Abstract
Signal transducer and activator of transcription 3 (STAT3) is a key transcriptional mediator for many cytokines and is essential for normal embryonic development. We have generated a unique strain of mice with tissue-specific disruption of STAT3 in bone marrow cells during hematopoiesis. This specific STAT3 deletion causes death of these mice within 4–6 weeks after birth with Crohn's disease-like pathogenesis in both the small and large intestine, including segmental inflammatory cell infiltration, ulceration, bowel wall thickening, and granuloma formation. Deletion of STAT3 causes significantly increased cell autonomous proliferation of cells of the myeloid lineage, both in vivo and in vitro. Most importantly, Stat3 deletion during hematopoiesis causes overly pseudoactivated innate immune responses. Although inflammatory cytokines, including tumor necrosis factor α and IFN-γ, are overly produced in these mice, the NAPDH oxidase activity, which is involved in antimicrobial and innate immune responses, is inhibited. The signaling responses to lipopolysaccharide are changed in the absence of STAT3, leading to enhanced NF-κB activation. Our results suggest a model in which STAT3 has critical roles in the development and regulation of innate immunity, and deletion of STAT3 during hematopoiesis results in abnormalities in myeloid cells and causes Crohn's disease-like pathogenesis.
The innate immune system initiates immediate host responses to microbial antigens and acts as an effector by stimulating adaptive immune responses (1). The mucosal immune system in the digestive tract is among the first lines of defense against microbial pathogens, which involve both innate and adaptive immune responses (2). The digestive tract is not normally affected by inflammation despite its exposure to many diverse antigens, indicating that there must be an essential mechanism that can suppress inflammation by enabling the mucosal immune system to tolerate foreign antigens. One important question is how the mucosal immune system in the gastrointestinal tract responds to a variety of antigens and how the decision is made between tolerance and antigen clearance. Disruption of this balance can lead to disorders in the mucosal immune system that cause severe human diseases (3).
For example, in Crohn's disease and several other inflammatory bowel diseases (IBDs), chronic inflammation can occur in parts of the gastrointestinal tract. Regarding molecular etiopathology of Crohn's disease and other IBDs, abnormalities in both innate and adaptive immune responses have been suggested (4, 5). It is likely that the loss of tolerance and uncontrolled responses to microbial antigens in the mucosal immune system is a major cause of Crohn's disease and other IBDs (6). Recently, several groups have shown that the NOD2 gene on chromosome 16 is implicated in Crohn's disease (7–9). NOD2 is a member of a gene family that mediates response to bacterial lipopolysaccharide (LPS) (10). Even more interesting, the NOD2 gene is expressed in monocytes, but not in lymphocytes (11), suggesting specific abnormalities of myeloid cells have decisive functions in the development of Crohn's disease.
Signal transducers and activators of transcription (STATs) are not only mediators of signaling during immune responses, but also have roles in development and cell differentiation (12–16). They reside in the cytoplasm and become tyrosine-phosphorylated by Janus kinases and other protein tyrosine kinases (17, 18). Tyrosine phosphorylation of STATs leads to their dimerization, nuclear translocation, and transcriptional activation (19–22). It is well established that STATs are involved in multiple steps in adaptive immune responses (23, 24). It will be interesting to investigate functions of STATs in controlling the innate immune response.
In this article, we present evidence that STAT3, which is a transcriptional mediator for the IL-6 family cytokines, and many others such as IL-10, epidermal growth factor, and granulocyte–colony-stimulating factor (CSF) (25–28) may have an essential regulatory function in the innate immune system. In particular, STAT3 may play a critical role in the control of mucosal immune tolerance. We generated a unique strain of mice with tissue-specific deletion of STAT3 during hematopoiesis. We found that these mice had phenotypes of dramatic expansion of myeloid lineages, causing massive infiltration of the intestine with neutrophils, macrophages, and eosinophils closely resembling Crohn's disease pathology. This Crohn's disease-like pathogenesis is probably caused by a pseudoactivated innate immune response to LPS as a result of the STAT3 deletion during hematopoiesis. We propose a model that STAT3 mediates mucosal immune tolerance during the innate immune response to microbial antigens.
Methods
Colony Formation Assay.
Bone marrow cells were seeded in 1% methyl-cellulose in Iscove's modified Dulbecco's medium supplemented with 15% FBS, 1% BSA, 10 μg/ml bovine pancreatic insulin, 200 μg/ml human transferrin, 2 mM l-glutamine, 0.1 mM 2-mercaptoethanol, 50 ng/ml recombinant mouse stem cell factor, 10 ng/ml recombinant mouse IL-3, and 10 ng/ml recombinant human IL-6.
Bone Marrow Transfer.
Recipients (B6) were lethally irradiated (1,100 rad) and received 200,000 donor bone marrow cells through tail vein injection. Eight weeks later bone marrow reconstitution was analyzed by a genomic PCR that detects a donor-derived allele (F allele) with the primers described above.
In Vitro Cultures of Macrophages.
Single cell suspensions of spleens were cultured in αMEM and 20 ng/ml CSF-1 as described (30).
Cytometric Bead Array.
To measure cytokine levels, serum samples (50 μl) were added to capture beads (BD Bioscience, San Diego) and mixed with cytokine antibodies (anti-IFN-γ, anti-IL-4, anti-tumor necrosis factor α, and anti-IL-5); phycoerythrin detection reagent was added to the tube. After a 2-h incubation samples were analyzed with fluorescence-activated cell sorting (FACS). Absolute values were obtained by comparisons with standards.
Statistical Analysis.
P values were calculated with a nonpaired Student's t test.
Additional Methods.
For descriptions of other methods used see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Results
A Mouse Strain with a Conditional STAT3 Disruption Developed Gradual Lethality After Birth.
We have generated a STAT3 allele in which exons 18–20 are flanked by loxP sequences (STAT3-F, Fig. 6, which is published as supporting information on the PNAS web site). Removal of exons 18–20, which encode the Src homology 2 domain of STAT3, is expected to eliminate the function of the protein. To study immunological functions of STAT3, we chose here a mouse strain for tissue-specific gene deletion where Cre expression is driven by a TIE2 gene promoter/enhancer cassette (Tie2-Cre, Fig. 6). The TIE2 gene promoter drives Cre expression in bone marrow and endothelial cells (32).
In two steps of breeding STAT3-loxP with Tie2-Cre, we obtained mice that are homozygous for STAT3-loxP (F allele) and Tie2-Cre+ (C allele), which are conditional STAT3 knockout mice and designated as STAT3-CFF. These mice were obtained at the expected Mendelian ratio and appeared normal at birth, indicating the absence of severe embryonic deficiencies of STAT3-CFF mice. However, at 3–4 weeks of age, offspring with the STAT3 deletion were smaller with reduced body weight compared with their siblings. All STAT3-CFF animals appeared fragile and weak by 4–6 weeks after birth. Of eight animals tested, none survived >8 weeks, whereas WT (STAT3-C, or STAT3-FF) or heterozygous (STAT3-CF/+) littermates were viable and developed normally.
Crohn's Disease-Like Pathogenesis in STAT3-CFF Mice.
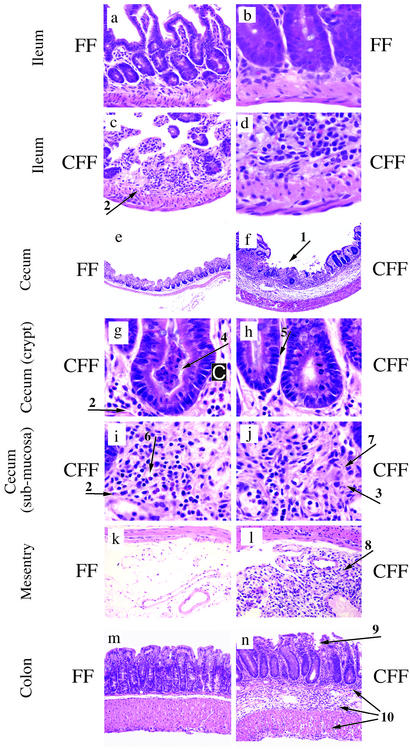
To determine the possible cause of death, necropsies were performed. Observation of the peritoneum revealed that the ileum was fused to the peritoneal wall in severely sick STAT3-CFF animals. This finding is an indication of a previous perforation or transmural inflammation of the gut. Histological sections of the ileum of control (Fig. 1 a and b) and STAT3-CFF animals (Fig. 1 c and d) indicated that the ileum was dramatically affected by STAT3 deletion. Significantly, changes were present in all four major layers: mucosa, submucosa, smooth muscle, and serosa, ulceration (Fig. 1c, arrow 1), and loss of mucosal texture and transmural inflammation (Fig. 1c, arrow 2). Higher-magnification views revealed striking neutrophilic and monocytic infiltration in the submucosal, muscular and serosa layers (Fig. 1d). Granuloma-like structures were also observed.
Figure 1.
STAT3 deficiency during hematopoiesis results in Crohn's disease-like pathology. (a–j) Ulcers (arrow 1), transmural infiltration (arrow 2), and granuloma-like structures (arrow 3) in the ileocecal area of STAT3-CFF mice in c, d, and f–j. For comparison, the normal phenotype is depicted in a, b, and e. (c and d) Ileum of STAT3-CFF mouse. (e) Cecum of a control littermate and (f) of a STAT3-CFF mouse. (Magnifications: ×40, a–d; ×10, e and f.) (g–j) Higher-magnification views (×100, hematoxylin and eosin). (g) Crypt abscesses with necrotic neutrophils and monocytes (arrow 4), (h) high frequency of mitosis in the epithelium (arrow 5), (i) a marked infiltration of neutrophils, macrophages, and eosinophils (arrow 6) and few lymphocytes, edema of the lamina propria, (j) epithelioid cells with eosinophilic cytoplasm (arrow 7) and other inflammatory cells in the lamina propria can be identified. (k) Intestinal mesentry of control and (l) STAT3-deleted mice. Infiltration of neutrophils, macrophages, eosinophils (arrow 8 in l), and other cell types in the mesenteric fat tissue of STAT3-CFF mouse (×40, hematoxylin and eosin). Six sex-matched pairs of control (STAT3-FF) and STAT3-CFF littermates between 4 and 6 weeks of age were analyzed with similar results. IBD also spreads to the colon (n). Note bowel wall thickening with mucosal erosion (arrow 9) and transmural inflammation (arrow 10) in STAT3-CFF mice (×40, hematoxylin and eosin).
The histology of the cecum area showed similar changes. Ulceration and a transmural inflammation of all layers occurred. The total thickness of the bowel wall was markedly increased (compare Fig. 1 e and f). Many further typical signs for IBD were found in this area, such as crypt abscesses with necrotic neutrophils and monocytes (arrow 4 in Fig. 1 g and h) and marked infiltration of neutrophils, macrophages and eosinophils (arrow 2 in Fig. 1i). Epitheloid cells with an eosinophilic cytoplasm were aggregated and reminiscent of giant fused cells that have been observed in Crohn's disease (arrow 7 in Fig. 1j). Having established the severe leukocyte infiltration of the intestine, we examined the possible route of entry of inflammatory cells and investigated the intestinal mesentry (Fig. 1 k and l). The fat tissue of the mesentry was massively invaded with neutrophils, macrophages, eosinophils (arrow 8 in Fig. 1l), and other cell types (compare with control, Fig. 1k, and STAT3-CFF, Fig. 1l).
Another area of the gastrointestinal tract that is susceptible to IBD is the colon. We demonstrate that this area of the gut is also affected by pathological phenotypes in a representative 4-week-old STAT3-CFF mouse. In comparison to the control (Fig. 1m), the colon of STAT3-CFF animals (Fig. 1n) showed bowel wall thickening, mucosal erosion, transmural inflammation affecting all layers, edema in the submucosa, and serosa thickening.
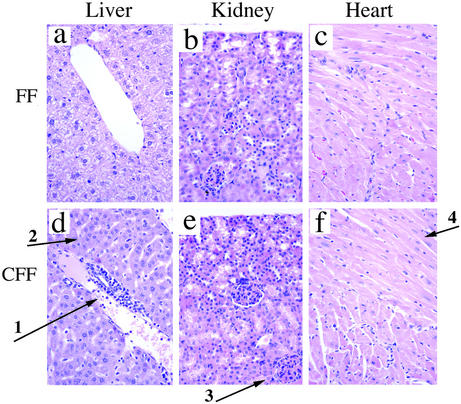
We have also examined other organs (liver, kidney, and heart) for pathological features (Fig. 2). In the liver, infiltration of granulocytes was observed around the portal vein (arrow 1 in Fig. 2d). Similar to the gastrointestinal tract, neutrophils and monocytes could be distinguished in the infiltrate. Heart and kidney appeared normal in these animals (Fig. 2 e and f).
Figure 2.
The inflammatory phenotype is not restricted to the gut but also occurs in liver. (a–c) Tissue sections of liver, kidney, and heart of control littermates. (Magnifications: ×40.) (d–f) STAT3-CFF littermates. (d) The liver of STAT3-CFF mice was infiltrated around the portal vein by neutrophils, monocytes, and other cell types (arrow 1); hepatocytes were of normal appearance (arrow 2) (×40, hematoxylin and eosin). (e) Kidney had normal glomeruli (arrow 3) and tubular structures without inflammatory cell infiltration (×40, hematoxylin and eosin). (f) Heart showed normal myocardial fibers (arrow 4) without inflammatory cell infiltration (×40, hematoxylin and eosin). Five age- and sex-matched pairs were analyzed. Pathologic alteration of the liver was observed in all STAT3-CFF animals examined.
Specific STAT3 Deletion in Myeloid Cells in Mice with Homozygous F Allele and Tie2-Cre (STAT3-CFF).
As shown above, the STAT3 deletion mediated by Tie2-Cre in STAT3-CFF mice leads to severe Crohn's disease-like phenotypes. Interestingly, myeloid cells, rather than lymphocytes, are the major cell populations in the inflammatory infiltrates of the gastrointestinal tract. This observation indicates that STAT3 deletion causes abnormalities in myeloid cells.
To determine cell and stage specificity of the Tie2-Cre used to delete STAT3 in this study, we analyzed a reporter mouse. The ZEG reporter mouse, which expresses GFP as a measure of Cre activity, was crossed with Tie2-Cre mice. Bone marrow cells of ZEG+Tie2-Cre+ pups were isolated and classified with cell type and stage-specific antibodies. Then we examined these cells for GFP expression by FACS analysis. We found that maturation marker-positive cells (Mac1+ for myeloid) show high GFP expression. More than 80% of cells expressed GFP in each case (Fig. 7, which is published as supporting information on the PNAS web site, and results not shown). Different precursor cell populations, such as c-kit+ lineage marker (B220, CD4, CD8, TER119, Mac-1)− cells, ScaI+ lineage marker (B220, CD4, CD8, TER119, Mac-1)−, and B220−CD43+ cells also expressed GFP (>75%, >60%, and >75% of the cells, respectively, Fig. 7 and results not shown). Similarly, high activity of Tie2Cre was detected in CD144+PECAM-1+ in the spleen (endothelial cells, data not shown). Therefore this Tie2Cre strain mediates gene deletion of loxP-containing alleles during hematopoietic development starting at early precursor stages.
As mentioned above, we expect that Cre expression should cause deletion of floxed STAT3 and the genotype (CFF) leads to conditional STAT3 gene ablation (Fig. 6). To observe whether gene deletion occurs early in the animal's life, spleens of 2-day-old pups were analyzed. Cells were fractioned, and the cell pellet containing red blood cells and the interphase containing white blood cells were compared (Fig. 7). A blot with STAT3 antibody demonstrated only minor deletion in the fraction enriched for red blood cells and efficient deletion of STAT3 in the fraction enriched for white blood cells (Fig. 7) at this age. Furthermore, specific cell types from the bone marrow of 3-week-old animals were analyzed to address whether STAT3 gene deletion occurred in bone marrow cells of different lineages and at different stages of development. Different cell populations were isolated by FACS sorting. Sorted Mac1+ (myeloid cells) and c-kit+ lineage marker precursor cells were analyzed by Western blotting with an anti-STAT3 antibody. Expression of STAT3 was found in myeloid and precursor cells in control littermates (STAT3-FF) (Fig. 7) and was deleted by Tie2-Cre to undetectable levels in these cell types in STAT3-CFF mice (Fig. 7).
These results were confirmed by performing a genomic PCR analysis with DNA isolated from sorted cell populations (Fig. 6).
STAT3 Deletion Causes Abnormal Development of Myeloid Cells in STAT3-CFF Mice.
The striking immune response phenotype observed in STAT3-CFF mice could be caused by a role of STAT3 in the development of the innate immune system. In particular, STAT3 may control myeloid cell proliferation and differentiation and sensitivities to microbial antigens.
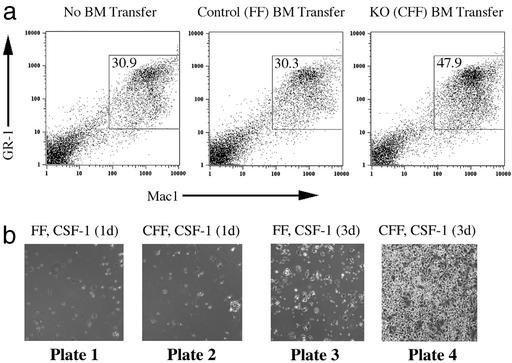
To examine these possibilities we first determined the cellular composition in the bone marrow by FACS analyses using a panel of antibodies against cell type and stage-specific surface antigens. GR-1+Mac1+ cells represent the neutrophil lineage. We observed an expansion of this cell type typically from 20% in controls and heterozygous (STAT3-CF+) animals to 35% in STAT3-CFF homozygous knockout bone marrow in all mice (n = 15) analyzed at 3–6 weeks of age (Fig. 3a). A similarly skewed cellular distribution is observed at an age when there are no outward signs of disease (3 weeks) or at later stages (4–6 weeks). By comparison, the relative abundance of TER119+ cells (erythroid) is not changed in bone marrow from these mice (Fig. 3a). These experiments revealed an abnormal expansion of a myeloid lineage in the bone marrow in the absence of STAT3. Colony formation assays revealed that the number of proliferating precursors was slightly increased in the bone marrow (Fig. 3b), indicating that STAT3 may have a minor effect on control of the number of progenitors generated in the bone marrow, but a major effect on expansion and differentiation at a later stage.
Figure 3.
In the absence of STAT3, hematopoietic development is skewed toward the myeloid lineage at an early stage. (a) The presence of the myeloid lineage (GR-1+Mac1+) and erythroid lineage (TER119+) in the bone marrow of sex-matched littermates was analyzed by FACS staining. The genotype of the control is STAT3-FF. Heterozygous is STAT3-CF+ and knockout (KO) is STAT3-CFF. A result with typical ratios of GR-1+Mac1+ and TER119+ cells is shown. Three repeats with heterozygous animals and at least 15 repeats with control and STAT3-CFF mice gave similar ratios between the individual cell lineages. (b) Bone marrow (BM) was cultured for 7 days in methyl-cellulose in the presence of cytokines (IL-3, IL-6, stem cell factor, and insulin). The numbers of colonies formed were counted. Results obtained with four sex-matched pairs of control (STAT3-FF) and STAT3-CFF littermates are shown. The difference in the colony numbers of control and STAT3-CFF mice was not statistically significant. (c) FACS analyses with antibodies against c-Kit and different lineage markers (Mac1, B220, CD4, CD8, and TER119). The percentage of c-Kit+ lineage marker− cells in the bone marrow of STAT3-CFF mice was evaluated and compared with sex-matched littermates. The results of five independent experiments are shown.
Cell Autonomous Overproduction of Myeloid Cells with STAT3 Deletion.
We next asked whether myeloid overproliferation is a cell-autonomous function. Bone marrow of STAT3-deficient mice was transferred to lethally irradiated WT mice. The bone marrow transfer rescued the survival of the animals, indicating a successful procedure. Eight weeks after transfer, the bone marrow of the recipients was reconstituted and was derived mainly from transferred cells. A genomic PCR control showed that the majority of bone marrow cells had the genotype of the donor (results not shown). Mice reconstituted with STAT3-deficient bone marrow (STAT3-CFF) showed an increased proportion of the myeloid lineage (Mac1+) in bone marrow and spleen (1.6- and 2.5-fold increase, respectively) that was very similar to what was seen in the original STAT3-deficient mice. As a control, transfer of control bone marrow (STAT3-FF) yielded ratios of Mac1+ cells after 8 weeks as found in untreated mice (Fig. 4a).
Figure 4.
STAT3 regulates the generation of the myeloid lineage in a cell autonomous fashion in vivo and in vitro. (a) Bone marrow (BM) was transferred from a donor with control genotype (Center, STAT3-FF) or from a STAT3-CFF donor (Right) into lethally irradiated recipients. The presence of donor-derived bone marrow was determined after 8 weeks. Bone marrow was analyzed for the presence of GR-1+Mac1+ cells and compared with bone marrow of an age-matched untreated animal (Left). Experiments with three controls and three STAT3-CFF animals showed an increased percentage of GR-1+Mac1+ cells in the absence of STAT3. (b) Bone marrow from control (plates 1 and 3, STAT3-FF) and STAT3-deleted (plates 2 and 4, STAT3-CFF) littermates was cultured for 1 and 3 days in the presence of CSF-1 (30 ng/ml). Light microscopic pictures were taken showing a dramatic increase in the generation of attached cells in cultures of STAT3-deficient bone marrow. FACS with Mac1 antibody confirmed that these cells were macrophages. Three repeats showed similar results. (Magnification: ×2.)
We confirmed the cell autonomous proliferation of myeloid cells in in vitro studies of their growth. Spleen cells from STAT3-CFF mice and control mice were cultured in the presence of CSF-1 to stimulate the expansion of macrophages. At early time points (Fig. 4b, plates 1 and 2) the number of attached Mac1+ cells was low regardless of the genotype. Stimulation with CSF-1 for 3 days led to an increase of attached Mac1+ cells, indicating that macrophages were generated in the cultures. When STAT3-deficient splenocytes were cultured, a much higher number of attached Mac1+ cells were present compared with controls (Fig. 4b, compare plates 3 and 4). Thus, the propensity of generating macrophages in response to CSF-1 is negatively regulated by STAT3.
The STAT3 Gene Deletion Causes Dysfunctional Innate Immunity.
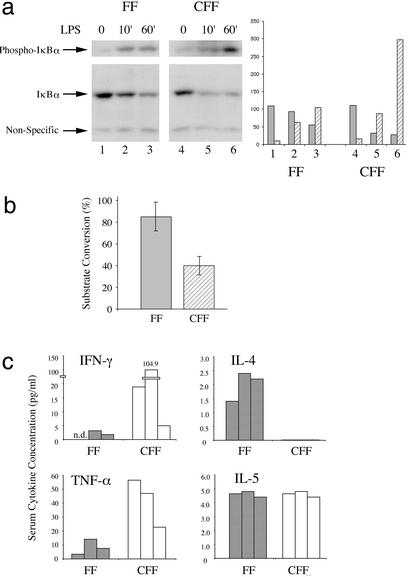
To elucidate the mechanisms that could be involved in the generation and propagation of Crohn's disease in the gut and activation of the innate immune system, we asked how Toll-like receptor (TLR) signaling in STAT3-CFF mice would be regulated. TLRs have been recognized as an important family of receptors for the activation of innate immune cells in response to bacteria and other pathogens (33). LPS, for example activates TLR4. Phosphorylation of IκB, degradation of IκB, and consequent activation of NF-κB are downstream events of TLR stimulation. Activation and differentiation of dendritic cells by TLR signaling is an initial event of innate immune responses. LPS treatment of bone marrow-derived dendritic cells induced IκB phosphorylation and its degradation (Fig. 5a, compare lanes 1–3). In comparison to control, IκB phosphorylation normalized to IκB protein level was induced 3-fold more in STAT3-CFF mice after a 1-h stimulation (Fig. 5a, lanes 4–6). Gel-shift experiments with an NF-κB DNA binding site showed that the DNA binding activity of NF-κB family members was enhanced in the absence of STAT3 after stimulation (Fig. 8, which is published as supporting information on the PNAS web site). A similar result was obtained on stimulating splenic B cells (Fig. 8).
Figure 5.
STAT3 regulates crucial functional responses of the innate immune system. (a) IκB protein and phosphorylation levels were measured by Western blotting with specific antibodies to IκB and phospho-IκB before and after 10-min and 1-h LPS (10 ng/ml) stimulation of bone marrow-derived myeloid cells cultured in granulocyte/macrophage-CSF as indicated (FF, control mice; CFF, STAT3-CFF mice). (b) NADPH oxidase activity was measured in neutrophils isolated from whole blood samples of 4- to 5-week-old mice. Five controls (shaded bar) were compared with five sex-matched littermates (hatched bars). The difference between control and STAT3 knockout mice was statistically significant (five pairs analyzed). P value of Student's t test was P < 0.004. (c) Sera from 4- to 5-week-old mice were collected. IFN-γ, IL-4, tumor necrosis factor α (TNF-α), and IL-5 levels were measured. The results with three matched pairs of control (shaded bars) and STAT3-CFF littermates (open bars) are shown.
Previous studies have shown that the generation of reactive oxygen species by NADPH oxidase is an important response induced by the innate immune system. NADPH oxidase activity is increased in normal mice during an inflammatory process (34–36). We measured NADPH oxidase activity of neutrophils isolated from whole blood samples of WT control mice by using Fc-Oxyburst analysis (37). In this assay, NADPH oxidase activity is >90% positive. By contrast, in the STAT3-deficient mice NADPH oxidase activity was reduced to 45% positive (Fig. 5b).
This finding indicates that STAT3-CFF mice have decreased host defense ability. As a consequence, it is possible that these STAT3-deficient mice are prone to further up-regulate their response to an insult. It is expected that the concentrations of inflammatory cytokines will increase during this response. We tested several cytokines in the serum of the mice. Tumor necrosis factor α and IFN-γ were present at increased levels in the STAT3-deficient mice (Fig. 5c), indicating an ongoing deregulated T helper 1-type immune response.
Discussion
In this study we generated mice lacking STAT3 in the hematopoietic system to analyze functions of STAT3 in vivo. Unlike previous strains with a conditional STAT3 gene knockout, our strain has a rather complete deletion of STAT3 in the bone marrow cells, which causes novel and severe phenotypes, including a widespread inflammatory disease in the digestive tract and develops close to 100% lethality at an early age (4–6 weeks after birth).
The pathological abnormalities resemble some of the hallmarks for Crohn's disease (38), making these mice a valuable model to understand the mechanisms underlying Crohn's disease and related intestinal bowel diseases. Presumably mucosal interstitia of the gastrointestinal tract are continuously exposed to a variety of foreign antigens. However, no severe inflammatory response against these foreign antigens occurs, indicating that the mucosal immune system in the gastrointestinal tract has developed unique mechanisms for immune tolerance. When inflammatory responses occur during invasion by a pathogen, the same mechanism may also control the immune response to a limited location and time and enable the system to turn off the response when pathogens are cleared and to suppress the hypersensitive innate immune responses to regular microflora in the gastrointestinal tract. Crohn's disease and other IBDs likely result from dysfunction of this “turn-off” mechanism. Currently little is known about the molecular mechanisms by which mucosal immune tolerance is regulated.
Our model is that STAT3 is a key regulator of innate immune responses. We identified at least three stages that involve STAT3 in orchestrating tolerance in the mucosal immune system of the gut. First, dendritic cells and B cells that lack STAT3 show an enhanced immediate response to bacterial LPS by generating higher NF-κB activities after stimulation. Because these cell types are critical in the initial uptake of antigen and trigger further responses of other cell types, the observed increased TLR signaling could explain how the system initially becomes sensitive to the otherwise innocuous microflora in the gastrointestinal tract. We have found that in STAT3-deficient dendritic cells the LPS-induced IκBα phosphorylation, leading to subsequent NF-κB activation, is ≈3-fold higher in STAT3-CFF mice. Whereas such a change may be considered to be moderate, it could be of striking significance in a system such as the gut, where TLR stimulation could occur chronically and repeatedly. The down-regulation of LPS-induced NF-κB activation by STAT3 could be one important factor in the maintenance of tolerance and could be of general relevance for Crohn's disease. This notion is supported by the recent discovery that NOD2 has been identified as the first susceptibility gene for Crohn's disease (7–9). NOD2 was shown to also regulate NF-κB activation.
Second, deletion of STAT3 causes overproliferation of macrophages and neutrophils, which could be the second factor responsible for an enhanced response of the innate immune system. This event is triggered by the cytokines macrophage-CSF and granulocyte-CSF, respectively. Our finding that STAT3 negatively regulates proliferation in response to these cytokines is consistent with a recent report of Lee et al. (39), in which a negative role of STAT3 in the growth response to granulocyte-CSF is suggested. However, the study by Lee et al. did not expand into IBDs. We show here that myeloid cells are recruited in high numbers to inflammatory sites in STAT3-CFF mice. The importance of the inhibition of myelopoiesis by STAT3 lies in the fact that it could moderate and prevent overgeneration of these cells under nonpathological conditions and during clearance of the pathogen. Furthermore, our analysis and our unpublished results show that STAT3 inhibits not only the generation of neutrophils but also other types of myeloid cells (monocytes, myeloid dendritic cells, and osteoclasts). These findings challenge the current paradigm that positions STAT3 as an oncogene for promoting cell proliferation. In contrast, we propose here that STAT3 is a negative regulator of cell growth under physiological conditions.
Third, we found that STAT3 regulates critical effector functions of the activated innate immune system. This finding is documented by two examples. First, deletion of STAT3 causes compromised NADPH oxidase activities in neutrophils (Fig. 5b), thereby hampering the efficacy of the innate immune system to battle infectious microbes. Difficulties in clearance of microbial antigens will further induce stronger and continuous responses, resulting in escalating inflammatory reactions. Second, the literature on mice whose NADPH oxidase subunits were gene targeted is in line with our reasoning, because those mice develop a chronic inflammatory syndrome similar to chronic granulomatous disease in the absence of functional NADPH oxidase (40).
Takeda et al. (41) provided an earlier example of effector functions regulated by STAT3 in myeloid cells that causes a mild IBD (29). This IBD was attributed to defects in the function of macrophages in response to IL-10. In contrast, we presented data here suggesting that STAT3 deletion causes additional abnormalities in the regulation of innate immune responses. The changes in LPS signaling, macrophage-CSF signaling, and NADPH oxidase function all could significantly contribute to the observed inflammatory phenotype. Although the enhanced phenotype of the current STAT3-CFF mice could be caused by differences in strain backgrounds and efficiencies in gene deletion, it is more likely caused by the fact that in the current model STAT3 was not only deleted in macrophages and neutrophils, but also other cell types that develop innate immune responses and respond to microbial challenge (endothelial cells, dendritic cells, and B cells). By using an IL-10-neutralizing antibody we obtained evidence for an IL-10-independent function of STAT3 in the LPS response of B cells (results not shown). Therefore we believe that the negative role of STAT3 for innate immunity is widespread and not restricted to the previously proposed mechanism that operates specifically in macrophages and down-regulates cytokine production through IL-10 during the later stage of infection.
Our results further contrast the view that activated CD4 T lymphocytes are the major players during the inflammatory responses in Crohn's disease and related animal models. Our studies have indicated that activated myeloid cells have a more prominent role than lymphocytes in the initiation of the disease. There are few lymphocytes present in the infiltrated areas, yet the inflammatory responses are severe and sufficient for generation of the disease phenotypes. This observation is supported by the studies on NOD2, the susceptibility gene for Crohn's disease. Interestingly, NOD2 is expressed only in monocytes but not in lymphocytes, consistent with the essential roles of myeloid cells in the disease. Furthermore we analyzed T cell responses of STAT3-CFF mice in a tumor challenge experiment and found no evidence for an increased proliferation of T cells cultured ex vivo from draining lymph nodes (data not shown). This result may indicate that T cell responses per se are not enhanced in the absence of STAT3, consistent with previous reports on STAT3-deficient T cells (31).
Our major conclusion is that STAT3 regulates innate immune responses at several levels, through down-regulating NF-κB activation by TLRs, through inhibiting the growth response to cytokines such as granulocyte-CSF and macrophage-CSF, and through regulating an effector function of neutrophils, the NADPH oxidase activity. This finding suggests that several pathways important for the tolerance of the innate immune system are coordinately regulated by STAT3 and may explain the severity of the Crohn's-like inflammatory disease observed in STAT3-deficient mouse strain in the present study.
Supplementary Material
Acknowledgments
We thank Drs. Joe Madri and Jordan Porber for discussions, Drs. A. Nagy and C. Lobe for Z/EG mice, Marie Robert for help with diagnosis, and Qian Gao and Michael Wolfgang for reading the manuscript and providing comments. T. Welte is an Austrian Programme for Advanced Research and Technology Fellow of the Austrian Academy of Sciences (Osterreichische Akademie Der Wissenschaften). R.A.F. is an Investigator of the Howard Hughes Medical Institute. X.-Y.F. was a recipient of a Career Development Award from the National Institutes of Health. This work was supported by National Institutes of Health Grants AI34522 and AR44906 (to X.-Y.F.).
Abbreviations
- IBD
inflammatory bowel disease
- LPS
lipopolysaccharide
- STAT
signal transducer and activator of transcription
- CSF
colony-stimulating factor
- FACS
fluorescence-activated cell sorting
- TLR
Toll-like receptor
Footnotes
Present address: Division of Urology and Human and Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, WI 53226-0509.
References
- 1.Medzhitov R, Janeway C A., Jr Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss I J, Blumberg R S. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 3.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde K H. Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugot J P, Zouali H, Lesage S, Thomas G. Int J Colorectal Dis. 1999;14:2–9. doi: 10.1007/s003840050175. [DOI] [PubMed] [Google Scholar]
- 5.Laroux F S, Pavlick K P, Wolf R E, Grisham M B. News Physiol Sci. 2001;16:272–277. doi: 10.1152/physiologyonline.2001.16.6.272. [DOI] [PubMed] [Google Scholar]
- 6.Shanahan F. Lancet. 2002;359:62–69. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- 7.Hugot J P, Chamaillard M, Zouali H, Lesage S, Cezard J P, Belaiche J, Almer S, Tysk C, O'Morain C A, Gassull M, et al. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, Bonen D K, Inohara N, Nicolae D L, Chen F F, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr R H, et al. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 9.Hampe J, Cuthbert A, Croucher P J, Mirza M M, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson A J, et al. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 10.Inohara N, Ogura Y, Nunez G. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 11.Ogura Y, Inohara N, Benito A, Chen F F, Yamaoka S, Nunez G. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 12.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 13.Akira S. Stem Cells. 1999;17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 14.Su W C, Kitagawa M, Xue N, Xie B, Garofalo S, Cho J, Deng C, Horton W A, Fu X Y. Nature. 1997;386:288–292. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- 15.Fu X Y. Cell Death Differ. 1999;6:1201–1208. doi: 10.1038/sj.cdd.4400613. [DOI] [PubMed] [Google Scholar]
- 16.O'Shea J J, Gadina M, Schreiber R D. Cell. 2002;109:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 17.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 18.Fu X Y. J Leukocyte Biol. 1995;57:529–535. doi: 10.1002/jlb.57.4.529. [DOI] [PubMed] [Google Scholar]
- 19.Fu X Y. Cell. 1992;70:323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- 20.Schindler C, Shuai K, Prezioso V R, Darnell J E., Jr Science. 1992;257:809–813. [PubMed] [Google Scholar]
- 21.Shuai K, Stark G R, Kerr I M, Darnell J E., Jr Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 22.Shuai K, Horvath C M, Huang L H, Qureshi S A, Cowburn D, Darnell J E., Jr Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 23.Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 24.Welte T, Leitenberg D, Dittel B N, al-Ramadi B K, Xie B, Chin Y E, Janeway C A, Jr, Bothwell A L, Bottomly K, Fu X Y. Science. 1999;283:222–225. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- 25.Wegenka U M, Buschmann J, Lutticken C, Heinrich P C, Horn F. Mol Cell Biol. 1993;13:276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Z, Wen Z, Darnell J E., Jr Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 27.Tian S S, Lamb P, Seidel H M, Stein R B, Rosen J. Blood. 1994;84:1760–1764. [PubMed] [Google Scholar]
- 28.Hirano T, Ishihara K, Hibi M. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 29.Martin H M, Rhodes J M. Curr Opin Infect Dis. 2000;13:503–509. doi: 10.1097/00001432-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, et al. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 32.Koni P A, Joshi S K, Temann U A, Olson D, Burkly L, Flavell R A. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janeway C A, Jr, Medzhitov R. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 34.Babior B M, Lambeth J D, Nauseef W. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 35.Burg N D, Pillinger M H. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 36.Sadler K L, Badwey J A. Hematol Oncol Clin North Am. 1988;2:185–200. [PubMed] [Google Scholar]
- 37.Wang T, Malawista S E, Pal U, Grey G, Meek J, Akkoyunlu M, Thomas V, Fikrig E. J Infect Dis. 2002;186:274–280. doi: 10.1086/341451. [DOI] [PubMed] [Google Scholar]
- 38.Cotran R S, Kumar V, Robbins S L. Pathologic Basis of Disease. Philadelphia: Saunders; 1994. [Google Scholar]
- 39.Lee C, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho R A, Levy D E. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 40.Jackson S H, Gallin J I, Holland S M. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.