Abstract
Clinical expression of Hirschsprung disease (HSCR) requires the interaction of multiple susceptibility genes. Molecular genetic analyses have revealed that interactions between mutations in the genes encoding the RET receptor tyrosine kinase and the endothelin receptor type B (EDNRB) are central to the genesis of HSCR. We have established two locus noncomplementation assays in mice, using allelic series at Ednrb in the context of Ret kinase-null heterozygotes, to understand the clinical presentation, incomplete penetrance, variation in length of aganglionic segment, and sex bias observed in human HSCR patients. Titration of Ednrb in the presence of half the genetic dose of Ret determines the presentation of an enteric phenotype in these strains, revealing or abrogating a sex bias in disease expression depending on the genotype at Ednrb. RET and EDNRB signaling pathways are also critical for the normal development of other tissues, including the kidneys and neural crest-derived melanocytes. Our data demonstrate that interaction between these genes is restricted to the enteric nervous system and does not affect renal, coat color, and retinal choroid development.
Complex inheritance minimizes the impact of genetic variation and suppresses the potential expression of deleterious phenotypes by requiring the combined influence of mutant alleles at multiple loci to reveal a disease phenotype. Hirschsprung disease (HSCR) or aganglionic megacolon is a complex trait requiring the interaction of multiple genes for disease expression. Mutations in eight genes have already been identified in patients with HSCR and include RET, GDNF, NRTN, EDNRB, EDN3, ECE1, SOX10, and SMADIP1 (1–13). HSCR also displays several genetic hallmarks, including incomplete penetrance and pleiotropic effects of mutant genotypes, a marked sex difference in clinical expression, and variation in penetrance with extent of aganglionosis. Genetic and epigenetic modification of known mutations may provide a parsimonious explanation for these findings, suggesting that the majority of HSCR cases are likely to arise from the combined effects of multiple susceptibility genes. Although studies in mice have uncovered the disease phenotypes resulting from mutations within HSCR genes, we know little of the influence of variation in multiple genes or genetic background. Variants that abrogate a gene's capacity to moderate disease expression reveal interactions intrinsic to the associated trait. We have begun to dissect these interactions in enteric nervous system (ENS) development and HSCR and report specific genetic buffering interactions in ENS development and HSCR.
HSCR is a congenital malformation with an incidence in the general population of 1/5,000 live births (14, 15) and is characterized by an absence of neural crest (NC)-derived intrinsic ganglia along a variable length of the distal intestinal tract. The pathways mediated by the RET receptor tyrosine kinase and the G-protein-coupled endothelin receptor type B (EDNRB) are critical for the normal development of the ENS and are known to be central to the genesis of HSCR. These pathways are also pleiotropic, and their signaling during embryogenesis demonstrates marks tissue-specific sensitivity.
HSCR-like phenotypes have been reported in a wide array of higher organisms, including horse, pig, rat, and mouse (16–23). Mouse models, in particular, have been crucial to the identification and functional analysis of genes underlying HSCR. Homozygous Ret kinase-null mice demonstrate complete enteric aganglionosis, defects in sympathetic innervation, and bilateral renal agenesis (18). In contrast, an absence of EDNRB signaling in the embryo results in severe epidermal hypopigmentation and bilateral absence of the retinal choroid layer but is also accompanied by intestinal aganglionosis (distal gut only) (16, 17). Mutations have since been identified in the human RET (5, 24) and EDNRB genes (6, 25). These mouse strains are the current models for HSCR. However, they also demonstrate several key differences from human disease. First, intestinal aganglionosis is restricted to the homozygous null genotypes in Ret and Ednrb, yet HSCR patients frequently are heterozygous for mutations in these genes. Patients homozygous for mutations within EDNRB have WS4 (Waardenburg–Shah syndrome; Online Mendelian Inheritance in Man, no. 277580; www.ncbi.nlm.nih.gov/htbin-post/Omim/dispmim?277580) (26), presenting with hypopigmentation anomalies, akin to the Ednrb mouse models (26). Second, these mouse strains display full phenotypic penetrance in homozygotes. However, human mutations in these genes are incompletely penetrant and display significant intra- and interfamilial variation (14, 27). Third, the mouse models do not demonstrate a sex bias, whereas human mutations in the same genes result in a 2- to 4-fold predominance of male affecteds. The overlapping role of RET and EDNRB signaling in ENS development raises the question whether these pathways are functionally independent. This is doubtful, because our recent studies of HSCR in an Old Order Mennonite kindred have demonstrated interaction between alleles of RET and EDNRB (28).
These data prompted us to examine the potential for noncomplementation between these loci using extant mutations in the mouse. We have analyzed allelic series at Ednrb (wild-type, +; piebald, s; piebald lethal, sl) in Ret kinase-null heterozygotes (kinase-null allele, −) in a series of two-locus noncomplementation tests to uncover genetic interactions. The hypomorphic piebald allele of Ednrb (Ednrbs) results in a 75% reduction in the corresponding transcript in homozygotes (17), although the causative molecular variant remains to be identified. Ednrbs/Ednrbs mice are characterized by a small hypopigmented belly spot and occasional dorsal spotting and have a low incidence of megacolon (10%) in the mouse-fancier strain in which it arose (29). The spontaneous null allele of Ednrb (Piebald lethal, Ednrbs-l) results from a deletion of all coding exons (17). Ednrbs-l/Ednrbs-l mice develop megacolon and are almost completely devoid of coat color, except for an occasional pigmented blaze on the head or rump (17, 30). Ednrbs-l/Ednrbs mice are also hypopigmented (40–60% white spotting) with a reported incidence of megacolon of 8.3% (1/12) at 12 mo (30). The Ret targeted mutation deletes a critical lysine (Lys-748) at the beginning of the tyrosine kinase domain, abolishing the kinase activity of its receptor protein product and truncating RET at this point (18). We have assumed this allele to be a genetic and functional null, consistent with the report of an identical phenotype in mice harboring another targeted Ret null allele, terminating the protein product at the signal peptide (31). However, it remains possible that the truncated protein retains weak dominant negative activity. Ret−/Ret− mice display renal agenesis or severe dysgenesis and an absence of intrinsic ganglion cells throughout their digestive tracts and die in the first 24 h postpartum (18). All of the mutations described above are complete recessives with respect to the enteric phenotype.
We have established oligogenic mouse strains, titrating the genetic contribution of Ednrb in the context of Ret kinase-null heterozygotes. Placing the hypomorphic Ednrbs allele, as a homozygote or transheterozygote with the Ednrb null allele (Ednrbs-l) in combination with a Ret kinase-null heterozygote, results in mice presenting with a stark HSCR phenotype before weaning. Our data demonstrate that some of these oligogenic models recapitulate the sex bias observed in human HSCR, and that decreasing Ednrb in these mice abolishes this bias. These data indicate that the effect of mutations at Ret may be modulated by mutations in other HSCR susceptibility genes, and that interaction between Ret and Ednrb is a causative mechanism of HSCR (28). Furthermore, despite the fact that these pathways individually influence several aspects of development, interaction between these loci appears restricted to the ENS.
Materials and Methods
Generation of Intercross Mice.
We established a series of matings titrating the genetic contribution of Ednrb in the context of Ret kinase-null heterozygotes (Ret−/Ret+). Ret−/Ret+; Ednrb+/Ednrb+ mice were mated with Ret+/Ret+; Ednrbs/Ednrbs mice, to assess the potential for interaction between these loci. Similarly, Ret−/Ret+; Ednrb+/Ednrb+ mice were mated with Ret+/Ret+; Ednrbs-l/Ednrbs mice. Compound heterozygous offspring (Ret−/Ret+; Ednrbs/Ednrb+, n = 109, age >12 weeks nor Ret−/Ret+;/Ednrb+, n > 35, age >12 weeks) did not demonstrate an enteric phenotype. We used progeny from these matings in a second mating series (Ret−/Ret+; Ednrbs/Ednrb+ × Ret+/Ret+; Ednrbs/Ednrbs and Ret−/Ret+; Ednrbs-l/Ednrb+ × Ret+/Ret+; EdnrbsEdnrbs), generating 275 and 105 offspring, respectively. Details of strain maintenance, signs of distress, and the proportions of each genetic class produced by the mating programs can be found in Supporting Text and Table 2, which are published as supporting information on the PNAS web site, www.pnas.org. All animal studies were performed under protocols approved by the Johns Hopkins University Animal Care and Use Committee. Staining of intestines for acetylcholinesterase (AchE) activity was performed as described (28).
Quantification of Pigmented Surfaces.
Digital images of the dorsal and ventral surfaces of each mouse were taken by using a Sony DSC S-70 digital camera. Values for complete dorsal and ventral surface areas and corresponding values for hypopigmented areas were determined by using NIH image software (http://rsb.info.nih.gov/nih-image), as described (32). Values for the percentage of each surface and percentage of the total surface area occupied by hypopigmented hair were then calculated (see Table 3 and Fig. 6, which are published as supporting information on the PNAS web site).
Hematoxylin/Eosin (H&E) Staining of Paraffin-Embedded Tissues.
All tissues for H&E staining were fixed for 2 h at 4°C in 10% buffered formalin. Five-micrometer sections were cut from paraffin-embedded tissues and stained with H&E (standard protocols). Sections were examined by using bright-field microscopy under a Zeiss (Axiophot) photomicroscope.
In Situ Hybridization on Frozen Sections.
Wild-type embryos were obtained from timed matings established with CD1 mice, and 12:00 p.m. (noon) of the day that vaginal plugs were observed defined as 0.5 days postcoitum (dpc). Nonradioactive cryosection in situ hybridization was performed as described (33). Digoxigenin-labeled antisense probes were made with templates 0.96-kb Ednrb (nt 555-1514, pWP40 KpnI T7 RNA polymerase), and 2.5 kb Ret (pmcRet7 NotI T7 RNA polymerase).
Statistical Analyses.
Determination of statistical significance in comparisons of extent of aganglionosis and white spotting were assayed by using Student's t test, assuming unequal variances. Determination of statistical significance in comparisons of variances in aganglionic and white-spotting phenotypes were assayed by using an F test of variances. Significance was attributed for values P < 0.05 in either test.
Results
Noncomplementation of Ret Kinase-Null Heterozygotes by Mutant Alleles at Ednrb.
Singly, the null alleles of Ret and Ednrb are strictly recessive for the enteric phenotype. The hypomorphic Ednrbs allele, as a heterozygote, homozygote, or transheterozygote with the Ednrb null allele (Ednrbs-l), does not reduce EDNRB signaling sufficiently to result in an enteric phenotype, despite progressively stronger effects on coat pigmentation. When mutations in both Ednrb and Ret are combined, EDNRB levels in the presence of half the genetic dose of Ret determine the enteric phenotype. Fig. 1D reports the incidence of megacolon for each genotype generated in this study.
Figure 1.
Oligogenic HSCR mice present with abdominal distension, demonstrating spastic distal bowel and dilatation of the proximal bowel and cecum. Dorsal surfaces (A), ventral surfaces (B), and intestinal tracts (C, stomach to recto-anal junction) from Ret−/Ret+; Ednrbs/Ednrbs (Upper) and Ret+/Ret+; Ednrbs/Ednrbs (Lower) mice are shown. (D) Incidence of megacolon in two locus Ret-Ednrb mouse models. Genotypes at Ednrb and Ret are listed left to right, upper, and in the left column, respectively (see Supporting Text for Fig. 1, which is published as supporting information on the PNAS web site). dws, dorsal white spotting; vws, ventral white spotting; s, stomach; si, small intestine; ce, cecum; co, colon; r, rectum; fp, fecal pellet; dc, dilated colon, +, wild-type; −, Ret−; s-l, Ednrbs-l; s, Ednrbs; nd, not done.
Onset and Clinical Presentation of Disease.
All animals were examined daily from birth. Mice displaying abdominal distension or signs of distress (see Supporting Text) were identified and killed immediately (Fig. 1). Gross examination of intestinal tracts of such mice revealed a variable length of spastic distal colon with dilated proximal colon and ileocecal junction (Fig. 1). All animals were genotyped at weaning (3–4 weeks) and killed between the ages of 4–6 weeks with the exception of three mice listed below. Among mice derived from Ret−/Ret+; Ednrbs/Ednrb+ × Ret+/Ret+; Ednrbs/Ednrbs backcross matings, only animals of genetic class Ret−/Ret+; Ednrbs/Ednrbs displayed abdominal distension and distress, comprising 53/275 progeny generated. Anticipating equal numbers of each genotype, this number is significantly lower than expected (P < 0.002, χ2 = 5.48) and is consistent with loss in utero or in the early postnatal period (see Table 2). A small number of animals (≈12) were unavailable for study due to infanticide or because they died before the third postnatal day and were cannibalized by their mother. Likewise, among mice derived from Ret−/Ret+; Ednrbs-l/Ednrb+ × Ret+/Ret+; Ednrbs/Ednrbs matings, 100% (22/22) of those displaying abdominal distension or distress were of the genotype Ret−/Ret+; Ednrbs-l/Ednrbs and comprised 22/105. Anticipating equal numbers of each genotype, this number is not significantly lower than expected (P < 0.33, χ2 = 0.96) (see Table 2). The apparent increased lethality in the former cross may reflect the larger number of animals assessed (n = 275) compared with the latter cross (n = 105) or the differing genetic backgrounds of the parental strains (see Supporting Text).
Human HSCR patients also demonstrate a marked sex bias with a 2- to 4-fold higher incidence in males, depending on the length of the affected segment (15). Consequently, we compared the penetrance of the overt disease phenotype (abdominal distension and distress) among males and females of genotype Ret−/Ret+; Ednrbs/Ednrbs. One hundred percent of males (28/28) of this genotype were visibly affected on open field examination, compared with 68% of females (17/25). Gross examination of intestinal tracts from the remaining eight Ret−/Ret+; Ednrbs/Ednrbs females (five females killed at 4–6 weeks, one at 10 weeks, and two aged 12 weeks) also revealed a variable length of spastic distal colon. To determine the impact of further reducing the genetic contribution of Ednrb on presentation of disease, we compared the incidence of distension and distress among males and females of genotype Ret−/Ret+; Ednrbs-l/Ednrbs. Disease expression was fully penetrant in both sexes of this genotype.
Pathological Presentation of Disease.
HSCR is defined by the absence of NC-derived enteric ganglia (aganglionosis) from the myenteric (Auerbach) and submucosal (Meissner) plexuses of the gut (34). We performed AchE staining of whole-mounted intestinal tracts and H&E staining of paraffin-embedded tissue sections from mice of all genotypes generated, to assess the presence or absence of enteric ganglia. The normal reticulate plexuses of intrinsic ganglia were absent from variable lengths of the intestinal tract of Ret−/Ret+; Ednrbs/Ednrbs (Fig. 2) and Ret−/Ret+; Ednrbs-l/Ednrbs (data not shown) mice, progressing from the recto-anal junction (constant inferior limit) and extending proximally. The myenteric plexus was replaced by large AchE-positive extrinsic nerve fibers. The plexus was restored as fibers contacted an increasing density of ganglia, although the upper limit of normal innervation was highly heterogeneous. The hypertrophic AchE-positive neurites observed in whole-mounts of affected guts (Fig. 2) are characteristic of HSCR and contrast with the normal reticulate pattern of enteric innervation observed in age-matched Ret+/Ret+; Ednrb+/Ednrb+, Ret−/Ret+; Ednrb+/Ednrb+ (data not shown), and Ret+/Ret+; Ednrbs/Ednrbs controls (Fig. 2). One explanation for the observation of incomplete penetrance in Ret−/Ret+; Ednrbs/Ednrbs females is that the unaffected mice do not display intestinal aganglionosis. However, all Ret−/Ret+; Ednrbs/Ednrbs females demonstrated intestinal aganglionosis. Likewise, all Ret−/Ret+; Ednrbs/Ednrbs mice lacked ganglion cells throughout a variable length of their distal gut. Finally, the extent of aganglionosis observed in overtly unaffected versus affected Ret−/Ret+; Ednrbs/Ednrbs females was within the range of extents displayed by overtly affected females (data not shown). Thus, additional genes or effects must be necessary for clinical phenotype expression.
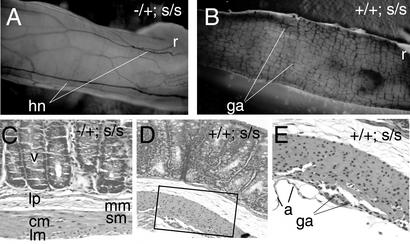
Figure 2.
Ret−/Ret+; Ednrbs/Ednrbs mice lack intrinsic ganglia along a variable length of the distal colon. (A and B) AchE-stained whole-mount intestinal tracts from oligogenic (Ret−/Ret+; Ednrbs/Ednrbs) mice (A) and a piebald homozygous (Ret+/Ret+; Ednrbs/Ednrbs) mouse (B). (A) Distal intestinal segment from a Ret−/Ret+; Ednrbs/Ednrbs mouse, demonstrating an absence of ganglia and presence of hypertrophic AchE-positive neurites. (B) Normal myenteric plexus in the Ret+/Ret+; Ednrbs/Ednrbs mouse. (C–E) H&E-stained paraffin-embedded sections through mouse intestines. (C) Ret−/Ret+; Ednrbs/Ednrbs. (D) Ret+/Ret+; Ednrbs/Ednrbs at ×20. (E) A ganglion cell (×40) observed (boxed) in D (Ret+/Ret+; Ednrbs/Ednrbs). +, wild-type; −, Ret−; s-l, Ednrbs-l; s, Ednrbs; v, villus; lp, lamina propria; mm, muscularis mucosa; sm, submucosa; cm, circular muscle; lm, longitudinal muscle; a, adventitia; hn, hypertrophic neurite; ga, ganglion cell; r, recto-anal junction.
Sex Difference in Disease Severity.
These observations led us to hypothesize that the length of the aganglionic segment in Ret−/Ret+; Ednrbs/Ednrbs mice may influence clinical expression and thus may differ between males and females. To determine the impact on disease expression, we assayed the extent of aganglionosis among animals of genotype Ret−/Ret+; Ednrbs/Ednrbs in whole-mount intestinal segments (recto-anal junction to cecum, inclusive) stained for AchE activity. Extent of aganglionosis was defined as the distance between the recto-anal junction and correctly innervated bowel and is reported as a percentage of the recto-anal junction to cecum measurement. The mean extent of aganglionosis in Ret−/Ret+; Ednrbs/Ednrbs male mice was 41% (n = 26, see Figs. 1D and 3), in contrast to a mean of 28% (n = 23) among females of the same genotype: this difference is highly significant (P = 0.0004). The correlation between incomplete penetrance and a lesser extent of aganglionosis in Ret−/Ret+; Ednrbs/Ednrbs females, compared with Ret−/Ret+; Ednrbs/Ednrbs males, is consistent with our hypothesis. We further hypothesized that in mice displaying complete penetrance of disease expression (Ret−/Ret+; Ednrbs-l/Ednrbs), extent of aganglionosis would not differ significantly between males and females and/or would exceed an unknown threshold beyond which full penetrance would be assured.
Figure 3.
Ret−/Ret+; Ednrbs/Ednrbs mice demonstrate a marked sex bias in the extent of aganglionosis. (A) Distribution of extents of aganglionosis illustrating the increased severity of disease observed in Ret−/Ret+; Ednrbs/Ednrbs males compared with females of the same genotype. (B) Distribution of extents of aganglionosis observed in Ret−/Ret+; Ednrbs-l/Ednrbs males and females did not differ significantly between the sexes of this genotype. Table 1 reports the P values for corresponding Student's t test and F test. +, wild-type; −, Ret−; s-l, Ednrbs-l; s, Ednrbs.
The mean extent of aganglionosis in Ret−/Ret+; Ednrbs-l/Ednrbs males was 42.5% (n = 10) compared with 36.9% in Ret−/Ret+; Ednrbs-l/Ednrbs females (n = 12, Figs. 1D and 3). Consistent with our hypothesis, this difference was not statistically significant (P = 0.59). However, the phenotypic variances in Ret−/Ret+; Ednrbs-l/Ednrbs males and females are increased compared with their Ret−/Ret+; Ednrbs/Ednrbs counterparts (see Table 1). Consequently, although we observe an increase in the mean extent of aganglionosis in Ret−/Ret+; Ednrbs-l/Ednrbs females compared with Ret−/Ret+; Ednrbs/Ednrbs females, in the direction that is consistent with our hypothesis, the results are not significantly different (P = 0.236; t test). This may be in part due to lower statistical power. The elevated variance among Ret−/Ret+; Ednrbs-l/Ednrbs mice is consistent with segregation of an increased number of loci influencing the trait and may, in part, be due to presence of alleles contributed by differing strain backgrounds in Ret−/Ret+; Ednrbs-l/Ednrbs mice (129S1;LP/J;SSL/Le) compared with Ret−/Ret+; Ednrbs/Ednrbs (129S1;LP/J, for details, see Table 2). The increased phenotypic variance in Ret−/Ret+; Ednrbs-l/Ednrbs mice may also reflect the smaller sample size ascertained from this cross (n = 12, females; n = 10, males) compared with Ret−/Ret+; Ednrbs/Ednrbs mice (n = 23, females; n = 26, males).
Table 1.
Ret−/Ret+; Ednrbs-l/Ednrbs animals demonstrate no sex bias in the extent of aganglionosis, in contrast to Ret−/Ret+; Ednrbs/Ednrbs mice
| Sex | Genotype | Mean extent, % | Variance | Male −/+; s/s | Female −/+; s/s | Male −/+; s-l/s | Female −/+; s-l/s |
|---|---|---|---|---|---|---|---|
| Males | −/+; s/s | 41.0 | 179.8 | 0.0004* | 0.8787 | 0.5525 | |
| Females | −/+; s/s | 28.2 | 107.2 | 0.1120 | 0.1073 | 0.2359 | |
| Males | −/+; s-l/s | 42.5 | 610.8 | 0.0075† | 0.0004 | 0.5892 | |
| Females | −/+; s-l/s | 36.9 | 532.8 | 0.0119 | 0.0007† | 0.4085 |
P values corresponding to all comparisons of means (t tests) in Ret−/Ret+; Ednrbs/Ednrbs and Ret−/Ret+; Ednrbs-l/Ednrbs mice are reported in the upper right half of the table. Comparisons of variances (F tests) are reported in the lower left half of the table. +, wild-type; −, Ret−; s-l, Ednrbs-l; s, Ednrbs.
Significant difference in extent of aganglionosis between Ret−/Ret+; Ednrbs/Ednrbs males and females.
Significant difference in variance between Ret−/Ret+; Ednrbs-l/Ednrbs and Ret−/Ret+; Ednrbs/Ednrbs mice.
Tissue-Specific Sensitivity to RET and EDNRB Signaling.
RET and EDNRB signaling pathways are critical for the normal development of the central nervous system, peripheral nervous system, and ENS. RET is critical for development of the kidneys and EDNRB for development of NC-derived pigment cell populations, indicating a tissue-specific sensitivity to reduced levels of signaling through these pathways. The pathological influence of compound Ret/Ednrb genotypes on ENS development prompted us to determine their impact on melanocyte, retinal choroid, and kidney development.
Influence of Compound Genotypes on Melanocyte Development.
Defects in EDNRB signaling result in WS4, the concurrent presentation of HSCR and Waardenburg syndrome (27). EDNRB signaling is critical for normal development of epidermal melanocytes. RET signaling also promotes epidermal pigmentation, although its role has been the subject of considerably less study (35). To determine whether heterozygosity at Ret (Ret−/Ret+) increases the degree of hypopigmentation in mice harboring Ednrbs/Ednrbs or Ednrbs-l/Ednrbs, we compared the extent of white spotting between Ret+/Ret+; Ednrbs/Ednrbs and Ret−/Ret+; Ednrbs/Ednrbs mice and between Ret+/Ret+;/Ednrbs and Ret−/Ret+; Ednrbs-l/Ednrbs mice. Digital images of the dorsal and ventral surfaces of mice of each genotype were obtained (Fig. 4). Absolute values of percentage dorsal and ventral white spotting for animals of each genotype were calculated (see Materials and Methods), and their distributions are published as Table 3 and Fig. 6.
Figure 4.
Compound Ret/Ednrb genotypes exhibit no pathological impact on the development of epidermal melanocytes or the retinal choroid. Examples are shown of ventral (A and B) and dorsal (C and D) white spotting displayed by Ret−/Ret+; Ednrbs-l/Ednrbs (A and C), and Ret+/Ret+; Ednrbs-l/Ednrbs (B and D) mice. (E and F) H&E-stained sections through the eyes of Ret−/Ret+; Ednrbs-l/Ednrbs and Ret+/Ret+; Ednrbs-l/Ednrbs, respectively. dws, dorsal white spotting; vws, ventral white spotting; ch, choroid; RPE, retinal pigmented epithelium.
Values for dorsal spotting in Ret−/Ret+; Ednrbs/Ednrbs (n = 18, mean = 2.07%) and Ret+/Ret+; Ednrbs/Ednrbs (n = 23, mean = 2.37%) mice were not significantly different (P = 0.67). Values for dorsal spotting in Ret−/Ret+; Ednrbs-l/Ednrbs (n = 23, mean = 26.89%) and Ret+/Ret+; Ednrbs-l/Ednrbs (n = 29, mean = 24.32%) mice were also not significantly different (P = 0.37). Likewise, ventral spotting values were compared for the above genotype pairs and none differed significantly.
The retina contains two pigmented cell layers, the non-NC-derived retinal pigmented epithelium (RPE) and the NC-derived choroid layer. EDNRB-deficient mice lack the NC-derived retinal choroid. RET is expressed in the adjacent RPE, although this layer demonstrates no defect in mice deficient in the RET ligand, glial cell line-derived neurotrophic factor (GDNF; L. Shen, personal communication). To determine the impact of the compound genotypes Ret−/Ret+; Ednrbs/Ednrbs and Ret−/Ret+; Ednrbs-l/Ednrbs on the development of the choroid and RPE layers, we compared H&E-stained sections taken through the eyes of mice of the above genotypes (n = 6) with Ret+/Ret+; Ednrb+/Ednrb+ (n = 6) and Ret+/Ret+; Ednrbs-l/Ednrbs-l (n = 6) mice at 4 weeks (data not shown). Ret−/Ret+; Ednrbs/Ednrbs or Ret−/Ret+; Ednrbs-l/Ednrbs (Fig. 4E) mice develop normal pigmented choroid and RPE cell layers, identical to those observed in Ret+/Ret+; Ednrb+/Ednrb+, Ret+/Ret+; Ednrbs/Ednrbs and Ret+/Ret+; Ednrbs-l/Ednrbs (Fig. 4F) mice. These findings imply that interaction between Ret and Ednrb mutations has no significant pathological impact on the development of NC-derived epidermal or retinal melanocytes.
Combined Influence of Ret and Ednrb on Renal Development.
RET kinase-deficient mice (Ret−/Ret−) demonstrate a failure of renal induction, resulting in bilateral renal agenesis or severe renal dysgenesis (18). Although a small but significant number of Gdnf null heterozygotes demonstrate unilateral or bilateral renal dysgenesis (36–38), Ret−/Ret+ mice demonstrate normal bilateral kidney development (39), suggesting that 50% of wild-type RET signaling levels may be close to the threshold required for normal renal development. EDNRB is also expressed in the Wolffian duct of the metanephros (40), although mice lacking this receptor (Ednrbs-l/Ednrbs-l) demonstrate normal renal development. We examined H&E-stained sections through kidneys from Ret−/Ret+; Ednrbs/Ednrbs and Ret−/Ret+; Ednrbs-l/Ednrbs mice at 4 weeks and observed no defect in the kidney structure or organization (see Fig. 7, which is published as supporting information on the PNAS web site). These findings strongly suggest that interaction between the assayed mutant alleles of Ret and Ednrb does not significantly impact renal development.
Colocalization of Ret and Ednrb Transcripts in Migrating Enteric Neuroblasts.
It is as yet unclear whether the observed impact on enteric development is the result of biochemical interaction between components of the RET and EDNRB signaling pathways or redundancy in processes contributing to a common phenotype, the formation of an intact ENS. Although RET and EDNRB are both expressed in populations of NC-derived enteric neuroblasts, their temporal and spatial expression is also tightly regulated, and no report of their colocalization exists. We performed in situ hybridization comparing serial sections through wild-type embryos (11.5, 12.5, and 13.5 dpc), hybridized to digoxygenin-labeled Ret and Ednrb riboprobes. Fig. 5 demonstrates that Ret and Ednrb transcripts are present in a shared subpopulation of migrating enteric neuron precursors throughout the stomach and mid-gut intestinal loops of the intestinal tract throughout 11–13.5 dpc, consistent with the hypothesis that physical interaction between these signaling mechanisms may underlie the pathological observation.
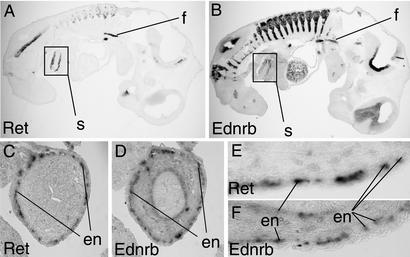
Figure 5.
Localization of Ret and Ednrb transcripts within the intestinal tract of 11.5 and 13.5 dpc embryos. (A and B) Embryos (11.5 dpc, ×25). (C–F) Embryonic gut (13.5 dpc, ×100). (A, C, and E) Migrant enteric neuroblast population in the embryonic foregut (A), stomach (A), and midgut loops (C and E) of wild-type embryos, visualized by using a digoxygenin (DIG)-labeled Ret antisense riboprobe on 20-μm (A) and 5-μm (C and E) sections. (B, D, and F) Sections adjacent to sections A, C, and E, respectively, hybridized to a DIG-labeled Ednrb antisense riboprobe. Ret and Ednrb riboprobes are clearly present in overlapping populations of enteric neuroblasts throughout these developmental stages. en, enteric neuron precursor; f, foregut; s, stomach.
Discussion
Interactions between RET and other loci, including EDNRB, may explain disease risk in subsets of HSCR families (25, 28, 41–44). We have used two-locus noncomplementation of existing mouse mutations in Ret and Ednrb to test these observations. We have demonstrated that certain compound genotypes of the two major HSCR genes Ret and Ednrb, which independently fail to yield intestinal aganglionosis, can result in an enteric defect presenting in the early postnatal stages. Furthermore, we report a sex bias in the penetrance of the Ret−/Ret+; Ednrbs/Ednrbs genotype. Formation of the ENS necessitates integration of a complex web of interactions, a series of checks and balances whose role it is to ensure the establishment and maintenance of a homeostatic system. As with all complex traits, the genesis of HSCR requires the interaction of molecular variants at multiple loci. These data reveal a relationship between Ret and Ednrb in buffering genetic variation in the formation of the ENS and between their mutant alleles in the genesis of an HSCR phenotype. Interaction between Ret and Ednrb mutations in these mouse strains also reveals the potential impact of other factors, e.g., the influence of gender and molecular variants at other loci, in enhancing or suppressing phenotypic variation.
Our oligogenic models display several striking characteristics. First, intestinal aganglionosis in mice combining independently benign genotypes of Ret and Ednrb requires an interaction to explain disease transmission, consistent with observations in human HSCR families. Second, mice with the compound genotype Ret−/Ret+; Ednrbs/Ednrbs demonstrate incomplete penetrance, manifest as reduced clinical expression in aganglionic females, consistent with observations in human HSCR. This may reflect gender-specific differences during development, e.g., in gene expression profile during embryogenesis and consequently in the microenvironment of the forming ENS. Third, the observed bias is gene-dosage dependent, because, once the contribution of Ednrb was further diminished (Ret−/Ret+; Ednrbs-l/Ednrbs), the sex bias is extinguished, although we did not determine the influence of further reducing RET signaling in these mice. Although our interpretation must be tempered by differences in genetic backgrounds of the intercross strains, our data suggest that sex may be revealed or masked as a HSCR susceptibility factor, depending on the magnitude of effects at the mutant loci involved. Likewise, the sex bias is diminished in HSCR patients harboring severe coding sequence mutations and presenting with the more severe long-segment HSCR (L-HSCR) (15).
The greater extent of aganglionosis observed in males of genotype Ret−/Ret+; Ednrbs/Ednrbs compared with females is not reflected in the clinical literature. However, an absence of comparable data (quantitative measures of segment length and genotypes at relevant loci) means that one cannot compare the proportion of gut affected in males and females harboring equivalent mutations. Decreased penetrance in Ret−/Ret+; Ednrbs/Ednrbs female mice also correlates with the decreased extent of aganglionosis observed in these mice compared with Ret−/Ret+; Ednrbs/Ednrbs males. However, the observation of aganglionosis in all females failing to present with signs of distress or distension indicates that there is not a 1:1 correlation between aganglionosis and clinical presentation and suggests that our understanding of HSCR pathology is still incomplete. It is also unclear what impact these genetic lesions have on the normal function (motility, secretion, blood flow, and mucosal growth) of remaining intrinsic ganglia or their response, e.g., to endocrine factors, and how this may influence gut function.
We have demonstrated that there is no evidence for a significant impact of compound Ret/Ednrb genotypes on epidermal melanocytes or renal development, compared with either hetero- or homozygous-null Ret and Ednrb genotypes in isolation. Thus, pleiotropic activities of these genes are sensitive to interaction with other pathways in a cell-type-dependent manner.
It is not unexpected that mutant alleles of genes involved in the same biochemical system can fail to complement each other (intrinsic noncomplementation) (45, 46). Somewhat less common is the observation that mutant alleles of genes involved in biochemically distinct pathways fail to complement each other (extrinsic noncomplementation) in the genesis of a common phenotype. Intrinsic noncomplementation is common in HSCR, because the ligands of RET and EDNRB are also susceptibility genes. Our data support multiple hypotheses, including the model of intrinsic noncomplementation. Biochemical crosstalk between the G-protein-coupled receptor EPO-R and the receptor tyrosine kinase c-KIT (47) provides a clear precedent for interaction between analogous pathways, suggesting that interaction between RET and EDNRB may potentially define a novel intrinsic complementation mechanism. The tissue-specific nature of this interaction suggests that the cell type in which one chooses to address questions of biochemical interaction between RET and EDNRB is thus critically important.
Supplementary Material
Acknowledgments
We thank the current members of the Chakravarti lab for helpful discussions and comments on this manuscript and V. Pachnis and W. J. Pavan for cDNA fragments used to generate riboprobes for Ret and Ednrb, respectively. This work is supported by National Institutes of Health Grant HD28088 (to A.C.).
Abbreviations
- HSCR
Hirschsprung disease
- EDNRB
endothelin receptor type B
- dpc
days postcoitum
- AchE
acetylcholinesterase
- H&E
hematoxylin/eosin
- ENS
enteric nervous system
- NC
neural crest
- RPE
retinal pigmented epithelium
References
- 1.Amiel J, Attie T, Jan D, Pelet A, Edery P, Bidaud C, Lacombe D, Tam P, Simeoni J, Flori E, et al. Hum Mol Genet. 1996;5:355–357. doi: 10.1093/hmg/5.3.355. [DOI] [PubMed] [Google Scholar]
- 2.Amiel J, Espinosa-Parrilla Y, Steffann J, Gosset P, Pelet A, Prieur M, Boute O, Choiset A, Lacombe D, Philip N, et al. Am J Hum Genet. 2001;69:1370–1377. doi: 10.1086/324342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidaud C, Salomon R, Van Camp G, Pelet A, Attie T, Eng C, Bonduelle M, Amiel J, Nihoul-Fekete C, Willems P J, et al. Eur J Hum Genet. 1997;5:247–251. [PubMed] [Google Scholar]
- 4.Cacheux V, Dastot-Le Moal F, Kaariainen H, Bondurand N, Rintala R, Boissier B, Wilson M, Mowat D, Goossens M. Hum Mol Genet. 2001;10:1503–1510. doi: 10.1093/hmg/10.14.1503. [DOI] [PubMed] [Google Scholar]
- 5.Edery P, Lyonnet S, Mulligan L M, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder B A, Munnich A. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- 6.Edery P, Attie T, Amiel J, Pelet A, Eng C, Hofstra R M, Martelli H, Bidaud C, Munnich A, Lyonnet S. Nat Genet. 1996;12:442–444. doi: 10.1038/ng0496-442. [DOI] [PubMed] [Google Scholar]
- 7.Doray B, Salomon R, Amiel J, Pelet A, Touraine R, Billaud M, Attie T, Bachy B, Munnich A, Lyonnet S. Hum Mol Genet. 1998;7:1449–1452. doi: 10.1093/hmg/7.9.1449. [DOI] [PubMed] [Google Scholar]
- 8.Hofstra R M, Valdenaire O, Arch E, Osinga J, Kroes H, Loffler B M, Hamosh A, Meijers C, Buys C H. Am J Hum Genet. 1999;64:304–308. doi: 10.1086/302184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusafuka T, Puri P. Pediatr Surg Int. 1997;12:19–23. doi: 10.1007/BF01194795. [DOI] [PubMed] [Google Scholar]
- 10.Lyonnet S, Edery P, Mulligan L M, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder B A, Munnich A. C R Acad Sci III. 1994;317:358–362. [PubMed] [Google Scholar]
- 11.Southard-Smith E M, Angrist M, Ellison J S, Agarwala R, Baxevanis A D, Chakravarti A, Pavan W J. Genome Res. 1999;9:215–225. [PubMed] [Google Scholar]
- 12.Pingault V, Bondurand N, Kuhlbrodt K, Goerich D E, Prehu M O, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, et al. Nat Genet. 1998;18:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 13.Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, et al. Nat Genet. 2001;27:369–370. doi: 10.1038/86860. [DOI] [PubMed] [Google Scholar]
- 14.Badner J A, Sieber W K, Garver K L, Chakravarti A. Am J Hum Genet. 1990;46:568–580. [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarti A, Lyonnet S. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Sly W S, Valle D, Beaudet A L, editors. New York: McGraw–Hill; 2000. , Chap. 251. [Google Scholar]
- 16.Baynash A G, Hosoda K, Giaid A, Richardson J A, Emoto N, Hammer R E, Yanagisawa M. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda K, Hammer R E, Richardson J A, Baynash A G, Cheung J C, Giaid A, Yanagisawa M. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 18.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 19.Ceccherini I, Zhang A L, Matera I, Yang G, Devoto M, Romeo G, Cass D T. Hum Mol Genet. 1995;4:2089–2096. doi: 10.1093/hmg/4.11.2089. [DOI] [PubMed] [Google Scholar]
- 20.Gariepy C E, Cass D T, Yanagisawa M. Proc Natl Acad Sci USA. 1996;93:867–872. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metallinos D L, Bowling A T, Rine J. Mamm Genome. 1998;9:426–431. doi: 10.1007/s003359900790. [DOI] [PubMed] [Google Scholar]
- 22.Santschi E M, Purdy A K, Valberg S J, Vrotsos P D, Kaese H, Mickelson J R. Mamm Genome. 1998;9:306–309. doi: 10.1007/s003359900754. [DOI] [PubMed] [Google Scholar]
- 23.Stockhofe-Zurwieden N, Buijs R M, De Jong M. Dtsch Tierarztl Wochenschr. 2001;108:267–269. [PubMed] [Google Scholar]
- 24.Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kaariainen H, et al. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 25.Puffenberger E G, Hosoda K, Washington S S, Nakao K, deWit D, Yanagisawa M, Chakravart A. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 26.McCallion A S, Chakravarti A. Pigment Cell Res. 2001;14:161–169. doi: 10.1034/j.1600-0749.2001.140305.x. [DOI] [PubMed] [Google Scholar]
- 27.Torfs C. The Third International Meeting: Hirschsprung Disease and Related Neurocristophathies. France: Evian; 1998. [Google Scholar]
- 28.Carrasquillo M, McCallion A S, Puffenberger E G, Kashuk C S, Nouri N, Chakravarti A. Nat Genet. 2002;32:237–244. doi: 10.1038/ng998. [DOI] [PubMed] [Google Scholar]
- 29.Bielschowsky M, Schofield G C. Proc Univ Ortag Med Sch. 1960;38:14–15. [Google Scholar]
- 30.Lane P W. J Hered. 1966;57:29–31. doi: 10.1093/oxfordjournals.jhered.a107457. [DOI] [PubMed] [Google Scholar]
- 31.Enomoto H, Crawford P A, Gorodinsky A, Heuckeroth R O, Johnson E M, Milbrandt J. Development (Cambridge, UK) 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- 32.Rhim H, Dunn K J, Aronzon A, Mac S, Cheng M, Lamoreux M L, Tilghman S M, Pavan W J. Genome Res. 2000;10:17–29. [PubMed] [Google Scholar]
- 33.Wilkinson D G, Nieto M A. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- 34.McCallion A S, Chakravarti A. Inborn Errors of Development. San Francisco: Oxford Univ. Press; 2002. [Google Scholar]
- 35.Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Dai Y, Nakayama S, Toriyama K, Tamada Y, Takahashi M, Nakashima I. Oncogene. 2001;20:7536–7541. doi: 10.1038/sj.onc.1204918. [DOI] [PubMed] [Google Scholar]
- 36.Moore M W, Klein R D, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt L F, Ryan A M, Carver-Moore K, Rosenthal A. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 37.Pichel J G, Shen L, Sheng H Z, Granholm A C, Drago J, Grinberg A, Lee E J, Huang S P, Saarma M, Hoffer, et al. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez M P, Silos-Santiago I, Frisen J, He B, Lira S A, Barbacid M. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 39.Schuchardt A, D'Agati V, Pachnis V, Costantini F. Development (Cambridge, UK) 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- 40.Brand M, Le Moullec J M, Corvol P, Gasc J M. J Clin Invest. 1998;101:549–559. doi: 10.1172/JCI524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auricchio A, Griseri P, Carpentieri M L, Betsos N, Staiano A, Tozzi A, Priolo M, Thompson H, Bocciardi R, Romeo G, et al. Am J Hum Genet. 1999;64:1216–1221. doi: 10.1086/302329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson P J, Anvret M, Molander M L, Nordenskjold A. Hum Genet. 1998;103:145–148. doi: 10.1007/s004390050797. [DOI] [PubMed] [Google Scholar]
- 43.Bolk S, Pelet A, Hofstra R M, Angrist M, Salomon R, Croaker D, Buys C H, Lyonnet S, Chakravarti A. Proc Natl Acad Sci USA. 2000;97:268–273. doi: 10.1073/pnas.97.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriel S B, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, Attie-Bitach T, Olson J M, Hofstra R, Buys C, et al. Nat Genet. 2002;31:89–93. doi: 10.1038/ng868. [DOI] [PubMed] [Google Scholar]
- 45.Hartman J L, Garvik B, Hartwell L. Science. 2001;291:1001–1004. doi: 10.1126/science.291.5506.1001. [DOI] [PubMed] [Google Scholar]
- 46.Kacser H, Burns J A. Genetics. 1980;97:639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu H, Klingmuller U, Acurio A, Hsiao J G, Lodish H F. Proc Natl Acad Sci USA. 1997;94:1806–1810. doi: 10.1073/pnas.94.5.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.