Abstract
During organogenesis, immunosurveillance, and inflammation, chemokines selectively recruit leukocytes by activating seven-transmembrane-spanning receptors. It has been suggested that an important component of this process is the formation of a haptotactic gradient by immobilization of chemokines on cell surface glycosaminoglycans (GAGs). However, this hypothesis has not been experimentally demonstrated in vivo. In the present study we investigated the effect of mutations in the GAG binding sites of three chemokines, monocyte chemoattractant protein-1/CC chemokine ligand (CCL)2, macrophage-inflammatory protein-1β/CCL4, and RANTES/CCL5, on their ability to recruit cells in vivo. These mutant chemokines retain chemotactic activity in vitro, but they are unable to recruit cells when administered intraperitoneally. Additionally, monomeric variants, although fully active in vitro, are devoid of activity in vivo. These data demonstrate that both GAG binding and the ability to form higher-order oligomers are essential for the activity of particular chemokines in vivo, although they are not required for receptor activation in vitro. Thus, quaternary structure of chemokines and their interaction with GAGs may significantly contribute to the localization of leukocytes beyond migration patterns defined by chemokine receptor interactions.
Cellular migration of leukocyte populations occurs during both routine immunosurveillance and inflammation and is orchestrated by several families of proteins, including proinflammatory cytokines, adhesion molecules, and matrix metalloproteases. However, chemokines control the direction of cell migration and provide a trigger for cell activation. The immobilization of chemokines on glycosaminoglycans (GAGs) of the extracellular matrix and endothelial cell surfaces is thought to be an essential part of this process (1).
Chemokines are a superfamily of small proteins (<100 residues), which number ≈40 in humans (2). Their activities are mediated through G protein-coupled seven-transmembrane receptors, of which 19 have been identified. Many chemokines bind several receptors and multiple chemokines often bind the same receptor, resulting in a highly complex network of interactions (3). The majority of chemokines [macrophage-inflammatory protein (MIP)-1α/CC chemokine ligand (CCL)3 and MIP-1β/CCL4 being exceptions] are basic proteins, whereas the extracellular domains of their receptors are acidic; thus, it is not surprising that chemokines are also able to bind linear sulfated GAGs such as heparin and heparan sulfate. Immobilization of chemokines to endothelial surfaces by proteoglycans has been demonstrated both in vitro (4) and in vivo (1, 5). This interaction is thought to facilitate the retention of chemokines on cell surfaces and enable localized high concentrations of chemokines to form, even in the presence of shear forces caused by blood flow in capillary beds. However, the biological relevance of the GAG interaction has not been demonstrated.
Chemokines have been traditionally divided into four families (CXC, CC, C, and CX3C) based on the patterns of amino-terminal cysteine residues. Despite sequence identities that can be as low as 20%, a remarkably conserved three-dimensional tertiary structure exists across the four families. Many chemokines also form dimers or higher-order oligomers. However, the dissociation constants for dimerization are at least two orders of magnitude higher than the concentrations required for maximal biological activity in vitro (6). Furthermore, several studies have shown that variants of chemokines with altered dimer interfaces remain monomeric even at high protein concentrations and yet are indistinguishable from wild-type chemokines in receptor binding and activation assays in vitro. For example, N-methylation of Leu-25 in the CXC chemokine IL-8/CXC chemokine ligand 8 produces a monomer that is fully functional in vitro (7). Similarly, mutation of Pro-8 to Ala in both monocyte chemoattractant protein (MCP)-1/CCL2 (8) and MIP-1β/CCL4 (9) produces monomeric variants that are indistinguishable from wild type. Thus, like GAG binding, the physiological significance of quaternary structure has not been fully elucidated.
In this study, the in vivo role of chemokine GAG binding and oligomerization was addressed by using variants engineered for reduced ability to bind GAGs and to oligomerize. We have studied three chemokines: MCP-1/CCL2, which binds selectively to CC chemokine receptor (CCR)2; MIP-1β/CCL4, which binds selectively to CCR5; and RANTES/CCL5, which binds to CCR1, CCR3, and CCR5.
Materials and Methods
Production of Chemokines.
Recombinant wild-type RANTES (10), MCP-1 (11), and MIP-1β (9) were produced as described. The [44AANA47]-RANTES mutant was generated by PCR mutagenesis in a two-step reaction by using two oligonucleotide pairs: GTTGGCACACACTTGCGCGTTCGCCGCGGTGACAAAGACGAC in combination with an N-terminal forward primer and GTCGTCTTTGTCACCGCGGCGAACGCGCAAGTGTGTGCCAAC in combination with a C-terminal reverse primer. The PCR product was purified and digested with BspHI and XhoI restriction endonucleases, cloned into the NcoI and XhoI sites of pET24d, and transformed into TG1-competent cells. After confirmation of the correct DNA sequence, the resulting vector was transformed into BL21(DE3)pLysS Escherichia coli cells. The protein was expressed and purified as described (10). [18AA19]-MCP-1 and [45AASA48]-MIP-1β were produced by using described strategies (8, 9). [Nme-7T]-RANTES was synthesized by using tBoc chemistry in a manner similar to an N-methylated variant of IL-8 (7, 12). Eotaxin, MCP-3, and I-309 were purchased from PeproTech (Rocky Hill, NJ).
Heparin Binding Assays.
Heparin binding (13) was assessed by incubating increasing concentrations of chemokine with [3H]heparin (50 ng/ml) in 96-well filter plates for 1 h at 37°C. The solutions were transferred to 96-well plates fitted with Whatman cellulose phosphate filters and washed three times with PBS under vacuum. After the addition of 50 μl of scintillant, radioactivity was counted. An aggregation assay on immobilized heparin was performed as described (4) by using heparin beads incubated with 0.1 nM 125I-labeled chemokine (custom labeled by Amersham Pharmacia) in the presence of increasing concentrations of unlabeled chemokine.
Chemokine–Chinese Hamster Ovary (CHO) Cell Binding.
Wild-type CHO cells were used to assess RANTES binding to cell surface GAGs by flow cytometry. Cells were incubated at 4°C overnight with 1 and 0.1 μM RANTES or [44AANA47]-RANTES. A mouse monoclonal antibody directed against RANTES (a gift from Dr. Matthias Mack, University of Munich) was used as the primary detection antibody, and a goat anti-mouse IgG-FITC (Silenus, Hawthorn, Australia) was used to reveal binding. Fluorescence was measured by using a FACSCalibur (Becton Dickinson).
In Vitro Chemotaxis.
Chemotaxis was assayed in 96-well ChemoTx plates (Neuroprobe, Cabin John, MD) by using 8-μm pores for the L1.2/CCR5 transfectants and 5-μm pores for the THP-1 cells. A total of 3 × 105 cells (L1.2/CCR5 transfectants for RANTES and MIP-1β and the promonocytic THP-1 cell line for MCP-1) were placed in the upper chambers. Chemokines were placed in the lower wells with appropriate dilutions, and the chambers were incubated for 2 h at 37°C. The bottom wells containing the migrated cells were transferred to a black 96-well plate (Costar), frozen at −80°C for 2 h, and thawed, and the number of cells was measured by using the cell proliferation assay CyQUANT kit (Molecular Probes). Measurements were done in triplicate.
Peritoneal Cell Recruitment.
Eight- to 12-week-old female Balb/c mice (Janvier, Le Genest St Isle, France) were injected intraperitoneally with 200 μl of NaCl (0.9%, lipopolysaccharide-free) or chemokine diluted into 200 μl of NaCl (0.9%, lipopolysaccharide-free). At 18 h postinjection mice were killed by CO2 asphyxiation, the peritoneal cavity was washed three times with 5 ml of ice-cold PBS, and the total lavage was pooled for individual mice. Total cells collected were counted with a hemocytometer (Neubauer, Hausser Scientific Company, Horsham, PA).
Statistical Tests.
Statistically significant in vivo cell recruitment was tested by one-way ANOVA, with a Bonferroni post test to compare each treatment with baseline (NaCl). Levels of significance were assigned as follows: P > 0.05, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results and Discussion
Characterization of the GAG Binding Sites of Chemokines.
Residues important for the interaction of chemokines with GAGs have been defined for several chemokines by mutagenesis and in vitro heparin/heparan sulfate binding assays. In some cases, the binding site is spatially distinct from the G protein-coupled receptor binding site(s). Examples include K58 and H66 in the C-terminal domain of MCP-1 (13), K64 and R68 in the C-terminal domain of IL-8 (14), and a BBXB motif (B represents a basic residue) in the 20s loop of stromal cell-derived factor-1/CXC chemokine ligand 12 (15). For others there is partial overlap of the GAG and receptor binding sites. The principal heparin binding region of RANTES, MIP-1β, and MIP-1α involves a classical BBXB cluster in the 40s loop (16–18) that is also implicated in receptor binding. A GAG mutant of RANTES with a triple mutation, [44AANA47]-RANTES, retains high-affinity binding to CCR5 but has a 200-fold decrease in CCR1 binding (16, 19). In the case of MIP-1β and MIP-1α, mutation of the BBXB motif in the 40s loop causes a significant decrease in receptor binding affinity (17, 18). For MCP-1, we show here that R18 and K19 participate in GAG binding in addition to residues K58 and H66 in the C-terminal helix (13). The single mutations, R18A and K19A, make small contributions to receptor binding (11), and the double mutant [18AA19]-MCP-1 shows only a 20-fold decrease in affinity for CCR2 (IC50 = 1.4 nM versus 0.08 nM for wild type; results not shown).
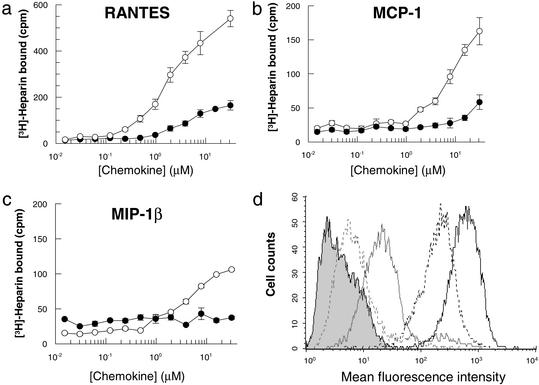
As illustrated in Fig. 1, a tritiated heparin binding assay (13) demonstrated that RANTES has the highest heparin binding capacity among the three chemokines, with MIP-1 β being the weakest, in accordance with its acidic pI. However, the fact that MIP-1β (and MIP-1α) binds heparin at all suggests that the chemokine/heparin interaction is not due only to nonspecific electrostatic interactions; it has specificity. As previously reported, [44AANA47]-RANTES retains some residual affinity for heparin (16). The same is true for [18AA19]-MCP-1. By contrast, heparin binding of [45AASA48]-MIP-1β is completely abrogated (Fig. 1c).
Figure 1.
(a–c) Comparison of the ability of wild-type chemokines RANTES, MIP-1β, and MCP-1 (open circles) and the mutants [44AANA44]-RANTES, [18AA19]-MCP-1, and [45AASA48]-MIP-1β (filled circles) to bind [3H]heparin. RANTES binds approximately three and five times as much heparin as MCP-1 and MIP-1β, respectively. These results are consistent with their elution pattern on heparin Sepharose affinity chromatography (results not shown). The three mutants show significant losses of heparin binding, but [44AANA47]-RANTES and [18AA19]-MCP-1 retain 25–30% binding capacity, whereas heparin binding is completely abrogated in the [45AASA48]-MIP-1β mutant. (d) Flow cytometric analysis of the binding of wild-type RANTES and [44AANA47]-RANTES to cell surface GAGs using CHO cells. Bound protein was detected with an anti-RANTES mAb; goat anti-mouse IgG-FITC was used to reveal bound protein. Wild-type RANTES is shown in black and [44AANA47]-RANTES is shown in gray. The concentrations used were 1 μM (solid lines) and 0.1 μM (dotted lines). The shaded area represents secondary antibody binding control.
One potential caveat to these in vitro assays is in the use of heparin rather than the perhaps more physiologically relevant cell surface GAGs such as heparan sulfate. However, heparin is believed to be structurally and chemically similar enough to heparan sulfate to serve as a good substitute, at least in a first approach. Furthermore, chemokine binding to extracellular matrix and other structures has been shown to correlate strongly with the avidity of binding to heparin in vitro (20). Along the same lines, Fig. 1d shows that wild-type RANTES binds strongly to CHO cells lacking chemokine receptors, whereas [44AANA47]-RANTES binds poorly, paralleling its reduced heparin-binding capacity. Thus, the heparin assays appear to be reasonable predictors of the behavior of the mutants toward other types of GAG surfaces.
In Vitro and in Vivo Activity of GAG Binding Site Mutants.
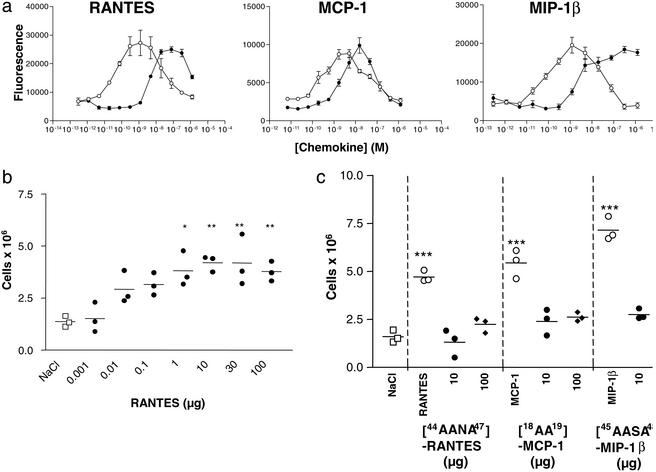
Importantly, the GAG binding site mutants maintain the ability to induce chemotaxis in vitro (Fig. 2a). The in vitro assay involves the establishment of an artificial gradient, produced in a standard chemotaxis apparatus by placing chemokine in a lower chamber and cells in an upper chamber, separated by a porous membrane. Restricting the chemokine to a microchamber, coupled with a lack of flow conditions, apparently eliminates the necessity for immobilization on GAGs. Thus, the three mutants, despite their significantly compromised GAG binding, show robust chemotaxis profiles in vitro (Fig. 2a). The losses in potency can be attributed to the small losses of receptor affinity and to the impaired interaction with GAGs on the recruited cells, because GAGs can enhance the localization of chemokine to these cells in vitro (21). Thus, these chemokine variants show significant reductions in GAG binding but retain chemotactic activity in vitro, making them ideal reagents for testing the importance of GAG binding in vivo.
Figure 2.
(a) In vitro chemotaxis assays show that each GAG-binding mutant (filled circles) retains the ability to elicit a robust chemotactic response compared with the wild-type chemokines (open circles). Chemotaxis of the [44AANA47]-RANTES and [45AASA48]-MIP-1β mutants was measured on L1.2/CCR5 transfectants, and [18AA19]-MCP-1 was measured by using the promonocytic THP-1 cell line, which expresses CCR2. The three mutants have small losses of receptor affinity (3-, 20-, and 77-fold for [44AANA44]-RANTES, [18AA19]-MCP-1, and [45AASA48]-MIP-1β, respectively), as reflected in their EC50 values. (b) An in vivo dose–response curve for RANTES shows that recruitment of cells into the peritoneum is maximal at the 10-μg dose. Cells were counted 18 h after administration of chemokine. (c) Intraperitoneal recruitment elicited by the mutants (filled circles) in comparison to wild-type chemokines (open circles). In view of the small reduction in efficacy observed in vitro, two of the mutants, [44AANA47]-RANTES and [18AA19]-MCP-1, were tested at a 100-μg dose (filled diamonds) but still did not recruit.
To test the role of GAG binding in vivo, wild-type or mutant chemokine was injected into the peritoneal cavity of Balb/c mice. Maximal recruitment was determined to be 18 h postinjection of wild-type chemokine for a 10-μg dose, because the number of cells increased linearly over the range of 10 ng to 10 μg, with no further increase up to 100 μg (Fig. 2b); thus, 10 μg per mouse was used in all subsequent experiments. As shown in Fig. 2c, all three wild-type chemokines induced a robust increase in the number of recruited cells to a level approximately two- to threefold over the control. However, no recruitment was observed for the GAG binding site mutants compared with the saline controls (Fig. 2c). Because these mutants had some reduction in chemotactic activity in vitro, two ([44AANA47]-RANTES and [18AA19]-MCP-1) were tested at a dose of 100 μg, which is 10,000-fold higher than that at which the wild-type chemokines showed measurable recruitment; yet still no activity was observed (Fig. 2c). These results demonstrate that interaction with GAGs in vivo is indeed essential, in contrast to the in vitro chemotaxis assay.
The Role of Oligomerization.
Next, the relevance of oligomerization to in vivo chemotaxis was addressed. These studies were motivated by the observation that certain chemokines form dimers or tetramers in solution, whereas others form large oligomers that attain a mass between 100 and 200 kDa (6). Additionally, heparin has been shown to induce higher-order aggregates in solution in vitro (4), and β-chemokines secreted from cytotoxic T cells have been observed as large as 400–600 kDa in complex with sulfated proteoglycans (22). Chemokine oligomerization can also be observed on immobilized heparin or cell surface proteoglycans (4). Although chemokine oligomerization is not important for receptor activation as indicated by several in vitro studies using monomeric variants (8, 9, 12), these heparin/proteoglycan interactions suggest that it may be important in vivo via a mechanism involving GAG binding.
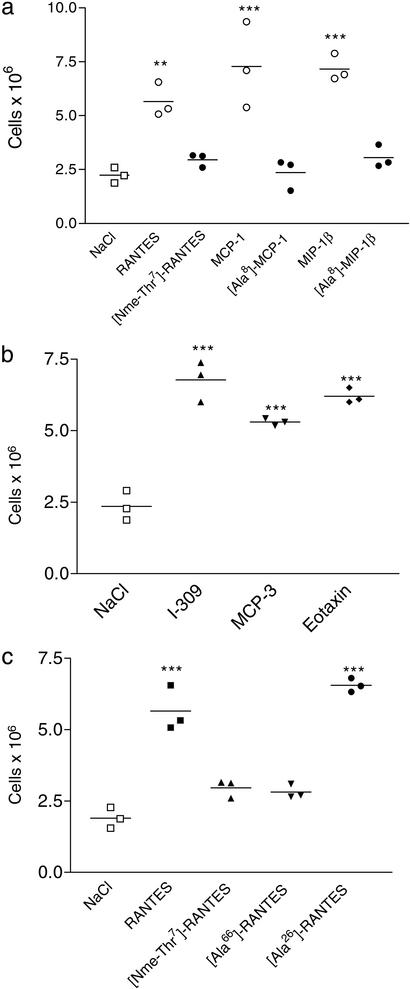
To investigate this hypothesis, we examined the in vivo chemotaxis behavior of previously reported monomeric mutants of MCP-1 ([Ala-8]-MCP-1), MIP-1β ([Ala-8]-MIP-1β), and a chemically synthesized variant of RANTES that was N-methylated on the amide nitrogen of Thr-7 ([Nme-7T]-RANTES). The first two mutants are monomeric at or above 1.0 mM concentrations (8, 9). The RANTES analogue was predicted to be monomeric because the N-methyl group should prevent hydrogen bonding across the β-sheet dimer interface, similar to a previously reported IL-8 variant (7). This hypothesis was confirmed by analytical ultracentrifugation, which suggested a mass of 10.6 kDa at ≈10 μM chemokine (data not shown), consistent with a predominantly monomeric state (expected mass = 7,865 Da). Moreover, [Nme-7T]-RANTES was unable to oligomerize on immobilized GAGs (Fig. 3b) in contrast to wild-type RANTES and other chemokines (4). These monomeric variants retain the ability to bind heparin, but with reduced capacity relative to the wild-type proteins that form dimers at the concentrations used in this assay (Fig. 3a). As has been reported for [Ala-8]-MCP-1 (8) and [Ala-8]-MIP-1β (9), which show wild-type affinities in receptor binding assays, [Nme-7T]-RANTES retains wild-type receptor binding to CCR1 and CCR5 (Fig. 3c). Full activity in in vitro chemotaxis is shown for all three of these proteins (Fig. 3d and ref. 8). Nevertheless, despite the ability to bind and activate their receptors as well as an ability to bind to heparin, the inability to recruit cells in vivo (Fig. 4a) demonstrates that oligomerization is critical for these three chemokines. Interestingly, like [Nme-7T]-RANTES, the GAG binding mutant, [44AANA47]-RANTES, is also unable to oligomerize on immobilized heparin (data not shown).
Figure 3.
Characterization of monomeric variants. (a) Binding of [Nme-7T]-RANTES, [Ala-8]-MCP-1, and [Ala-8]-MIP-1β (filled circles) to [3H]heparin in comparison to wild-type chemokines (open circles). (b) Ability of [Nme-7T]-RANTES (filled circles) to oligomerize on heparin beads compared with wild-type RANTES (open circles). (c) Equilibrium competition binding assays of [Nme-7T]-RANTES (filled circles) and wild-type RANTES (open circles) using CHO membranes expressing CCR1 and CCR5. (d) In vitro chemotaxis activity of L1.2/CCR5 transfectants in response to [Nme-7T]-RANTES, [Ala-8]-MIP-1β (filled circles), and wild-type chemokines (open circles).
Figure 4.
Ability of oligomerization mutants to recruit cells into the peritoneal cavity. (a) Wild-type chemokine (open circles) administered at a dose of 10 μg shows a robust response, whereas the monomeric mutants [Nme-7T]-RANTES, [Ala-8]-MCP-1, and [Ala-8]-MIP-1β (filled circles) are devoid of activity when administered at the same dose. (b) Three naturally occurring monomeric chemokines, I-309, MCP-3, and eotaxin, are active in the recruitment assay (10-μg dose). (c) Comparison of wild-type RANTES, a synthetic monomer ([Nme-7T]-RANTES), a dimeric RANTES mutant ([Ala-66]-RANTES), and a tetrameric mutant ([Ala-26]-RANTES) in the recruitment assay (10-μg dose). Of the mutants, only the tetramer recruits, suggesting a minimal oligomerization state for in vivo activity.
A minimal oligomerization state is also suggested by the in vivo activity of other RANTES mutants. [Ala-66]-RANTES has been reported to form dimers whereas [Ala-26]-RANTES forms tetramers, but neither forms higher-order oligomers in solution (6). Interestingly, the dimeric form of RANTES was devoid of activity, whereas the tetramer was fully active (Fig. 4c), indicating that a tetramer may be required for in vivo activity of this chemokine. On the other hand, certain chemokines, such as MCP-3/CCL7, eotaxin/CCL11, and I-309/CCL-1, appear to be naturally occurring monomers, because structural and biophysical studies have failed to provide evidence of oligomerization (23–25). Nevertheless, eotaxin has activity when administered into guinea pig skin (26), and we have shown here that it is also active in the peritoneal cavity recruitment assay (Fig. 4b). Similarly, MCP-3 and I-309 are fully active (Fig. 4b). However, it is not known whether these chemokines oligomerize on cell surface proteoglycans.
Thus, oligomerization may be important only for some chemokines, and this requirement, or lack thereof, may have functional implications and/or depend on the site of production. The simplest explanation for these results is that chemokines that are functional in recruiting cells from the circulation into the underlying tissue may require oligomerization to function under flow conditions, whereas those produced within the extravascular space may not. A more intriguing hypothesis is that chemokines whose GAG binding sites overlap with receptor binding sites, as is the case for RANTES, MCP-1, and MIP1-β, may require oligomerization to bind GAGs through some of the subunits while exposing other subunit binding sites to the receptor. Interaction of chemokine through the exposed binding site could then trigger release of chemokine monomers, allowing the entire binding site, which is partially buried in the oligomers, to interact with the receptor (8).
Summary
We believe that this is the first formal demonstration that GAG binding is a prerequisite of chemokine activity in vivo, even though it is not required for receptor activation. Without this tethering mechanism, chemokines would be washed away from the local production site, especially under flow conditions, diluted to a concentration below the threshold required for binding, and distributed uniformly throughout the vasculature such that no localized chemotactic signal is generated for leukocytes to follow. These results also suggest an additional level of specificity to this system, generally perceived as redundant, where certain chemokines need to oligomerize for their in vivo function. Not only do these results show that GAG binding and chemokine oligomerization are essential for the chemotactic properties of chemokines, but they suggest plausible mechanisms for functional specificity involving GAGs that may be operative in other complex cytokine networks. Finally, the results suggest new strategies based on these mechanistic observations to interfere with chemokine function for the treatment of disease.
Acknowledgments
We dedicate this work to Dr. Ian Clark-Lewis, who passed away December 30, 2002. T.M.H. gratefully acknowledges support from the National Institutes of Health and the American Heart Association.
Abbreviations
- GAGs
glycosaminoglycans
- MIP
macrophage-inflammatory protein
- MCP
monocyte chemoattractant protein
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CHO
Chinese hamster ovary
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rot A. Eur J Immunol. 1993;23:303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3.Proudfoot A E. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoogewerf A J, Kuschert G S, Proudfoot A E I, Borlat F, Clark L I, Power C A, Wells T N C. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 5.Middleton J, Neil S, Wintle J, Clark L I, Moore H, Lam C, Auer M, Hub E, Rot A. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 6.Czaplewski L G, McKeating J, Craven C J, Higgins L D, Appay V, Brown A, Dudgeon T, Howard L A, Meyers T, Owen J, et al. J Biol Chem. 1999;274:16077–16084. doi: 10.1074/jbc.274.23.16077. [DOI] [PubMed] [Google Scholar]
- 7.Rajarathnam K, Sykes B D, Kay C M, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I. Science. 1994;264:90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- 8.Paavola C D, Hemmerich S, Grunberger D, Polsky I, Bloom A, Freedman R, Mulkins M, Bhakta S, McCarley D, Wiesent L, et al. J Biol Chem. 1998;273:33157–33165. doi: 10.1074/jbc.273.50.33157. [DOI] [PubMed] [Google Scholar]
- 9.Laurence J S, Blanpain C, Burgner J W, Parmentier M, Liwang P J. Biochemistry. 2000;39:3401–3409. doi: 10.1021/bi9923196. [DOI] [PubMed] [Google Scholar]
- 10.Proudfoot A E, Borlat F. Methods Mol Biol. 2000;138:75–87. doi: 10.1385/1-59259-058-6:75. [DOI] [PubMed] [Google Scholar]
- 11.Hemmerich S, Paavola C, Bloom A, Bhakta S, Freedman R, Grunberger D, Krstenansky J, Lee S, McCarley D, Mulkins M, et al. Biochemistry. 1999;38:13013–13025. doi: 10.1021/bi991029m. [DOI] [PubMed] [Google Scholar]
- 12.Rajarathnam K, Clark-Lewis I, Sykes B D. Biochemistry. 1995;34:12983–12990. doi: 10.1021/bi00040a008. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarty L, Rogers L, Quach T, Breckenridge S, Kolattukudy P E. J Biol Chem. 1998;273:29641–29647. doi: 10.1074/jbc.273.45.29641. [DOI] [PubMed] [Google Scholar]
- 14.Kuschert G S, Hoogewerf A J, Proudfoot A E I, Chung C W, Cooke R M, Hubbard R E, Wells T N C, Sanderson P N. Biochemistry. 1998;37:11193–11201. doi: 10.1021/bi972867o. [DOI] [PubMed] [Google Scholar]
- 15.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier J L, Delepierre M, Baleux F, Lortat-Jacob H, et al. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 16.Proudfoot A E, Fritchley S, Borlat F, Shaw J P, Vilbois F, Zwahlen C, Trkola A, Marchant D, Clapham P R, Wells T N. J Biol Chem. 2001;276:10620–10626. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]
- 17.Laurence J S, Blanpain C, De Leener A, Parmentier M, Liwang P J. Biochemistry. 2001;40:4990–4999. doi: 10.1021/bi002593w. [DOI] [PubMed] [Google Scholar]
- 18.Graham G J, Wilkinson P C, Nibbs R J, Lowe S, Kolset S O, Parker A, Freshney M G, Tsang M L, Pragnell I B. EMBO J. 1996;15:6506–6515. [PMC free article] [PubMed] [Google Scholar]
- 19.Martin L, Blanpain C, Garnier P, Wittamer V, Parmentier M, Vita C. Biochemistry. 2001;40:6303–6318. doi: 10.1021/bi002670n. [DOI] [PubMed] [Google Scholar]
- 20.Koopmann P D D, Imai T, Whichard L P, Yoshi O, Krangel M S. Clin Immunol. 2001;99:43–52. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, Palmer A C V, Banerjee B, Fritchley S J, Kirby J A. J Biol Chem. 2000;275:11721–11727. doi: 10.1074/jbc.275.16.11721. [DOI] [PubMed] [Google Scholar]
- 22.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 23.Kim K S, Rajarathnam K, Clark-Lewis I, Sykes B D. FEBS Lett. 1996;395:277–282. doi: 10.1016/0014-5793(96)01024-1. [DOI] [PubMed] [Google Scholar]
- 24.Crump M P, Rajarathnam K, Kim K S, Clark-Lewis I, Sykes B D. J Biol Chem. 1998;273:22471–22479. doi: 10.1074/jbc.273.35.22471. [DOI] [PubMed] [Google Scholar]
- 25.Keizer D W, Crump M P, Lee T W, Slupsky C M, Clark-Lewis I, Sykes B D. Biochemistry. 2000;39:6053–6059. doi: 10.1021/bi000089l. [DOI] [PubMed] [Google Scholar]
- 26.Jose P J, Griffiths-Johnson D A, Collins P D, Walsh D T, Moqbel R, Totty N F, Truong O, Hsuan J J, Williams T J. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]