Abstract
Although radioimmunotherapy with radiolabeled intact monoclonal antibodies has demonstrated efficacy in the treatment of lymphoma, it provides low tumor-to-normal-tissue radionuclide target ratios and unwanted prolonged radiation exposure to the bone marrow. To overcome these obstacles, the administration of the radionuclide was separated from that of the antibody by using an anti-IL-2 receptor α antibody single chain Fv-streptavidin fusion protein, followed by radiolabeled biotin to treat lymphoma or leukemia xenografted mice. This Pretarget approach provided extremely rapid and effective tumor targeting, permitting the use of short-lived α-emitting radionuclides. With the β-emitter 90Y, all of the 10 lymphoma-xenografted mice were cured. With the α-emitter 213Bi, significant efficacy was obtained in treating leukemic mice, and, furthermore, when combined with immunotherapy, 7 of 10 leukemic mice were cured. Thus, Pretarget radioimmunotherapy is very promising and could represent the next generation in the treatment of lymphoma and leukemia.
Radioimmunotherapy (RIT) with radiolabeled antibodies has already demonstrated efficacy in the treatment of lymphoma (1–4). However, the long serum half-lives of antibodies yield low tumor-to-normal-tissue ratios and prolongs radiation exposure to normal organs and to radiosensitive bone marrow, which limits the radiation dose that can be safely administered (5, 6). Furthermore, the large size of antibodies yields only slow access to malignant cells in large tumors, precluding the use of short-lived radionuclides including most available α-emitting radionuclides.
To overcome some of the obstacles encountered by conventional RIT, several pretargeting techniques have been developed (7–10). In these techniques, antibody and radionuclides are administered separately, and radioactivity is rapidly and selectively accumulated in tumors, with a parallel reduction of radioactivity in normal tissues. Some of the methods are based on the extremely high affinity of biotin binding to avidin/streptavidin (SA; Ka = 10−15) and rapid pharmacokinetics of the small molecule biotin (10–17). Recently, a promising approach, Pretarget, was reported by the NeoRx Corporation (14–18). In this approach, SA is initially targeted to the antigens on the tumor cell surface by using chemically linked antibody–SA conjugate (antibody–SA) or genetically fused antibody single chain Fv SA fusion protein (scFvSA). This step is followed by a synthetic clearing agent (sCA) to remove unbound antibody-SA or scFvSA from the circulation, which is then followed by radiolabeled 1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid (DOTA)-biotin. This low molecular weight cytotoxic molecule reaches the tumor rapidly, where it is captured by the prelocalized antibody–SA or scFvSA. Unbound radiolabeled DOTA-biotin is concomitantly eliminated in the urine.
Preclinical studies using this Pretarget approach have been performed in different solid tumor models including those involving colon, breast, lung, and epidermoid carcinomas and lymphoma (14–16). The results showed favorable, specific, and rapid targeting of radionuclide to tumors, with very low normal tissue uptakes. Therapeutic studies showed that 70–100% of xenografted mice were cured with 90Y-DOTA-biotin, and the toxicity was significantly lower when compared with 90Y directly labeled intact antibodies. Clinical trails also validated the feasibility of the Pretarget approach (19–22).
Alpha particles are high linear energy transfer helium nuclei, with relatively short effective path lengths in tissues, that are capable of powerful, yet selective, cytotoxicity (23). A single atom emitting an α-particle can kill a target cell (24). Therefore, α-particles are very attractive for cancer therapy, especially for isolated malignant cells as observed in leukemia (17, 25, 26). However, α-emitting radionuclides such as 213Bi or 211At, when conjugated to intact antibody, may be of only limited value in the therapy of tumor masses due to their short physical half-lives, t1/2 = 47 min and 7.2 h, respectively, and the time required to achieve a useful tumor-to-normal-tissue ratio of the radionuclide after administration (27, 28). In contrast, the Pretarget approach, which shows rapid pharmacokinetics for the radionuclide, makes α-emitters feasible for tumor therapy.
The observation that IL-2 receptor α (IL-2Rα, CD25) is not expressed by normal resting cells, but is expressed by a high proportion of the abnormal cells in certain forms of lymphoid neoplasia, provides the rationale for the use of the IL-2Rα as a target for therapeutic agents (29). We treated mice in an adult T cell leukemia (ATL) model with the Pretarget approach using 213Bi-DOTA-biotin after a chemically linked humanized anti-Tac antibody (HAT)-SA conjugate that recognizes CD25 (17). The growth of the malignancy was significantly inhibited, and the survival of the leukemia-bearing mice was prolonged significantly when compared with 213Bi directly labeled to HAT. Considering the clinical application of the system, we have generated a genetically engineered anti-Tac scFvSA. In this study, we investigated the use of the anti-Tac scFvSA in concert with DOTA-biotin armed with α- or β-emitting radionuclides with this Pretarget approach in two CD25-expressing tumor models. In the s.c. lymphoma model, rapid, specific, high tumor uptake of the radioactivity and dramatically high tumor-to-normal-tissue ratios were achieved, which resulted in a cure of the tumor-bearing mice when the β-emitting radionuclide 90Y was used. By using the α-emitting radionuclide 213Bi, very effective results were also demonstrated in the parallel leukemia model that had isolated malignant cells. These studies provide the scientific basis for a clinical trial of this very promising approach, which may yield a breakthrough in the RIT of cancer.
Materials and Methods
Monoclonal Antibody.
HAT, which recognizes IL-2Rα, was obtained from Hoffmann–La Roche (Nutley, NJ; ref. 30). Conjugation of HAT to CHX-A" was performed as described (31).
Preparation of scFvSA.
The anti-Tac and B9E9 (anti-CD20) scFvSAs were expressed as genetic fusion of the single chain variable region (scFv) of the murine anti-Tac or B9E9 antibodies, respectively, to the full-length, genomic SA of Streptomyces avidinii as described (18, 32). These soluble tetrameric targeting agents have a well-defined homogenous composition. In this study, B9E9 scFvSA was used as a control.
Radiolabeling.
The anti-Tac scFvSA was labeled with 125I at a specific activity of 111 kBq/μg (3 μCi/μg; 1 Ci = 37 GBg) by using the Chloramine-T method. Biotinidase-resistant DOTA-biotin and HAT-CHX-A" were labeled with 111In at specific activities of 370 kBq/μg (10 μCi/μg) and 37 kBq/μg (1 μCi/μg), respectively, for biodistribution experiments (16, 33). DOTA-biotin was labeled with either 213Bi or 90Y at specific activities of 18.5–37 MBq/μg (0.5–1 mCi/μg) for therapeutic studies as described (17).
Tumor Cell Lines and Mouse Models.
SUDHL-1 (a kind gift from S. Morris, St. Jude Children's Research Hospital, Memphis, TN) is an anaplastic large cell lymphoma (ALCL) cell line. The ATL cell population MET-1 was established from the peripheral blood of a patient with acute ATL, and the cells were maintained by serial transfer in severe combined immunodeficient/nonobese diabetic (SCID/NOD) mice (34). Both cell lines express CD25 on their cell surface and do not express CD20.
Female nude mice were inoculated s.c. with 1 × 107 SUDHL-1 cells in the right flank (35). Biodistribution and therapy studies were performed when xenografted tumors typically reached about 0.5 cm in maximal diameter.
The ATL model was established by i.p. injection of 1.5 × 107 MET-1 cells into SCID/NOD mice as described previously (17, 34). The therapy experiment was performed on these mice when their serum soluble IL-2Rα levels were >1,000 pg/ml.
Immunoreactivity Assay and Internalization Study.
Immunoreactivity and internalization of the anti-Tac scFvSA were evaluated by using SUDHL-1 cells and compared with that of unmodified HAT by using the methods described (36, 37).
Clearance of Radiolabeled scFvSA.
To evaluate the effect of the sCA, which consists of a bifunctional moiety with multiple N-acetyl-galactosamine residues linked to biotin, on the clearance of circulating scFvSA, six nude mice were injected i.v. with 600 μg of 125I-labeled anti-Tac scFvSA. Eighteen hours later, three of the mice were injected i.v. with 100 μg of sCA. Serial blood samples were taken and counted in a γ-counter.
Biodistribution Study.
SUDHL-1 tumor-bearing mice were injected i.v. with 600 μg of the anti-Tac scFvSA. After allowing 18 h for distribution and tumor localization, 100 μg of sCA was injected i.v. Four hours later, 1 μg of 111In-DOTA-biotin was injected i.v. At intervals after injection of 111In-DOTA-biotin, mice (n = 4 per time point) were killed, and the organ distribution was evaluated.
For comparison, mice (n = 4 per time point) bearing the same tumor were injected i.v. with 10 μg of 111In directly labeled HAT, and the biodistribution was evaluated.
The percentage of the injected dose (ID) per gram of tissue was calculated for each organ. Seven days before administration of the scFvSA, the mice were fed a biotin-free diet (Purina) to reduce their endogenous biotin level. All animal experiments were performed under a National Institutes of Health Animal Committee approved protocol.
Therapy Study.
There are four groups (n = 10 except for nsPRIT group) in the 90Y therapy study, performed in SUDHL-1 tumor-bearing mice by using the same Pretarget approach as used in the biodistribution study. Group 1, Pretarget RIT (PRIT), was treated with 29.6 MBq (800 μCi) of 90Y-DOTA-biotin after the anti-Tac scFvSA targeting and sCA. Group 2, nonspecific PRIT (nsPRIT, n = 9), received 29.6 MBq (800 μCi) of 90Y-DOTA-biotin after the administrations of the B9E9 scFvSA and sCA. Group 3, no-radionuclide PRIT (nrPRIT), received the same anti-Tac scFvSA targeting, sCA, and DOTA-biotin, but without radioactivity. Group 4 did not receive treatment and served as a control.
There were 5 groups (n = 10) in the 213Bi therapy study, performed in MET-1 leukemia-bearing mice. Group 1, PRIT, was treated with 9.25 MBq (250 μCi) of 213Bi-DOTA-biotin following the same anti-Tac scFvSA Pretarget approach. Group 2, nsPRIT, received 9.25 MBq (250 μCi) of 213Bi-DOTA-biotin after administrations of B9E9 scFvSA and sCA. Group 3, immunotherapy (HAT), received 100 μg of HAT weekly for 3 mo. Group 4, combination therapy (PRIT + HAT), received combined therapy with PRIT and HAT. Group 5 did not receive treatment.
Monitoring of Tumor Growth.
SUDHL-1 tumor growth was monitored by measuring tumor size in two orthogonal dimensions twice per week for 2 wk after treatment, and then once per week. The volume was calculated by using the formula 1/2(long dimension)(short dimension)2.
Measurements of the serum concentrations of soluble IL-2Rα and/or human β2μ were performed by using an ELISA to monitor the growth of the leukemia. The ELISA kits were purchased from R & D Systems.
Statistical Analysis.
The serum levels of β2μ and tumor size at different time points for the different treatment groups were analyzed by using the Student t test for unpaired data. In terms of the mouse survival, the statview program was used to generate Kaplan–Meier cumulative survival plots.
Results
The Pretarget approach fulfilled all of the requirements for the effective RIT in two xenograft models, one of lymphoma and the other of leukemia.
Immunoreactivity of the Anti-Tac scFvSA.
The proportion of the radiolabeled anti-Tac scFvSA that bound to SUDHL-1 cells was similar to that observed with radiolabeled HAT. The maximal bindings for anti-Tac scFvSA and HAT were 84.8% and 77.8%, respectively. The slow internalization rates of anti-Tac scFvSA and HAT by SUDHL-1 cells (data not shown) were similar to those observed with leukemic cells as reported (17).
Effect of the sCA on the Clearance of Circulating scFvSA.
After i.v. injection, the terminal serum half-life of anti-Tac scFvSA was 11 h. The scFvSA was rapidly removed from the blood by a single injection of sCA. Radioactivity dropped from 6.19% ID/g to 1.42% ID/g in the blood within 4 h after sCA administration.
Biodistribution.
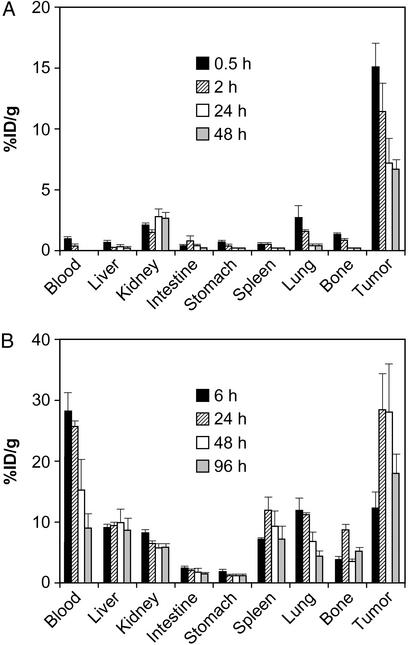
The biodistribution of 111In-DOTA-biotin with the Pretarget approach in SUDHL-1 tumor-bearing mice is shown in Fig. 1A. The highest concentration was at the tumor at all time points examined. Tumor uptake of 111In-DOTA-biotin reached >15% ID/g, and all tumor-to-normal-tissue ratios were >7 at 0.5 h after injection. Tumor-to-blood ratios were from 15 at 0.5 h to >1,000 at 48 h postinjection. In contrast, radiolabeled HAT showed persistent high localization in normal tissues, and the tumor-to-blood ratio was only ≈1 at 24 h (Fig. 1B).
Figure 1.
Biodistributions of radioactivity in SUDHL-1 lymphoma-bearing mice. (A) Pretarget approach. (B) Directly labeled HAT. Bars represent the mean ± SD of the percentage of ID per gram of tissue (%ID/g).
Therapy Study.
90Y therapy.
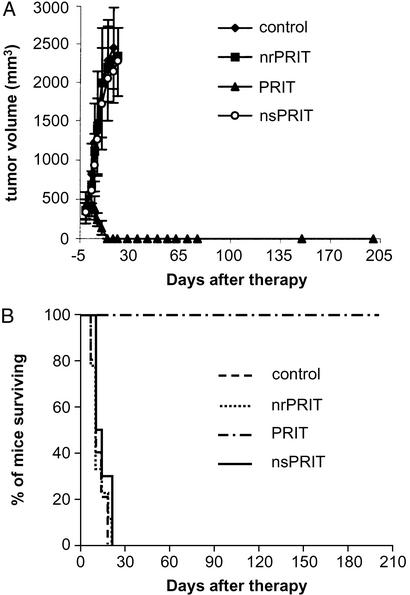
PRIT with 90Y-DOTA-biotin was performed in the SUDHL-1 lymphoma model. SUDHL-1 tumors in the control group grew rapidly, from 0.35 cm3 one day before therapy, to ≈2 cm3 within 3 wk (Fig. 2A), and these mice were killed according to our animal protocol. In the PRIT group, tumor growth was halted, and the tumor sizes decreased to undetectable within 2 wk (Fig. 2A). All of the 10 mice in this group became tumor free and remained healthy for >6 mo, significantly prolonged survival when compared with the other three groups (Fig. 2B; P < 0.0001).
Figure 2.
Therapy of SUDHL-1 lymphoma-bearing mice with 90Y-DOTA-biotin in the Pretarget approach. Tumor volume (A) and Kaplan–Meier survival (B) plots are shown. nrPRIT, no-radionuclide PRIT.
To confirm the specificity of the therapeutic effect, we compared specific anti-Tac scFvSA PRIT with the nsPRIT by using the irrelevant B9E9 scFvSA as the targeting agent. The tumor size and survival in the nsPRIT group was significantly different from that in the specific PRIT group (Fig. 2; P < 0.0001). No efficacy was observed in the nsPRIT or the nrPRIT group compared with the control group (Fig. 2; P > 0.1).
213Bi therapy.
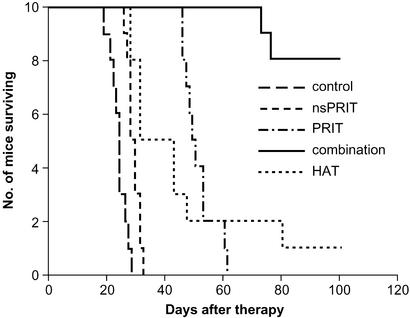
PRIT with 213Bi-DOTA-biotin was performed in the MET-1 leukemia model. In this model, there are isolated leukemic cells in all organs, a pattern that contrasts with the tumor mass present in the SUDHL-1 lymphoma model. Tumor growth was inhibited significantly with the PRIT as seen by the effect on the serum levels of the surrogate tumor marker, human β2μ (Table 1). At 14 days after therapy, the level of β2μ was 0.106 μg/ml in the PRIT group as compared with 10.36 μg/ml in the control group (Table 1, P < 0.00001). Furthermore, survival of the mice in the PRIT group was significantly prolonged as compared with the control group (Fig. 3; P < 0.0001). The median survival duration was 51.3 days in the PRIT group as compared with 23.8 days in the control group.
Table 1.
Serum levels of sIL-2Rα and β2μ in the 213Bi therapeutic study
| Group | Days after therapy
|
|||
|---|---|---|---|---|
| −2
|
14
|
28
|
||
| sIL-2Rα, pg/ml | β2μ, μg/ml | β2μ, μg/ml | β2μ, μg/ml | |
| Control | 39,980 ± 15,740 | 0.520 ± 0.350 | 10.360 ± 2.930 | NA |
| nsPRIT | 40,001 ± 13,920 | 0.675 ± 0.469 | 4.355 ± 1.711* | 12.877 ± 3.242168 |
| PRIT | 39,997 ± 12,444 | 0.611 ± 0.272 | 0.106 ± 0.174**,** | 4.812 ± 2.517168 |
| Combination | 39,115 ± 13,246 | 0.485 ± 0.204 | 0.000 ± 0.000**,** | 0.103 ± 0.313 |
| HAT | 39,779 ± 11,581 | 0.343 ± 0.307 | 2.925 ± 2.493** | 8.178 ± 5.653168 |
NA, not available (no mice surviving).
, P < 0.0001 compared with control group;
, P < 0.00001 compared with control group;
, P < 0.00001 compared with nsPRIT;
, P < 0.0005 compared with combination group.
Figure 3.
Kaplan–Meier survival plot of MET-1 leukemia-bearing SCID/NOD mice with 213Bi-DOTA-biotin in the Pretarget approach.
The specificity of the therapeutic effect was confirmed, by comparison with the nsPRIT, by using B9E9 scFvSA as the targeting agent. The serum level of β2μ was significantly lower with specific PRIT than with nsPRIT (P < 0.00001), and there was a significant prolongation of survival of the mice in the PRIT group when compared with the nsPRIT group (Fig. 3; P < 0.0001).
Immunotherapy with nonradiolabeled HAT administered weekly at doses of 100 μg for 3 mo demonstrated efficacy in this model (17, 34). Tumor growth was inhibited (Table 1, P < 0.00001 at 14 days after therapy), and survival of the mice was prolonged (Fig. 3; P < 0.0001) significantly when compared with the control group.
Combination therapy, involving 9.25 MBq (250 μCi) of 213Bi in the Pretarget protocol as used above followed by weekly doses of HAT for 3 mo, showed improved therapeutic results when compared with either PRIT or HAT alone. The serum levels of β2μ were significantly lower in the combination group when compared with those in the PRIT or HAT groups at 28 days after therapy (Table 1, P < 0.0005). Furthermore, there was a significant prolongation of survival of the mice in the combination group as compared with either the PRIT or HAT groups (Fig. 3; P < 0.0001). At 100 days after therapy, β2μ could not be detected in 7 of the 8 mice alive in the combination group, but only 1 mouse with high serum β2μ level in the HAT group was alive and none were alive in the other groups.
Discussion
There are a number of components that must be considered in designing an optimal systemic RIT strategy including: (i) the selection of the monoclonal antibody and thus the antigenic target, (ii) the choice of the delivery system used to target the radionuclide to the tumor cell, and (iii) the choice of the radionuclide. In the present study, we have chosen the human IL-2Rα receptor subunit identified by the anti-Tac antibody as our target. The scientific basis for this choice is that IL-2Rα is not expressed by most resting cells whereas this receptor is expressed by the abnormal cells in certain forms of lymphoid neoplasia (29). Furthermore, the slow rate of internalization of the anti-CD25 antibody fulfills a critical requirement of this strategy (17).
A second issue in designing an optimal RIT reagent is the choice of the method used to deliver the radionuclide to the tumor cell. In our previous clinical trials, we used intact antibodies to deliver the radionuclide 90Y (2). There are a number of limitations in this approach. Only modest tumor-to-normal-tissue ratios are achieved. In addition, the long serum half-life of the intact monoclonal antibody prolongs radiation exposure to normal organs, including the radiosensitive bone marrow, which limits the radiation dose that can be safely administered (5, 6). Furthermore, because of the slow equilibration of intact antibodies with cells in tumor masses, one cannot use short-lived α-emitting radionuclides, but is limited to longer-lived β-emitting radionuclides that deliver only low-dose irradiation that may be insufficient to kill the tumor cell. To circumvent these obstacles, the approach used in the present study involves a multistep targeting system that separates the delivery of the radionuclide from the delivery of the antibody. Using this approach, we delivered large quantities of radioactivity to the tumor, with the remaining radionuclide being rapidly cleared by the kidneys. In our study, when compared with standard RIT, we were able to achieve dramatic increases in the tumor-to-normal-tissue ratios of radionuclide delivered and therefore could administer 5- to 10-fold more radionuclide to the tumor with the same bone marrow exposure when compared with that achieved when intact antibodies are used as carriers of the radionuclide.
The third component of an optimal RIT regimen requires consideration of the nature of the radionuclide and the overall application that is intended. In this and other studies, we observed an advantage of the β-emitting radionuclide 90Y with its crossfire when large tumor masses such as those observed with a lymphoma were treated (14, 16). In contrast, short-lived α-emitting radionuclides such as 213Bi were ineffective with large tumor masses (28). However, as the tumor mass decreases, the benefit of the crossfire effect also decreases. Thus, in considering treatment of small tumors, including micrometastases as well as individual tumor cells, such as the leukemic cells of the MET-1 model of ATL, therapeutic efficacy may become limited, due to the fact that high-energy β-emitting radionuclides, with their long irradiation path, deliver a high proportion of their radiation to the tissue surrounding the tumor. Additionally, the number of relatively weak β-emission traversals required to kill the tumor cell may not be achieved with single cells as is true with leukemia. For such forms of malignancy the future development of isotopic multistep approaches, such as the Pretarget method, may focus on α-emitting radionuclides to most effectively kill tumor cells without damaging adjacent normal tissues.
Results of the Pretarget approach in the SUDHL-1 lymphoma model with 90Y and in the MET-1 model of ATL with 213Bi were encouraging. However, with the latter model system involving T cell leukemia, not all of the malignant T cells were eliminated by a single course of therapy. Relevant to this issue is an emerging paradigm that suggests for cancer therapy that the efficacy of two therapeutic agents with different models of action may be greater than additive against tumor cells. In our combination trial, we achieved the complementary actions of receptor saturating doses of HAT to yield antibody dependent cellular cytotoxicity and cytokine deprivation-mediated leukemic cell death, along with the tumor cytoreduction provided by irradiation mediated by the radionuclide 213Bi delivered to leukemic cell surfaces. This combination yielded the highest efficacy observed with acceptable toxicity.
In conclusion, the Pretarget approach, with β-emitting radionuclides for the large tumor masses that are seen with lymphomas and with α-emitting radionuclides for the therapy of the isolated cells in leukemia and micrometastases, provides a major opportunity to dramatically increase the radiation that can be safely delivered to the tumor; thus, it could provide a breakthrough for the RIT of cancer. Furthermore, our emerging understanding of the IL-2/IL-2R system in normal and leukemic cells opens the possibility for the use of this approach in the IL-2R directed therapy of leukemia and lymphoma. These findings support the use of this Pretarget approach with the anti-Tac scFvSA targeting agent followed by radiolabeled DOTA-biotin alone, or together with saturating doses of HAT, in a clinical trial involving patients with IL-2Rα expressing leukemias and lymphomas.
Acknowledgments
This work was supported in part by funding from NeoRx to J.S. and D.B.A.
Abbreviations
- RIT
radioimmunotherapy
- SA
streptavidin
- scFvSA
antibody single chain Fv SA fusion protein
- sCA
synthetic clearing agent
- DOTA
1,4,7,10-tetraazacyclododecane-N,N′,N",N‴-tetraacetic acid
- IL-2Rα
IL-2 receptor α
- ATL
adult T cell leukemia
- HAT
humanized anti-Tac antibody
- PRIT
Pretarget RIT
- nsPRIT
nonspecific PRIT
- SCID/NOD
severe combined immunodeficient/nonobese diabetic
- ID
injected dose
References
- 1.Knox S J, Goris M L, Trisler K, Negrin R, Davis T, Liles T M, Grillo-Lopez A, Chinn P, Varns C, Ning S C, et al. Clin Cancer Res. 1996;2:457–470. [PubMed] [Google Scholar]
- 2.Waldmann T A, White J D, Carrasquillo J A, Reynolds J C, Paik C H, Gansow O A, Brechbiel M W, Jaffe E S, Fleisher T A, Goldman C K. Blood. 1995;86:4063–4075. [PubMed] [Google Scholar]
- 3.Kaminski M S, Zasadny K R, Francis I R, Milik A W, Ross C W, Moon S D, Crawford S M, Burgess J M, Petry N A, Butchko G M, et al. N Engl J Med. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 4.Press O W, Eary J F, Appelbaum F R, Martin P J, Badger C C, Nelp W B, Glenn S, Butchko G, Fisher D, Porter B, et al. N Engl J Med. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 5.Knox S J. Cancer Res. 1995;55:5832s–5836s. [PubMed] [Google Scholar]
- 6.Cassidy J, Newell D R, Wedge S R, Cummings J. Cancer Surv. 1993;17:315–341. [PubMed] [Google Scholar]
- 7.Goodwin D A. Eur J Nucl Med. 1984;9:209–215. doi: 10.1007/BF00448541. [DOI] [PubMed] [Google Scholar]
- 8.Hnatowich D J, Virzi F, Rusckowski M. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 9.Bardies M, Bardet S, FaivreChauvet A, Peltier P, Douillard J Y, Mahe M, Fiche M, Lisbona A, Giacalone F, Meyer P, et al. J Nucl Med. 1996;37:1853–1859. [PubMed] [Google Scholar]
- 10.Kalofonos H P, Rusckowski M, Siebecker D A, Sivolapenko G B, Snook D, Lavender J P, Epenetos A A, Hnatowich D J. J Nucl Med. 1990;31:1791–1796. [PubMed] [Google Scholar]
- 11.Paganelli G, Magnani P, Zito F, Villa E, Sudati F, Lopalco L, Rossetti C, Malcovati M, Chiolerio F, Seccamani E, et al. Cancer Res. 1991;51:5960–5966. [PubMed] [Google Scholar]
- 12.Yao Z, Zhang M, Kobayashi H, Sakahara H, Nakada H, Yamashina I, Konishi J. J Nucl Med. 1995;36:837–841. [PubMed] [Google Scholar]
- 13.Zhang M, Yao Z, Saga T, Sakahara H, Nakamoto Y, Sato N, Nakada H, Yamashina I, Konishi J. J Nucl Med. 1998;39:30–33. [PubMed] [Google Scholar]
- 14.Axworthy D B, Reno J M, Hylarides M D, Mallett R W, Theodore L J, Gustavson L M, Su F, Hobson L J, Beaumier P L, Fritzberg A R. Proc Natl Acad Sci USA. 2000;97:1802–1807. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Press O W, Corcoran M, Subbiah K, Hamlin D K, Wilbur D S, Johnson T, Theodore L, Yau E, Mallett R, Meyer D L, Axworthy D. Blood. 2001;98:2535–2543. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 16.Yao Z, Zhang M, Axworthy D B, Wong K J, Garmestani K, Park L, Park C W, Mallett R W, Theodore L J, Yau E K, et al. Cancer Res. 2002;62:5755–5760. [PubMed] [Google Scholar]
- 17.Zhang M, Yao Z, Garmestani K, Axworthy D B, Zhang Z, Mallett R W, Theodore L J, Goldman C K, Brechbiel M W, Carrasquillo J A, Waldmann T A. Blood. 2002;100:208–216. doi: 10.1182/blood-2002-01-0107. [DOI] [PubMed] [Google Scholar]
- 18.Goshorn S, Sanderson J, Axworthy D, Lin Y, Hylarides M, Schultz J. Cancer Biother Radiopharmacol. 2001;16:109–123. doi: 10.1089/108497801300189209. [DOI] [PubMed] [Google Scholar]
- 19.Breitz H B, Fisher D R, Goris M L, Knox S, Ratliff B, Murtha A D, Weiden P L. Cancer Biother Radiopharmacol. 1999;14:381–395. doi: 10.1089/cbr.1999.14.381. [DOI] [PubMed] [Google Scholar]
- 20.Breitz H B, Weiden P L, Beaumier P L, Axworthy D B, Seiler C, Su F M, Graves S, Bryan K, Reno J M. J Nucl Med. 2000;41:131–140. [PubMed] [Google Scholar]
- 21.Weiden P L, Breitz H B, Press O, Appelbaum J W, Bryan J K, Gaffigan S, Stone D, Axworthy D, Fisher D, Reno J. Cancer Biother Radiopharmacol. 2000;15:15–29. doi: 10.1089/cbr.2000.15.15. [DOI] [PubMed] [Google Scholar]
- 22.Knox S J, Goris M L, Tempero M, Weiden P L, Gentner L, Breitz H, Adams G P, Axworthy D, Gaffigan S, Bryan K, et al. Clin Cancer Res. 2000;6:406–414. [PubMed] [Google Scholar]
- 23.Humm J L, Chin L M. Radiat Res. 1993;134:143–150. [PubMed] [Google Scholar]
- 24.Nikula T K, McDevitt M R, Finn R D, Wu C, Kozak R W, Garmestani K, Brechbiel M W, Curcio M J, Pippin C G, Tiffany-Jones L, et al. J Nucl Med. 1999;40:166–176. [PubMed] [Google Scholar]
- 25.McDevitt M R, Ma D, Lai L T, Simon J, Borchardt P, Frank R K, Wu K, Pellegrini V, Curcio M J, Miederer M, et al. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 26.Macklis R M, Kinsey B M, Kassis A I, Ferrara J L, Atcher R W, Hines J J, Coleman C N, Adelstein S J, Burakoff S J. Science. 1988;240:1024–1026. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann F, Horak E M, Garmestani K, Wu C, Brechbiel M W, Kozak R W, Tso J, Kosteiny S A, Gansow O A, Nelson D L, et al. Cancer Res. 1994;54:4362–4370. [PubMed] [Google Scholar]
- 28.Horak E, Hartmann F, Garmestani K, Wu C, Brechbiel M, Gansow O A, Landolfi N F, Waldmann T A. J Nucl Med. 1997;38:1944–1950. [PubMed] [Google Scholar]
- 29.Waldmann T A, Pastan I H, Gansow O A, Junghans R P. Ann Intern Med. 1992;116:148–160. doi: 10.7326/0003-4819-116-2-148. [DOI] [PubMed] [Google Scholar]
- 30.Queen C, Schneider W P, Selick H E, Payne P W, Landolfi N F, Duncan J F, Avdalovic N M, Levitt M, Junghans R P, Waldmann T A. Proc Natl Acad Sci USA. 1989;86:10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirzadeh S, Brechbiel M W, Atcher R W, Gansow O A. Bioconjugate Chem. 1990;1:59–65. doi: 10.1021/bc00001a007. [DOI] [PubMed] [Google Scholar]
- 32.Schultz J, Lin Y, Sanderson J, Zuo Y, Stone D, Mallett R, Wilbert S, Axworthy D. Cancer Res. 2000;60:6663–6669. [PubMed] [Google Scholar]
- 33.Camera L, Kinuya S, Pai L H, Garmestani K, Brechbiel M W, Gansow O A, Paik C H, Pastan I, Carrasquillo J A. Cancer Res. 1993;53:2834–2839. [PubMed] [Google Scholar]
- 34.Phillips K E, Herring B, Wilson L A, Rickford M S, Zhang M, Goldman C K, Tso J Y, Waldmann T A. Cancer Res. 2000;60:6977–6984. [PubMed] [Google Scholar]
- 35.Turturro F, Heineke H L, Drevyanko T F, Link C J, Jr, Seth P. Gene Ther. 2000;7:930–933. doi: 10.1038/sj.gt.3301186. [DOI] [PubMed] [Google Scholar]
- 36.Yao Z, Sakahara H, Zhang M, Kobayashi H, Nakada H, Yamashina I, Konishi J. Nucl Med Biol. 1995;22:199–203. doi: 10.1016/0969-8051(94)00092-x. [DOI] [PubMed] [Google Scholar]
- 37.Yao Z, Garmestani K, Wong K J, Park L S, Dadachova E, Yordanov A, Waldmann T A, Eckelman W C, Paik C H, Carrasquillo J A. J Nucl Med. 2001;42:1538–1544. [PubMed] [Google Scholar]