Abstract
β-Arrestins bind to activated G protein-coupled receptor kinase-phosphorylated receptors, which leads to their desensitization with respect to G proteins, internalization via clathrin-coated pits, and signaling via a growing list of “scaffolded” pathways. To facilitate the discovery of novel adaptor and signaling roles of β-arrestins, we have developed and validated a generally applicable interfering RNA approach for selectively suppressing β-arrestins 1 or 2 expression by up to 95%. β-Arrestin depletion in HEK293 cells leads to enhanced cAMP generation in response to β2-adrenergic receptor stimulation, markedly reduced β2-adrenergic receptor and angiotensin II receptor internalization and impaired activation of the MAP kinases ERK 1 and 2 by angiotensin II. This approach should allow discovery of novel signaling and regulatory roles for the β-arrestins in many seven-membrane-spanning receptor systems.
Despite the diversity of physiological roles played by the large and ubiquitous family of seven membrane-spanning (7MS) receptors, there is remarkable conservation of the molecular mechanisms that mediate and modulate their signaling. Three families of proteins seem to interact almost universally and in a stimulus-dependent way with the receptors. These are the heterotrimeric G proteins (hence the name G protein-coupled receptors), the G protein-coupled receptor kinases (GRKs), and the arrestins. Interaction of an activated 7MS receptor with G protein leads to dissociation of its α and βγ subunits, which activate a variety of effectors (1). Interaction of the activated receptors with GRKs leads to their phosphorylation on serine and threonine residues, which is followed by β-arrestin binding (2). Although the mediation of 7MS receptor signaling by G proteins is reasonably well understood, a full appreciation of the roles and mechanisms of receptor regulation by arrestins and GRKs is not yet at hand. Originally discovered as proteins that mediate the desensitization of receptors (2, 3), the last several years have seen rapid progress in uncovering an ever-increasing list of additional functions of this regulatory system. These include adaptor and scaffold functions that link the receptors to the clathrin-coated pit endocytosis machinery (4–6) as well as to a variety of signaling systems such as the mitogen-activated protein kinases (MAPKs) ERK 1 and 2 (7–10), JNK 3 (11), and p38 (12).
In contrast with the extraordinarily large superfamily of 7MS receptors, numbering about a thousand members in the human genome, both the G protein-coupled receptor kinase and arrestin families are quite small, with only seven and four genes known, respectively, in mammals. Of the four arrestin genes, two are expressed only in retina, whereas two others, termed β-arrestins 1 and 2 (also known as arrestin 2 and 3) are ubiquitously expressed (13–15). Over the past several years, the development of knockout (KO) mice in which the genes for β-arrestins 1 and 2 have been inactivated by homologous recombination, and of mouse embryonic fibroblasts (MEFs) derived therefrom, have greatly aided the study of the physiological and cellular roles of these multifunctional proteins (16). However, the likely possibility that the arrestins might play previously unsuspected roles in other signaling pathways in diverse cell types has indicated the need for a generally applicable and reliable method for selectively suppressing β-arrestin isoform expression in cultured cells. Highly specific posttranscriptional gene silencing using 21- to 23-nt small interfering RNA (siRNA) duplexes has extended the RNA interference (RNAi) technique to a wide range of common mammalian culture lines (17), suggesting that this might represent an appropriate approach to this problem. RNAi is the phenomenon by which double-stranded RNA, acting through an incompletely characterized nuclease complex, catalytically destroys mRNA transcripts containing an identical nucleotide sequence, thereby suppressing protein expression (18). Here we report the development of a highly effective and specific RNAi method for depleting β-arrestins from human cells. We rigorously validate this approach in a variety of systems representing each of the currently known classes of β-arrestin action: desensitization, internalization, and signaling.
Materials and Methods
Materials.
Tissue culture reagents were purchased from Invitrogen. A horseradish peroxidase-conjugated secondary antibody was from Amersham Pharmacia, and the Supersignal chemiluminescence reagent was from Pierce. The radiolabeled compounds [125I]Tyr-4-angiotensin II (AngII) and [3H]cAMP were obtained from NEN. Human AngII was from Peninsula Laboratories. All other reagents were purchased from Sigma.
Synthesis of siRNAs.
Chemically synthesized, double-stranded siRNAs, with 19-nt duplex RNA and 2-nt 3′ dTdT overhangs, were purchased from Dharmacon Research (Lafayette, CO) or Xeragon (Germantown, MD) in deprotected and desalted form. To design β-arrestin-specific siRNA duplexes, the mRNA sequences for human β-arrestins 1 and 2 were screened for unique 21-nt sequences in the National Center for Biotechnology Information database by using the blast search algorithm. The accession numbers in brackets given below are from GenBank. The siRNA sequences targeting β-arrestin 1 (NM_020251) and β-arrestin 2 (NM_004313) are 5′-AAAGCCUUCUGCGCGGAGAAU-3′ and 5′-AAGGACCGCAAAGUGUUUGUG-3′ and correspond to the positions 439–459 and 201–221 relative to the start codon, respectively. One small RNA duplex was synthesized and used as a control. This RNA, initially designed to target another β-arrestin 2 mRNA unique sequence (5′-AAGUGGACCCUGUAGAUGGCG-3′; position 101–120 from the start codon), has no silencing effects on β-arrestin expression.
Cell Culture and Transfection.
HEK293 cells were maintained as described (19). Forty to 50% confluent cells in 100-mm plates, split at least 24 h before transfection, were transfected with siRNA using the GeneSilencer transfection reagent (Gene Therapy Systems, San Diego) according to the modified manufacturer's instructions. Briefly, 50 μl of the GeneSilencer transfection reagent was added to 300 μl of MEM, while RNA mixtures containing 72 μl of 20 μM (≈20 μg) RNA, 240 μl of siRNA diluent, and 180 μl of MEM were prepared. Both solutions were allowed to stand 5–10 min at room temperature and mixed by inversion. After a 10–20-min incubation at room temperature, the entire transfection mixture was added to cells in a 100-mm plate containing 3–4 ml of fresh, serum-free MEM. After cells were incubated for 4 h at 37°C, an additional 4–5 ml of MEM with 20% FBS and 2% penicillin/streptomycin were added to the plate. After additional incubation for 48 h, cells were divided into 12-well plates for β-arrestin immunoblotting and further experiments. For assays requiring transient receptor expression, the appropriate amounts of the plasmid encoding the selected receptor were transfected either 2 days after RNA treatment (β2-AR, β2-adrenergic receptor) or simultaneously with RNA (AT1A-R, AngII type 1A receptor) as above. All assays were performed at least 3 days after RNA transfection.
β-Arrestin Immunoblotting.
Whole-cell lysates, normalized for equal amounts of protein, were separated by SDS/PAGE on 10% Tris-Glycine polyacrylamide gels (Invitrogen) and immunoblotted with a 1:3,000 dilution of the rabbit polyclonal anti-β-arrestin antibody A1CT (15). β-arrestins 1 and 2 immunoblots were quantified by densitometry with a Fluor-S MultiImager (Bio-Rad).
cAMP Accumulation Assays.
Isoproterenol-stimulated cAMP accumulation in cells was measured as described (20) and normalized to total forskolin-stimulated cAMP production.
Receptor Sequestration Assays.
Agonist-induced sequestration of transiently expressed Flag epitope-tagged β2-ARs and AT1A-Rs were defined as loss of cell surface receptors after agonist treatment, measured by immunofluorescence flow cytometry (21), and radioligand binding (22), respectively, as described.
ERK Activation Assays.
ERK 1/2 MAPK activation in cells was determined as increased levels of phosphorylated ERK 1/2 on various concentrations of agonist treatment, measured essentially as described (10). Dose–response curves and EC50s were analyzed with prism software (GraphPad, San Diego).
Results
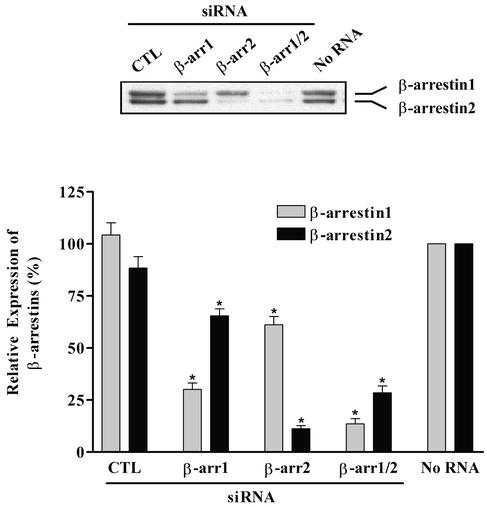
Two small RNA duplexes corresponding to the β-arrestin 1 and 2 genes were chemically synthesized and transfected into HEK293 cells to test their ability to silence β-arrestin expression. Protein expression levels of β-arrestins 1 and 2 in siRNA-transfected cells were analyzed by immunoblotting the whole-cell lysates (Fig. 1). Transfection of β-arrestins 1 or 2 siRNA reduces expression of the targeted β-arrestin by 70% and 90%, respectively, compared with either nonsilencing, control RNA- or mock-transfected cells. Both siRNAs also show significant isoform specificity. Moreover, dual transfection with both siRNAs significantly reduces the amount of β-arrestins 1 and 2. These results validate our siRNAs as potent reagents capable of selectively or collectively depleting β-arrestin levels in human cells.
Figure 1.
Analysis of β-arrestin expression in siRNA-transfected HEK293 cells. Whole-cell lysates were prepared from the indicated RNA-transfected HEK293 cells as described. Equal amounts of proteins (20 μg) in each sample were used to determine expression of β-arrestins 1 and 2 by immunoblotting with the polyclonal anti-β-arrestin antibody A1CT. Values shown are expressed as percent of the level of each β-arrestin in mock (No RNA)-transfected cells and represents the mean ± SE from seven independent experiments. Statistical significance was determined by using a one-way ANOVA to correct for multiple comparisons (PRISM software) between β-arrestin siRNA-transfected cells and control cells [both mock and nonsilencing, control RNA (CTL)-transfected] (*, P < 0.001). A representative immunoblot is shown (Upper).
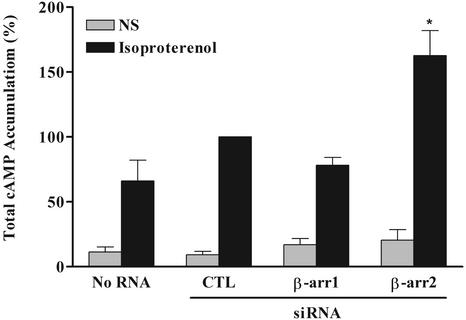
β-arrestins 1 and 2 have been ascribed roles in both 7MS receptor desensitization and internalization (2–6). We first tested the ability of cells transfected with siRNAs targeting the β-arrestins to support agonist-induced desensitization of the endogenously expressed β2-AR by measuring the accumulation of the second messenger cAMP. Stimulation of the β2-AR with isoproterenol for 2 min leads to maximal levels of cAMP accumulated in our HEK293 cells (data not shown). After agonist treatment for 2 min, total cAMP accumulation is significantly elevated in cells with β-arrestin 2 siRNA, whereas cells transfected with β-arrestin 1 siRNA show no change relative to controls (Fig. 2). These results demonstrate that reducing β-arrestin 2 levels (85–95%; Fig. 1) by RNAi provides a functional KO with respect to desensitization of the β2-AR. Additionally, these data are also in agreement with studies showing that the β2-AR preferentially recruits β-arrestin 2 following agonist stimulation (19).
Figure 2.
Effect of siRNA-mediated β-arrestin depletion on cAMP accumulation by endogenously expressed β2-AR. HEK293 cells were transfected with the indicated RNA oligonucleotides as described and subsequently stimulated with 10 μM isoproterenol for 2 min. Accumulation of cAMP was determined by competition with [3H]cAMP for PKA binding sites and then normalized to total forskolin (10 μM)-stimulated cAMP levels for each treatment. Data are expressed as percent of agonist-induced cAMP accumulation in control RNA-treated (CTL) cells and represent the mean ± SE of six independent experiments. NS, nonstimulated. Statistical significance was determined by using a one-way ANOVA to correct for multiple comparisons between β-arrestin siRNA-transfected cells and control cells. The asterisk indicates P < 0.05 vs. both control RNA- and mock (No RNA)-transfected cells.
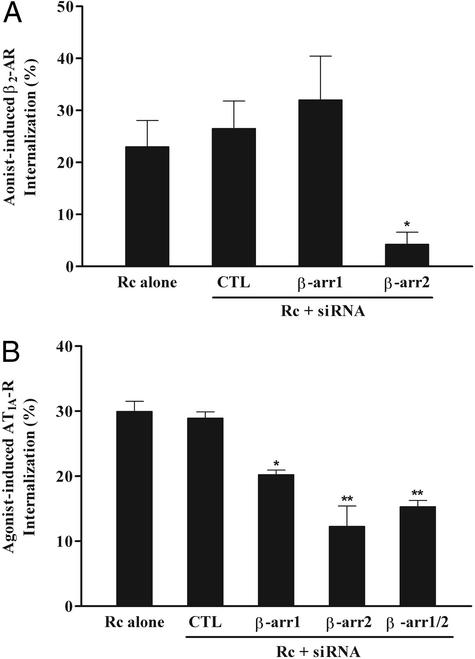
Next, we examined the effect of siRNA-suppressed β-arrestin expression on internalization of 7MS receptors by using transiently expressed β2-ARs and AT1A-Rs. Flag epitope-tagged β2-AR internalization was measured by flow cytometry after isoproterenol treatment for 30 min. Analogous to β2-AR desensitization, agonist-induced receptor internalization is dramatically attenuated in β-arrestin 2 siRNA-transfected cells, whereas β-arrestin 1 siRNA shows no significant effect on β2-AR internalization (Fig. 3A). These data are also consistent with our previous results in MEF lines originated from β-arrestin KO mice, suggesting that β-arrestin 2 is critical for regulating β2-AR sequestration, whereas β-arrestin 1 is largely dispensable for this function (16).
Figure 3.
Effect of siRNA-mediated suppression of β-arrestin levels on 7MS receptor internalization. (A) HEK293 cells were transfected with either control RNA (CTL) or the indicated β-arrestin siRNA and subsequently transfected again with plasmids encoding Flag epitope-tagged β2-AR as described. Serum-starved cells were exposed to 10 μM isoproterenol for 30 min at 37°C and analyzed for their plasma membrane content of the Flag epitope-tagged β2-AR by flow cytometry. Values shown are expressed as percentage of loss of agonist-induced cell surface receptors over nonstimulated cells. (B) HEK293 cells were transfected with appropriate amounts of AT1A-R encoding plasmids and the indicated siRNAs simultaneously. Receptor expression was equivalent (200–300 fmol per mg of protein) among all transfected cells. Cells were cooled down in ice-cold, serum-free media and subsequently stimulated with 0.2 nM 125I-labeled AngII for 5 min at 37°C. Percent of agonist-stimulated AT1A-R sequestration was determined as acid-resistant cpm divided by the total cpm bound. Data represent the mean ± SE from three independent experiments done in triplicate. Statistical significance was determined by using a one-way ANOVA to correct for multiple comparisons between β-arrestin siRNA-transfected cells and control cells [both receptor-transfected cells without RNA (Rc alone) and control RNA-treated cells] (*, P < 0.05; **, P < 0.01).
Sequestration of the AT1A-R, a representative of a 7MS receptor group interacting with both β-arrestins 1 and 2 with approximately equal affinity (19), was measured 5 min after AngII treatment by using ligand-binding assays. The AT1A-R shows a very different pattern in siRNA effects on its agonist-induced internalization (Fig. 3B) from that of the β2-AR (Fig. 3A). β-arrestin 1 siRNA-transfected cells show a modest, but significant, reduction (≈35%) in AT1A-R sequestration, whereas transfection of β-arrestin 2 siRNA, more effective in suppressing the β-arrestin level (Fig. 1), results in ≈60% attenuation of receptor internalization. The combination of both siRNAs, however, fails to further inhibit AT1A-R sequestration. Although the dual transfection decreases levels of both β-arrestins 1 and 2, it is not as effective as single transfection, particularly for β-arrestin 2 (Fig. 1). Thus, residual amounts of β-arrestin 2 in the double-transfected cells may prevent further inhibition of AT1A-R internalization, or alternatively, there may be a β-arrestin-independent pathway for AT1A-R internalization. Furthermore, at longer stimulation times (>15 min), the level of receptor sequestration in the β-arrestin-suppressed cells becomes equal to the controls (data not shown), confirming that at these levels (Fig. 1) of residual β-arrestins, the major effect is to slow the rate of internalization of the AT1A-R.
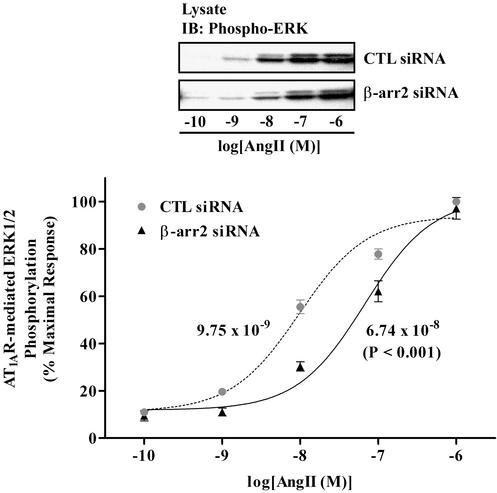
Besides its classical roles in desensitization and internalization of 7MS receptors, β-arrestin has recently been demonstrated to facilitate propagation of signals from the receptor to a variety of MAPK signaling systems such as the MAPKs ERK 1/2 (7–10), JNK 3 (11), and p38 (12) by scaffolding those kinases. To test whether siRNA-mediated depletion of β-arrestin alters MAPK signaling from the activated 7MS receptor, we examined ERK 1/2 activation by transiently expressed AT1A-R. As shown in Fig. 4, the maximal activation of ERK 1/2 in response to high concentrations of AngII (>10−6 M) is not different between β-arrestin 2 siRNA- and nonsilencing, control RNA-transfected cells. The dose–response curve, however, is shifted to higher concentrations of ligands by siRNA-mediated β-arrestin 2 depletion compared with that of control cells. The EC50 of ERK 1/2 activation in response to AngII is 7-fold higher in β-arrestin 2 siRNA-transfected cells than in control siRNA-transfected cells. These results directly demonstrate that β-arrestin acts as a positive regulator in 7MS receptor-mediated MAPK signaling.
Figure 4.
Effect of siRNA-inhibited expression of β-arrestin on ERK activation in response to AT1A-R stimulation. HEK293 cells transiently expressing AT1A-R (≈300 fmol per mg of protein) by cotransfection with either control RNA (CTL) or the β-arrestin 2 siRNA were serum-starved for 1–2 h and stimulated with the indicated concentrations of AngII for 5 min at 37°C. Phosphorylation of ERK in whole-cell lysates, normalized to equal amounts of proteins in each sample, was detected by immunoblotting with an antiphospho-ERK 1/2 antibody as described. Equal loading of total ERK proteins were also confirmed by immunoblotting with an anti-ERK 1/2 antibody (data not shown). Each data point is expressed as percent of the maximal phosphorylation of ERK in response to 10−6 M AngII in control cells and represents the mean ± SE from seven independent experiments. Dose–response curves and EC50s between control RNA- and β-arrestin 2 siRNA-treated samples were obtained and analyzed by a two-way ANOVA to determine statistical significance for shift of the curve (P < 0.001) by using PRISM software. A representative immunoblot is shown (Upper).
Discussion
Since their discovery more than a decade ago as molecules that desensitize 7MS receptors (14, 15), the roles of β-arrestins in these receptor systems have strikingly expanded (23, 24). Overexpression of wild-type or dominant-negative mutant β-arrestins have been commonly used strategies to characterize currently known β-arrestin functions. However, because endogenously expressed levels of β-arrestins may not be limiting, overexpression of the wild-type may fail to potentiate β-arrestin-dependent cellular responses. Moreover, since β-arrestins are noncatalytic proteins that bind various signaling molecules through distinct epitopes (24), dominant-negative mutants, developed for one purpose (e.g., to block sequestration), may fail or give misleading results when used to assess another.
Depletion of β-arrestin by various methods has also been used as an alternative approach to overcome such obstacles in studying its roles in 7MS receptor systems. One such method is antisense oligonucleotides. This technique, however, only modestly attenuates β-arrestin expression and is associated with low isoform-selectivity (25). Recently developed β-arrestin KO mice and MEF lines derived from them have been valuable tools to expand our understanding of physiological and cellular functions of β-arrestin (16). However, MEF lines are not applicable to the study of potentially variable roles of these multifunctional proteins in diverse cell types. Thus, we have chosen to use RNAi, with its potential for high silencing efficiency and selectivity in multiple cell types (17), as a new method to investigate the consequences of reduced β-arrestin levels in cultured cells.
Here, we show that transfection of siRNA designed to target β-arrestins 1 or 2 markedly and reliably attenuates expression of each β-arrestin, interfering with their currently known functions in 7MS receptor systems. Although some cross-reactivity to the opposite β-arrestin is observed (Fig. 1), our siRNAs show highly isoform-selective effects on β2-AR desensitization (Fig. 2) and sequestration (Fig. 3A); introduction of β-arrestin 2 siRNA attenuates both desensitization and sequestration of the β2-AR, whereas β-arrestin 1 siRNA has no effect. Thus, the siRNAs used here provide functionally selective depletion of a designated isoform of β-arrestin and are thereby useful to elucidate nonredundant, isoform-specific functions of β-arrestins.
While the advantages of using RNAi to examine roles of specific proteins are numerous and represent a significant improvement over alternative methods (18), there are caveats that need to be addressed when using this technique. Foremost among these is the impossibility of complete silencing of the target protein in siRNA-transfected cells. Studies using confocal microscopy strongly suggest that this phenomenon is limited by the transfection efficiency of the experimental cell population (17, 18). The method used in the present study makes it possible to achieve over 90% transfection efficiency in our HEK293 cells (data not shown). Furthermore, the extent of reduction of β-arrestin levels that we observed was sufficient in all cases to cause functional impairment of β-arrestin-mediated mechanisms. Secondly, using multiple siRNAs simultaneously may yield different results than either reagent individually (18). Such is the case for the dual transfection of β-arrestins 1 and 2 siRNAs. Although both β-arrestins are significantly depleted, the combination is not quite as effective at silencing β-arrestin 2 as the β-arrestin 2 siRNA alone. Undoubtedly, future advances in understanding the mechanisms underlying RNAi will result in improvements in this area.
To date, the best system for examining the functional consequences of loss of β-arrestins has been MEF lines established from β-arrestin KO mice (16). Our siRNA treatment produces patterns in desensitization (Fig. 2) and internalization (Fig. 3) of 7MS receptors similar to those in these MEF cells. We observed significant impairment in desensitization and sequestration of the β2-AR by depletion of β-arrestin 2, but not β-arrestin 1, as well as in internalization of the AT1A-R by suppression of both β-arrestins 1 and 2 expression. The data here is also in accord with the previous finding that β-arrestin 2 is recruited to the β2-AR with a 10-fold-higher affinity than β-arrestin 1, whereas the AT1A-R recruits both β-arrestins with approximately equal efficacy (19).
Depletion of a single β-arrestin isoform by RNAi significantly reduces AT1A-R internalization after short time exposure (5 min) to AngII but shows no effect with longer stimulation time (>15 min; data not shown). These results suggest that the siRNA-mediated reduction of the β-arrestin level functions to slow the rate of internalization of the AT1A-R rather than to block this process. Furthermore, it has been demonstrated that the AT1A-R is also internalized through β-arrestin-independent pathways (26). MEF cells from mice devoid of β-arrestins also fail to completely abolish the sequestration of the AT1A-R (16).
We found that siRNA-mediated depletion of β-arrestin2 shifts the dose–response curve for ERK activation in response to AT1A-R stimulation to the right but has no effect on the maximal response to a high concentration of agonist. Because MAPKs can be activated through multiple signaling pathways upon 7MS receptor activation (27, 28), it likely is not possible to abolish their activation by knocking out only a single pathway such as a β-arrestin-dependent pathway. Nonetheless, our results directly demonstrate that β-arrestin acts as a signal transducer for 7MS receptor-mediated MAPK activation, a conclusion that is also supported by previous findings that overexpression of β-arrestin potentiates ERK activation by various 7MS receptors (9, 10). In the future, it will be of interest to determine whether suppression of both β-arrestin isoforms has even more profound effects on ERK activation.
Our results demonstrate that siRNA-mediated suppression of the β-arrestin levels is sufficient to alter the currently known β-arrestin functions in 7MS receptor systems: desensitization, internalization, and signaling. A growing list of newly discovered roles of β-arrestins as adapter proteins and scaffolds for propagating signals in a variety of 7MS receptor systems (24) suggests that our appreciation of the functions of these proteins will most likely continue to expand. Thus, the results reported here introduce the RNAi technique as a reliable and powerful approach to expose unrevealed, physiological, and cellular functions of these versatile proteins in the future.
Acknowledgments
We thank Donna Addison and Julie Turnbough for excellent secretarial assistance. This work was supported by National Institutes of Health Grant RO1 HL16037 (to R.J.L.). R.J.L. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- 7MS
seven membrane-spanning
- siRNA
small interfering RNA
- RNAi
RNA interference
- MEF
mouse embryonic fibroblast
- β2-AR
β2-adrenergic receptor
- AngII
angiotensin II
- AT1A-R
AngII type 1A receptor
- MAPK
mitogen-activated protein kinase
- KO
knockout
References
- 1.Hall R A, Premont R T, Lefkowitz R J. J Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 4.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 5.Laporte S A, Oakley R H, Zhang J, Holt J A, Ferguson S S, Caron M G, Barak L S. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald P H, Cote N L, Lin F T, Premont R T, Pitcher J A, Lefkowitz R J. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 7.DeFea K A, Zalevsky J, Thoma M S, Dery O, Mullins R D, Bunnett N W. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttrell L M, Roudabush F L, Choy E W, Miller W E, Field M E, Pierce K L, Lefkowitz R J. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tohgo, A., Choy, E. W., Gesty-Palmer, D., Pierce, K. L., Laporte, S., Oakley, R. H., Caron, M. G., Lefkowitz, R. J. & Luttrell, L. M. (2002) J. Biol. Chem., 10.1074/jbc.M212231200. [DOI] [PubMed]
- 10.Tohgo A, Pierce K L, Choy E W, Lefkowitz R J, Luttrell L M. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 11.McDonald P H, Chow C W, Miller W E, Laporte S A, Field M E, Lin F T, Davis R J, Lefkowitz R J. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Cheng Z, Ma L, Pei G. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 13.Krupnick J G, Benovic J L. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 14.Lohse M J, Benovic J L, Codina J, Caron M G, Lefkowitz R J. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 15.Attramadal H, Arriza J L, Aoki C, Dawson T M, Codina J, Kwatra M M, Snyder S H, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 16.Kohout T A, Lin F S, Perry S J, Conner D A, Lefkowitz R J. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbashir S M, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 18.McManus M T, Sharp P A. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 19.Oakley R H, Laporte S A, Holt J A, Caron M G, Barak L S. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 20.Tovey K C, Oldham K G, Whelan J A. Clin Chim Acta. 1974;56:221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Barak L S, Tiberi M, Freedman N J, Kwatra M M, Lefkowitz R J, Caron M G. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 22.Laporte S A, Servant G, Richard D E, Escher E, Guillemette G, Leduc R. Mol Pharmacol. 1996;49:89–95. [PubMed] [Google Scholar]
- 23.Miller W E, Lefkowitz R J. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 24.Luttrell L M, Lefkowitz R J. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 25.Mundell S J, Loudon R P, Benovic J L. Biochemistry. 1999;38:8723–8732. doi: 10.1021/bi990361v. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Ferguson S S, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 27.Pierce K L, Luttrell L M, Lefkowitz R J. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell L M. Can J Physiol Pharmacol. 2002;80:375–382. doi: 10.1139/y02-045. [DOI] [PubMed] [Google Scholar]