Abstract
Analysis of mRNA from multiple sclerosis lesions revealed increased amounts of transcripts for several genes encoding molecules traditionally associated with allergic responses, including prostaglandin D synthase, histamine receptor type 1 (H1R), platelet activating factor receptor, Ig Fc ɛ receptor 1 (FcɛRI), and tryptase. We now demonstrate that, in the animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), mediated by T helper 1 (Th1) T cells, histamine receptor 1 and 2 (H1R and H2R) are present on inflammatory cells in brain lesions. Th1 cells reactive to myelin proteolipid protein expressed more H1R and less H2R than Th2 cells. Pyrilamine, an H1R antagonist, blocked EAE, and the platelet activating factor receptor antagonist CV6209 reduced the severity of EAE. EAE severity was also decreased in mice with disruption of the genes encoding Ig FcγRIII or both FcγRIII and FcɛRI. Prostaglandin D synthase and tryptase transcripts were elevated in EAE brain. Taken together, these data reveal extensive involvement of elements of the immune response associated with allergy in autoimmune demyelination. The pathogenesis of demyelination must now be viewed as encompassing elements of both Th1 responses and “allergic” responses.
Multiple sclerosis (MS) and its animal model, originally called experimental allergic encephalomyelitis, a name later changed to experimental autoimmune encephalomyelitis (EAE; refs. 1–3), are generally regarded to be mediated by T helper 1 (Th1) T cells (4, 5). We have recently shown that the boundary between allergy and autoimmunity can be blurred: It is possible to induce “horror autotoxicus” with anaphylaxis against certain self antigens, exemplified by myelin peptides (3). Further, Th2 T cells are capable of inducing EAE with features that include eosinophilic inflammation, sometimes also present in MS (6, 7). In addition, it is known that mast cells and other elements that can participate in allergic responses are present in MS lesions (8–11), whereas platelet activating factor and mast cell tryptase are elevated in the spinal fluid during MS relapses (12, 13).
We recently performed large scale sequencing of >11,000 transcripts from libraries derived from MS lesions, as well as gene microarray analyses of transcripts from MS lesions. We reported in these two papers (Table 1) increased levels of prostaglandin D synthase (PGDS), histamine receptor 1 (H1R), platelet activating factor receptor (PAFR), Ig Fc ɛ receptor 1 (FcɛRI), and tryptase III in MS lesions (14, 15). Moreover, we and others have shown that it is possible to ameliorate EAE with drugs that are termed “antihistamines,” but that block serotonin receptors and muscarinic cholinergic receptors, as well as histamine receptors (3, 16, 17).
Table 1.
Genes related to allergy up-regulated in MS
We report here strong evidence for roles for H1R, PAFR, and Ig Fc receptors in autoimmune demyelination. Specific pharmacological targeting of H1R and the PAFR, receptors for the main mediators of murine anaphylaxis, resulted in amelioration of EAE. Mice with deletions of the Ig Fc γ receptor III (FcγRIII), and of both this receptor and FcɛRI, develop attenuated EAE. H1R is elevated on Th1 T cell lines (TCL) causing EAE. Responses to self that include many elements of classical “allergic” responses thus seem to play a pathogenic role in EAE, and these elements therefore represent a previously uncharacterized collection of potential targets for treatment of MS.
Materials and Methods
FcγRIII and FcR γ Chain-Knockout Mice.
The production of mice with targeted mutations that result in failure of production of the α chain of the FcγRIII (FcγRIII−/− mice; ref. 18) or the FcR γ chain (FcR γ chain −/− mice; ref. 19), and many of the phenotypic characteristics of these mice, have been described in detail. For these studies, we used 8- to 12-wk-old female FcγRIII−/− mice that were backcrossed for six generations with C57BL/6 mice, and used C57BL/6 mice as FcγRIII+/+ mice. Female FcR γ chain −/− and +/+ mice were generated by breeding the F2 offspring of crosses between chimeras and C57BL/6 mice (15, 19, 20). All these mice were purchased from The Jackson Laboratory.
Immunization Protocol.
EAE was induced with myelin proteolipid protein (PLP) 139–151 in 8- to 12-wk-old SJL mice (The Jackson Laboratory) as described (3). Mice were assessed daily for clinical signs of EAE (3). For each mouse, a remission was defined as decrease of the score of at least one point for at least 2 consecutive days. For RNA extraction and transcription analysis, animals were killed at different time points during the course of EAE, and brains and spinal cords were removed and kept frozen at −80°C until use. In the pharmacological studies, the H1R antagonist pyrilamine (Sigma) and the PAF antagonist CV6209 (Biomol, Plymouth Meeting, PA) were injected daily i.p. in PBS starting 2 days after the induction of EAE. In FcγRIII−/− and FcγRIII+/+, and in FcR γ chain −/− and +/+ mice, EAE was induced with myelin oligodendrocyte glycoprotein (MOG) 35–55 as described (15). For each mouse with EAE, a complete remission was defined as absence of disease for at least 2 consecutive days. Blood was collected from the tail 6 wk after the immunization and analyzed for antibody responses. Mice were challenged with i.p. injection of 0.1 mg of MOG 35–55 6 wk after the induction of EAE, and the presence of anaphylactic reactions was evaluated by measurement of body temperature with a rectal probe (Physitemp, Clifton, NJ; ref. 3). All animal protocols were approved by the Institutional Animal Care and Use Committee and the Division of Laboratory Medicine at Stanford, in conformance with National Institutes of Health guidelines.
Th1 and Th2 T Cell Lines to PLP 139–151.
Th1 and Th2 T cell lines (TCL) were obtained as previously described by us (21). For quantitative PCR analysis, TCL were harvested 1 wk after stimulation with γ-irradiated spleen cells and PLP 139–151. RNA was isolated by using a Stratagene microRNA isolation kit according to the manufacturer's instructions.
Sample Preparation.
Brain and spinal cord were homogenized in Trizol solution (Invitrogen), and RNA was isolated according to the manufacturer's instructions under RNase-free conditions. RNA was resuspended in 200–500 μl of diethyl pyrocarbonate (DEPC) treated water and stored at −80°C until use. RNA was reverse transcribed to cDNA by using Superscript II reverse transcriptase (Invitrogen). Briefly, 3 μg of RNA was mixed with reaction mix (final concentration: 1× first strand buffer, 0.5 mM each dNTP, 100 ng of random hexamer, 0.01 M DTT and DEPC-treated water to 20 μl; Invitrogen). After a 5-min incubation at 65°C followed by chilling on ice, 200 units of Superscript II was added, and the mixture was incubated at 25°C for 10 min, 42°C for 50 min, and 70°C for 15 min. cDNA was stored at −20°C until use.
Quantitative PCR.
Expression levels of target genes were analyzed by quantitative PCR using a Lightcycler (Roche). Primer sequences are shown in Table 2. Primers for multiexon genes were designed to span introns and be RNA specific [mouse mast cell protease 7 (MMCP-7), PGDS, and actin]. To ensure RNA specificity, the primers were optimized on template from reverse transcriptase reactions with or without reverse transcriptase enzyme (data not shown). The primers were used as follows: 1 μl cDNA from the Superscript II reaction was mixed with a final concentration of 1× Quantitect SYBR green reagent (Qiagen, Valencia, CA), 1 μM forward primer, 1 μM reverse primer, and diethyl pyrocarbonate-treated water in a total volume of 20 μl. The PCR conditions for H1R, H2R, PGDS, MMCP-7, and PAFR were as follows: activation at 95°C for 15 s followed by 60 cycles of 94°C for 15 s, 54°C for 20 s, and 72°C for 19 s. A melting curve of the PCR product was obtained by heating at 65°C for 15 s, then increasing to 95°C at a rate of 0.1°C/s while recording SYBR green fluorescence. The PCR conditions for actin differed in that the annealing temperature was 55°C and the extension time was 12 s. Quantification was performed by using the relative standard curve method (22).
Table 2.
Primer sequences for quantitative PCR
| Gene | Accession no. | Primer | Sequence |
|---|---|---|---|
| β-actin | X03672 | F | GAACCCTAAGGCCAACGCT |
| R | CACGCACGATTTCCCTCTC | ||
| H1R | AF387892 | F | TTGAACCGAGAGCGGA |
| R | TGCCCTTAGGAACGAAT | ||
| H2R | NM_008286 | F | TGGCACGGTTCATTCC |
| R | GCAGTAGCGGTCCAAG | ||
| PAFR | AF004858 | F | CTACAACGAGGGCGAC |
| R | GGGACAAAGAGATGCCA | ||
| PDGS | D88329 | F | CTGGTTCCGGGAGAAG |
| R | AGCGTACTCGTCATAGTT | ||
| MMCP-7 | L00653 | F | ACACGAGAAGGCATTG |
| R | AGGTACTGCTTACGGAG |
F, forward; R, reverse.
Measurement of Serum Ig Responses.
Peptide-specific IgG1 and IgG2a antibodies were measured in mouse serum samples by ELISA as described (23). Briefly, for IgG1 and IgG2a ELISA, 96-well microtiter plates (Nunc MaxiSorp) were coated overnight at 4°C with 0.1 ml of MOG 35–55 diluted in 0.1 M NaHCO3 buffer (pH 9.5) at a concentration of 0.010 mg/ml. The plates were blocked with PBS/3% BSA for 2 h. Samples were diluted in blocking buffer at 1:100 for IgG1 and IgG2a ELISA and incubated for 2 h at room temperature. Antibody binding was tested by the addition of alkaline phosphatase-conjugated monoclonal goat anti-mouse IgG1 and IgG2a (Southern Biotechnology Associates), each at 1:1000 dilution in blocking buffer. Enzyme substrate was added, and plates were read at 405 nm on a micro plate reader. Total IgE was measured by sandwich ELISA (PharMingen) following the manufacturer's instructions (24).
Pathological Studies.
For histological evaluation of EAE in the different knockout mice, three to seven animals per group were killed 6 wk after the induction of EAE, and brain and spinal cord were removed and fixed in 10% formalin. Four-micrometer to 6-μm sections were prepared from paraffin-embedded tissues and analyzed as described (14) for inflammatory lesions after hematoxylin and eosin staining by an observer (R.A.S.) unaware of the identity of individual sections.
For histamine receptor detection in EAE brains, immunohistochemistry was performed as described with rabbit polyclonal antibodies (Rockland, Gilbertsville, PA) generated against the extracellular domain (amino-terminal) peptides of H1R (SSASEDKMCEGN) and H2R (SCCLDSIALKVT). After mice were killed and perfused with cold PBS, tissues were embedded in OCT and quick-frozen. Four-micrometer to 6-μm cryostat sections were fixed with acetone. Staining with anti-H1R and –H2R antibodies at a 1:500 dilution was performed as described (14) by using avidin-biotin immunoperoxidase reagents (Vector Laboratories). Sections were counterstained with hematoxylin.
Results
Transcriptional Profiles of Allergy-Related Genes in the Central Nervous System of Mice with EAE.
To assess whether we could use the animal model of MS, EAE, to understand the pathobiology of the proteins encoded by allergy related genes whose transcripts were elevated in the human MS samples as previously reported, we first analyzed the transcription profiles of these genes in brain and spinal cord of mice with EAE. A relapsing-remitting model of EAE was induced in SJL mice (H-2s) with PLP 139–151 in complete Freund's adjuvant (CFA), and animals were scored daily for clinical signs of disease (3). Brain and spinal cord were removed during the acute phase, remissions, or relapses of EAE, and RNA was extracted and analyzed by real time quantitative PCR (25, 26). MMCP-7, PAFR, and lipocalin-type PGDS were all detected and quantified in these tissues.
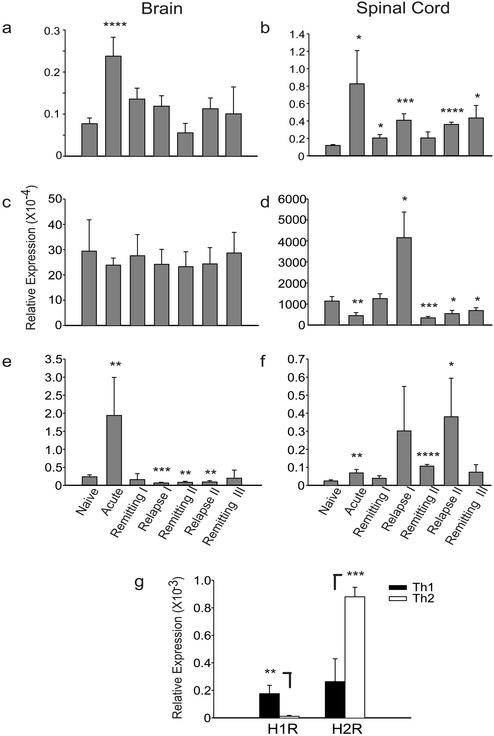
PAF plays a major role in murine anaphylaxis, where, depending on the conditions of immunization and antigen challenge, the role of the IgG1-FcγRIII-macrophage-PAF axis can be more important than that of the IgE-FcɛRI-mast cell-histamine axis (21, 27, 28). (“Axis” here implies a “pathway” involving the named participants.) PAF may also contribute to anaphylaxis in man (27). Moreover, PAF may have a role in MS. In the cerebrospinal fluid and plasma of patients with the relapsing-remitting form of MS, PAF is elevated, and its level correlates with the number of gadolinium MRI-enhancing lesions in the brain (12). Quantitative PCR studies showed that PAFR transcripts were elevated 3- and 6-fold in brain and spinal cord, respectively, in the acute phase of EAE compared with naive mice (P = 0.00006 in brain and P = 0.03 in spinal cord by ANOVA for acute vs. naive), and remained elevated throughout the course of the disease (Fig. 1 a and b). Interestingly, transcripts for PAFR decreased in spinal cord during the remission phase of the disease and increased during the relapsing phase (3-fold in the first relapse, P = 0.005 by ANOVA; 3-fold in the second relapse, P = 0.0001 by ANOVA), suggesting a role for PAFR in the pathogenesis of a relapse.
Figure 1.
Allergy-related gene expression in CNS of mice with EAE and in Th1 and Th2 TCL activated against a myelin peptide. (a–f) EAE was induced with PLP 139–151 in SJL mice; brain and spinal cord were removed at different time points of the disease and analyzed by quantitative PCR. The relative expression of PAFR (a and b), PGDS (c and d), and MMCP-7 (e and f) was quantified by using primers specific for the target (see Materials and Methods) and normalization against β-actin. Means of qPCR values of three to five animals ± SD per time point are represented. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001 by ANOVA vs. naive. (g) The relative expression of H1R and H2R was quantified in a Th1 and Th2 type TCL specific for PLP 139–151. Data are representative of two consecutive experiments. *, P < 0.05, **, P < 0.01 by ANOVA Th1 vs. Th2.
Prostaglandin D2 (PGD2) is a major lipid mediator released from mast cells in the acute phase of allergic reactions and seems to be involved in the regulation of allergic inflammation (29, 30). In the brain, PGD2 is also involved in sleep induction (31). In a murine asthma model, mice transgenic for lipocalin-type PGDS overproduce PGD2, resulting in increased levels of Th2 cytokines and enhanced accumulation of eosinophils and lymphocytes in the lung (29). PGD2 is also preferentially produced by hematopoietic-PGDS in antigen-stimulated human Th2 cells but not Th1 cells (32). Although the expression pattern of l-PGDS does not change in brain tissue from EAE animals, where there is already a high background level due to its pleiotropic functions in brain (Fig. 1c), a significant up-regulation occurs in the spinal cord during the relapse phase (Fig. 1d; 3.6-fold increase in the first relapse compared with naive, P = 0.013 by ANOVA). Accordingly, PGDS may have a role in initiating the relapsing phase of disease.
MMCP-7 is a mouse homologue of human tryptase III (32), which was found to be up-regulated in acute MS plaques (15). Tryptase has also been shown to be elevated in cerebrospinal fluid of patients with MS (13). MMCP-7 is predominantly expressed by mast cells (33, 34). In V3 mice with mastocytosis, after sensitization with IgE and subsequent challenge with antigen, MMCP-7 may contribute to anaphylaxis (35). MMCP-7 is significantly up-regulated in brain (8-fold) and spinal cord (3-fold) in the acute phase of EAE (P = 0.009 and P = 0.008 by ANOVA for acute vs. naive in brain and spinal cord, respectively; Fig. 1 e and f). Relapsing animals also showed increased expression of MMCP-7 in the spinal cord (13-fold during the first relapse and 16-fold during the second one; P = 0.08 and P = 0.045 by ANOVA for the first and second relapse, respectively, vs. naive). At least one in vivo substrate of MMCP-7 is believed to be fibrinogen (36). Perivascular fibrinogen/fibrin deposits are found in EAE and inflammatory MS lesions (37, 38). Interestingly, dermatan sulfate and batroxobin, which degrade fibrinogen and suppress fibrin deposition, respectively, were shown to ameliorate EAE (39, 40).
Expression of H1R and H2R on Myelin-Specific T Cells.
We explored the expression of some of the genes related to allergy in murine Th1 and Th2 TCL activated against PLP 139–151 (21). Compared with Th2 cells, encephalitogenic Th1 cells showed increased levels of transcripts for H1R (16-fold increase in Th1 vs. Th2; P = 0.009 by ANOVA), whereas Th2 cells showed increased transcripts of H2R (3-fold increase in Th2 vs. Th1; P = 0.004 by ANOVA; Fig. 1b).
Immunohistochemical Detection.
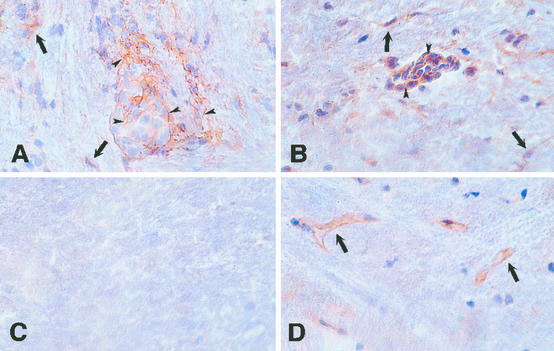
We analyzed the expression of H1R and H2R during EAE by immunohistochemistry, using two polyclonal antibodies generated in rabbits against the extracellular domain of these receptors (see Materials and Methods). In naive SJL mouse brain, H1R and H2R are expressed, as previously described (41–45), on rare astrocytes and on epithelial cells of the choroid plexus, whereas H2R was preferentially expressed on the endothelial cells of the blood vessels. In brains obtained from mice with EAE, H1R and H2R are expressed on the surface of mononuclear and other cells in the lesions (Fig. 2), indicating specific expression of these receptors in the inflammatory EAE infiltrates.
Figure 2.
Expression of H1R and H2R in the CNS of SJL mice with EAE induced with PLP 139–151. Brains were obtained 20 days after disease induction, and cryostat sections were stained with rabbit polyclonal antibodies against H1R and H2R. H1R (A) and H2R (B) are expressed on mononuclear cells (arrowheads) in perivascular inflammatory foci. Parenchymal cells consistent with microglia, astrocytes, and infiltrating inflammatory cells (arrows) are also stained. In brains of naive SJL mice, H1R (C) is not detected, although rare astrocytes and choroid plexus cells were stained (not shown). H2R (D) is expressed on microvascular endothelial cells (arrows). Original magnifications: A and C, ×240; B and D, ×320.
EAE in FcγRIII−/− and FcR γ chain −/− Mice.
We next studied the contribution of different allergy pathways in the development of EAE. MOG peptide 35–55 was used to induce EAE (15) in mice with a disruption of the alpha chain of FcγRIII (FcγRIII−/−; ref. 18) and in mice with disruption of the γ chain common to FcγRIII and FcɛRI (FcR γ chain −/−; ref. 19; see Materials and Methods). We have previously shown that C57BL/6 mice (H-2b) immunized with MOG 35–55 develop anaphylactic shock when reexposed to this self myelin peptide (3). To explore the contribution of the Fc receptors to the development of anaphylaxis, we also challenged these two different strains of knockout mice with 0.1 mg of MOG 35–55 i.p., 6 wk after primary immunization, at a time when anaphylactic reactions to this peptide are known to occur (3).
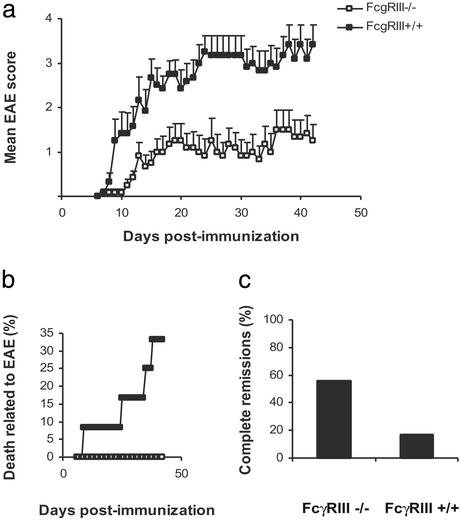
EAE was significantly ameliorated in mice lacking the low affinity IgG1 receptor FcγRIII (Fig. 3). For example, the incidence of EAE (9 of 12 in FcγRIII−/− vs. 12 of 12 in FcγRIII+/+), mean peak of disease at day 15 (0.75 ± 0.25 in FcγRIII−/− vs. 2.67 ± 0.43 in FcγRIII+/+; P = 0.0035 by Mann–Whitney rank sum test), mean peak disease severity (2.42 ± 0.61 in FcγRIII−/− vs. 4.17 ± 0.24 in FcγRIII+/+; P = 0.0055 by t test) and EAE-related death (0 of 12 in FcγRIII−/− vs. 4 of 12 in FcγRIII+/+) were significantly reduced in the knockout mice. The evaluation of the relapse/remission rate showed that the majority of FcγRIII−/− mice had more remissions when compared with the wild-type animals, with the majority of EAE mice presenting periods of complete remission (5 of 9 in FcγRIII−/− vs. 2 of 12 of the FcγRIII+/+). Histopathologic analysis revealed fewer inflammatory foci within the CNS of the knockout mice both in parenchyma and in meninges (4.2 ± 1.9 vs. 14.5 ± 3 in the meninges of FcγRIII−/− vs. FcγRIII+/+, P = 0.0187 by t test; 0.6 ± 0.4 vs. 5.5 ± 1.2 in the parenchyma of FcγRIII−/− vs. FcγRIII+/+, P = 0.0036 by t test), revealing that FcγRIII might be involved both in parenchymal and meningeal infiltration of inflammatory cells. FcγRIII−/− presented a lower incidence of anaphylaxis at challenge with MOG 35–55 compared with wild types (6 of 12 in FcγRIII−/− vs. 7 of 8 in FcγRIII+/+), despite the higher titers of IgG1 and IgE observed in this group (Table 3). Because FcγRIII receptor is necessary for the expression of IgG1-mediated anaphylaxis (20), the presence of anaphylactic shock in mice lacking this receptor suggests that both IgG1 and IgE might mediate anaphylaxis to MOG 35–55. Nevertheless, together with an impairment of other immune processes (18), the abrogation of IgG1-mediated anaphylaxis is correlated with relative resistance to EAE in FcγRIII mice.
Figure 3.
Amelioration of EAE in FcγRIII-deficient mice. EAE was induced in FcγRIII−/− (n = 12) and FcγRIII+/+ (n = 12) with MOG 35–55. FcγRIII−/− mice have a significantly milder disease compared with FcγRIII+/+ mice (a, data are shown as mean ± SEM), and they are protected from EAE-related death (b, 0 of 12 in the FcγRIII−/− mice vs. 4 of 12 in the FcγRIII+/+). (c) EAE is more remitting in FcγRIII−/− mice, with 56% (5 of 9) presenting periods of complete remissions compared with 17% (2 of 12) of the wild-type mice.
Table 3.
Serum antibody responses and allergic reactions to MOG 35–55 in FcγRIII−/−, in FcRγ chain −/− and their controls
| Strain | Antibody responses*
|
No. of mice with allergic reactions at challenge† | ||
|---|---|---|---|---|
| IgG1, OD405 | IgG2a, OD405 | Total IgE, μg/ml | ||
| FcγRIII−/− | 1.094 ± 0.473 | 0.123 ± 0.053 | 4.2 ± 0.23 | 6/12 |
| FcγRIII+/+ | 0.259 ± 0.047 | 0.048 ± 0.021 | 1.45 ± 0.47 | 7/8 |
| FcR γ chain −/− | 1.343 ± 0.506 | 0.140 ± 0.074 | 4.1 ± 0.17 | 0/11 |
| FcR γ chain +/+ | 0.434 ± 0.179 | 0.071 ± 0.022 | 3.45 ± 0.51 | 5/9 |
Serum from individual mice (5–12 per group) was collected 6 wk after the induction of EAE and tested individually by ELISA. Numbers represent mean ± SEM.
Challenge was 6 wk after the induction of EAE with MOG 35–55 (0.1 mg) in PBS i.p., and presence of allergic reactions was confirmed by a reduction in body temperature of at least 0.5°C (see Materials and Methods).
As previously shown by us and others (15, 46), amelioration of EAE was even more striking in mice lacking both FcγRIII and FcɛRI (FcR γ chain −/−; Table 4). In these mice, incidence of EAE (5 of 11 in FcR γ chain −/− vs. 12 of 12 in +/+; P = 0.0046 by Fisher's exact test), mean disease severity at day 16 (0.64 ± 0.39 in FcR γ chain −/− vs. 2.42 ± 0.4 in +/+; P = 0.005 by Mann–Whitney rank sum test) and mean peak of disease severity (1.18 ± 0.49 in FcR γ chain −/− vs. 3.58 ± 0.36 in +/+, P = 0.0017 by Mann–Whitney rank sum test) were significantly reduced. All mice with deletion of these receptors had a remitting course (5 of 5 in FcR γ chain −/− vs. 6 of 12 in +/+). Only two mice with deletion of both FcγRIII and FcɛRI had one relapse, each, during the observation period of 6 wk compared with the wild-type mice where, of the mice surviving the acute phase (10 of 12), all had relapses. Histopathologic analysis revealed a paucity of CNS infiltrates in knockout mice compared with wild-type mice (1.57 ± 1.2 vs. 45 ± 13.6 in the meninges of FcR γ chain −/− vs. +/+, P = 0.0167 by Mann–Whitney rank sum test; 0.29 ± 0.3 vs. 46.3 ± 22.8 in the parenchyma of FcR γ chain −/− vs. +/+, P = 0.0167 by Mann–Whitney rank sum test). Moreover, FcR γ chain −/− mice were completely protected against anaphylactic shock to MOG 35–55 (Table 3), whereas 56% (5 of 9) of wild-type mice had anaphylactic reactions.
Table 4.
EAE in FcR γ chain −/− and +/+ mice
| Strain | Incidence, % | EAE onset, day* | EAE score (day 16)* | Peak disease severity* | Death rate, % | Complete remissions, % |
|---|---|---|---|---|---|---|
| FcR γ chain −/− | 45 (5/11)† | 11 ± 0.5 | 0.64 ± 0.4‡ | 1.18 ± 0.5§ | 0 (0/11) | 100 (5/5) |
| FcR γ chain +/+ | 100 (12/12) | 12.4 ± 0.6 | 2.42 ± 0.4 | 3.58 ± 0.4 | 25 (3/12) | 50 (6/12) |
Data are shown as mean ± SEM values.
P = 0.0046 (Fisher's exact test).
P = 0.005 (Mann–Whitney).
P = 0.0017 (Mann–Whitney). All P values are in comparison with the FcR γ chain +/+ group.
Modulation of EAE with H1R Blockade and PAFR Blockade.
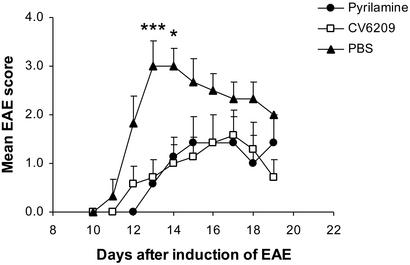
We then tested the functional roles of H1R and PAF in EAE. We targeted pharmacologically PAF and histamine, the main vasoactive mediators of murine anaphylaxis, and evaluated the development of EAE. EAE was induced in SJL (H-2s) mice with PLP 139–151, and, on the second day after the induction of the disease, we started a daily i.p. treatment with the PAFR antagonist CV 6209, or with the pure H1R antagonist pyrilamine. CV 6209 has been previously used to block anaphylaxis in mice (27, 47), whereas cyproheptadine, an anti-H1R, anti-5-HT2 and anti-muscarinic receptor, has been shown by us and others to ameliorate EAE (3, 16, 17). Treatment with either of these drugs ameliorated EAE (on day 13, mean EAE score was 0.57 ± 0.2 in the pyrilamine-treated group, 0.71 ± 0.36 in the CV 6209-treated group, and 3 ± 0.52 in the vehicle-treated group; P = 0.007 and P = 0.0034 by t test for pyrilamine and CV 6209, respectively, vs. vehicle; Fig. 4), suggesting a role for H1R and PAFR in the development of EAE.
Figure 4.
Modulation of EAE with H1R antagonist and PAFR antagonist. EAE was induced in SJL/J mice with PLP 139–151. The H1R antagonist pyrilamine (0.6 mg per mouse; n = 8), PAFR antagonist CV6209 (1 μg per mouse; n = 8), or vehicle alone (PBS; n = 7) were given daily i.p. starting on day 2 after the induction of EAE. *, P < 0.05; ***, P < 0.005 by t test for treatment groups vs. naive.
Discussion
A number of molecules that can play important roles in allergic responses were shown to participate in EAE, a model for Th1-mediated autoimmunity (4). Thus, by large scale transcriptional analysis, we showed increased transcription of H1R, PAFR, tryptase, FcɛRI, and PGDS in MS lesions (14, 15). In the animal model of MS, EAE, transcripts for tryptase, PAFR, and PGDS were elevated in the CNS during the disease. Moreover, H1R was elevated on Th1 cells reactive to myelin, and immunohistochemical staining of EAE brain revealed H1R and H2R expression on inflammatory cells of the infiltrates. EAE was ameliorated in mice with disruptions of the α chain of FcγRIII (FcγRIII−/−) or of the γ chain common to FcγRIII and FcɛRI (FcR γ chain −/−), which have a partial or a complete abrogation of anaphylactic responses. Last, pharmacological blockade of histamine and PAF, the main mediators of murine anaphylaxis, with H1R antagonists and PAFR antagonists significantly blunted EAE. We conclude that, even if Th1 lymphocytes represent major contributors to the pathogenesis of EAE and MS, molecules involved in the allergic response can potently modulate the disease.
Since Rivers' description of experimental allergic encephalomyelitis 70 years ago (48), our concepts of allergy and autoimmunity have been highly dichotomous. However, this distinction has been increasingly blurred as drugs commonly used for the treatment of allergic diseases have been shown to ameliorate EAE (3, 16, 17, 49). Moreover, expression of EAE can be reduced in mast cell deficient mice (50). In addition, allergy to self peptides has been described, and apparently depends on whether or not a self antigen is expressed in the thymus (3). An association between sensitivity to histamine and susceptibility to EAE has also been described (51–53). Bordetella pertussis toxin (PTX), which increases vasoactive amine sensitization (VAAS), is needed as an adjuvant to induce EAE in those strains of mice that are not physiologically very sensitive to histamine (51, 53–55). Bordetella pertussis histamine sensitization (Bphs) is the gene controlling PTX-induced VAAS, and susceptibility to EAE and other autoimmune disease is linked to a susceptible allele for this gene (56–58). Interestingly, Teuscher and colleagues (59) reported recently that Bphs is H1R and that, in mice with disruption of this gene, EAE is reduced. Thus, broad evidence suggests that allergy and Th2 responses modulate immune responses, and that histamine under certain conditions can polarize the immune response toward Th1 (60). Furthermore, histamine and PAF might contribute to facilitate the entry of autoreactive T cells into the CNS by increasing blood–brain barrier permeability (44, 52). Our findings suggest that several components of classical allergic responses also can significantly influence the pathogenesis of autoimmune disease in the EAE model. Allergy-related molecules might represent a rich source of new targets for the treatment of EAE and MS.
Acknowledgments
We thank Karim Dabbagh and Tomoko Shiba for fruitful discussions and Mary Jane Eaton for help with immunohistochemistry. This investigation was supported in part by a postdoctoral fellowship from the National Multiple Sclerosis Society (R.P.) and by support from the National Multiple Sclerosis Society (to R.A.S.), the National Science Foundation (to J.J.D.), the Stanford Graduate Fellowships Program in Science and Engineering (to J.J.D.), the National Institutes of Health (to L.S. and S.J.G.), and the Phil N. Allen Fund (to L.S.).
Abbreviations
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- Th1
T helper 1
- PGDS
prostaglandin D synthase
- H1R
histamine receptor type 1
- PAFR
platelet activating factor receptor
- FcɛRI
Fc ɛ receptor I
- FCγRIII
Fc γ receptor III
- MOG
myelin oligodendrocyte glycoprotein
- PLP
myelin proteolipid protein
- TCL
T cell line
- MMCP
mouse mast cell protease
References
- 1.Steinman L. J Exp Med. 2001;194:F27–F30. doi: 10.1084/jem.194.5.f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman L. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 3.Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab E M, Tsai M, Galli S J, Steinman L. Nat Immunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 5.Steinman L, Martin R, Bernard C C, Conlon P, Oksenberg J R. Annu Rev Neurosci. 2003;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 6.Lafaille J J, Keere F V, Hsu A L, Baron J L, Haas W, Raine C S, Tonegawa S. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladue R P, Carroll L A, Milici A J, Scampoli D N, Stukenbrok H A, Pettipher E R, Salter E D, Contillo L, Showell H J. J Exp Med. 1996;183:1893–1898. doi: 10.1084/jem.183.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson Y. Acta Neurol Scand. 1974;50:611–618. doi: 10.1111/j.1600-0404.1974.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 9.Toms R, Weiner H L, Johnson D. J Neuroimmunol. 1990;30:169–177. doi: 10.1016/0165-5728(90)90101-r. [DOI] [PubMed] [Google Scholar]
- 10.Brenner T, Soffer D, Shalit M, Levi-Schaffer F. J Neurol Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim M Z, Reder A T, Lawand R, Takash W, Sallouh-Khatib S. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 12.Callea L, Arese M, Orlandini A, Bargnani C, Priori A, Bussolino F. J Neuroimmunol. 1999;94:212–221. doi: 10.1016/s0165-5728(98)00246-x. [DOI] [PubMed] [Google Scholar]
- 13.Rozniecki J J, Hauser S L, Stein M, Lincoln R, Theoharides T C. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 14.Chabas D, Baranzini S E, Mitchell D, Bernard C C, Rittling S R, Denhardt D T, Sobel R A, Lock C, Karpuj M, Pedotti R, et al. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 15.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 16.Dietsch G N, Hinrichs D J. J Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- 17.Linthicum D S. Immunobiology. 1982;162:211–220. doi: 10.1016/S0171-2985(11)80001-X. [DOI] [PubMed] [Google Scholar]
- 18.Hazenbos W L, Gessner J E, Hofhuis F M, Kuipers H, Meyer D, Heijnen I A, Schmidt R E, Sandor M, Capel P J, Daeron M, et al. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 19.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 20.Miyajima I, Dombrowicz D, Martin T R, Ravetch J V, Kinet J P, Galli S J. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garren H, Ruiz P J, Watkins T A, Fontoura P, Nguyen L T, Estline E R, Hirschberg D L, Steinman L. Immunity. 2001;15:15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 22.Vincent V A, DeVoss J J, Ryan H S, Murphy G M., Jr J Neurosci Res. 2002;69:578–586. doi: 10.1002/jnr.10329. [DOI] [PubMed] [Google Scholar]
- 23.Slavin A, Ewing C, Liu J, Ichikawa M, Slavin J, Bernard C C. Autoimmunity. 1998;28:109–120. doi: 10.3109/08916939809003872. [DOI] [PubMed] [Google Scholar]
- 24.Spergel J M, Mizoguchi E, Brewer J P, Martin T R, Bhan A K, Geha R S. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentle A, Anastasopoulos F, McBrien N A. Biotechniques. 2001;31:502. doi: 10.2144/01313st03. , 504–506, 508. [DOI] [PubMed] [Google Scholar]
- 26.Rajeevan M S, Vernon S D, Taysavang N, Unger E R. J Mol Diagn. 2001;3:26–31. doi: 10.1016/S1525-1578(10)60646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strait R T, Morris S C, Yang M, Qu X W, Finkelman F D. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 28.Choi I H, Shin Y M, Park J S, Lee M S, Han E H, Chai O H, Im S Y, Ha T Y, Lee H K. J Exp Med. 1998;188:1587–1592. doi: 10.1084/jem.188.9.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. J Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, et al. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 31.Hayaishi O. FASEB J. 1991;5:2575–2581. [PubMed] [Google Scholar]
- 32.Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, Nagata K. J Immunol. 2000;164:2277–2280. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- 33.McNeil H P, Reynolds D S, Schiller V, Ghildyal N, Gurley D S, Austen K F, Stevens R L. Proc Natl Acad Sci USA. 1992;89:11174–11178. doi: 10.1073/pnas.89.23.11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens R L, Friend D S, McNeil H P, Schiller V, Ghildyal N, Austen K F. Proc Natl Acad Sci USA. 1994;91:128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghildyal N, Friend D S, Stevens R L, Austen K F, Huang C, Penrose J F, Sali A, Gurish M F. J Exp Med. 1996;184:1061–1073. doi: 10.1084/jem.184.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Wong G W, Ghildyal N, Gurish M F, Sali A, Matsumoto R, Qiu W T, Stevens R L. J Biol Chem. 1997;272:31885–31893. doi: 10.1074/jbc.272.50.31885. [DOI] [PubMed] [Google Scholar]
- 37.Sobel R A, Schneeberger E E, Colvin R B. Am J Pathol. 1988;131:547–558. [PMC free article] [PubMed] [Google Scholar]
- 38.Sobel R A, Mitchell M E. Am J Pathol. 1989;135:161–168. [PMC free article] [PubMed] [Google Scholar]
- 39.Inaba Y, Ichikawa M, Koh C S, Inoue A, Itoh M, Kyogashima M, Komiyama A. Cell Immunol. 1999;198:96–102. doi: 10.1006/cimm.1999.1588. [DOI] [PubMed] [Google Scholar]
- 40.Inoue A, Koh C S, Shimada K, Yanagisawa N, Yoshimura K. J Neuroimmunol. 1996;71:131–137. doi: 10.1016/s0165-5728(96)00150-6. [DOI] [PubMed] [Google Scholar]
- 41.Fukui H, Inagaki N, Ito S, Kubo A, Kondoh H, Yamatodani A, Wada H. Agents Actions. 1991;33,Suppl.:161–180. doi: 10.1007/978-3-0348-7309-3_12. [DOI] [PubMed] [Google Scholar]
- 42.Arbones L, Picatoste F, Garcia A. Brain Res. 1988;450:144–152. doi: 10.1016/0006-8993(88)91554-5. [DOI] [PubMed] [Google Scholar]
- 43.Hosli L, Hosli E, Schneider U, Wiget W. Neurosci Lett. 1984;48:287–291. doi: 10.1016/0304-3940(84)90052-1. [DOI] [PubMed] [Google Scholar]
- 44.Karlstedt K, Sallmen T, Eriksson K S, Lintunen M, Couraud P O, Joo F, Panula P. J Cereb Blood Flow Metab. 1999;19:321–330. doi: 10.1097/00004647-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Karnushina I L, Palacios J M, Barbin G, Dux E, Joo F, Schwartz J C. J Neurochem. 1980;34:1201–1208. doi: 10.1111/j.1471-4159.1980.tb09960.x. [DOI] [PubMed] [Google Scholar]
- 46.Abdul-Majid K B, Stefferl A, Bourquin C, Lassmann H, Linington C, Olsson T, Kleinau S, Harris R A. Scand J Immunol. 2002;55:70–81. doi: 10.1046/j.1365-3083.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 47.Terashita Z, Imura Y, Takatani M, Tsushima S, Nishikawa K. J Pharmacol Exp Ther. 1987;242:263–268. [PubMed] [Google Scholar]
- 48.Rivers T H, Sprunt D H, Berry G P. J Exp Med. 1933;58:39–53. doi: 10.1084/jem.58.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimitriadou V, Pang X, Theoharides T C. Int J Immunopharmacol. 2000;22:673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 50.Secor V H, Secor W E, Gutekunst C A, Brown M A. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linthicum D S, Munoz J J, Blaskett A. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 52.Bebo B F, Jr, Lee C H, Orr E L, Linthicum D S. Immunol Cell Biol. 1996;74:225–230. doi: 10.1038/icb.1996.41. [DOI] [PubMed] [Google Scholar]
- 53.Linthicum D S, Frelinger J A. J Exp Med. 1982;156:31–40. doi: 10.1084/jem.156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Black W J, Munoz J J, Peacock M G, Schad P A, Cowell J L, Burchall J J, Lim M, Kent A, Steinman L, Falkow S. Science. 1988;240:656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- 55.Teuscher C, Blankenhorn E P, Hickey W F. Cell Immunol. 1987;110:294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 56.Meeker N D, Stafford A N, Lunceford J K, Avner P, Ma R Z, Teuscher C. Mamm Genome. 1999;10:858–863. doi: 10.1007/s003359901104. [DOI] [PubMed] [Google Scholar]
- 57.Blankenhorn E P, Butterfield R J, Rigby R, Cort L, Giambrone D, McDermott P, McEntee K, Solowski N, Meeker N D, Zachary J F, et al. J Immunol. 2000;164:3420–3425. doi: 10.4049/jimmunol.164.6.3420. [DOI] [PubMed] [Google Scholar]
- 58.Teuscher C. Immunogenetics. 1985;22:417–425. doi: 10.1007/BF00418088. [DOI] [PubMed] [Google Scholar]
- 59.Ma R Z, Gao J, Meeker N D, Fillmore P D, Tung K S, Watanabe T, Zachary J F, Offner H, Blankenhorn E P, Teuscher C. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- 60.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet O A, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis C A. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]