Abstract
Vitellogenin is a female-specific glucolipoprotein yolk precursor produced by all oviparous animals. Vitellogenin expression is under hormonal control, and the protein is generally synthesized directly before yolk deposition. In the honeybee (Apis mellifera), vitellogenin is not only synthesized by the reproductive queen, but also by the functionally sterile workers. In summer, the worker population consists of a hive bee group performing a multitude of tasks including nursing inside the nest, and a forager group specialized in collecting nectar, pollen, water, and propolis. Vitellogenin is synthesized in large quantities by hive bees. When hive bees develop into foragers, their juvenile hormone titers increase, and this causes cessation of their vitellogenin production. This inverse relationship between vitellogenin synthesis and juvenile hormone is opposite to the norm in insects, and the underlying proximate processes and life-history reasons are still not understood. Here we document an alternative use of vitellogenin by showing that it is a source for the proteinaceous royal jelly that is produced by the hive bees. Hive bees use the jelly to feed larvae, queen, workers, and drones. This finding suggests that the evolution of a brood-rearing worker class and a specialized forager class in an advanced eusocial insect society has been directed by an alternative utilization of yolk protein.
Invertebrate and vertebrate vitellogenins (1) constitute a multigene superfamily together with insect apolipophorin II/I, human apolipoprotein B, and the large subunit of mammalian microsomal triglyceride transfer protein (2). Honeybee vitellogenin is considered to be a 180-kDa monomer (3). In queens, hive bees, and wintering workers (winter bees), vitellogenin is the predominant hemolymph protein (4–7) (30–50% of total). The rate of synthesis in a worker is negligible at the time of emergence, but increases rapidly within 2–3 days (6). Vitellogenin synthesis peaks during the period when the bee normally nurses brood (5–15 days of age) (6–9). In this period, the rate of synthesis equals the amount needed to provision 30–100 eggs daily (10).
The high rate of vitellogenin synthesis in hive bees has been enigmatic for almost three decades since the protein was documented not to be present in jelly fed to the larvae (11). In the actual study, small amounts of vitellogenin were observed in homogenates of the hypopharyngeal glands (HPGs), the paired acinous jelly-producing glands located in the head of the worker. However, they concluded that this observation was due to contamination and that vitellogenin was not used as brood food. At the same time, it was suggested elsewhere that the activity of the HPGs and the production of vitellogenin were coregulated by the corpora allata brain complex (5). The high rate of vitellogenin production during the nursing phase was thus explained as a regulatory side effect. Based on the observation of vitellogenin in drone hemolymph, it was later proposed that the synthesized vitellogenin was recycled by the fat body as a compensatory strategy (12).
From an evolutionary point of view, it is unlikely that the costly production of vitellogenin in nurse bees has no specific biological role. If the trait had not been under positive selection, one would expect that mutational events and subsequent genetic drift would have led to considerable variation of the trait within or between nurse bee populations. However, a high rate of vitellogenin synthesis is one of the key defining characteristics of nurse bees (4–7, 10). Furthermore, failing to detect vitellogenin in jelly does not rule out that the protein is incorporated and rapidly lysed within the HPGs' acini. The consequences of assuming vitellogenin to be a major source for the jelly produced by hive bees were recently tested by a data-driven differential equations model (13). The model describes the dynamics of the protein in the individual bee as a function of its task profile under various intracolonial regimes, and explains the available empirical data on vitellogenin profiles in workers. Because of the evolutionary implications associated with a nonoogenic utilization of vitellogenin in an advanced eusocial insect, the above considerations motivated us to experimentally readdress the role of vitellogenin in jelly production.
Our experimental work was guided by the facts that the transport of vitellogenin into ovaries is exclusively reported to be a receptor-mediated process (2) and that jelly contains a substantial amount of Zn (14). Vitellogenin is the dominant Zn-carrier in honeybee hemolymph (G.V.A., Z. L. P. Simões, A.H., K.N., K. Schrøder, Ø. Mikkelsen, T. Kirkwood, and S.W.O., unpublished data), and the capacity of this protein to carry Zn over oocyte membranes by receptor-mediated cotransport is documented in several genera (15). This observation led us to search for a vitellogenin-binding HPG-membrane protein by ligand blot techniques customized for visualizing insect vitellogenin receptors while simultaneously assaying a positive (queen ovary) and a negative control (worker rectum). To substantiate that the receptor actually transports vitellogenin into the HPGs for further processing, 14C vitellogenin was produced by in vitro synthesis and then injected into the hemolymph of hive bees.
Materials and Methods
Ligand Blotting.
Polyclonal vitellogenin antibodies against honeybee vitellogenin were raised in rabbits as described (16). The antiserum was found to specifically recognize vitellogenin in immunoblots of honeybee egg homogenate and worker hemolymph as described (17), at a concentration of <1:25,000 by using 5 μg of protein per lane. Its specificity was in both cases found to be equal to the antiserum produced and applied by Pinto et al. (4).
Honeybee queens stored at −80°C were thawed at room temperature before their ovaries were dissected in insect saline. Honeybee workers of unknown age were removed from the brood nest and anaesthetized on ice before dissection of the rectum and head. Only HPGs containing jelly were used. The rectum was cut open and rinsed in insect saline. Bound vitellogenin was removed from all samples by use of the protocol for ovary membrane isolation described in ref. 17. Solubilized membrane preparations of queen ovaries, worker rectums, and worker HPGs were then made as described in ref. 18. Subsequent immunoblotting did not reveal any signs of 180-kDa vitellogenin in the preparations.
Samples of 2.0–5.0 μg of protein were subjected to one-dimensional SDS electrophoresis as described (19), except that slab gels were 0.75 mm thick and reducing conditions were achieved by boiling the samples for 5 min in the presence of DTT (Bio-Rad). After electrophoresis, separated samples were transferred to nitrocellulose paper. The paper was incubated with native vitellogenin from worker hemolymph before being incubated with vitellogenin antibody at a concentration of 1:75,000 as described (19). Bound vitellogenin was visualized by biotinylated secondary antibodies and the Vectastain ABC-AmP Detection System (Vector Laboratories).
Radiolabeling Assay.
Each of the fat bodies of 30 newly mated queens was incubated in 200 μl of Kaatz medium (20) where 50 μg/ml l-phenylalanine was substituted by l-phenylalanine [14C (U)] (Moravek Biochemicals, Brea, CA); BSA was substituted by 50 μl/ml FCS; 10 units/ml nystatin, 50 μg/ml gentamisin, and vitamins as in Grace's medium (Sigma) were added; and proteins >100 kDa were removed by filter centrifugation (Centriplus YM-100, centrifugal filter unit, Millipore). Every 6 h, each fat body received 10 μl of queen head extract (21) as described (10) and 20 μl of medium without l-phenylalanine [14C (U)]. After 48 h, the cultures were frozen, thawed, and homogenized. The homogenate was centrifuged at 10,000 × g for 10 min. The supernatant was briefly stored at −20°C.
The supernatant was separated by SDS electrophoresis under nonreducing conditions. Bands were visualized by using nonfixing Sypro-Tangerine protein gel stain (Molecular Probes). Vitellogenin was recognized as a single band at 180 kDa, aligning with the most abundant protein in egg homogenate and a 180-kDa molecular mass standard (Sigma). The vitellogenin band was removed, and the protein was recovered by electroelution (Elutrap, Schleicher & Schuell). The sample was concentrated to 200 μl by filter centrifugation (Centriplus YM-100, centrifugal filter unit, Millipore), and the protein was renaturated in suspension as described (22). The final sample was dissolved in insect saline to an approximate activity of 10,000 dmp/μl. The presence of vitellogenin was confirmed by immunoblotting, and the purity of the sample was densitometrically estimated to be >95%.
Four queenright colonies were kept in Apidea hives (Transidea, Bern, Switzerland) with three frames of brood and two frames of pollen and honey. Two hundred newly emerged workers sampled from five source colonies were marked and introduced into each hive. After 8 days, 100 marked workers were collected from the brood nest of each hive, fixed to a piece of styrophor with two crossed needles, and pacified at 8°C. In this position, they were injected with 0.5 or 1.0 μl of 14C vitellogenin. The injection was made dorsally between the fifth and sixth abdominal segment with a Hamilton microsyringe (30-gauge needle, Becton Dickinson). Individuals showing signs of hemolymph leakage after withdrawal of the needle were discarded. The bees stayed in the fixed position for 2 h before being remarked with a new color code and frozen or transferred back to their hives together with 50 newly emerged workers marked with a separate color.
After 12 h, the colonies were anesthetized with CO2. Workers and queens were packed in plastic bags labeled according to colony. The larvae were removed from their cells and pooled. Cells containing jelly were flushed with 50 μl of distilled water, and the volume of jelly was equated with the excess volume of water. All samples were stored at −20°C. The activities of the samples were determined by biooxidation and flow cytometry (Harvey oxidizer OX-500-2T). In addition, we separately measured the activities of heads, thoraxes, and abdomens dissected from a random sample of 10 injected workers. To avoid leakage of hemolymph from the body compartments the workers were dissected while frozen.
Results
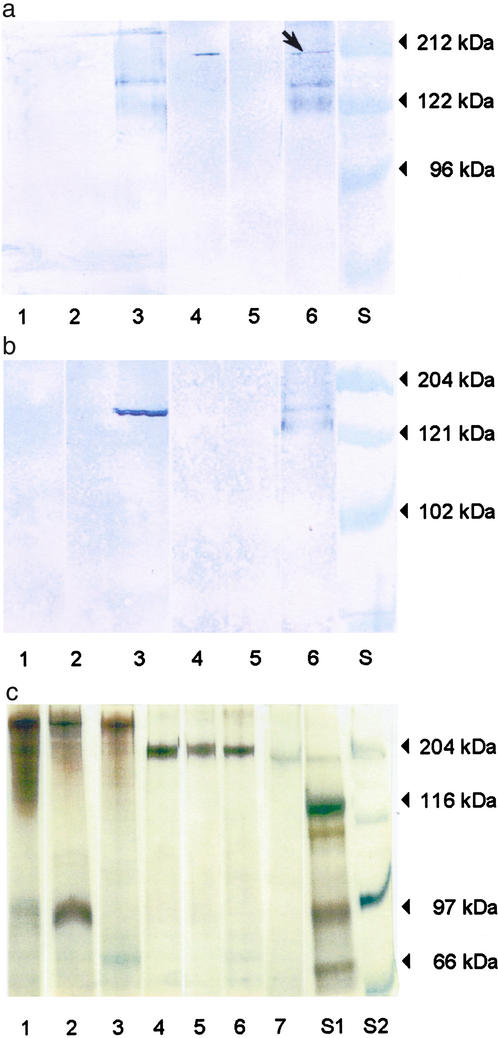
We are able to show that HPG membrane homogenates from worker honeybees contain a single vitellogenin receptor protein that migrates with an apparent molecular weight of 205 kDa on SDS/polyacrylamide gel under nonreducing conditions (Fig. 1a, lane 6). The queen ovary membrane contains a receptor protein of the same size, whereas the worker rectum does not (Fig. 1a, lane 4 vs. lane 5). The size is comparable with the vitellogenin receptor proteins identified for the fever mosquito, Aedes aegypti (205 kDa) (23) and the polychaetous annelid Nereis virens (190 kDa) (18). No binding of vitellogenin was observed in the presence of 15 mM EDTA, which is known to block binding to the receptor (19) (Fig. 1a, lanes 1–3). In accordance with previous observations (18, 19, 23), we were unable to visualize the receptor under reducing conditions (Fig. 1b, lanes 1–3). This finding indicates that intact disulfide bonds in the receptor are required for binding activity (19). Fig. 1c shows the protein profiles of the membrane homogenates under reducing as well as nonreducing conditions, visualized by silver staining. Honeybee vitellogenin is included for comparison (lane 7).
Figure 1.
Visualization of the honeybee vitellogenin receptor. Solubilized membrane samples of queen ovary (lanes 1 and 4), worker rectum (lanes 2 and 5), and worker HPGs (lanes 3 and 6). S, molecular mass standards. (a) Ligand blotting results under nonreducing conditions. Lanes 1–3, with 15 mM EDTA added during incubation with native vitellogenin; lanes 4–6, without EDTA; 2 μg of protein per lane. The arrow indicates the vitellogenin receptor. (b) Lanes 1–3, ligand blotting under reducing conditions; lanes 4–6, immunoblotting under nonreducing conditions. Lanes 1, 2, 4, and 5 used 2 μg of protein per lane; lanes 3 and 6 used 3 μg of protein per lane. (c) Proteins visualized by silver staining. Lanes 1–3 and 4–7, samples prepared under reducing and nonreducing conditions, respectively. Lane 7, honeybee egg homogenate, where the dominant band is vitellogenin. All samples used 1 μg of protein per lane.
Under reducing conditions, the HPG membrane homogenate yields two close bands recognized by vitellogenin antibodies at ≈150 kDa (Fig. 1b, lane 3). Under nonreducing conditions, the antibodies visualize two bands at ≈150 and 125 kDa, respectively (Fig. 1b, lane 6). Under reducing conditions, membrane association and transport affect protein characteristics like solubility and size (24), and the presence of distinct bands might thus be an experimental artifact. In any case, the result confirms previous reports on the existence of a 150-kDa subunit of vitellogenin or a second honeybee vitellogenin of this size (4).
The distribution of 14C after 12 h shows that a noticeable proportion (14–38%) of the activity lost from injected workers is recovered from the colony (Table 1). 14C was found in jelly (1,875 ± 122 dpm/g wet weight) and colony members including larvae (177 ± 13 dpm/g wet weight) and queens (324 ± 36 dpm/g wet weight). The duration of our experiment was substantially longer than what is needed to obtain a uniform hemolymph distribution of injected material (25). Thus, when corrected for hemolymph volume, the radiolabel activity in the three main body compartments is expected to be the same unless vitellogenin is not specifically absorbed or bound in tissues or organs. However, we find that, relative to hemolymph volume, the mean amount of radiolabel in the heads of injected workers is twice that of the abdomens and thoraxes (Fig. 2). This finding suggests that vitellogenin or vitellogenin-derived products accumulate in the heads of the injected bees.
Table 1.
Radioactive label data (dpm⋅103)
| Colony | Colony size | Injected* | Initial activity† | Remaining‡ | Transferred§ |
|---|---|---|---|---|---|
| 1 | ≈700 | 55/30 | 746.08 ± 0.70 | 550.97 ± 0.74 | 26.44 ± 0.09 |
| 2 | ≈900 | 50/25 | 665.50 ± 0.61 | 526.56 ± 0.73 | 53.15 ± 0.12 |
| 3 | ≈800 | 50/25 | 665.50 ± 0.61 | 473.55 ± 0.69 | 56.83 ± 0.13 |
| 4 | ≈1,000 | 40/20 | 532.40 ± 0.49 | 427.62 ± 0.65 | 24.37 ± 0.08 |
Variance calculations include instrumental error, background activity is subtracted, and the instrumental efficiency is >95%.
No. of workers introduced to the experimental colonies after injection of 1.0 and 0.5 μl of 14C vitellogenin, respectively.
Bootstrap estimates (sum ± SEM) of the total activity in the introduced bees extrapolated from the activity levels of 20 workers killed immediately after injection (1,000 iterations).
Activity present in injected bees after 12 h (sum ± SEM).
Brood food, larvae, and noninjected adults.
Figure 2.
Distribution of radioactive label in injected bees 12 h after injection (mean ± SEM), n = 10. ⋄, Original data. ♦, Relative to hemolymph volume as described in ref. 25.
Discussion
It has been suspected for some time that vitellogenin has functions other than reproduction in the honeybee (10, 11). This paper reports evidence that vitellogenin binds to the HPGs of young bees, and that a labeled amino acid incorporated into vitellogenin is transferred to other colony members including larvae, workers, and queens. The HPGs are specialized for jelly production in young workers. In this stage, the organs are responsible for a substantial fraction of net protein synthesis (26), and the increase in size of the HPG acini in nurses is caused by the accumulation of a secretion reservoir of jelly (27). Binding of vitellogenin to the HPG membrane (Fig. 1) and accumulation of vitellogenin or vitellogenin-derived products in the heads of young workers (Fig. 2) thus suggest that vitellogenin is involved in brood-food production. Furthermore, the fact that the introduced activity is especially recovered in jelly and colony members, including adult bees, is in accordance with a previous report describing the flow of jelly in honeybee colonies (8). From this we conclude that vitellogenin is at least in part directly used in production of jelly. The extent of this utilization may be documented in the future by comparing vitellogenin with proteins of known function serving as positive and negative controls.
The transfer of activity in our experiment is lower than what is observed when using free amino acids metabolized at very low rates (8). Furthermore, a considerable proportion of the activity is not recovered in our study (Table 1). This may indicate that activity is released as 14CO2, and that vitellogenin is also metabolized for other purposes than jelly production. This hypothesis was originally suggested by Engels et al. (10), and may in part explain the high rate of vitellogenin production in nonlaying wintering queens (10), the high level of vitellogenin in wintering workers (7), and the presence of vitellogenin in drones (12). However, further studies are required to show whether vitellogenin is a storage protein used for a variety of metabolic purposes.
The range of strategies for larvae and queen provisioning in the social Hymenoptera includes more or less masticated prey items, pollen and honey mixed with glandular secretions, crop regurgitate from adults, larvae saliva, trophic eggs, and protinaceous HPG secretions (28, 29). By being shunted into the production of jelly in the HPGs, vitellogenin serves the same function as it does in worker-laid eggs being eaten by larvae (e.g., species of Myrmica) or fed to the queen to enhance egg production (e.g., Myrmica and Meliponini) (28, 29).
Trophic eggs may qualify as social exploitation of vitellogenin, but the vitellogenin-to-jelly mechanism provides a novel strategy that represents a more refined physiological specialization. It is premature to make decisive statements about the selective assets of the vitellogenin-to-jelly invention. However, in all of the main groups of social insects there has been an increase in the time and energy invested by adults to refine and concentrate foods (28, 30). This progress has in many cases been accompanied by the evolution of a specialized temporal nursing caste (28). If the vitellogenin-to-jelly mechanism caused the nurse bee to become more physiologically dedicated to the nursing role, it probably made brood feeding more efficient. Moreover, it seems hard to combine an efficient physiology capable of continuous delivery of large amounts of jelly based on a high rate of vitellogenin synthesis with one dedicated to intensive energy-consuming foraging. Selection for the ability to incorporate the predominant yolk protein in the production of HPG secretions is therefore likely to have contributed to the evolution of the observed temporal caste structure in honeybees.
The vitellogenin-to-jelly mechanism might also have contributed to the physiological specialization of the short-lived honeybee forager. In the social Hymenoptera, nurses may store considerable amounts of the colony's nutritional resources in the form of body fat or storage proteins (28). In general, this is not the case for foragers, and age-based division of labor, with performance of risky tasks delayed until late in life by workers with depleted nutrient stores, has been suggested to have evolved as an energy saving mechanism in insect colonies (31, 32). For example, in species exhibiting a temporal caste structure such as the ant Pogonomyrmex owyheei and the wasp Polybia occidentalis, workers are drained of nutritional reserves before they start foraging (28, 31). In the case of honeybees, turning off vitellogenin synthesis in the forager (4) and reprogramming its HPGs to synthesise honey-processing enzymes at low rates (27) is probably a means to economize the colony's protein household, as this will prevent buildup of a vitellogenin store that will be lost when the forager perishes in the field (13). Moreover, this seems to have opened up for further specialization of the forager physiology. It was recently found that the rise in juvenile hormone titer associated with the hive bee to forager transition causes apoptosis of the hemocyte population in the forager hemolymph through the regulatory chain juvenile hormone 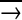 vitellogenin →+ Zn →- hemocyte apoptosis (G.V.A., Z. L. P. Simões, A.H., K.N., K. Schrøder, Ø. Mikkelsen, T. Kirkwood, and S.W.O., unpublished data). Hemocytes have important immunological functions (33), which implies that through inhibition of the vitellogenin synthesis the depletion of the hemolymph protein pool and the down-regulation of the somatic maintenance machinery are concertedly regulated. A functional immune system is apparently costly in social insects (33), and a down-regulation of somatic maintenance probably causes further nutrient deprivation as well as a lowered nutrient demand. This pattern may not be restricted to honeybees. In the ant Pogonomyrmex owyheei, workers that initiate foraging also show progressed somatic senescence (28).
vitellogenin →+ Zn →- hemocyte apoptosis (G.V.A., Z. L. P. Simões, A.H., K.N., K. Schrøder, Ø. Mikkelsen, T. Kirkwood, and S.W.O., unpublished data). Hemocytes have important immunological functions (33), which implies that through inhibition of the vitellogenin synthesis the depletion of the hemolymph protein pool and the down-regulation of the somatic maintenance machinery are concertedly regulated. A functional immune system is apparently costly in social insects (33), and a down-regulation of somatic maintenance probably causes further nutrient deprivation as well as a lowered nutrient demand. This pattern may not be restricted to honeybees. In the ant Pogonomyrmex owyheei, workers that initiate foraging also show progressed somatic senescence (28).
If honeybee vitellogenin can be characterized as a storage protein being used for a range of metabolic purposes, the vitellogenin-to-jelly invention would also have made possible the establishment of a very simple and flexible ambient condition-driven mechanism for transforming a nurse bee into a bee with large enough protein and lipid stores to survive several months on honey only. Brood production ceases under unfavorable ambient conditions (29), and this gives rise to the accumulation of vitellogenin in the workers in temperate zones (7). The observed accumulation is likely to be a direct result of continued synthesis of vitellogenin in individuals with a low juvenile hormone titer feeding on the remaining protein sources in the colony. This would at least be sufficient for an automatic buildup of a population of individuals that can survive without access to pollen until the ambient conditions improve once more (13).
The circumstantial evidence presented above indicates that the vitellogenin-to-jelly mechanism might have been a key innovation in the evolution of the regulatory anatomy of the honeybee society. We expect that future comparative work on other Hymenopterans addressing the relationships between the degree of exploitation of the vitellogenin machinery, larval feeding strategies, and social level will give us a better understanding of the proximate settings and life history conditions that have determined the use of vitellogenin within this insect order.
Acknowledgments
We thank Z. L. P. Simões for antiserum. We also thank K. Hartfelder, P. Kryger, and anonymous referees for comments on the manuscript, and Ø. Halskau for discussions on methods. G.V.A.'s contribution was financially supported by the Norwegian Research Council, Project No. 133680/110.
Abbreviation
- HPG
hypopharyngeal gland
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Byrne B M, Gruber M, Ab G. Prog Biophys Mol Biol. 1989;53:33–69. doi: 10.1016/0079-6107(89)90005-9. [DOI] [PubMed] [Google Scholar]
- 2.Mann C J, Anderson T A, Read J, Chester S A, Harrison G B, Kochl S, Ritchie P J, Bradbury P, Hussain F S, Amey J, et al. J Mol Biol. 1999;285:391–408. doi: 10.1006/jmbi.1998.2298. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler D E, Kawooya J K. Arch Insect Biochem Physiol. 1990;14:253–267. doi: 10.1002/arch.940140405. [DOI] [PubMed] [Google Scholar]
- 4.Pinto L Z, Bitondi M M G, Simões Z L P. J Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- 5.Engels W. Am Zool. 1974;14:1229–1237. [Google Scholar]
- 6.Engels W, Fahrenhorst H. Wilhelm Roux Arch. 1974;174:285–296. doi: 10.1007/BF00573233. [DOI] [PubMed] [Google Scholar]
- 7.Fluri P, Lüscher M, Wille H, Gerig L. J Insect Physiol. 1982;28:61–68. [Google Scholar]
- 8.Crailsheim K. J Comp Physiol B. 1992;162:681–689. [Google Scholar]
- 9.Seeley T D. Behav Ecol Sociobiol. 1982;11:287–293. [Google Scholar]
- 10.Engels W, Kaatz H H, Zilikens A, Simões Z L P, Truve A, Braun R, Dittrich F. In: Advances in Invertebrate Reproduction 5. Hashi M, Yamashita O, editors. Amsterdam: Elsevier Science; 1990. pp. 495–502. [Google Scholar]
- 11.Rutz W, Lüscher M. J Insect Physiol. 1974;20:897–909. doi: 10.1016/0022-1910(74)90179-6. [DOI] [PubMed] [Google Scholar]
- 12.Trenczek T, Zillikens A, Engels W. J Insect Physiol. 1989;35:475–481. [Google Scholar]
- 13.Amdam G V A, Omholt S W. J Theor Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- 14.Osanai M. Zoology. 1960;9:1–8. [Google Scholar]
- 15.Falchuk K H, Montorzi M. BioMetals. 2001;14:385–395. doi: 10.1023/a:1012994427351. [DOI] [PubMed] [Google Scholar]
- 16.Jensen P V, Børgesen L W. Arth Struct Dev. 2000;29:171–184. doi: 10.1016/s1467-8039(00)00021-9. [DOI] [PubMed] [Google Scholar]
- 17.Dhadialla T S, Raikhel A S. Arch Insect Biochem Physiol. 1991;18:55–70. doi: 10.1002/arch.940180106. [DOI] [PubMed] [Google Scholar]
- 18.Hafer J, Fischer A, Ferenz H J. J Comp Physiol B. 1992;162:148–152. [Google Scholar]
- 19.Daniel T O, Schneider W J, Goldstein J L, Brown M. J Biol Chem. 1983;258:4606–4611. [PubMed] [Google Scholar]
- 20.Kaatz H H, Hagedorn H H, Engels W. In Vitro Cell Dev Biol. 1985;21:347–352. [Google Scholar]
- 21.Song Q, Gilbert L I. Insect Biochem Mol Biol. 1995;25:591–602. doi: 10.1016/0965-1748(94)00100-v. [DOI] [PubMed] [Google Scholar]
- 22.Hrzenjak A, Frank S, Maderegger B, Serk S, Kostner G M. Protein Eng. 2000;13:661–666. doi: 10.1093/protein/13.9.661. [DOI] [PubMed] [Google Scholar]
- 23.Dhadialla T S, Hays A R, Raikhel A S. Insect Biochem Mol Biol. 1992;22:803–816. [Google Scholar]
- 24.Fu H, Subramanian R R, Masters S C. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 25.Crailsheim K. J Insect Physiol. 1985;31:707–713. [Google Scholar]
- 26.Takenaka T, Kaatz H H. In: Chemistry and Biology of Social Insects. Eder J, Rembold H, editors. München, Germany: Peperny; 1987. pp. 166–167. [Google Scholar]
- 27.Knecht D, Kaatz H H. Apidologie. 1990;21:457–468. [Google Scholar]
- 28.Brian M W. Social Insects: Ecology and Behavioural Biology. New York: Chapman and Hall; 1983. pp. 146–160. [Google Scholar]
- 29.Engels W, Imperatriz-Fonseca V L. In: Social Insects: An Evolutionary Approach to Castes and Reproduction. Engels W, editor. New York: Springer; 1990. pp. 167–230. [Google Scholar]
- 30.Velthuis H H W. Bee World. 1992;73:77–89. [Google Scholar]
- 31.O'Donnell S, Jeanne R L. Experientia. 1995;51:749–752. [Google Scholar]
- 32.Jeanne R L. Monitore Zool Ital. 1986;20:119–133. [Google Scholar]
- 33.Moret Y, Schmid-Hempel P. Science. 2002;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]