Abstract
Nonthymic epithelial cells were compared with thymic epithelial cells for their role in T cell repertoire selection. Tetraparental aggregation chimeras were generated from T and B cell-deficient mice (H-2d SCID or H-2b Rag−/−) and thymus-deficient nude mice (H-2b or H-2d). These tetraparental mice showed primary protective CD8+ T cell responses, after lymphocytic choriomeningitis virus infection, that were peptide-specifically restricted to either thymic or nonthymic epithelial MHC at comparable levels. These chimeras also mounted neutralizing IgG responses dependent on cognate CD4+ T helper cell activity restricted to nonthymic epithelial MHC. Therefore, in contrast to earlier results with irradiation or thymus chimeras, these relatively undisturbed tetraparental mice reveal that the MHC of nonthymic epithelial cells efficiently selects a functional T cell repertoire.
It is well established that the thymus is essential for T cell receptor (TCR) rearrangement and T cell maturation (1). It is also widely accepted that the MHC of radio-resistant cells of the thymus (presumably thymic epithelial cells) selects the T cell repertoire. This conclusion is based on a series of classical irradiation bone marrow and thymus chimera experiments (reviewed in refs. 2 and 3). Several groups have shown that (H-2a × H-2b)F1-bone marrow cells reconstituting lethally irradiated parental (H-2a)-mice generate H-2a-restricted but virtually no H-2b-restricted virus-specific cytotoxic T lymphocytes (CTL) in a primary immune response (2, 3). Although this view has since been accepted by most immunologists and included in textbooks, exceptions have been reported (4–9). However, examples of such nonthymic epithelial MHC-restricted T cells have been rare, have not been defined at the peptide level, and have usually reflected weak responses that needed priming and several rounds of in vitro restimulation before they could be measured. Also, injection of allogeneic fibroblasts or thymic epithelial cells into the thymus of mice (10–12) and fetal thymic organ culture experiments (13) indicated positive selection of thymocytes restricted to the MHC of these (assumed to be exclusively thymic) cells. Surprisingly, experiments with nude mice reconstituted with a completely allogeneic day-14 fetal thymus graft demonstrated that the T cell repertoire was almost exclusively specific for the recipient MHC haplotype (14).
Therefore, to clarify the respective roles of the MHC of thymic epithelial versus nonthymic epithelial cells in T cell repertoire selection, we have generated tetraparental aggregation chimeras from T and B cell-deficient mice (H-2d SCID or H-2b Rag−/−) and thymus-deficient nude mice (H-2b or H-2d). In the resulting SCIDd ↔ nudeb tetraparental mice, thymic epithelial cells are exclusively of SCID H-2d haplotype, and T and B cells exclusively of nude H-2b haplotype. In the Rag−/−b ↔ nuded tetraparental chimeras, thymic epithelial cells are exclusively of Rag−/− H-2b haplotype, and T and B cells exclusively of nude H-2d haplotype. These relatively undisturbed (i.e., nonirradiated and nonreconstituted) and well mixed tetraparental adult chimeras allow a unique opportunity to study whether, during a primary immune response, the T cell repertoire is restricted exclusively to the MHC of thymic epithelial cells or to both parental haplotypes. Moreover, we analyzed whether these mice could clear lymphocytic choriomeningitis virus (LCMV) as efficiently as control mice and whether a protective CD4+ T helper cell-dependent antibody response was present against LCMV or vesicular stomatitis virus (VSV). Because B cells in these tetraparental mice express nonthymic epithelial MHC (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org), a protective CD4+ T helper cell-dependent antibody response would be direct evidence for the presence of functional effector T cells restricted to nonthymic epithelial MHC.
Materials and Methods
Mice.
C57BL/6 (H-2b), BALB/c (H-2d), C57BL/6-Rag1−/−, and C57BL/6-Rag2−/− (Rag−/−b) were obtained from the Institute of Laboratory Animal Science, University of Zurich. C57BL/6-nude (nudeb) and BALB/c-nude (nuded) were purchased from Biological Research Laboratories, Füllinsdorf, Switzerland. C.B-17-SCID (SCIDd) mice were purchased from IFFA CREDO, L'Arbresle, France. All mice were kept under specific pathogen-free (SPF) conditions.
Aggregation Chimeras.
Mouse chimeras were generated by aggregation of eight-cell embryos recovered from genetically immunodeficient mutants: Nuded and SCIDd (both albino, Gpi-1a and H-2d); nudeb and Rag−/−b (all black, Gpi-1b and H-2b). Embryos of each strain were obtained by mating homozygous unreconstituted parents. Females were superovulated according to standard procedures with 5 units of PMSG/hCG (pregnant mare serum gonadotropin/human chorionic gonadotropin) and additionally stimulated by the Whitten effect to improve their response. On day 3 of gestation, each embryo was flushed from the oviduct and its zona pellucida was removed by brief incubation in pronase solution. After washing, embryos were transferred in drops of M16 culture medium under liquid paraffin. Double-embryo aggregates of the following combinations were produced by gently pushing two uncompacted morulae together: SCIDd ↔ nudeb and Rag−/−b ↔ nuded. After 30 h of incubation at 37°C and 5% CO2 in air, most aggregates formed early blastocysts. Morphologically normal embryos were transferred into the uteri of pseudopregnant histocompatible CB6F1 surrogate foster mothers under SPF conditions. Offspring were born after 18 days of gestation, and chimeras were recognized by the presence of albino and pigmented skin patches a few days later. Additionally, several tissues were tested for chimerism by GPI (glucose-6-phosphate isomerase)-isoenzyme gel electrophoresis (15). The contributions from both parental strains were approximately equal, indicating that there was no strong strain-specific selective advantage during embryonic development.
Cell Lines, ELISA, 51Cr-Release Assay, VSV-IND Neutralization Assays, and Virus.
EL-4 (H-2b) and P815 (H-2d) cells were obtained from American Type Culture Collection. The LCMV nucleoprotein-specific ELISA, the 51Cr-release assay, the VSV-IND (VSV Indiana serotype) neutralization assay, LCMV-WE (LCMV strain WE), and VSV-IND have been described (16–21).
Immunohistology.
Thymi were immersed in Hanks' balanced salt solution (HBSS) and snap-frozen, and 5-μm cryostat sections were cut and fixed in acetone for 10 min. Sections were incubated with the following antibodies: rat monoclonals against murine CD4 (YTS 191), CD8 (YTS 169), CD45R/B220 (RA3-6B2) and CD11b (M1/70), biotinylated mouse monoclonal antibodies against murine MHC class I H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), MHC class II IAb (AF6-120.1), and IAd (AMS-32.1), followed by incubation with streptavidin-alkaline phosphatase (all from PharMingen). Primary antibodies were detected by sequential incubation with goat antibodies against species-specific immunoglobulins, followed by alkaline phosphatase-labeled donkey anti-goat antibodies (Jackson ImmunoResearch). Alkaline phosphatase was visualized using naphthol AS-BI (6-bromo-2-hydroxy-3-naphtholic acid 2-methoxy anilide) phosphate and new fuchsin as substrate, yielding a red reaction product. Endogenous alkaline phosphatase was blocked by levamisole. Sections were counterstained with hemalum.
Confocal Fluorescence Microscopy.
Thymic epithelial cells were stained with a polyclonal rabbit anti-cytokeratin antiserum (wide-spectrum screening; dilution 1:1,500; Dako). Primary rabbit antibodies were detected by sequential incubation with affinity-purified, rhodamine-labeled goat anti-rabbit Ig antibodies followed by rhodamine-labeled donkey anti-goat Ig antibodies (dilutions 1:100 in TBS containing 5% normal mouse serum; Jackson ImmunoResearch). MHC class II antigens were revealed with biotinylated mouse anti-IAb antibodies (clone AF6-120.1; dilution 1:200; PharMingen) or biotinylated mouse anti-IAd antibodies (clone AMS-32.1; dilution 1:60; PharMingen) and fluorescein streptavidin (dilution 1:200; Dako). The appropriate primary and secondary reagents were mixed and incubated in three steps of 30 min each; anti-MHC class II antibodies were added twice. Slides were mounted with Dako medium. Images were recorded with a confocal laser scanning system TCS-SP2 (laser technique of Leica, Mannheim, Germany) and processed using openlab software (Improvision, Lexington, MA).
Flow Cytometric Analysis.
Peripheral blood or splenic cells were stained with the following antibodies: anti-CD8Zα-APC (53-6.7), anti-CD8b.2-PE (53-5.8), anti-B220-PE (RA3-6B2), anti-CD11b-PE (M1/70), anti-H-2Dd-FITC (34-2-12), anti-H-2Db-PE (KH95), anti-H-2Kb-biotin (AF6-88.5), streptavidin-PerCP, and streptavidin-APC (all from PharMingen). For double tetramer stains, peripheral blood or splenic cells (7.5 × 105) were stained with equal amounts of APC-labeled LCMV-WE GP33 tetramer (GP33-Db) and PE-labeled LCMV-WE NP118 tetramer (NP118-Ld), and incubated for 20 min at 37°C. One microgram of anti-CD8α-FITC antibody (53-6.7) was added to each sample and incubated for another 20 min at 4°C. All samples were acquired on a FACSCalibur and analyzed using cellquest software (BD Biosciences).
Results
Tetraparental Aggregation Chimeras Show a Well Mixed Chimerism.
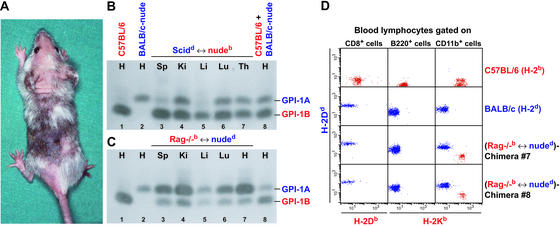
Tetraparental aggregation chimeric mice (chimeras) of the following combinations were generated: SCIDd ↔ nudeb (Fig. 1A) and Rag−/−b ↔ nuded. All chimeras had a well mixed chimerism, shown by the presence of both GPI isoforms (GPI-1A and GPI-1B) in tissue homogenates of spleen, kidney, liver, lung, thymus, and heart (Fig. 1 B and C). Blood lymphocytes of Rag−/−b ↔ nuded chimeras were tested for H-2d and H-2b expression by FACS analysis. Whereas CD8+ and B220+ cells expressed only the parental BALB/c-nude H-2d, CD11b+ macrophages were distributed into two populations expressing either the parental BALB/c-nude H-2d or the parental Rag−/− H-2b (Fig. 1D). Like CD8+ T cells, CD4+ T cells expressed only H-2d (data not shown). Equivalent expression patterns were observed with SCIDd ↔ nudeb chimeras (data not shown). Nonmucosal CD8+ T cells in these chimeras were all CD8αβ as shown by costaining with CD8α- and CD8β-specific antibodies (data not shown).
Figure 1.
Phenotypic analysis of tetraparental chimeric mice. (A) Picture of a 6-week-old SCIDd ↔ nudeb chimera. Distribution of GPI isoforms (GPI-1A and GPI-1B) in different tissues (H, heart; Sp, spleen; Ki, kidney; Li, liver; Lu, lung; Th, thymus) of a SCIDd ↔ nudeb chimera (B, lanes 3–7) and a Rag−/−b ↔ nuded chimera (C, lanes 3–7). As controls, heart preparations of a C57BL/6 (GPI-1B) (B and C, lane 1), BALB/c-nude (GPI-1A) (B and C, lane 2), and a 50:50 mixture of both (B and C, lane 8) were used. The experiment was repeated three times with similar results. (D) Blood FACS analysis of 6- to 8-week-old chimeric and control mice. Blood lymphocytes of naive C57BL/6, BALB/c, and Rag−/−b ↔ nuded chimeras #7 and #8 were gated on CD8+, B220+, or CD11b+ cells and stained with H-2Db-, H-2Kb-, and H-2Dd-specific antibodies. The experiment was repeated five times with similar results.
No Rescue of Nude Thymic Rudiments in Tetraparental Chimeras.
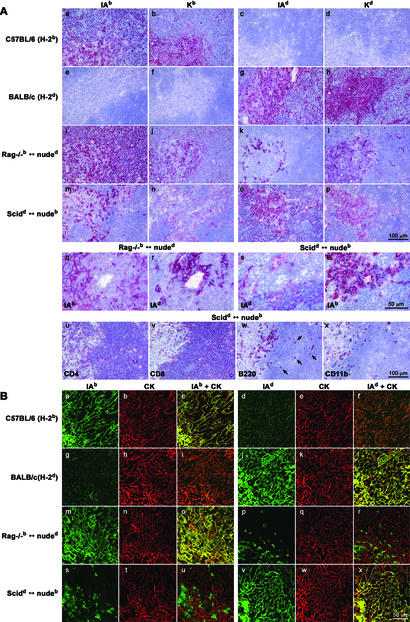
Expression of MHC class I and II, CD4, CD8, B220, and CD11b in chimeric and control thymi was assessed by immunohistological analysis of frozen serial sections (Fig. 2A). Whereas analysis of MHC class I and II expression of the Rag−/− H-2b or SCID H-2d haplotype (representing thymic epithelial haplotype) revealed a typical thymic network (Fig. 2A i–j and q, o–p and s), MHC class I and II expression patterns of the nude haplotype revealed the presence of individual cells rather than this thymic pattern (Fig. 2A k–l and r, m–n and t). The presence of CD4+, CD8+, B220+, and CD11b+ cells in SCIDd ↔ nudeb chimeras was similar to control mice (Fig. 2A u–x; data not shown). Some B220+ cells of C57BL/6-nude or BALB/c-nude origin, respectively, were found in both the thymic medulla and the cortex (arrows in Fig. 2Aw; data not shown).
Figure 2.
Immunohistological (A) and confocal immunofluorescence (B) analysis of the thymus from LCMV-memory chimeras. (A) Frozen thymic sections of LCMV-memory C57BL/6, BALB/c, Rag−/−b ↔ nuded, and SCIDd ↔ nudeb were stained with monoclonal antibodies specific for MHC class I H-2Kb and H-2Kd and MHC class II IAb and IAd (a–t). The thymus of (SCIDd ↔ nudeb) chimera was additionally stained with monoclonal antibodies specific for CD4, CD8, B220, and CD11b (u–x). Arrows in w indicate B220+ cells in the thymic cortex. The experiment was repeated three times with similar results. (B) Two-color thymus histology from LCMV-memory C57BL/6, BALB/c, Rag−/−b ↔ nuded, and SCIDd ↔ nudeb. Sections were stained with monoclonal antibodies specific for MHC class II IAb (a, g, m, and s, green) and cytokeratin (CK) (b, h, n, and t, red; overlay in c, i, o, and u) or MHC class II IAd (d, j, p, and v, green) and CK (e, k, q, and w, red; overlay in f, l, r, and x). Nonepithelial cells of hematopoietic nude origin are CK-negative, MHC class II-positive (r and u, green). Cells double positive for MHC class II (green) and CK (red) stain in yellow (c, l, o, and x). The experiment was repeated three times with similar results.
To exclude potential rescue of nude thymic rudiments (22–26) and confirm that mature thymic epithelial cells in these chimeras express the Rag−/− or SCID-haplotype exclusively, but not the nude haplotype, two-color thymus histology was performed (Fig. 2B). Chimeras and control mice were analyzed for expression of MHC class II and cytokeratin (CK), the latter being a characteristic marker for epithelial cells. Sections of chimeras and control mice revealed an intense yellow stain when thymic epithelial MHC (Rag−/− or SCID-haplotype for chimeras) and cytokeratin stains were superimposed, showing that both markers coincided on the thymic epithelial network (Fig. 2B c and o, l and x). When nude MHC and cytokeratin stains were superimposed, cytokeratin-negative, nude MHC class II-positive cells were found (Fig. 2B r and u, green). Therefore, these cells must represent nonthymic epithelial cells of hematopoietic nude origin that have migrated into the thymus. In summary, in all chimeras tested we found no evidence that nude thymic rudiments were rescued to become mature thymic epithelial cells.
Chimeras Mount Primary Protective Virus-Specific CD8+ T Cell Responses Restricted to both Thymic and Nonthymic Epithelial MHC.
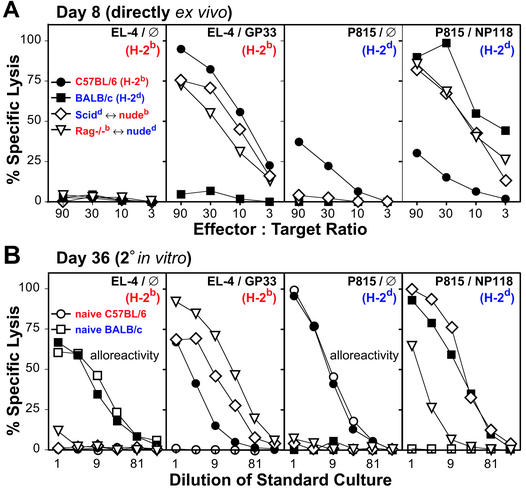
The effector function of T cells of nude origin maturing in a thymic environment composed of epithelial cells expressing nonmatching MHC molecules was evaluated during an immune response against LCMV. Chimeras aged 6–8 weeks showing comparable furry/nude and pigmented/albino skin patches (Fig. 1A) were infected intravenously with 200 plaque-forming units (pfu) of LCMV-WE. Eight days later, mice were hemisplenectomized and cytotoxic CD8+ T cell activity was measured directly ex vivo in a 5-h 51Cr-release assay on H-2b (EL-4) and H-2d (P815) target cells prepulsed with the immunodominant LCMV peptides LCMV-GP33–41 (H-2Db) and LCMV-NP118–126 (H-2Ld) (Fig. 3A) or the subdominant LCMV peptide LCMV-NP396–404 (H-2Db) (data not shown). The chimeras exhibited strong primary CTL activity specific for all three peptides tested. The CTL responses observed in chimeras were similar to those of LCMV-infected C57BL/6 or BALB/c mice (Fig. 3A). Chimeras were efficiently protected against viral infection, as indicated by the absence of detectable virus in spleen and other organs 8 days postinfection (data not shown).
Figure 3.
Primary ex vivo and secondary in vitro CTL response of LCMV-infected chimeras. Eight-week-old C57BL/6 (●), BALB/c (■), SCIDd ↔ nudeb (◊), and Rag−/−b ↔ nuded (▿) were infected intravenously with 200 pfu of LCMV-WE. (A) On day 8 postinfection, mice were hemisplenectomized and single-cell suspensions were tested directly ex vivo for 5 h in a standard 51Cr-release assay on LCMV-GP33-loaded (EL-4/GP33) or control (EL-4/∅) EL-4 targets (H-2b) and on LCMV-NP118-loaded (P815/NP118) or control (P815/∅) P815 targets (H-2d). (B) On day 36 postinfection, mice were killed and pooled lymph node cells were restimulated in vitro for 5 days with LCMV-GP33-loaded irradiated C57BL/6 splenocytes or LCMV-NP118-loaded irradiated BALB/c splenocytes as stimulator cells. Cultures were tested for 5 h in a standard 51Cr-release assay on LCMV-GP33-loaded (EL-4/GP33) or control (EL-4/∅) EL-4 targets and on LCMV-NP118-loaded (P815/NP118) or control (P815/∅) P815 targets. Similar results were obtained by using restimulated splenocytes as effectors (data not shown). Because of alloreactivity, C57BL/6 effectors lysed equally well peptide-loaded and control P815 targets. The same was true for BALB/c effectors with peptide-loaded or control EL-4 targets. Therefore, results of C57BL/6, or BALB/c effectors, with NP118-loaded P815 targets, or GP33-loaded EL-4 targets, are omitted for clarity. The experiment was repeated six times with similar results.
On day 36 postinfection, lymph node cells from the hemisplenectomized mice were restimulated in vitro for 5 days with peptide-labeled H-2d or H-2b spleen cells (Fig. 3B). The strong CTL activity of chimeric lymph node cells was comparably restricted to both thymic and nonthymic MHC (Fig. 3B). As expected, no alloreactivity against chimeric MHC was seen (Fig. 3B). In contrast, in a standard mixed lymphocyte culture assay, alloreactivity to third-party H-2k was found for control and chimeric effector cells (data not shown).
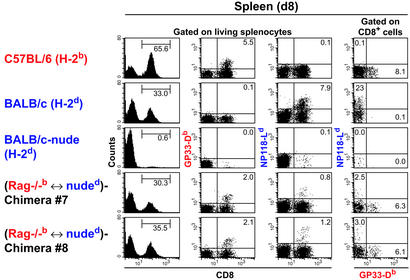
To characterize the CD8+ T cell repertoire in more detail, day-8 and -13 effector T cells were also analyzed at the receptor level by using tetramer staining (Fig. 4 and data not shown for day 13). Chimeras with strong primary CTL activity restricted to each of the parental H-2 haplotypes always showed two distinct effector T cell populations, which either bound LCMV-GP33 (H-2Db) tetramer or LCMV-NP118 (H-2Ld) tetramer, but not both (Fig. 4, columns 2–4, and data not shown). Whereas LCMV-NP118 (H-2Ld) tetramer bindings on day 8 were lower in chimeras than in BALB/c controls (1% in chimeras versus 8% in BALB/c controls; Fig. 4, third column), by day 13, LCMV-NP118 (H-2Ld) tetramer bindings were comparable [8–10% in chimeras versus 7% in F1 (B6×BALB/c) controls versus 16% in BALB/c controls; FACS data not shown].
Figure 4.
Tetramer analysis of LCMV-immune T cells of tetraparental chimeras. Eight-week-old C57BL/6, BALB/c, BALB/c-nude, and Rag−/−b ↔ nuded chimeras #7 and #8 were infected with 200 pfu of LCMV-WE intravenously. Eight days postinfection, mice were hemisplenectomized and 7.5 × 105 splenocytes were tested for binding to LCMV-GP33 tetramer (GP33-Db) and LCMV-NP118 tetramer (NP118-Ld). Histogram plots show the percentage of CD8+ cells amongst living splenocytes (first column). Dot plots gated on living splenocytes show double staining with a CD8-specific antibody and GP33-Db (second column) or double staining with a CD8-specific antibody and NP118-Ld (third column). Numbers in upper right quadrants represent percentage of tetramer and CD8 double-positive splenocytes. Dot plots gated on CD8+ splenocytes show staining with equal amounts of GP33-Db and NP118-Ld (fourth column). Numbers in upper left and lower right quadrants represent percentage of CD8+ splenocytes binding to either NP118-Ld or to GP33-Db, respectively. The experiment was repeated three times with similar results.
Nonthymic Epithelial MHC-Restricted CD4+ T Cells Cooperate Efficiently with B Cells and Mediate Protective IgG Responses.
Because B cells of tetraparental chimeras express MHC class II of the nonthymic haplotype, cooperation is only possible when CD4+ T cells are restricted to nonthymic MHC (see Table 2). Therefore, to assess whether CD4+ T cells restricted to nonthymic MHC are present and functional in these chimeras, VSV-neutralizing IgG or LCMV-NP-specific IgG titers [which have both been shown previously to be strictly dependent on cognate MHC class II-restricted CD4+ T helper cell activity (27, 28)] were monitored after VSV or LCMV infection, respectively. VSV-infected chimeras generated protective neutralizing IgG responses (Table 1) and LCMV-infected chimeras generated LCMV-NP-specific IgG responses (Fig. 5) comparable to control mice. These normal B cell responses confirm the presence of functional CD4+ T cells restricted to nonthymic epithelial MHC.
Table 1.
Nonthymic epithelial MHC-restricted CD4+ T cells mediate protective B cell IgG responses in tetraparental chimeras after intravenous infection with 2 × 106 pfu VSV-IND
| Mouse* | VSV-IND neutralizing IgG titer† (serum dilution)
|
|
|---|---|---|
| Day 8 | Day 20 | |
| C57BL/6 (H-2b) | 1/40,000 | 1/40,000 |
| BALB/c (H-2d) | 1/80,000 | 1/40,000 |
| Rag−/−b ↔ nuded | 1/80,000 | 1/80,000 |
| Rag−/− (H-2b) | <1/40 | <1/40 |
Rag−/−b ↔ nuded tetraparental chimeras and control mice were infected i.v. with 2 × 106 pfu of VSV-IND.
VSV-IND-neutralizing IgG titers were monitored on days 8 and 20 postinfection. The highest serum dilution that neutralizes 50% of input virus is expressed. At least four individual mice were tested in each group. Values represent averages; variations were always less than one dilution step of two.
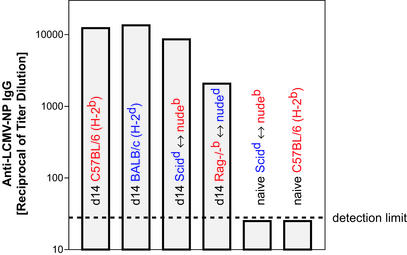
Figure 5.
CD4+ T helper cells cooperate efficiently with B cells of nonthymic H-2 haplotype in LCMV-immune chimeras. Eight-week-old C57BL/6, BALB/c, SCIDd ↔ nudeb, and Rag−/−b ↔ nuded were infected with 200 pfu of LCMV-WE intravenously. On day 14 postinfection, serum was prediluted 30-fold and tested for the presence of LCMV-NP-specific IgG in a standard ELISA. Naive SCIDd ↔ nudeb and naive C57BL/6 were used as negative controls and signals were always below detection level. The same was true for noninfected Rag−/−b ↔ nuded chimeras or day-14 LCMV-infected BALB/c-nude or for C.B-17-SCID or Rag-deficient mice (data not shown). The experiment was repeated three times with similar results.
Discussion
In summary, these chimeras showed protective virus-specific primary CD8+ T cell responses restricted to both thymic and nonthymic MHC at comparable levels. Virus-neutralizing IgG responses, strictly dependent on CD4+ T cell help restricted to nonthymic epithelial MHC, were generated in these chimeras as efficiently as in control mice. Taken together, these results demonstrate that cells other than thymic epithelial cells are efficient in selecting a mature and functional T cell repertoire.
These findings challenge the widely accepted postulate that MHC restriction is determined predominantly by the MHC of thymic epithelial cells (thymic nurse cells) (29) or the radio-resistant portion of the thymus (reviewed in refs. 2 and 3).
The present study was prompted by data obtained from experiments using nude mice reconstituted with day-14 fetal thymus grafts from histoincompatible donors (14, 30). These nude thymus chimeras generated nude MHC-restricted effector T cells, but no thymic MHC-restricted effector T cells. The conclusion from these earlier studies was either that there was rescue of the nude thymic rudiment or that cells other than thymic epithelia were efficient in and essential for positive selection of MHC-restricted T cell specificities. More recently, tetraparental chimeras between thymus-competent and nude donors of distinct MHC haplotypes revealed that the thymic rudiment of the nude donor could not be rescued anatomically to form mature thymic epithelial cells in a tetraparental chimeric situation (25, 26), as we confirm here (Fig. 2). In addition, histological analysis of more than 10 chimeric thymi showed no evidence of mature thymic epithelial cells, cysts, or other rudiments of nude origin in well mixed chimeras.
The discrepancies between the present results and those obtained with F1(A × B) → A-irradiation bone marrow chimeras and/or F1(A × B) nude grafted with an A-thymus yielding virtually exclusively A-restricted but not B-restricted T cell responses are particularly important (2, 31, 32). We believe they are best explained as follows. It is possible that lethal or supralethal irradiation is not capable of eliminating all host cells that contribute to T cell receptor (TCR) interactions resulting in survival of such T cells, regardless of whether they are encountered only in the thymus or also in the periphery. For example, radio-resistant follicular dendritic cells in the spleen and lymph nodes, fibroblasts, or other mesenchymal cells would fulfill such requirements. Also, lymphohemopoietically derived cells, including macrophages and dendritic cells, can probably not be eliminated completely, and current detection limits cannot exclude the presence of 0.5–2% of “contaminating” cells. Therefore, precursor T cells migrating into the thymus of such F1(A × B) → A-irradiation bone marrow chimeras will predominantly see A-expressing cells and therefore will first and preferentially be restricted to A. Those A-restricted T cells will strongly proliferate in the thymus and leave the thymus as A-restricted T cells. B-expressing cells from F1(A × B) bone marrow must first migrate into the thymus, leading to a time disadvantage compared with A-expressing cells already present in the A recipient. Because the proliferation rate in the thymus is enormous, A-restricted T cells will have a huge advantage over B-restricted T cells. For example, the numerical advantage of A-restricted T cells in an A thymus of an F1(A × B) → A-irradiation chimera or in an F1(A × B) nude grafted with an A thymus may readily reach factors of 10–30 (even after a subsequent second depletion of T cells) within three to five cell divisions (corresponding to only 1–3 days) (6, 33, 34).
In the case of nude mice reconstituted with a fully allogeneic fetal thymus (14), precursor T cells migrating into the thymus predominantly encounter thymic epithelial MHC but may not survive thereafter in the periphery (35–37). In contrast, those T cells being selected by nonthymic epithelial MHC on cells from nude origin survive because once they leave the thymus they will contact only cells expressing nonthymic MHC. Our results show that dendritic cells, macrophages, and other host cells of both nude and nonnude MHC are present and infected to induce about equal CTL responses. This finding is compatible with the present view that peripheral amplification and survival of mature T cells are strongly MHC-dependent (35–37).
The advantage of the chimeras described here over F1(A × B) → A-irradiation bone marrow chimeras or F1(A × B) nude grafted with an A thymus is that cells expressing either thymic epithelial or nonthymic epithelial MHC (except T and B cells) are present in the thymus and the periphery in roughly equal numbers from the beginning, i.e., from the time point when precursor T cells migrate into the thymus. Unless one postulates the novel selective influence of the MHC of T and B cells instead of that of thymic epithelial cells, our data indicate that selection and/or MHC-dependent amplification and survival of a mature T cell repertoire restricted to either thymic or nonthymic epithelial MHC is equivalent.
In conclusion, selection of a mature and functional T cell repertoire involves multiple stages: (i) T cell receptor (TCR) rearrangement, followed by (ii) positive selection leading to self-MHC-restricted T cells, and finally (iii) the continuous interaction between MHC-restricted mature T cells and cells expressing the T cell-restricting MHC molecules, ensuring their amplification and survival. Whereas thymic epithelium is crucial for TCR rearrangement, the results presented here show that the MHC of thymic epithelial cells is not required for efficient T cell repertoire selection during the two latter stages.
Supplementary Material
Acknowledgments
We thank E. Horvath, K. Rappold, K. Osei-Boadum, Y. Tan, and N. Wey for technical help, and Drs. A. Macpherson, K. McCoy, and M. van den Broek for helpful comments on the manuscript. This work was supported by the Swiss National Foundation for Science and the Kanton of Zurich.
Abbreviations
- LCMV
lymphocytic choriomeningitis virus
- LCMV-GP
LCMV glycoprotein
- LCMV-NP
LCMV nucleoprotein
- VSV
vesicular stomatitis virus
- CTL
cytotoxic T lymphocyte
- GPI
glucose-6-phosphate isomerase
- pfu
plaque-forming units
References
- 1.Miller J. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 2.Moller G, editor. Immunol Rev. 1978;42:3–270. [Google Scholar]
- 3.von Boehmer H. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P, Mirkwood G. J Exp Med. 1978;148:84–92. doi: 10.1084/jem.148.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty P C, Bennink J C. J Exp Med. 1979;149:150–157. doi: 10.1084/jem.149.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo D L, Schwartz R H. Nature. 1980;287:44–46. doi: 10.1038/287044a0. [DOI] [PubMed] [Google Scholar]
- 7.Wagner H, Rollinghoff M, Rodt H, Thierfelder S. Eur J Immunol. 1980;10:521–525. doi: 10.1002/eji.1830100707. [DOI] [PubMed] [Google Scholar]
- 8.Bix M, Raulet D. Nature. 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 9.Hugo P, Kappler J W, McCormack J E, Marrack P. Proc Natl Acad Sci USA. 1993;90:10335–10339. doi: 10.1073/pnas.90.21.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugo P, Kappler J W, Godfrey D I, Marrack P C. Nature. 1992;360:679–682. doi: 10.1038/360679a0. [DOI] [PubMed] [Google Scholar]
- 11.Pawlowski T, Elliott J D, Loh D Y, Staerz U D. Nature. 1993;364:642–645. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]
- 12.Vukmanovic S, Grandea A G, III, Faas S J, Knowles B B, Bevan M J. Nature. 1992;359:729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahama Y, Suzuki H, Katz K S, Grusby M J, Singer A. Nature. 1994;371:67–70. doi: 10.1038/371067a0. [DOI] [PubMed] [Google Scholar]
- 14.Zinkernagel R M, Althage A, Waterfield E, Kindred B, Welsh R M, Callahan G, Pincetl P. J Exp Med. 1980;151:376–399. doi: 10.1084/jem.151.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eppig J J, Kozak L P, Eicher E M, Stevens L C. Nature. 1977;269:517–518. doi: 10.1038/269517a0. [DOI] [PubMed] [Google Scholar]
- 16.Battegay M, Moskophidis D, Waldner H, Brundler M A, Fung-Leung W P, Mak T W, Hengartner H, Zinkernagel R M. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 17.Speiser D E, Stubi U, Zinkernagel R M. Nature. 1992;355:170–172. doi: 10.1038/355170a0. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann M F, Kundig T M, Kalberer C P, Hengartner H, Zinkernagel R M. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyburz D, Aichele P, Speiser D E, Hengartner H, Zinkernagel R M, Pircher H. Eur J Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 20.Charan S, Zinkernagel R M. J Immunol. 1986;136:3057–3061. [PubMed] [Google Scholar]
- 21.McCaren L C, Holland J J, Syverton J T. J Exp Med. 1959;109:475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holub M, Rossmann P, Tlaskalova H, Vidmarova H. Nature. 1975;256:491–493. doi: 10.1038/256491a0. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C, Whitney R A, Jr, Hansen C T. Nature. 1975;257:681–682. doi: 10.1038/257681a0. [DOI] [PubMed] [Google Scholar]
- 24.Kindred B. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- 25.Blackburn C C, Augustine C L, Li R, Harvey R P, Malin M A, Boyd R L, Miller J F, Morahan G. Proc Natl Acad Sci USA. 1996;93:5742–5746. doi: 10.1073/pnas.93.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodewald H R, Paul S, Haller C, Bluethmann H, Blum C. Nature. 2001;414:763–768. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- 27.Leist T P, Cobbold S P, Waldmann H, Aguet M, Zinkernagel R M. J Immunol. 1987;138:2278–2281. [PubMed] [Google Scholar]
- 28.Ochsenbein A F, Pinschewer D D, Odermatt B, Ciurea A, Hengartner H, Zinkernagel R M. J Immunol. 2000;164:6296–6302. doi: 10.4049/jimmunol.164.12.6296. [DOI] [PubMed] [Google Scholar]
- 29.Wekerle H, Ketelsen U P. Nature. 1980;283:402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- 30.Zinkernagel R M, Althage A. Proc Natl Acad Sci USA. 1999;96:8092–8097. doi: 10.1073/pnas.96.14.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer A. J Immunol. 1988;140:2481–2483. [PubMed] [Google Scholar]
- 32.Matzinger P. Immunol Rev. 1993;135:81–117. doi: 10.1111/j.1600-065x.1993.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 33.Zinkernagel R M. J Exp Med. 1982;156:1842–1847. doi: 10.1084/jem.156.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemischka I R, Raulet D H, Mulligan R C. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 35.Rocha B, Vassalli P, Guy-Grand D. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.