Abstract
Cellular adhesive events affect cell proliferation and differentiation decisions. How cell surface events mediating adhesion transduce signals to the nucleus is not well understood. After cell–cell or cell–substratum contact, cytosolic proteins are recruited to clustered adhesion receptor complexes. One such family of cytosolic proteins found at sites of cell adhesion is the Zyxin family of LIM proteins. Here we demonstrate that the family member Ajuba was recruited to the cell surface of embryonal cells, upon aggregate formation, at sites of cell–cell contact. Ajuba contained a functional nuclear export signal and shuttled into the nucleus. Importantly, accumulation of the LIM domains of Ajuba in the nucleus of P19 embryonal cells resulted in growth inhibition and spontaneous endodermal differentiation. The differentiating effect of Ajuba mapped to the third LIM domain, whereas regulation of proliferation mapped to the first and second LIM domains. Ajuba-induced endodermal differentiation of these cells correlated with the capacity to activate c-Jun kinase and required c-Jun kinase activation. These results suggest that the cytosolic LIM protein Ajuba may provide a new mechanism to transduce signals from sites of cell adhesion to the nucleus, regulating cell growth and differentiation decisions during early development.
INTRODUCTION
How cells respond to environmental signals affects proliferation, differentiation, locomotion, and survival decisions, functions that are critically important during development, adult homeostasis, and response to injury and that are often altered in pathological processes. Growth factors and cytokines, adhesion to the extracellular matrix, and cell–cell adhesion all regulate cell and tissue growth. These signals are transduced from cell surface receptor complexes to the nucleus via cytosolic intermediates, either directly or through a relay involving multiple interacting proteins. In the nucleus, these signal-transducing proteins affect transcriptional regulation of a panel of genes, either directly (e.g., cytokine signals and STAT proteins), through interacting proteins (e.g., wnt signals and β-catenin), or by both mechanisms (e.g., TGF signals and Smad proteins), leading to specific cellular responses (Ihle et al., 1997; Miller et al., 1999; Massague and Chen, 2000). Although our understanding of growth factor and cytokine receptor signal transduction to the nucleus has increased greatly, how information from sites of cell adhesion is communicated to the nucleus remains less well understood.
Cells adhere to the extracellular matrix at focal sites of contact. The major families of cell surface adhesion receptors that recognize and attach to the extracellular matrix are the integrins and the syndecan family of proteoglycans (Hynes, 1992; Woods and Couchman, 1998). At sites of cell–cell contact, adhesion is mediated primarily by intercellular interactions mediated by members of the cadherin family of proteins (Takeichi, 1995; Steinberg and McNutt, 1999). Both adhesive events are generally analogous in that they recruit cytosolic proteins to clustered receptor complexes. These associated proteins anchor the complex to the underlying actin cytoskeleton. Cell adhesive events also result in remodeling of the actin cytoskeleton underlying the adhesion site, giving rise to focal adhesions and adherens junction structures. How adhesive events lead to local cytoskeletal reorganization is not clear, but the actions of the Rho family of GTPases, inositol phosphates, and calcium appear to be important regulators (Braga et al., 1997; Hall, 1998; Vasioukhin et al., 2000). Finally, knowledge of cell contact or adhesion needs to be relayed to the nucleus, affecting cell proliferation and fate decisions.
During development of multicellular organisms, cell adhesive events are crucial (Pfeifer, 1995; Gumbiner, 1996). For example, the cytoplasmic domain of E-cadherin associates with the actin cytoskeleton through interactions with the catenin protein family (Cowin and Burke, 1996). β-Catenin binds to the cytoplasmic domain of cadherin. α-Catenin binds to β-catenin, thereby providing a link to the actin cytoskeleton through its association with actin-binding proteins (Drubin and Nelson, 1996). β-Catenin also appears to play a regulatory, or signaling, role. Through an association with members of the Lef1/Tcf family of transcription factors present in the cytosol, β-catenin/Tcf complexes translocate into the nucleus and activate the expression of genes crucial for axis determination in early vertebrate development (Molenaar et al., 1996; Wylie et al., 1996; Brannon et al., 1997). In muscle cells, N-cadherin engagement affects growth and differentiation decisions (Goichberg and Geiger, 1998). Thus, determining how cellular adhesive events lead to specific cellular responses is clearly important for understanding early development and tissue homeostasis.
Several other proteins also accumulate at sites of cell–cell adhesion, some through an association with α-catenin, e.g., vinculin and α-actinin (Knudsen et al., 1995; Hazan et al., 1997), whereas others are recruited through associations with α-actinin. One such family of α-actinin–binding proteins are cytosolic LIM domain–containing proteins of the Zyxin family (Beckerle, 1997). In addition to Zyxin, this family includes thyroid hormone receptor–interacting protein 6 (Trip6), LIM-containing lipoma-preferred partner (LPP), Ajuba, and the human LIMD1 gene product (Lee et al., 1995; Petit et al., 1996; Goyal et al., 1999; Kiss et al., 1999). These proteins are composed of three related tandem LIM domains at the C terminus associated with unique and distinctive N-terminal, or PreLIM, domains. Although these proteins all appear to associate with the cytoskeleton at focal adhesion sites and sites of cell–cell contact, their cellular functions are largely unknown (Beckerle, 1988; Crawford and Beckerle, 1991; Wang et al., 1999; Petit et al., 2000).
The PreLIM domains are rich in proline residues, with stretches conforming to consensus SH3 recognition sites (Feng et al., 1994; Alexandropoulos et al., 1995). In fact, the PreLIM domains of Zyxin and Ajuba have been shown to interact with the SH3 domains of Vav and Grb2, respectively (Hobert et al., 1996; Goyal et al., 1999). The functional significance of the Zyxin/Vav association is not known, but the association between Ajuba and the cytosolic adapter protein Grb2 results in activation of the MAPK ERK (Goyal et al., 1999). Expression of Ajuba in Xenopus oocytes promotes meiotic maturation through activation of ERK in a Grb2- and Ras-dependent manner, indicating that Ajuba affects intracellular signaling pathways (Goyal et al., 1999).
Recently, Zyxin was shown to contain a functional NES and to shuttle between the nucleus and the cytoplasm (Nix and Beckerle, 1997). The biological implications of translocation of Zyxin into the nucleus are unknown, but it may play a role in transducing signals from sites of adhesion to the nucleus. Thus, we sought to determine the subcellular trafficking of Ajuba. Specifically, we asked whether Ajuba translocates from adhesive sites or the cytosol into the nucleus and, importantly, whether this intracellular trafficking affects the regulation of cell growth and differentiation decisions.
MATERIALS AND METHODS
Reagents, Antiserum, and Cell Lines
Retinoic acid, anisomycin, and myelin basic protein were purchased from Sigma Chemical (St. Louis, MO). Leptomycin B was provided by Minoru Yoshida (University of Tokyo). Zyxin and Trip6 cDNAs were provided by M. Beckerle (University of Utah, Salt Lake City, UT). The plasmid pCDNA3-JNK1 (APF) containing a kinase-dead form of JNK1 was provided by Dr. R. Davis (University of Massachusetts Medical School, Worcester, MA) (Derijard et al., 1994). pGEX c-Jun was provided by Dr. M. Karin (University of California, San Diego). The human IL2 receptor α cDNA was provided by Susan LaFlamme (Albany Medical College, Albany, NY) (LaFlamme et al., 1992).
Ajuba polyclonal rabbit antiserum was generated against a GST–PreLIM domain fusion protein and affinity purified. C-Myc epitope polyclonal rabbit antiserum was generated and provided by Dr. A. Shaw (Washington University). Monoclonal myc epitope antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). The mAbs MC-480 (SSEA 1), TROMA-1, and 2H3 were obtained from the National Institutes of Health developmental studies hybridoma bank (Iowa City, Iowa). Anti-JNK1, anti-p38, and anti-ERK2 antisera were from Santa Cruz Biotechnology. Anti-FLAG antiserum was from Kodak Laboratories (Rochester, NY). NIH 3T3 fibroblasts, P19 embryonal carcinoma cells, and embryonal stem cells were obtained from the American Type Culture Collection (Rockville, MD).
Cell Culture, Cell Transfection, Proliferation, and Differentiation Assays
NIH 3T3 fibroblasts were maintained in DMEM (Life Technologies, Grand Island, NY) containing 10% FCS in a 5% CO2–humidified chamber. P19 cells were cultured in DMEM supplemented with 10% FCS in a 5% CO2–humidified chamber. Transfection of fibroblasts and P19 cells was carried out with the use of the Lipofectamine reagent according to the manufacturer's instructions (Life Technologies). To select stable clones after transfection, P19 cells were cultured in medium containing 0.4 mg/ml G418 (Life Technologies).
For proliferation assays, P19 cells were washed three times in DMEM/FCS, and 1 × 105 cells were cultured in 3 ml of P19 growth medium. Triplicate wells for each time point were established at time zero. Each day for 4 d, the total number of viable cells per well was determined by counting after the addition of trypan blue. Results were plotted with the use of the Delta Graph program (SSPS, Inc., Chicago, IL). To induce endodermal differentiation, P19 cells were cultured as monolayers on tissue culture plates in growth medium supplemented with 10 nM retinoic acid (all-trans; Sigma) for 2–4 d (Roy et al., 1995). For cells treated with Leptomycin B, 20 μM Leptomycin B was added to cultures and incubated for 30 min at 37°C in 5% CO2.
Plasmids
All Ajuba cDNAs were subcloned in the eukaryotic expression plasmid pMEX.Neo. Those constructs subcloned as PCR products were subjected to DNA sequencing. All constructs contained a hexa-myc epitope tag at the N-terminal end and a neomycin cassette for selection with G418. The JNK1-APF insert was subcloned into the pBabe.puro vector. This vector allowed for alternate selection with puromycin. Some constructs were subcloned into expression plasmids containing enhanced green fluorescent protein (GFP) (Clontech, Palo Alto, CA) to generate EGFP fusion proteins.
Protein Immunoblot Analysis
Cells were washed in PBS and then lysed in “lysis” buffer (1% Triton X-100 or 1% NP-40, 20 mM Tris-Cl, pH 7.4, 140 mM NaCl [TBS], 5 mM EDTA) or RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS, pH 7.4) containing 1 mM sodium vanadate, 2 mM PMSF, and 10 IU/ml aprotinin for 15 min on ice. Lysates were clarified by centrifugation at 10,000 × g for 20 min, and the detergent-soluble supernatant was saved for further analysis. Detergent-soluble proteins were separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose (Schleicher & Schuell, Keene, NH). Membranes were incubated in TBS/T blocking solution (TBS, pH 7.4, containing 5% [wt/vol] milk and 0.1% [vol/vol] Tween-20; or TBS/T, pH 7.4, containing 3.0% [wt/vol] BSA) followed by incubation with primary antibodies. After washing in TBS/T, membranes were incubated with HRP-coupled donkey anti-rabbit or anti-mouse immunoglobulin G (Amersham, Arlington Heights, IL). Membranes were washed and immunoreactive bands visualized with ECL chemiluminescence reagents (Amersham). Autorads were scanned into Adobe (Mountain View, CA) Photoshop for production of figures.
Protein Immunoprecipitation and Kinase Assays
Solid-phase kinase assays for ERK, JNK, and p38 were performed with the use of immunoprecipitates obtained from 1 mg of the total protein in cell lysates. The immunoprecipitates were washed twice with lysis buffer and then twice with kinase assay buffer (20 mM HEPES, pH 7.5, 20 mM MgCl2, 0.1 mM Na3VO4, 2 mM DTT, 20 mM B-glycerophosphate). Immunoprecipitates were resuspended in a final volume of 40 μl of the assay buffer. A 20-μl aliquot was used for Western blot analysis, and the other 20 μl was used for phosphorylation assay. GST–c-Jun, myelin basic protein (Sigma), and GST–ATF2 were used as substrates for JNK, ERK, and p38, respectively. Reactions were started by the addition of [γ-32P]ATP (10 μCi/reaction, 100 μM final concentration) and incubated for 30 min at 30°C. Reactions were terminated by the addition of Laemmli buffer (200 mM Tris-HCl, pH 6.8, 0.4 mM DTT, 8% SDS, 0.08% bromphenol blue, 40% glycerol). After separation of proteins by SDS-PAGE under reducing conditions and staining with Coomassie Brilliant Blue R-250, gels were dried and autoradiography was performed.
Indirect Immunofluorescence
Cells were grown in chamber slides (Becton-Dickinson, Franklin Lakes, NJ) and fixed for 10 min with 3% paraformaldehyde. Slides were rinsed three times with modified Shield's medium/piperazine-N,N′-bis[2-ethanesulfonic acid] (MSM-PIPES; 18 mM MgSO4, 5 mM CaCl2, 40 mM KCl, 24 mM NaCl, 5 mM PIPES, pH 6.8, 0.5% Triton X-100, 0.5% NP-40) after fixation. The fixed cells were incubated with primary antibodies and then washed three times with MSM-PIPES buffer. Fluorescein- or rhodamine-conjugated goat anti-rat immunoglobulin G (for TROMA-1) or anti-mouse immunoglobulin M (for SSEA-1, myc, and IL2R) was then added. The cells were washed three times with blotting buffer (560 mM NaCl, 10 mM KPO4, pH 7.5, 0.1% Triton X-100, 0.02% SDS). The images were photographed with the use of a digital camera attached to the fluorescence microscope (Nikon, Garden City, NY) and processed as Adobe Photoshop files.
RESULTS
Ajuba Was Present in Embryonal Cells, and Endodermal Differentiation of Embryonal Carcinoma Cells Was Associated with Increased Ajuba Protein Expression
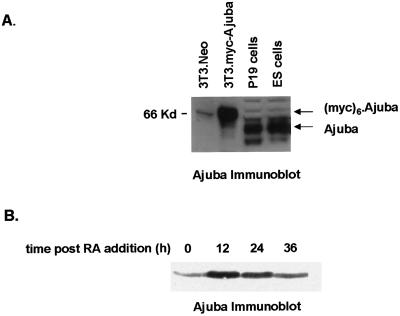
Early during murine fetal development (ED3.5 blastocyst and ED7.5 embryo), Ajuba was expressed in the cells of all three germ layers and the placenta. Soon thereafter, expression was dramatically restricted to the developing skin, genitourinary system, and neuroectoderm (our unpublished results). These observations suggested that Ajuba might play a role in early developmental decisions. To explore this possibility in a model system, we first determined whether embryonic cell lines expressed Ajuba protein. Immunoblot analysis of cell extracts from the P19 embryonal carcinoma cell line and embryonal stem cells (ES cells) with Ajuba antiserum demonstrated that protein was indeed present in both cells (Figure 1A, lanes 3 and 4). NIH 3T3 fibroblasts do not express Ajuba, and thus no protein was detected with Ajuba antiserum (Figure 1A, lane 1). After transfection of these cells with myc-tagged Ajuba, Ajuba protein expression was readily detected (Figure 1A, lane 2); the increase in apparent size reflected the addition of a hexa-myc N-terminal epitope tag.
Figure 1.
Ajuba is expressed in embryonal cells, and its level increases during endodermal differentiation. (A) Immunoblot analysis for Ajuba expression in cell extracts from NIH 3T3 fibroblasts (lane1), NIH 3T3 fibroblasts containing myc-Ajuba (lane 2), P19 embryonal carcinoma cells (lane 3), and embryonal stem cells (lane 4). P19 cells were incubated in medium containing 10 nM atRA, and cell extracts were prepared before and 12, 24, and 36 h after treatment. (B) Anti-Ajuba immunoblotting was performed at each time point. A total of 25 μg of protein was loaded in each lane.
Embryonal carcinoma cells are an excellent ex vivo model of murine preimplantation development. These cells are potentially totipotent. When injected into blastocysts, they can contribute to all tissues (Rudnicki and McBurney, 1987). In culture, the addition of the all-trans retinoic acid (atRA) to P19 embryonal cells induces growth inhibition and differentiation. At low doses of atRA (10–20 nM), P19 cells differentiate from an ectodermal phenotype into endodermal-like cells, whereas at higher concentrations (100 nM), terminal neuroectodermal differentiation results (Roy et al., 1995). Thus, P19 cells could serve as an excellent model system to determine the cellular functions of Ajuba in early developmental decisions.
Therefore, we asked whether during atRA-induced endodermal differentiation of P19 cells the level of Ajuba protein was altered. Immunoblot analysis for Ajuba protein expression at progressive times after low-dose (10 nM) atRA addition was performed. By 24 h after atRA addition, P19 cells began to express markers indicative of endodermal differentiation and lost markers specific for ectodermal cells (Figure 2D, panels A–D). By 12 h after atRA addition, the level of Ajuba protein was increased approximately fivefold (Figure 1B, lane 2). At 24 h, the Ajuba protein level was still increased, but by 36 h it had returned to a level approximating that in parental cells (Figure 1B, lanes 3 and 4). Thus, Ajuba protein was present in embryonal P19 cells, and the level of expression was transiently increased after atRA-induced endodermal differentiation, suggesting that Ajuba may play a role in this process.
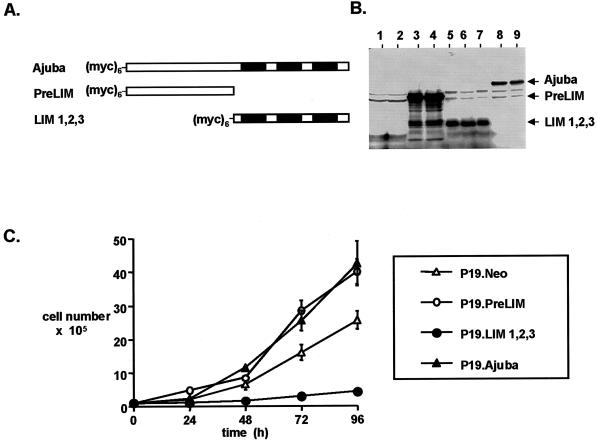
Figure 2.
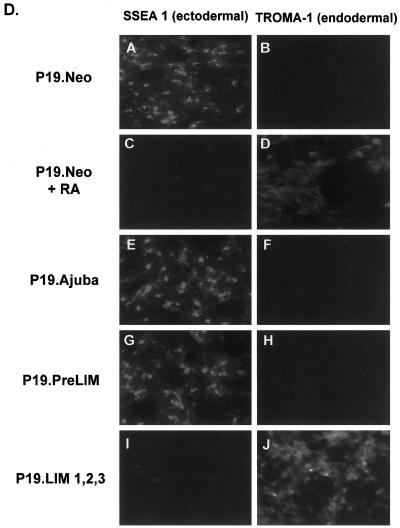
Ajuba affects proliferation and differentiation decisions of P19 embryonal carcinoma cells. (A) Stick figure representation of the various Ajuba constructs used to establish stable clones of P19 cells. All constructs contained a hexa-myc epitope tag at the N terminus. Filled boxes indicate individual LIM domains. (B) Anti-myc immunoblot of cell extracts from P19 clones containing various Ajuba isoforms. Lanes 1 and 2, Neo-resistant control cells; lanes 3 and 4, myc-PreLIM; lanes 5, 6, and 7, myc-LIM 1,2,3; lanes 8 and 9, myc-Ajuba. (C) Proliferation curve of P19 clones overexpressing different domains of Ajuba. Analyses of multiple clones were performed, and a representative clone for each cell line is presented. Triplicate wells for each time point were established at time zero. Error bars represent the SD about the mean. (D) Indirect immunofluorescence analysis of P19 cell lines with the mAb SSEA 1 (panels A, C, E, G, and I) and the mAb TROMA-1 (panels B, D, F, H, and J). Panels A and B, P19.Neo control cells; panels C and D, P19.Neo cells treated with 10 nM atRA to induce endodermal differentiation; panels E and F, P19.Ajuba cells; panels G and H, P19.PreLIM cells; panels I and J, P19.LIM 1,2,3 cells.
Ajuba Affected Proliferation and Differentiation Decisions of P19 Embryonal Cells
To determine whether Ajuba affected endodermal differentiation decisions of P19 cells, we generated stable P19 clones constitutively overexpressing full-length Ajuba, the PreLIM domain of Ajuba (PreLIM), the three LIM domains of Ajuba (LIM 1,2,3), and control cells transfected with empty vector alone (Neo). To distinguish exogenous, plasmid-derived Ajuba protein from endogenous protein, a hexa-myc epitope tag was added to the N terminus of all plasmid-derived forms of Ajuba (Figure 2A). Multiple clones of each cell line were isolated for further analyses. Anti-myc immunoblot analysis of cell extracts from these cells demonstrated that myc-tagged Ajuba isoforms of the appropriate size were present (Figure 2B).
The proliferation of these cell lines, in the presence of serum, was determined. A representative proliferation curve of one clone for each P19 cell line is shown in Figure 2C. Cells overexpressing full-length Ajuba or the PreLIM domain of Ajuba exhibited increased proliferation compared with control P19.Neo cells. Surprisingly, cells containing the three LIM domains of Ajuba grew very slowly.
We next determined if Ajuba overexpression affected the state of differentiation of P19 cells. The mAb SSEA 1 recognizes a cell surface protein present on ectodermal cells. TROMA-1 is a mAb that recognizes cytokeratin EndoA, a marker for the primitive endoderm, and the 2H3 mAb identifies a neurofilament protein specifically expressed in neuron-like cells. Parental P19 cells are ectodermal and stained positively for SSEA 1, negatively for TROMA-1, and negatively for 2H3 (Figure 2D, panels A and B). After the addition of low-dose atRA, P19 cells differentiated into cells of an endodermal lineage. This resulted in loss of SSEA 1 expression and acquisition of TROMA-1 staining (Figure 2D, panels C and D). P19 cells expressing Ajuba or the PreLIM domain of Ajuba remained positive for SSEA 1 staining and negative for TROMA-1 and 2H3 staining (Figure 2D, panels E–H), indicating that they remained ectodermal. In contrast, cells expressing the LIM domains of Ajuba were negative for SSEA 1 staining, positive for TROMA-1 staining, and negative for 2H3 staining (Figure 2D, panels I and J), indicating that they had undergone spontaneous endodermal differentiation.
Thus, overexpression of wild-type Ajuba or the PreLIM domain of Ajuba in P19 cells resulted in enhanced proliferation but no change in cellular phenotype, whereas overexpression of the three LIM domains resulted in suppression of proliferation and spontaneous endodermal differentiation.
Spontaneous Induction of Endodermal Differentiation of P19 Embryonal Cells by Ajuba Was Not a Function Common to Other Family Members
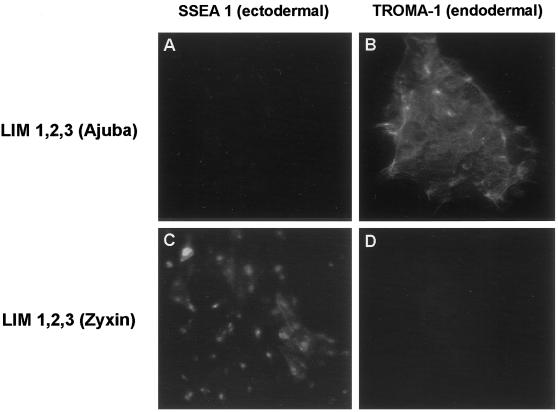
To determine if the effect of Ajuba on P19 embryonal cell development was specific to Ajuba or shared by other family members, we generated stable clones of P19 cells expressing the three LIM domains of human Zyxin. LIM 1,2,3 of Zyxin also contained a hexa-myc epitope tag at the N terminus. Like fibroblasts expressing the LIM domains of Ajuba (see Figure 4A, panel E), fibroblasts transfected with plasmids expressing myc-LIM 1,2,3 of Zyxin expressed a protein of the expected size that accumulated in the nucleus of cells (our unpublished results). P19 cells containing the LIM domains of Ajuba underwent spontaneous endodermal differentiation (Figure 3, A and B; SSEA 1 negative, TROMA-1 positive), whereas P19 cells containing the LIM domains of Zyxin remained ectodermal in phenotype (Figure 3, C and D; SSEA 1 positive, TROMA-1 negative). Thus, the LIM domains of Ajuba, but not the related family member Zyxin, induced spontaneous endodermal differentiation of P19 cells. Therefore, induction of endodermal differentiation of P19 cells by Ajuba was not a function shared by other family members, indicating selectivity for Ajuba in effecting endodermal differentiation of P19 cells.
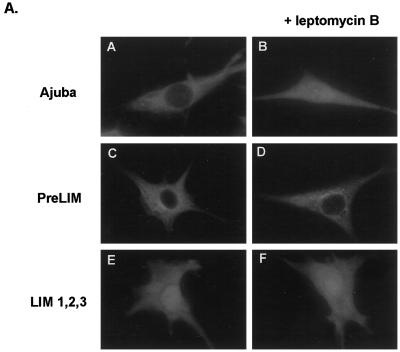
Figure 4.
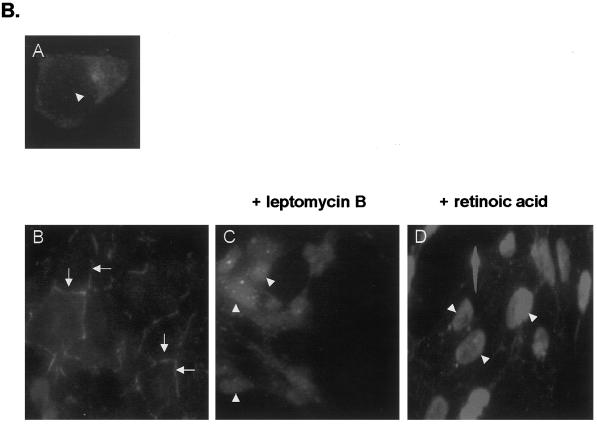
Ajuba shuttles from sites of cell–cell contact into the nucleus. (A) Indirect anti-myc immunofluorescence of fibroblasts transiently transfected with various forms of Ajuba. Panels A, C, and E, pretreatment; panels B, D, and F, after treatment with Leptomycin B for 30 min. Panels A and B, Fibroblasts transfected with myc-tagged Ajuba; panels C and D, fibroblasts transfected with the myc-tagged PreLIM domain of Ajuba; panels E and F, fibroblasts transfected with the myc-tagged LIM 1,2,3 domain of Ajuba. (B) Indirect anti-Ajuba immunofluorescence of P19 cells expressing endogenous Ajuba. Panel A, a single P19 cell devoid of any cell–cell contacts; panel B, an aggregate of P19 cells exhibiting multiple cell–cell contacts (arrows); panel C, an aggregate of P19 cells treated with Leptomycin B for 30 min; panel D, an aggregate of P19 cells treated with 10 nM atRA for 24 h. Arrowheads identify cell nuclei.
Figure 3.
Spontaneous endodermal differentiation of P19 cells by Ajuba is not a function common to all family members. Indirect immunofluorescence analysis of P19 cell lines with the mAb SSEA 1 (A and C) and the mAb TROMA-1 (B and D). A and B, P19 cells containing the three LIM domains of mouse Ajuba; C and D, P19 cells containing the three LIM domains of human Zyxin.
Cytosolic Ajuba Was Recruited to the Cell Surface upon Cell Aggregate Formation and Shuttled into the Nucleus after Treatment with Retinoic Acid
To understand how Ajuba could induce spontaneous endodermal differentiation of P19 embryonal cells, we first determined the subcellular localization of Ajuba isoforms. Previously, we had shown that removal of the PreLIM domain of Ajuba results in accumulation of the three LIM domains in the nucleus of cells (Goyal et al., 1999). Thus, we sought to determine if wild-type Ajuba shuttled between the cytosol and the nucleus. To test this hypothesis, we first treated fibroblast stably expressing different myc-tagged Ajuba isoforms with Leptomycin B, an inhibitor of Crm1-mediated nuclear export (Fukuda et al., 1997), and performed anti-myc immunofluorescence before and after Leptomycin B treatment. Ajuba was present in the cytosol of cells (Figure 4A, panel A). After the addition of Leptomycin B, Ajuba accumulated in the nucleus of cells to an extent equivalent to that observed in fibroblasts containing the LIM 1,2,3 domain of Ajuba (Figure 4A, compare panel B with panel E). In contrast, in cells containing only the PreLIM domain of Ajuba, Leptomycin B treatment did not alter the subcellular localization of this isoform (Figure 4A, panels C and D). Finally, Leptomycin B treatment of cells containing LIM 1,2,3 did not increase significantly the amount of nuclear protein (Figure 4A, panels E and F). This analysis indicated that Ajuba entered the nucleus and was rapidly exported out in a Leptomycin B–sensitive manner and that the LIM domains of Ajuba mediated nuclear entry.
Because the experiment described above was performed in NIH 3T3 fibroblasts that do not express endogenous Ajuba protein, we wanted to confirm these results in cells that naturally express Ajuba. For this experiment, anti-Ajuba immunofluorescence was performed on P19 cells before and after treatment with Leptomycin B or atRA. P19 cells grow as cell aggregates in culture. Ajuba in P19 cells growing without any cell contacts was distributed diffusely throughout the cytoplasm (Figure 4B, panel A). Interestingly, when aggregates of P19 cells were analyzed, Ajuba protein was found to accumulate at the cell surface, at sites of cell–cell contact (Figure 4B, panel B, arrows). When aggregates of P19 cells were treated with Leptomycin B, there was a dramatic loss of cell surface Ajuba, and protein accumulated in the nuclei (Figure 4B, panel C, arrowheads). Induction of endodermal differentiation of P19 cells with atRA was also associated with a redistribution of Ajuba from the cell surface and cytosol into the nucleus (Figure 4B, panel D, arrowheads). Thus, in P19 cells that naturally expressed endogenous Ajuba, the protein was recruited to the cell surface, at sites of cell–cell contact, when cells formed aggregates. Both inhibition of Crm1-mediated nuclear export and induction of endodermal differentiation with atRA resulted in nuclear accumulation of Ajuba, indicating that Ajuba entered the nucleus and that nuclear accumulation of Ajuba may be associated with endodermal differentiation of embryonal cells.
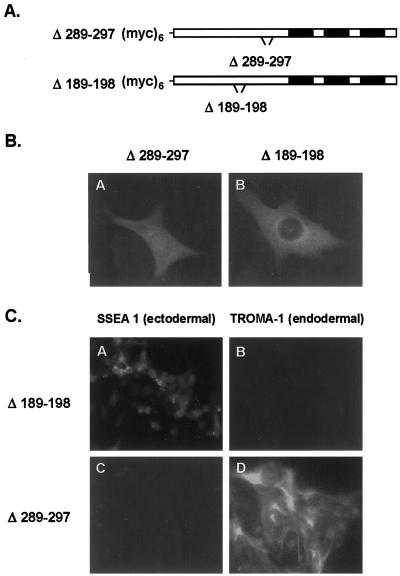
The PreLIM Region of Ajuba Contained a Functional Leucine-rich NES
Within the Ajuba protein sequence, there were two potential leucine-rich NESs (H X1–4 H X2–3 L X H, where H represents a hydrophobic residue) (Bogard et al., 1996). Both were in the PreLIM domain of Ajuba at positions 189–198 and 289–297. To determine if either of these sites was functional as a NES, we generated deletion mutants of Ajuba that lacked either domain: myc-Δ189–198 and myc-Δ289–297 (Figure 5A). When expressed in fibroblasts, both constructs expressed myc-tagged proteins of the appropriate size. Fibroblasts were transiently transfected with each plasmid, and anti-myc immunofluorescence was performed. Removal of amino acids 289–297 resulted in accumulation of Ajuba protein in the nucleus in a pattern similar to that seen in fibroblasts containing the LIM 1,2,3 domain of Ajuba (compare Figure 5B, panel A, with Figure 4A, panel E). The subcellular distribution of the Δ189–198 protein was not altered from that observed in cells containing wild-type Ajuba (compare Figure 5B, panel B, with Figure 4A, panel A). As in cells containing wild-type Ajuba, Leptomycin B treatment of cells containing Δ189–197 resulted in nuclear accumulation of the protein (our unpublished results). Thus, Ajuba contained a functional leucine-rich NES sequence at positions 289–297 that was required to export protein from the nucleus. When this sequence was deleted, Ajuba accumulated in the nucleus, indicating that Ajuba entered the nucleus and was rapidly exported out.
Figure 5.
The PreLIM region of Ajuba contains a NES, and nuclear accumulation of Ajuba induces spontaneous endodermal differentiation of P19 cells. (A) Stick figure representation of the putative leucine-rich domains deleted from full-length Ajuba: amino acids 189–198 and 289–297. Both domains contain a hexa-myc epitope tag at the N terminus. Filled boxes indicate individual LIM domains. (B) Indirect anti-myc immunofluorescence of fibroblasts transiently transfected with these mutants. Panel A, Δ289–297; panel B, Δ189–198. (C) Indirect immunofluorescence analysis of P19 cell lines with the mAb SSEA 1 (panels A and C) and the mAb TROMA-1 (panels B and D). Panels A and B, P19 cells containing Δ189–198; panels C and D, P19 cells containing Δ289–297.
Because accumulation of LIM 1,2,3 of Ajuba in the nucleus of P19 cells resulted in spontaneous endodermal differentiation, we next asked whether deletion of the NES (Δ289–297), which results in nuclear accumulation of Ajuba, affected P19 cell differentiation. Stable P19 clones containing Δ289–297, or as a control Δ189–198, were generated. The proliferation rate of P19 cells containing Δ289–297, but not Δ189–197, was reduced compared with that of P19.Neo control cells but not to the same extent as P19.LIM 1,2,3 cells. Like P19.LIM 1,2,3 cells (Figure 2D, panels I and J), P19.Δ289–297 cells were negative for SSEA 1 staining and positive for TROMA-1 staining (Figure 5C, panels C and D), indicating that they had undergone spontaneous endodermal differentiation. P19.Δ189–198 cells, which did not accumulate protein in the nucleus, remained ectodermal in phenotype (Figure 5C, panels A and B; SSEA 1 positive, TROMA-1 negative). Thus, P19 cells containing Ajuba lacking its NES, Δ289–297, accumulated protein in the nucleus and underwent spontaneous endodermal differentiation. This suggested that nuclear accumulation of the LIM domains of Ajuba was required to induce endodermal differentiation.
Nuclear Accumulation of Ajuba LIM Domains Was Required to Induce Endodermal Differentiation of P19 Embryonal Cells
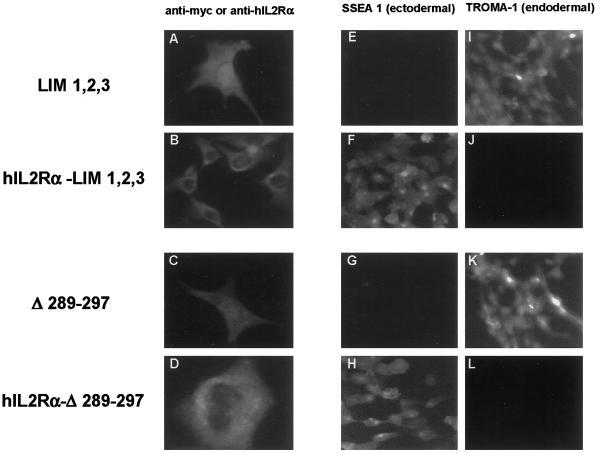
To determine if nuclear accumulation of the LIM domains of Ajuba was indeed required to induce endodermal differentiation of P19 embryonal cells, we targeted isoforms of Ajuba that accumulated in the nucleus and induced endodermal differentiation to cell membranes. This was accomplished by generating fusion proteins containing the extracellular and transmembrane domains of human IL2 receptor α chain (hIL2Rα) fused in frame to Ajuba isoforms (LaFlamme et al., 1992). Plasmids expressing hIL2Rα fused to LIM 1,2,3, to Δ289–297, and to control wild-type Ajuba were constructed. Stable P19 clones containing these fusion proteins were generated and characterized. To demonstrate membrane localization of these fusion proteins, two approaches were undertaken. First, anti-myc or anti-hIL2Rα immunofluorescence analysis of transiently transfected fibroblasts was performed (Figure 6, A–D). Compared with cells containing LIM 1,2,3 and Δ289–297, in cells containing hIL2Rα–LIM 1,2,3 and hIL2Rα–Δ289–297 fusion proteins there was no evidence of nuclear staining (Figure 6, A versus B and C versus D, respectively). Second, we prepared membrane and nuclear fractions from P19 cells containing the fusion proteins and from control cells containing the corresponding nonfusion protein and performed anti-Ajuba immunoblotting on protein extracts from these subcellular fractions. In the nuclear fractions of P19.Δ289–297 and P19.LIM 1,2,3 cells, protein was present, whereas in nuclear extracts from P19.hIL2Rα–Δ289–297 and P19.hIL2Rα–LIM 1,2,3 cells, protein was largely absent (our unpublished results). By these two criteria, it appeared that we had redirected expression of endodermal differentiating forms of Ajuba from the nucleus to cell membranes, thereby preventing nuclear accumulation of these isoforms.
Figure 6.
Nuclear accumulation of Ajuba LIM domains is required to induce endodermal differentiation of P19 cells. Indirect immunofluorescence of transiently transfected fibroblasts (A–D) or stable P19 clones (E–L) with anti-myc antiserum (A and C), anti-human IL2 receptor α antiserum (B and D), mAb SSEA 1 (E–H), or mAb TROMA-1 (I–L). Cells contain LIM 1,2,3 (A, E, and I), hIL2Rα–LIM 1,2,3 (B, F, and J), Δ289–297 (C, G, and K), or hIL2Rα–Δ289–297 (D, H, and L).
We next compared the differentiation state of P19 cells containing the hIL2Rα fusion proteins with that of the corresponding P19 cells containing nonfusion protein. P19.LIM 1,2,3 and P19.Δ289–297 cells spontaneously differentiated into endodermal cells (SSEA 1 negative, TROMA-1 positive; Figure 6, E and I and G and K, respectively). In contrast, P19.hIL2Rα–LIM 1,2,3 and P19.hIL2Rα–Δ289–297 cells remained ectodermal (SSEA 1 positive, TROMA-1 negative; Figure 6, F and J and H and L, respectively). This result indicated that for Ajuba to induce endodermal differentiation of P19 cells, the LIM domains must accumulate in the nucleus.
The Individual LIM Domains of Ajuba Exhibited Differential Effects on Developmental Decisions of P19 Embryonal Cells
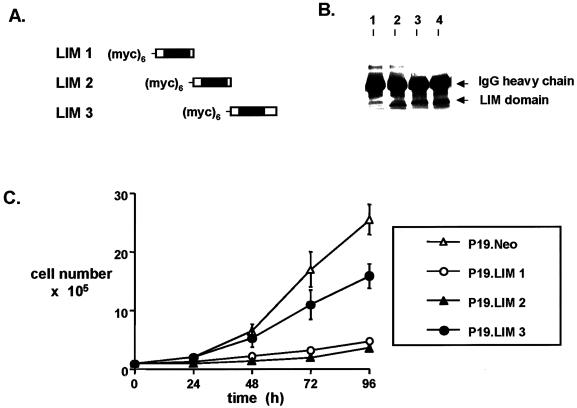
To determine whether individual LIM domains of Ajuba exhibited specific effects on P19 cell development, we generated stable P19 clones containing each individual LIM domain. All contained a myc epitope tag at the N terminus (Figure 7A). Anti-myc immunoprecipitation and immunoblotting of cell extracts demonstrated that each individual LIM domain was expressed in P19 cells (Figure 7B). When transiently transfected into fibroblasts, anti-myc immunofluorescence analysis demonstrated that each individual LIM domain was found to accumulate in the nucleus, similar to cells containing LIM 1,2,3. The proliferation rate of P19 clones containing individual LIM domains was compared with that of control P19.Neo cells (Figure 7C). P19 cells containing either the first or second LIM domain of Ajuba exhibited dramatically suppressed rates of proliferation, similar to proliferation of P19.LIM 1,2,3 cells (Figure 2C). Cells containing the third LIM domain proliferated at rates approaching that observed with control cells, albeit somewhat slower.
Figure 7.
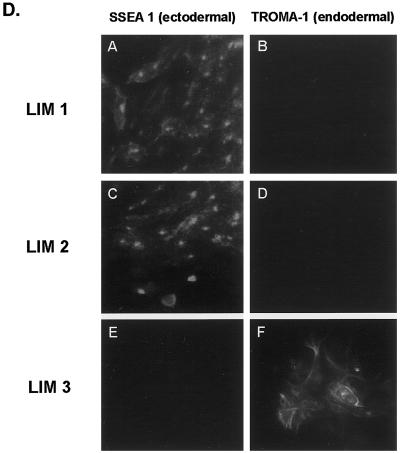
Individual LIM domains of Ajuba exhibit differential effects on developmental decisions by P19 cells. (A) Stick figure representation of the individual LIM domains of Ajuba overexpressed in P19 cells. All domains contain a hexa-myc epitope tag at the N terminus. (B) Anti-myc immunoprecipitation followed by anti-myc immunoblotting of cell extracts from P19 clones containing individual LIM domains. Lane 1, untransfected control; lane 2, myc-LIM 1; lane 3, myc-LIM 2; lane 4, myc-LIM 3. (C) Proliferation curve of P19 cell clones containing individual LIM domains of Ajuba. Analyses of multiple clones were performed, and a representative clone for each cell line is presented. Triplicate wells for each time point were established at time zero. Error bars represent the SD about the mean. (D) Indirect immunofluorescence analysis of P19 cell lines with the mAb SSEA 1 (panels A, C, and E) and the mAb TROMA-1 (panels B, D, and F). Panels A and B, P19 cells containing the LIM 1 domain of Ajuba; panels C and D, P19 cells containing the LIM 2 domain of Ajuba; panels E and F, P19 cells containing the LIM 3 domain of Ajuba.
We next determined the phenotype of each P19 cell line. P19 cells containing LIM 3 underwent spontaneous endodermal differentiation (SSEA 1 negative, TROMA-1 positive; Figure 7D, panels E and F). But P19 cells containing either the first or second LIM domain of Ajuba remained ectodermal (SSEA 1 positive, TROMA-1 negative; Figure 7D, panels A and B and C and D, respectively). Thus, the third LIM domain of Ajuba was necessary to induce endodermal differentiation of P19 cells, whereas the first and second LIM domains affected the proliferation of P19 cells without affecting differentiation decisions. Therefore, the LIM domains of Ajuba exhibited differential and specific effects on developmental decisions by P19 cells.
In P19 Cells Containing Forms of Ajuba That Induced Endodermal Differentiation, Increased JNK Activity Was Present and Was Required for Endodermal Differentiation
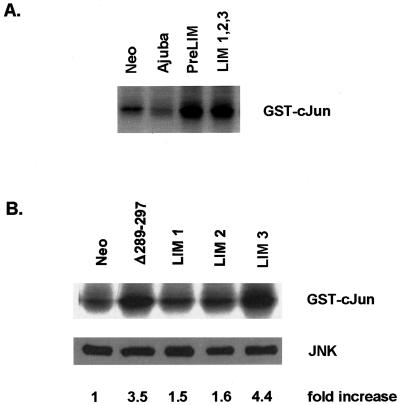
Previous reports have demonstrated that atRA-induced endodermal differentiation of P19 cells requires activation of JNK (Jho et al., 1997). Because Ajuba is a potent modulator of ERK activity in fibroblasts, we determined whether overexpression of the various isoforms of Ajuba in P19 cells affected the activity of the MAPK family of proteins (e.g., ERK, JNK, and p38) in these cells. The activity of each enzyme was determined from cells during exponential growth in serum. ERK and p38 kinase activity was not significantly affected by Ajuba overexpression in P19 cells under these conditions (our unpublished results). Interestingly, however, P19 cell lines containing forms of Ajuba that induced endodermal differentiation (e.g., LIM 1,2,3, Δ289–297, and LIM 3) all exhibited increased JNK activity (Figure 8A, lane 4; Figure 8B, lanes 2 and 5). Increased JNK activity was not restricted to only those cell lines that underwent endodermal differentiation, however. P19 cells containing the PreLIM domain of Ajuba also had increased JNK activity (Figure 8A, lane 3). Thus, in P19 cells containing forms of Ajuba that induced endodermal differentiation, increased JNK activity was also present, but increased JNK activity alone was not sufficient to induce differentiation.
Figure 8.
In P19 cells containing forms of Ajuba that induce endodermal differentiation, JNK activity is stimulated. In vitro kinase assay for JNK activity in P19 cells overexpressing various forms of Ajuba. Each panel represents different experiments. The increase in JNK activity relative to control P19.Neo cells (B, lane 1) was determined by scanning the in vitro kinase results and normalizing to the amount of JNK protein present in cell extracts, as determined by the anti-JNK immunoblot (B, lower panel). P19.Neo cells were arbitrarily set as 1 U. All others are expressed relative to P19.Neo cells. (A) Lane 1, P19.Neo control cells; lane 2, P19 cells overexpressing wild-type Ajuba; lane 3, P19 cells containing the PreLIM domain of Ajuba; lane 4, P19 cells containing the three LIM domains of Ajuba. (B) Lane 1, P19.Neo control cells; lane 2, P19 cells containing Δ289–297; lane 3, P19 cells containing the LIM 1 domain of Ajuba; lane 4, P19 cells containing the LIM 2 domain of Ajuba; lane 5, P19 cells containing the LIM 3 domain of Ajuba.
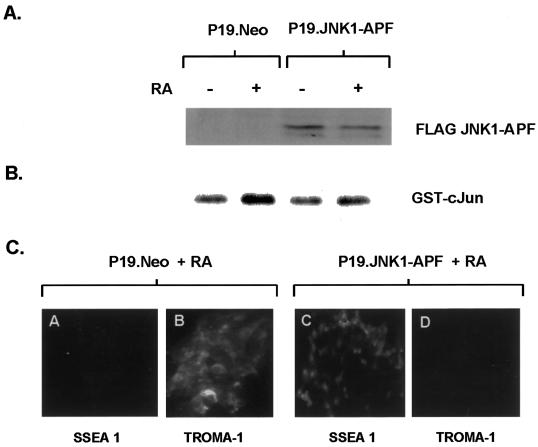
To determine if JNK activation was required for Ajuba-mediated P19 endodermal differentiation, we generated clones of P19 cells coexpressing Ajuba and a dominant inhibitory form of JNK, JNK1–APF (Derijard et al., 1994). First, stable clones of P19 cells containing FLAG-tagged JNK1–APF were generated. Expression of JNK1–APF was confirmed by immunoblotting of cell lysates with the anti-FLAG antibody (Figure 9A). Expression of JNK1–APF in P19 cells did not alter their proliferation rate in serum. To demonstrate that JNK1–APF expression inhibited JNK activity in P19 cells, P19.Neo and P19.JNK1–APF cells were treated with atRA (which induces JNK activity and endodermal differentiation) and JNK enzyme activity was determined. In contrast to control P19.Neo cells treated with atRA (Figure 9B, lanes 1 and 2), minimal activation of JNK occurred in P19.JNK1–APF cells in response to atRA (Figure 9B, lanes 3 and 4), indicating that JNK1–APF inhibited atRA-induced JNK activity. Finally, JNK1–APF expression also blocked atRA-induced endodermal differentiation of P19 cells (Figure 9C). P19.JNK1–APF cells remained SSEA positive and TROMA-1 negative (Figure 9C, panels C and D), whereas P19.Neo control cells became SSEA negative and TROMA-1 positive after atRA treatment (Figure 9C, panels A and B).
Figure 9.
Dominant inhibitory JNK blocks atRA-induced endodermal differentiation of P19 cells. (A) Anti-FLAG immunoblot analysis for expression of JNK1–APF. Lanes 1 and 2, P19.Neo control cells; lanes 3 and 4, P19 cells containing JNK1–APF. Cells were treated with 10 nM atRA for 2 d (lanes 2 and 4) or untreated (lanes 1 and 3). (B) In vitro kinase assay for JNK activity in P19 cells containing JNK1–APF (lanes 3 and 4) or control P19.Neo cells (lanes 1 and 2) before (lanes 1 and 3) and after (lanes 2 and 4) atRA treatment. (C) Indirect immunofluorescence analysis of P19 cell lines with the mAb SSEA 1 (ectodermal; panels A and C) and the mAb TROMA-1 (endodermal; panels B and D). Panels A and B, P19.Neo control cells treated with 10 nM atRA for 2 d; panels C and D, P19 cells containing JNK1–APF treated with 10 nM atRA for 2 d.
We next transfected P19.JNK1–APF cells with plasmids expressing Ajuba isoforms fused to GFP (GFP–Δ289–297, GFP–LIM 1,2,3, and GFP–LIM 3) and control GFP alone. Multiple GFP-positive clones of each were selected, and the phenotypes of cells were determined by immunophenotyping analysis. Control cells for this experiment were P19.Neo cells transfected with the same set of GFP–Ajuba fusion plasmids. When Δ289–297, LIM 1,2,3, or LIM 3 was coexpressed with JNK1–APF, no endodermal differentiation occurred, whereas control cells all underwent endodermal differentiation, as anticipated (Table 1). Thus, JNK1–APF inhibition of JNK activation in P19 cells blocked the ability of Ajuba isoforms to induce endodermal differentiation of P19 cells. Therefore, increased JNK activity in P19 cells containing endodermal differentiating isoforms of Ajuba was required for differentiation.
Table 1.
Activation of JNK was required for Ajuba-mediated induction of endodermal differentiation of P19 cells
| JNK activity | Endodermal differentiation
|
||
|---|---|---|---|
| P19.Neo | P19.JNK1–APF | ||
| Retinoic acid (10 nM) | + | + | − |
| Ajuba | − | − | − |
| PreLIM | + | − | nd |
| LIM 1,2,3 | + | + | − |
| Δ289–297 | + | + | − |
| LIM 3 | + | + | − |
JNK activity was determined by in vitro kinase assay as described. The state of differentiation of P19 cells was determined by immunofluorescence analysis: ectodermal, SSEA 1 positive, TROMA-1 negative; endodermal, SSEA 1 negative, TROMA-1 positive. nd, not determined.
DISCUSSION
Ajuba is a member of a family of cytosolic LIM domain–containing proteins. Members of this family of proteins are present in protein complexes mediating attachment of cell surface receptors to the actin cytoskeleton at sites of focal adhesion and cell–cell contact. A number of traits distinguish Ajuba from other family members, however. First, in contrast to all other family members, Ajuba does not localize to sites of focal adhesion (Crawford and Beckerle, 1991; Goyal et al., 1999; Wang et al., 1999; Petit et al., 2000). Like other family members, Ajuba was found at sites of cell–cell contact (Beckerle, 1988; Crawford and Beckerle, 1991; Wang et al., 1999; Petit et al., 2000). In the absence of cell–cell contact, Ajuba was diffusely distributed throughout the cytoplasm, however, suggesting that recruitment to the cell surface is an active process and may be regulated. Like Zyxin and LPP, Ajuba contained a functional leucine-rich NES and shuttled from the cell surface and the cytosol into the nucleus (Nix and Beckerle, 1997; Petit et al., 2000). Importantly, and in contrast to studies with Zyxin and LPP, we demonstrated that nuclear accumulation of the LIM domains of Ajuba in P19 embryonal cells resulted in inhibition of proliferation and altered cell fate. The LIM domains of Zyxin exhibited no such effect on P19 cell development, indicating specificity in this function for Ajuba. These developmental decisions affected by Ajuba mapped to specific LIM domains. The first two LIM domains affected cell proliferation, whereas the third LIM domain affected differentiation decisions. Finally, in contrast to other family members, Ajuba affects the activity of MAPK enzymes in cells (Goyal et al., 1999). In P19 embryonal cells, overexpression of certain Ajuba isoforms was associated with increased JNK activity. Increased JNK activity correlated with endodermal differentiation by these isoforms. Although JNK activation per se was not sufficient to induce endodermal differentiation of these cells, JNK activation was required for Ajuba-induced endodermal differentiation.
Other proteins present at sites of cell adhesion have been shown to shuttle between these sites and the nucleus, and in some cases they regulate cell fate decisions. The tight junction protein ZO-1 accumulates in the nucleus of cells in a cell density–dependent manner (Gottardi et al., 1996). Nuclear accumulation of ZO-1 occurred in subconfluent cells, whereas in confluent cells ZO-1 was present at cell–cell contacts. In contrast, Ajuba's expression pattern was cytosolic in dispersed P19 cells and recruited to the cell surface upon formation of cell aggregates. The syndecan-3–associated guanylate kinase CASK/LIN-2 interacts with the T-box transcription factor Tbr-1 (Hsueh et al., 2000). This association led to nuclear accumulation of CASK, DNA binding of the complex, and transcriptional activation of Tbr-1. The in vivo consequence of this interaction has not been determined, however. Furthermore, when CASK and Tbr-1 were coexpressed with syndecan-3 in COS cells, CASK expression remained cytosolic. β-Catenin associates with cadherin at sites of cell–cell contact and is an important mediator of cadherin-mediated adhesion (Gumbiner, 1993). It also has signaling activity in Xenopus and Drosophila embryonic development (Pfeifer, 1995; Brannon et al., 1997). This function of β-catenin occurs in the cytosol of cells, through an association with members of the Lef/Tcf family of transcription factors that facilitate translocation of β-catenin into the nucleus (Molenaar et al., 1996). It is unclear whether this signaling function of β-catenin is independent of its role in cadherin-mediated cell adhesion. However, overexpression of high levels of cadherin in cells can inhibit β-catenin signaling, suggesting that cadherin-mediated cell adhesion may indeed be linked, in some manner, to β-catenin signaling events (Heaseman et al., 1994). Ajuba also accumulated at sites of cell–cell adhesion and was shuttled into the nucleus. Therefore, Ajuba function may provide a new pathway whereby cell–cell adhesive events are transmitted to the nucleus to regulate cell proliferation and differentiation decisions.
Nuclear accumulation of the LIM domains of Ajuba was required to induce endodermal differentiation of P19 embryonal cells. Removal of the PreLIM domain of Ajuba or deletion of the NES within the PreLIM domain resulted in nuclear accumulation of protein and endodermal differentiation. In P19 cells containing forms of Ajuba that remained cytosolic, cells remained ectodermal in phenotype. Redirection of Ajuba LIM domains to the cell membranes blocked nuclear accumulation and abrogated endodermal differentiation. In actively dividing P19 cell aggregates, very little endogenous Ajuba was detectable in the nucleus. Because nuclear accumulation of Ajuba resulted in inhibition of cell proliferation, actively cycling cells would want to exclude Ajuba from the nucleus. Thus, nuclear entry/export of Ajuba may be tightly regulated during different phases of the cell cycle and in confluent sheets of growth-arrested adherent epithelial cells.
There are a number of possible mechanisms whereby Ajuba could alter the fate of P19 embryonal cells. The LIM domain defines a unique double zinc finger structure that is highly conserved among proteins present in organisms representing an extensive range of evolution (Dawid et al., 1998). They are thought to function as versatile protein modules, capable of acting within diverse cellular contexts and in multiple subcellular compartments (Dawid et al., 1998). Many have been shown to participate in direct protein–protein interactions. Nuclear LIM proteins have long been recognized as playing important roles in the control of gene expression and cell fate determination (Sanchez-Garcia and Rabbits, 1993). Although there is little evidence to support direct DNA-binding activity of LIM domains, nuclear magnetic resonance structural analysis of a LIM domain from CRP indicates that it is related to the zinc fingers of the GATA family of transcription factors (Perez-Alvardo et al., 1994). Thus, the LIM domains of Ajuba could, theoretically, directly bind DNA, thereby activating or repressing specific gene transcription. Both Trip6 and LPP have been shown to function as transcriptional activators when fused to the GAL4 DNA-binding domain (Wang et al., 1999; Petit et al., 2000). Alternatively, like other nuclear LIM proteins, the LIM domains of Ajuba may associate with DNA-binding proteins and alter their function (Sanchez-Garcia and Rabbits, 1993; van Meyel et al., 1999). The identification of cellular proteins interacting with the LIM domains of Ajuba should begin to address these possibilities. Trip6 was originally identified through a two-hybrid screen as interacting with thyroid hormone receptor in a hormone-dependent manner (Lee et al., 1995). Because thyroid hormone receptors are related to receptors for retinoic acid and low-dose atRA induces endodermal differentiation of P19 embryonal cells, Ajuba may induce endodermal differentiation of P19 cells through a retinoic acid–mediated signaling pathway or induction of retinoic acid–responsive genes (Roy et al., 1995). In support of this possibility, we found that expression of the three LIM domains of Trip6 in P19 embryonal cells also resulted in accumulation of protein in the nucleus and induced endodermal differentiation (our unpublished results).
In addition to nuclear accumulation of the LIM domains, increased JNK activity was required for Ajuba isoforms to induce endodermal differentiation of P19 cells. It has been reported that the endodermal differentiation in P19 cells induced by retinoic acid is also dependent on activation of a JNK1-dependent pathway (Jho et al., 1997). Increased JNK activity correlated with the ability of specific Ajuba isoforms to induce endodermal differentiation. However, endodermal differentiation does not always result when JNK activity is increased in P19 cells. For example, activation of JNK by anisomycin (a potent activator of JNK) did not induce endodermal differentiation. Likewise, P19 cells overexpressing the PreLIM domain of Ajuba also exhibited increased JNK activity but did not undergo endodermal differentiation. Thus, activation of JNK is necessary but not sufficient for endodermal differentiation of P19 cells induced by Ajuba. Most likely, nuclear accumulation of Ajuba LIM domains is also required for endodermal differentiation of P19 embryonal cells by Ajuba.
How overexpression of specific Ajuba isoforms in P19 cells leads to JNK activation is not clear. JNKs can be activated by Rac1 and cdc42, members of the Rho family of small GTP-binding proteins (Bagrodia et al., 1995; Coso et al., 1995; Minden et al., 1995). Cadherin-mediated cell–cell adhesion events are also regulated by the action of the Rho family of GTP-binding proteins (Braga et al., 1997; Hall, 1998). After aggregation of P19 cells, Ajuba was recruited to the cell surface, possibly at sites of cadherin-mediated cell–cell adhesion. Ajuba also enhances Ras signaling pathways (Goyal et al., 1999). Thus, it is tempting to speculate that Ajuba may also affect the activity of the Rho family of GTP-binding proteins and, thus, cell–cell adhesion events and activation of JNK. These putative functions for Ajuba further highlight its potential role as a major adapter protein in coupling cell–cell adhesive events to cellular signaling pathways.
The following lines of evidence indicated that the LIM domains of Ajuba mediated nuclear entry. First, removal of the PreLIM domain of Ajuba resulted in the accumulation of the LIM domains within the nucleus. Second, cells containing only the PreLIM domain of Ajuba did not exhibit nuclear accumulation of protein, even when treated with Leptomycin B, whereas Leptomycin B treatment of cells containing wild-type Ajuba led to nuclear accumulation of protein. Third, fusion of GFP to LIM 1,2,3 resulted in nuclear accumulation of GFP. Finally, deletion of the nine-amino acid leucine-rich NES (Δ289–297) also resulted in accumulation of protein in the nucleus. In fibroblasts overexpressing LIM 1,2,3 or Δ289–297, complete cytosolic absence of protein was not observed. Most likely this is due to overexpression of the proteins in these cells, because Leptomycin B treatment of P19 cells, which express endogenous Ajuba, resulted in nuclear accumulation of a larger fraction of cellular Ajuba protein. The minor amount of cytosolic Ajuba remaining in the cytosol of Leptomycin B–treated P19 cells raises the possibility that there may be distinct cellular “pools” of Ajuba. These results do not distinguish whether Ajuba present at the cell surface or cytosolic Ajuba or both are transported into the nucleus.
Within the LIM domains of Ajuba, there are no obvious nuclear localization signals. Thus, whether the LIM domains alone direct nuclear entry or interact with another cytosolic protein that then directs nuclear entry remains to be determined. Other proteins found at sites of cell adhesion, e.g., β-catenin and CASK/LIN-2, interact with cytosolic transcription factors that mediate their nuclear entry (Molenaar et al., 1996; Hsueh et al., 2000). Ajuba is rapidly serine phosphorylated after serum stimulation (L. Meek and G. Longmore, unpublished results). Whether posttranslational phosphorylation and dephosphorylation of Ajuba provides signals regulating its recruitment to the cell surface and nuclear entry is currently under investigation.
A leucine-rich sequence at positions 289–297 in the PreLIM domain of Ajuba mediated nuclear export. Support for this conclusion comes from the following observations. Leptomycin B treatment of cells containing wild-type Ajuba results in accumulation of protein in the nucleus. Leptomycin B specifically inhibits Crm1-mediated nuclear export (Kudo et al., 1999). Crm1 recognizes consensus leucine-rich NESs (Fukuda et al., 1997). Deletion of these amino acids in Ajuba results in nuclear accumulation, independent of Leptomycin B treatment. Removal of the PreLIM domain of Ajuba (including the NES sequences) also resulted in accumulation of the LIM domains in the nucleus, presumably as a result of the inability to be exported out of the nucleus after entry. Cytoplasm-to-nucleus shuttling of the Zyxin family of LIM proteins may be a general feature of all family members. Zyxin, LPP, and now Ajuba have all been shown to contain leucine-rich NESs that share amino acid sequence homology (Nix and Beckerle, 1997; Petit et al., 2000). Importantly, and in contrast to studies with other family members, we demonstrated that intracellular trafficking of Ajuba in embryonal cells has functional consequences on cell proliferation and differentiation decisions.
ACKNOWLEDGMENTS
We acknowledge the many investigators who willingly and readily supplied us with requested reagents. We also thank Drs. A. Chan, H. Piwnica-Worms, R. Kopan, and V. Braga for helpful comments and suggestions. This work was supported by grant CA85839 from the National Institutes of Health/National Cancer Institute and by a grant from the Edward Mallinckrodt, Jr., Foundation to G.D.L.
REFERENCES
- Alexandropoulos K, Cheng G, Baltimore D. Proline-rich sequences that bind to src homology 3 domains with individual specificities. Proc Natl Acad Sci USA. 1995;92:3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Beckerle MC. Identification of a new protein localized at sites of cell-substratum adhesion. J Cell Biol. 1988;103:1679–1687. doi: 10.1083/jcb.103.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle MC. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- Bogard HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type 1 rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the samois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cowin PM, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Crawford A, Beckerle M. Purification and characterization of zyxin, an 82,000-dalton component of adherent junctions. J Biol Chem. 1991;266:5847–5853. [PubMed] [Google Scholar]
- Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Feng S, Chen JK, Yu H, Simon JA, Schreiber SL. Two binding orientations for peptides to the src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for the intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Goichberg P, Geiger B. Direct involvement of N-cadherin mediated signaling in muscle differentiation. Mol Biol Cell. 1998;9:3119–3131. doi: 10.1091/mbc.9.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of the cell-cell contacts. Proc Natl Acad Sci USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Lin P, Kanungo J, Payne AS, Muslin AJ, Longmore GD. Ajuba, a novel LIM protein, interacts with Grb2, augments MAP kinase activity in fibroblasts, and promotes meiotic maturation of Xenopus oocytes in a Grb2- and Ras-dependent manner. Mol Cell Biol. 1999;19:4379–4389. doi: 10.1128/mcb.19.6.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- Heaseman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hobert O, Schilling JW, Beckerle MC, Ullrich A, Jallal B. SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein Zyxin. Oncogene. 1996;12:1577–1581. [PubMed] [Google Scholar]
- Hsueh Y-P, Wang T-F, Yang F-C, Sheng M. Nuclear translocation and transcription regulated by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ihle JN, Nosaka T, Thierfelder W, Quelle FW, Shimoda K. JAK and STATs in cytokine signaling. Stem Cells Suppl. 1997;1:105–111. doi: 10.1002/stem.5530150814. [DOI] [PubMed] [Google Scholar]
- Jho E-H, Davis RJ, Malbon CC. c-Jun amino-terminal kinase is regulated by Galpha12,13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J Biol Chem. 1997;272:24468–24474. doi: 10.1074/jbc.272.39.24468. [DOI] [PubMed] [Google Scholar]
- Kiss H, Kedra D, Yang Y, Kost-Alimova M, Kiss C, O'Brien KP, Fransson I, Klein G, Imreh S, Dumanski JP. A novel gene containing LIM domains (LIMD1) is located within a common eliminated region 1 (C3CER1) in 3p21.3. Hum Genet. 1999;105:552–559. doi: 10.1007/s004399900188. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of a-actinin with the cadherin/catenin cell-cell adhesion complex via a-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates Crm1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Choi H-S, Gyurist J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the wnt/beta-catenin and wnt/calcium pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and the c-Jun transcriptional activity by the small GTPases Rac and Cdc42. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nix DA, Beckerle MC. Nuclear-cytoplasmic shuttling of the focal contact protein, Zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol. 1997;138:1139–1147. doi: 10.1083/jcb.138.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvardo GC, Miles C, Michelson JW, Louis H, Winge DR, Beckerle MC, Summers MF. Structure of the carboxy-terminal LIM domain from the cysteine-rich protein CRP. Nat Struct Biol. 1994;1:388–398. doi: 10.1038/nsb0694-388. [DOI] [PubMed] [Google Scholar]
- Petit M, Mols R, Schoenmakers E, Mandahl N, Van De Ven W. LPP, the preferred fusion partner gene of HMGIC in lipomas, is a novel member of the LIM protein gene family. Genomics. 1996;36:118–129. doi: 10.1006/geno.1996.0432. [DOI] [PubMed] [Google Scholar]
- Petit MMR, Fradelizi J, Golsteyn RM, Ayoubi T, Menichi B, Louvard D, Van de Ven W, Friederich E. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol Biol Cell. 2000;11:117–129. doi: 10.1091/mbc.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M. Cell adhesion and signal transduction: the Armadillo connection. Trends Cell Biol. 1995;5:224–229. doi: 10.1016/s0962-8924(00)89015-7. [DOI] [PubMed] [Google Scholar]
- Roy B, Taneja R, Chambon P. Synergistic activation of RA-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha, beta, or gamma selective ligand in combination with a retinoid X receptor-specific ligand. Mol Cell Biol. 1995;15:6481–6487. doi: 10.1128/mcb.15.12.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, McBurney MW. In: Teratocarcinomas and Embryonic Stem Cells. Robertson EJ, editor. Oxford, UK: IRL Press; 1987. [Google Scholar]
- Sanchez-Garcia I, Rabbits TH. LIM domain proteins in leukemia and development. Cancer Biol. 1993;4:349–358. [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classical cadherins. Curr Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- van Meyel DJ, O'Keefe DD, Jurata LW, Thor S, Gill GN, Thomas JB. Chip and Apterous physically interact to form a functional complex during Drosophila development. Mol Cell. 1999;4:259–265. doi: 10.1016/s1097-2765(00)80373-1. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dooher JE, Zhao MK, Gilmore TD. Characterization of mouse Trip6: a putative intracellular signaling protein. Gene. 1999;234:403–409. doi: 10.1016/s0378-1119(99)00168-7. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecans: synergistic activation of cell adhesion. Trends Cell Biol. 1998;8:189–192. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J. Maternal beta-catenin establishes a “dorsal signal” in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]