by H3 histones, which were phosphorylated at Ser-10 and acetylated at Lys-9/14, but strikingly, these markings were not observed when this analysis was performed on undifferentiated myoblasts. Our finding of H3 acetylation after the process of differentiation is in accordance with a recently published study indicating that a MyoD fusion protein with an estrogen receptor hormone binding domain, which is stably expressed in MyoD−/−/Myf5−/− mouse embryo fibroblasts, can increase global acetylation of histone H3 at the myogenin promoter on its induction in DM (30). Finally, because E2F1 is down-regulated in differentiated cells, it was not surprising to find that the H3 histones at this gene were not phosphorylated (Ser-10) nor acetylated (Lys-9/14). Collectively, these results support the conclusion that the “silencing” of the myogenin gene in undifferentiated myoblasts is facilitated by the methylation of Lys-9 on H3 and that its activation in response to the differentiation of these cells is facilitated by the coupling of H3 phosphorylation and acetylation.
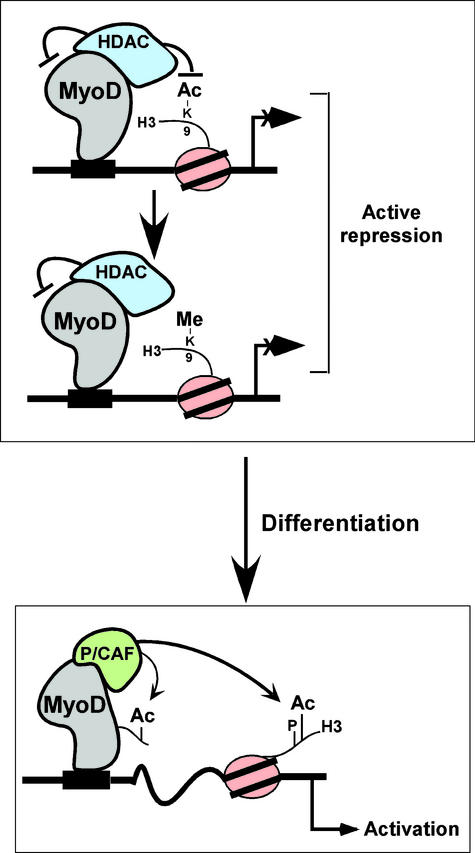
The results presented here indicate that both MyoD and HDAC1 help to establish an environment for repressing the myogenin gene in undifferentiated myoblasts (Fig. 4). Because our laboratory has previously shown that HDAC1 can bind directly to the helix–loop–helix domain of MyoD in vitro (7, 8), we postulate that MyoD may act by recruiting an HDAC complex to the myogenin promoter to maintain the deacetylation of H3 histones, thereby contributing to the methylation of Lys-9 (6). Whether this modification is mediated by the methylase SUV39h1 or provides a binding site for M31, a mouse heterochromatin protein 1 (HP1) homolog (31), is still unclear and is currently under investigation. Unprecedented is that once myoblasts undergo differentiation, MyoD actively engages in recruiting P/CAF, rather than an HDAC, to the same promoter. In practice, P/CAF could be involved in maintaining the acetylation of Lys-9/14 of H3 histones (Fig. 2 A and B) and/or the acetylation of MyoD. MyoD acetylation has been previously reported by others and us (7, 11), and the significance of this modification, at least in this context, is still unclear. Finally, the finding that histone H3 at the myogenin promotor in differentiated cells is phosphorylated on Ser-10 (Fig. 3) is consistent, because this marking, which is synergistically coupled to the acetylation of histone H3 and thereby inhibits the methylation of Lys-9 (15, 19, 29), recently has been shown to mediate transcriptional activation (13).
Figure 4.
Model for in vivo occupancy by MyoD and HDAC or P/CAF in muscle cells. (Upper) A MyoD–HDAC complex bound to an E-box sequence near the start site of the myogenin promoter in undifferentiated myoblasts. Deacetylation of histone H3 at Lys-9 by the MyoD associated HDAC is likely to precede the methylation of this amino acid, and both pathways ultimately result in the silencing of the myogenin gene. (Lower) The consequences of myoblast differentiation with MyoD–P/CAF complexes now binding to E-box sites. This occupancy contributes to the activation of the myogenin gene and coincides with the acetylation of Lys-9 and Lys-14 of histone H3, most likely before or after the phosphorylation of Ser-10.
Although MyoD is highly established in being a transcriptional activator of both muscle and nonmuscle genes, the results presented here suggest that it also may be involved in silencing the myogenin gene in skeletal muscle cells. Because myogenin expression in rostral somites of mice embryos and in the stem cells (satellites) of adult skeletal muscles seems to critically depend on the function of MyoD (5, 32, 33), this unexpected finding may reflect a general model for MyoD's function during muscle development.
Acknowledgments
We thank the laboratory of B. Dynlacht, especially Joe Rayman, for assistance in establishing the ChIP protocol in our own laboratory. This work was supported in part by grants from the National Institutes of Health (GM54014) and the American Heart Association.
Abbreviations
- HDAC
histone deacetylase
- ChIP
chromatin immunoprecipitation
- GM
growth medium
- DM
differentiation medium
References
- 1.Puri P L, Sartorelli V. J Cell Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Sabourin L A, Rudnicki M A. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, McKinsey T A, Zhang C-L, Olson E N. Mol Cell. 2000;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicki M A, Schnegelsberg P N, Stead R H, Braun T, Arnold H H, Jaenisch R. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 5.Lassar A B, Skapek S X, Novitch B. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C L, McKinsey T A, Olson E N. Mol Cell Biol. 2002;22:7302–7312. doi: 10.1128/MCB.22.20.7302-7312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mal A, Sturniolo M, Schiltz R L, Ghosh M, Harter M L. EMBO J. 2001;20:1739–1753. doi: 10.1093/emboj/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri P L, Iezzi S, Stiegler P, Chen T T, Schiltz R L, Muscat G E, Giordano A, Kedes L, Wang J Y, Sartorelli V. Mol Cell. 2001;8:885–897. doi: 10.1016/s1097-2765(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 9.Polesskaya A, Naguibneva I, Duquet A, Bengal E, Robin P, Harel-Bellan A. Mol Cell Biol. 2001;21:5312–5320. doi: 10.1128/MCB.21.16.5312-5320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polesskaya A, Duquet A, Naguibneva I, Weise C, Vervisch A, Bengal E, Hucho F, Robin P, Harel-Bellan A. J Biol Chem. 2000;275:34359–34364. doi: 10.1074/jbc.M003815200. [DOI] [PubMed] [Google Scholar]
- 11.Sartorelli V, Puri P L, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang J Y J, Kedes L. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 12.Roth S Y, Denu J M, Allis C D. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T, Allis C D. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 14.Lo W S, Trievel R C, Rojas J R, Duggan L, Hsu J Y, Allis C D, Marmorstein R, Berger S L. Mol Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 15.Cheung P, Tanner K G, Cheung W L, Sassone-Corsi P, Denu J M, Allis C D. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen S J, Schneider R, Bauer U M, Bannister A J, Morrison A, O'Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera R E, et al. Nature. 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- 17.Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 18.Bannister A J, Zegerman P, Partridge J F, Miska E A, Thomas J O, Allshire R C, Kouzarides T. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 19.Rea S, Eisenhaber F, O'Carroll D, Strahl B D, Sun Z W, Schmid M, Opravil S, Mechtler K, Ponting C P, Allis C D, et al. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Xia L, Wu D Y, Wang H, Chansky H A, Schubach W H, Hickstein D D, Zhang Y. Oncogene. 2002;21:148–152. doi: 10.1038/sj.onc.1204998. [DOI] [PubMed] [Google Scholar]
- 22.Mal A, Chattopadhyay D, Ghosh M K, Poon R Y C, Hunter T, Harter M L. J Cell Biol. 2000;149:281–292. doi: 10.1083/jcb.149.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neville C, Rosenthal N, McGrew M, Bogdanova N, Hauschka S. In: Methods in Muscle Biology. Emerson C P Jr, Sweeney H L, editors. New York: Academic; 1997. pp. 85–114. [PubMed] [Google Scholar]
- 24.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, Rayman J B, Dynlacht B D. Genes Dev. 2000;14:804–816. [PMC free article] [PubMed] [Google Scholar]
- 26.Shang Y, Hu X, DiRenzo J, Lazar M A, Brown M. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 27.Ng H H, Bird A. Trends Biochem Sci. 2000;25:121–126. doi: 10.1016/s0968-0004(00)01551-6. [DOI] [PubMed] [Google Scholar]
- 28.Corbeil H B, Whyte P, Branton P E. Oncogene. 1995;11:909–920. [PubMed] [Google Scholar]
- 29.Clayton A L, Rose S, Barratt M J, Mahadevan L C. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa H, Ishiguro K, Gaubatz S, Livingston D M, Nakatani Y. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- 31.Aagaard L, Laible G, Selenko P, Schmid M, Dorn R, Schotta G, Kuhfittig S, Wolf A, Lebersorger A, Singh P B, et al. EMBO J. 1999;18:1923–1938. doi: 10.1093/emboj/18.7.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yablonka-Reuveni Z, Rudnicki M A, Rivera A J, Primig M, Anderson J E, Natanson P. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun T, Bober E, Rudnicki M A, Jaenisch R, Arnold H H. Development (Cambridge, UK) 1994;120:3083–3092. doi: 10.1242/dev.120.11.3083. [DOI] [PubMed] [Google Scholar]