Abstract
Poor glucose tolerance and memory deficits, short of dementia, often accompanies aging. The purpose of this study was to ascertain whether, among nondiabetic, nondemented middle-aged and elderly individuals, poorer glucose tolerance is associated with reductions in memory performance and smaller hippocampal volumes. We studied 30 subjects who were evaluated consecutively in an outpatient research setting. The composition of the participant group was 57% female and 68.6 ± 7.5 years of age; the participants had an average education of 16.2 ± 2.3 years, a score on the Mini Mental State Examination of 28.6 ± 1.5, a glycosylated hemoglobin (HbA1C) of 5.88 ± 0.74%, and a body mass index of 24.9 ± 4.1 kg/m2. Glucose tolerance was measured by an i.v. glucose tolerance test. Memory was tested by using the Wechsler Paragraphs recall tests at the time of administering the i.v. glucose tolerance test. The hippocampus and other brain volumes were measured by using validated methods on standardized MRIs. Decreased peripheral glucose regulation was associated with decreased general cognitive performance, memory impairments, and atrophy of the hippocampus, a brain area that is key for learning and memory. These associations were independent of age and Mini Mental State Examination scores. Therefore, these data suggest that metabolic substrate delivery may influence hippocampal structure and function. This observation may bring to light a mechanism for aging brain injury that may have substantial medical impact, given the large number of elderly individuals with impaired glucose metabolism.
Twenty-five percent of individuals over 65 years of age have sufficient cognitive problems, short of dementia, to affect the quality of their lives (1, 2). The ability to learn consciously and recall new information, which is known as recent or declarative memory, is one of the areas most affected during aging. However, our knowledge about the medical factors that predispose a person to age-associated cognitive problems remains undeveloped.
There is a growing literature indicating that individuals with diabetes have impairments in recent memory (3–6). In addition, nondiabetic individuals with mild forms of impaired glucose tolerance (IGT) may also have cognitive impairments (7, 8). The prevalence of memory problems and IGT rise with age (1, 9, 10). In addition to genetic predisposition, obesity and low levels of physical activity have been identified as risk factors for IGT in adults and children (11, 12). With life expectancy and obesity on the rise, the prevalence of memory dysfunction and IGT will likely continue to climb. However, it remains to be established whether there are associations between peripheral glucose regulation and memory performance among nondemented middle-aged and elderly individuals.
The hippocampus, a brain structure deep in the temporal lobe, is key for recent memory formation (13–15). Hypothalamus-pituitary-adrenal axis hyperactivity have been associated with hippocampal atrophy in aging (16) and in Cushing's disease (17). Cortisol administration reduces glucose transport into neurons (18) and causes reductions in hippocampal glucose utilization (19), which may help explain why animals that have abnormal glucose metabolism have more hippocampal damage when exposed to high levels of corticosteroids (20). In addition to the higher prevalence of memory problems and IGT mentioned above, age-associated reductions in hippocampus volumes have also been reported (21). However, it is not known whether the hippocampus is affected by IGT.
In this study, we sought to characterize the association between peripheral glucose regulation and brain. We proposed that among nondiabetic normal elderly, poorer glucose tolerance would be associated with decreased recent memory performance and smaller hippocampal volumes. This study may help create the view that metabolic substrate delivery may influence brain structure and function, and that better lifetime management of blood sugar may improve memory in old age and perhaps even reduce the risk of hippocampal damage and possibly Alzheimer's disease. This study may also create the rationale for treating and preventing memory dysfunction with behavioral and pharmacological interventions aimed at improving glucose tolerance.
Methods
Patient Population.
We selected for evaluation 30 consecutively screened nondiabetic (fasting glucose level of <126 mg/dl) middle-aged and elderly individuals functioning within the normal range. All subjects gave informed written consent to participate in this project, which was approved by the Institutional Board of Research Associates of the New York University School of Medicine. Subjects represented a typical research clinic population and were not drawn randomly from the general population. Subjects underwent medical, neurological, psychiatric, neuropsychological, and MRI examinations. Individuals with current or historical evidence of significant neurological, medical, or psychiatric disease were excluded.
Assessment of Peripheral Glucose Regulation.
Glucose tolerance was assessed by a standardized i.v. glucose tolerance test (IVGTT) conducted at the New York University School of Medicine General Clinical Research Center (GCRC) at 9:30 a.m. after an overnight fast. The glucose dose administered was adjusted for body weight (0.3 g/kg, to a maximum of 25 g). Subjects arrived at the GCRC at 8:00 a.m. A catheter was placed in each forearm: one for the administration of the glucose and another for blood sampling. The site used for glucose administration was kept patent by using a heparin lock with 40 units/ml. The line for blood sampling was kept patent by infusing normal saline at a rate of 50 ml/hr. The sampling arm was kept warm with a heating pad. The lines were placed at least 45 min before the administration of the glucose to allow sufficient time for neuroendocrine parameters, such as cortisol, to return to baseline after the stress of the catheter insertions. After a 12-h fast, baseline bloods for glucose levels were obtained at 9:20 and 9:25 a.m., 10 and 5 min, respectively, before the glucose infusion. Glucose was administered as a 50% dextrose solution by i.v. push over 90 sec. Blood samples also were taken 1, 2, 3, 5, 7, 10, 15, 20, 30, 45, 60, 90, 120, 180, and 240 min after glucose infusion. We used three parameters as indices of glucose regulation: the average of the two baseline fasting glucose levels, the glucose level 2 h after glucose administration and the area under the glucose curve during the IVGTT. In addition, we also measured insulin levels.
Memory Testing.
The Wechsler Paragraph (22) test was used to assess recent memory. We obtained both the immediate and delayed (10 min) recall of the paragraph starting at 8:45 a.m., ≈30 min after catheter insertion on the morning of the IVGTT and ≈45 min before glucose infusion. In addition, to allow comparison with other studies, subjects received the Mini Mental Status Examination (MMSE; ref. 23), which is a measure of overall cognitive function.
MRI Evaluations.
Subjects were scanned by using a 1.5 T GE Advantage MR system (GE Medical Systems, Milwaukee, WI). Fast spin echo axial images (T1 and T2) were used to ensure subjects met the inclusion and exclusion criteria. Subjects with evidence of infarct, hydrocephalus, intra-cranial masses, or significant white matter lesions were excluded. For the anatomical measurements, a three-dimensional spoiled gradient recalled (SPGR) sequence in a sagittal plane was acquired by using TR 35/TE 9 ms, 60° flip angle, 1 signal average, 1.2-mm slice thickness with no gap, a 25-cm field of view, and a 256 × 128 matrix. These SPGR sagittal scans were used to create coronal images reformatted orthogonal to the plane through the inferior-most portion of the frontal and occipital lobes on the mid-sagittal plane. These reformatted 1.5 mm-thick coronal images were used for the temporal lobe regional volume measurements. We have used these scanning methods in many of our prior studies; details about the MRI acquisition and image reformatting are provided elsewhere (24). The hippocampus, parahippocampal gyrus, and superior temporal gyrus were manually outlined on the standardized reformatted coronal images. The hippocampus (cornu amonnis, dentate gyrus, and subiculum) was measured in its entire anterior–posterior extension combining our published method (described in ref. 25) with the use of multiple planes to separate reliably the hippocampus from the adjacent amygdala (for details, see ref. 24). The parahippocampal and superior temporal gyri were measured in the same sections as the hippocampus.
To correct for head size variations across individuals, a cerebral vault volume was obtained by measuring the volume of the compartment bounded by the dura and the tentorium cerebri (24). A measure of global brain atrophy was determined by using a threshold procedure to segment the cerebrospinal fluid (refer to refs. 24 and 25 for details) within the cerebral vault volume.
For all regional volumes, the right and left hemisphere values were averaged, and this measure was used in the analyses. In addition, all regions, including the global atrophy measure, were corrected for individual differences in head size by creating a ratio [(structure volume/cerebral vault volume) × 1,000].
Statistical Methods.
We assessed the relationships between the indices of glucose regulation and the cognitive and brain measures by means of Pearson r correlations. The subjects evaluated varied in age by as much as 36 years. Therefore, given the potential impact of age on both brain and regulation of peripheral glucose, we accounted for age. Incipient dementia would have a significant impact on memory and brain. Therefore, we used partial correlation analyses to assess the relationships between brain variables (memory and MRI volumes) and the glucose indices after accounting for age and age and MMSE as potential confounds. Significance was set at the P ≤ 0.05 level.
Results
The 30 subjects studied (13 males and 17 females) had a mean age of 68.6 ± 7.5 years (range: 53–89 years), an average education of 16.2 ± 2.3 years (range: 12–20), and a body mass index of 24.9 ± 4.1 kg/m2 (range: 19.1–36.5). The average percent glycosylated hemoglobin (HbA1C) was 5.88 ± 0.74% (range: 4.5–7.00), which is within the nondiabetic range. In addition, subjects had an MMSE score of 28.6 ± 1.5 (range: 26–30), and a mean score of 28.0 ± 7.4 (9–39) on the Wechsler paragraph delayed test.
In the analyses, we used the ratio of the regional brain volume to the overall cerebral vault volume for each subject. However, Table 1 shows the absolute values for the brain volumes (average, SD, and range) so as to allow for comparison of our hippocampal and other brain volumes to those of other published reports.
Table 1.
MRI-derived brain volumes
| Brain region | Mean ± SD, (range) in cm3 |
|---|---|
| Cerebral vault | 1201.6 ± 111.6 (984.5–1,376.4) |
| Hippocampus* | 2.5 ± 0.3 (2.0–3.1) |
| Parahippocampal gyrus* | 4.2 ± 0.7 (2.8–5.6) |
| Superior temporal gyrus* | 13.4 ± 1.6 (9.6–17.4) |
| Global atrophy | 226.4 ± 66.2 (109.9–403.9) |
Data are the raw volumes in cubic centimeters for the brain regions measured, including a measure of global atrophy.
, The average of right and left hemispheres.
The mean baseline glucose value, based on the average of the two independent baseline blood samples during the IVGTT, was 97.1 ± 15.4 mg/dl (range: 75.8–124.3 mg/dl). The 2-h serum glucose level after glucose injection during the IVGTT was 94.7 mg/dl ± 15.4 (72–141 mg/dl), and the mean area under the glucose curve during the IVGTT was 27,639.5 ± 3,385.4 (range 22,107.5–36,495.5 mg/dl−1/min−1).
All three indices of glucose regulation were inversely associated with scores on the Wechsler Paragraph Recall Test. Individuals with higher baseline and 2-h postinfusion glucose levels as well as larger area under the glucose curve had decreased immediate and delayed memory performance.
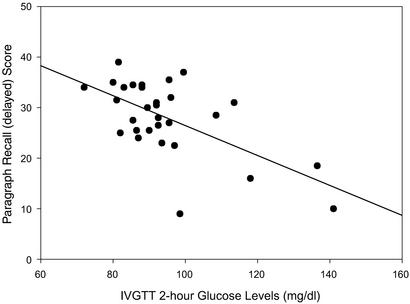
Table 2 summarizes the correlation coefficients between the brain variables (MRI-derived brain volumes and scores on cognitive tests) and the indices of glucose regulation. In addition, the score on the MMSE was also associated with the IVGTT 2-h glucose level and the area under the glucose curve. Fig. 1 represents the association between memory performance and the IVGTT 2-h glucose level (r = −0.62, P ≤ 0.05).
Table 2.
Pearson's correlation coefficients between glucose indices and brain or cognitive measures
| Hip. | Parahip. | STG | Global atrophy | Delayed recall | MMSE | |
|---|---|---|---|---|---|---|
| Baseline glucose | −0.43* | −0.25 | 0.04 | 0.18 | −0.37* | −0.15 |
| 2-h glucose | −0.42* | −0.06 | −0.06 | 0.07 | −0.62* | −0.43* |
| Glucose AUC | −0.30† | −0.11 | 0.07 | 0.03 | −0.53* | −0.41* |
Data are the associations (Pearson r coefficients) between the indices of glucose regulation and the adjusted regional brain volumes and cognitive variables. Hip., hippocampus/cerebral vault ratio; Parahip., parahippocampal gyrus/cerebral vault ratio; STG, superior temporal gyrus/cerebral vault ratio; MMSE, Mini Mental Status Examination; Glucose AUC, area under the glucose curve. N = 30;
, P < 0.05;
, P < 0.10.
Figure 1.
Relationship between recent memory performance and IVGTT 2-h glucose level.
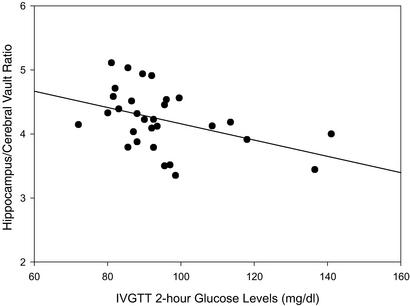
In addition, individuals with higher fasting glucose levels and elevated IVGTT 2-h glucose levels had significantly smaller hippocampi. Fig. 2 depicts the relationship between the 2-h postinfusion glucose levels and cerebral vault-adjusted hippocampal volumes (r = −0.42, P ≤ 0.05). This association to the hippocampus was anatomically specific, because no significant associations (all less than r = −0.25) were observed between the glucose indices and the parahippocampal gyrus, the superior temporal gyrus, or the measure of global atrophy.
Figure 2.
Relationship between adjusted hippocampal volume and 2-h glucose level.
Age was associated with greater cerebral vault-adjusted global brain atrophy (r = 0.48, P < 0.01) and tended to be inversely associated with adjusted hippocampal volumes (r = −0.34, P = 0.07). Very similar results were obtained for the partial correlations (after accounting for age and MMSE) between memory and brain variables and the indices of glucose regulation (Table 3).
Table 3.
Correlation coefficients between glucose indices and brain or cognitive measures (partial correlations controlling for age and MMSE)
| Hip. | Parahip. | STG | Atrophy | Delayed recall | |
|---|---|---|---|---|---|
| Baseline glucose | −0.44* | −0.24 | 0.07 | 0.23 | −0.35† |
| 2-h glucose | −0.40* | −0.01 | −0.03 | 0.16 | −0.57* |
| Glucose AUC | −0.29 | −0.09 | 0.15 | 0.14 | −0.48* |
Data are the associations (partial Pearson r coefficients) between the indices of glucose regulation and the adjusted regional brain volumes and cognitive variables after adjusting for age and Mini Mental Status Examination (MMSE). Hip., hippocampus/cerebral vault ratio; Parahip., parahippocampal gyrus/cerebral vault ratio; STG, superior temporal gyrus/cerebral vault ratio; glucose AUC, area under the glucose curve. N = 30;
, P < 0.05;
, P < 0.10.
To ensure that the reported relationships between indices of glucose regulation, memory performance, and hippocampal volumes are not due to a few individuals with high glucose values, we repeated those analyses after dropping the six subjects who either have a fasting glucose level or a 2-h glucose value of 110 mg/dl or higher. The results remained unchanged (data not shown).
We also measured insulin values during the IVGTT. However, insulin values were not related to memory, MMSE, age, or any of the brain volumes.
As expected, the delayed paragraph recall score was significantly and specifically correlated with hippocampus volume (r = 0.49, P < 0.01). The MMSE, which is a measure of overall cognitive function, was not related to any of the brain volumes.
Discussion
The present study provides evidence of a relationship between peripheral glucose regulation and specific brain findings in a sample of healthy middle-aged and elderly individuals functioning within the normal range. Memory impairments have been reported in individuals with Type 2 diabetes (3–6). However, it has been unclear whether similar deficits exist among nondiabetic elderly with varying degrees of impairment in glucose tolerance.
As a result of this study, we can report an association between peripheral glucose regulation and the volume of the hippocampus, a brain structure centrally involved in learning and memory. We found that among normal nondiabetic middle-aged and elderly individuals, those with poorer peripheral glucose regulation were more likely to have lower memory performance and smaller head size-adjusted hippocampal volumes. The fact that the maintenance of peripheral glucose levels would impact brain function, although not well described among normal elderly prior to this study, is not surprising, given that the glucose borne by the blood accounts for 99% of the brain energy requirements (26). What is surprising is that the impact seems to be restricted to the hippocampus, as no associations were found to the volume of other brain regions, including a measure of overall brain atrophy.
The hippocampus is more susceptible to damage by hypoglycemia and hypoxia than other brain regions (27–31). We propose that the anatomic specificity of our findings may be related to this higher hippocampal vulnerability. From animal studies we know that during a memory test (e.g., going through a maze), as the hippocampus is activated, localized drops in hippocampal glucose levels occur that are proportional to the difficulty of the maze (32). The drop in hippocampal glucose levels is deeper and lasts longer among older animals, perhaps indicating why older animals have impairments in memory performance (33).
We know that brain glucose transport is significantly reduced in diabetic animals (34, 35). Therefore, it is possible that among individuals with IGT, increased metabolic demand, such as occurs during regional activation (i.e., memory testing), results in regional low-grade hypoglycemia. We propose that memory deficits among elderly with poorer glucose tolerance may be caused by an inability to compensate for the drops in hippocampal glucose levels that occur with activation of those circuits during memory testing. Given the high vulnerability of the hippocampus, these subtle metabolic insults may, in the long run, lead to damage and volume loss. Although our subjects were not diabetic, it is also possible that the IGT leads to elevations in glucose levels sufficient to worsen the effects of other subtle brain insults by perhaps increasing excitotoxic free radical-induced damage (for a review, see ref. 36). Lastly, the hippocampus is known to be vulnerable to elevated cortisol levels, and there are known links between the HPA axis and the regulation of peripheral glucose (37). Therefore, future studies should study both systems in parallel.
This report is based on a relatively small number of subjects from 53 to 89 years of age. It is theoretically possible that the reported relationships between peripheral glucose regulation and hippocampal integrity (volumes and memory performance) are influenced by a small number of individuals who are in an early stage of dementia. One would expect that the oldest subjects or those with lower overall cognitive function (MMSE) to have poorer memory and smaller hippocampi. However, our findings were basically unchanged when we accounted for age and MMSE in the analyses.
In summary, our study reports a significant and anatomically specific relationship between several indices of peripheral glucose regulation and the volume of the hippocampus. These data suggest that metabolic substrate delivery may influence hippocampal structure and function. These findings also suggest that better lifetime management of blood sugar may improve memory in old age and perhaps even reduce the risk of Alzheimer's disease. Although we have speculated on a potential mechanism linking peripheral glucose regulation and hippocampal function and structure, empirical evidence remains to be developed. Also to be investigated is whether memory performance and hippocampal volumes can be improved by improving glucose tolerance. We hope that this report may bring to light a new mechanism for age-related brain injury that may have substantial medical impact, given the large number of elderly individuals with impaired glucose metabolism and memory dysfunction.
Acknowledgments
We thank Elissa Thorn for her tireless work in coordinating this study. This work was supported by National Institutes of Health/National Institute on Aging Grants RO1-AG17115, NCRR M01 RR00096, RO1-AG-12101, and P30 AG08051. Dr. Wolf's contribution was supported by Deutsche Forschungsgemeinschaft Grant WO 733/2-1.
Abbreviations
- IGT
impaired glucose tolerance
- IVGTT
i.v. glucose tolerance test
- MMSE
Mini Mental Status Examination
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Unverzagt F W, Gao S, Baiyewu O, Ogunniyi A O, Gureje O, Perkins A, Emsley C L, Dickens J, Evans R, Musick B, et al. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 2.Larrabee G J, Crook T H. Int Psychogeriatr. 1994;6:95–104. doi: 10.1017/s1041610294001663. [DOI] [PubMed] [Google Scholar]
- 3.Richardson J T E. Neurosci Biobehav Rev. 1990;14:385–388. doi: 10.1016/s0149-7634(05)80060-0. [DOI] [PubMed] [Google Scholar]
- 4.Stewart R, Liolitsa D. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 5.Strachan M, Deary I, Ewing F, Frier B. Diabetes Care. 2002;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 6.Biessels G-J, ter Braak E, Erkelens D, Hijman R. Neurosci Res Commun. 2001;28:11–22. [Google Scholar]
- 7.Vanhanen M, Koivisto K, Karjalainen L, Helkala E L, Laakso M, Soininen H, Riekkinen P., Sr NeuroReport. 1997;8:1527–1530. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan J R, Greenwood C E, Winocur G, Wolever T M S. Am J Clin Nutr. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- 9.Shimokata H, Muller D C, Fleg J L, Sorkin J, Ziemba A W, Andres R. Diabetes. 1991;40:44–50. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 10.Harris M I, Hadden W C, Knowler W C, Bennett P H. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 11.Astrup A. Pub Health Nutr. 2001;4:499–515. doi: 10.1079/phn2001136. [DOI] [PubMed] [Google Scholar]
- 12.Fagot-Campagna A. J Pediatr Endocrinol. 2000;13:1395–1402. doi: 10.1515/jpem-2000-s613. [DOI] [PubMed] [Google Scholar]
- 13.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H, Otto T, Cohen N J. Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 15.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Lupien S J, de Leon M J, De Santi S, Convit A, Tarshish C, Nair N P V, Thakur M, McEwen B S, Hauger R L, Meaney M J. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 17.Starkman M N, Gebarski S S, Berent S, Schteingart D E. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 18.Horner H C, Packan D R, Sapolsky R M. Neuroendocrinology. 1990;52:57–64. doi: 10.1159/000125539. [DOI] [PubMed] [Google Scholar]
- 19.de Leon M J, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, et al. J Clin Endocrinol Metab. 1997;82:3251–3259. doi: 10.1210/jcem.82.10.4305. [DOI] [PubMed] [Google Scholar]
- 20.Magarinos A M, McEwen B S. Proc Natl Acad Sci USA. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Convit A, de Leon M J, Hoptman M J, Tarshish C, De Santi S, Rusinek H. Psychiatr Q. 1995;66:343–355. doi: 10.1007/BF02238754. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale–Revised. San Antonio, TX: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 23.Folstein M. In: Assessment in Geriatric Psychopharmacology. Crook T, Ferris S H, Bartus R, editors. New Canaan, CT: Mark Powley Associates; 1983. pp. 47–51. [Google Scholar]
- 24.Convit A, McHugh P R, Wolf O T, de Leon M J, Bobinski M, De Santi S, Roche A, Tsui W. Psychiatry Res Neuroimag. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 25.Convit A, de Leon M J, Tarshish C, De Santi S, Tsui W, Rusinek H, George A E. Neurobiol Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 26.Sokoloff L. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 27.Mattson M P, Gurthrie P B, Kater S B. Prog Clin Biol Res. 1989;317:333–351. [PubMed] [Google Scholar]
- 28.McEwen B S. Mol Psychiatry. 1997;2:255–263. doi: 10.1038/sj.mp.4000254. [DOI] [PubMed] [Google Scholar]
- 29.Cervos-Navarro J, Kater S B. Crit Rev Neurobiol. 1991;6:149–182. [PubMed] [Google Scholar]
- 30.Chalmers J, Risk M T A, Kean D M, Grant R, Ashworth B, Campbell I W. Diabetes Care. 1991;14:922–925. doi: 10.2337/diacare.14.10.922. [DOI] [PubMed] [Google Scholar]
- 31.Ng T, Graham D I, Adams J H, Ford I. Acta Neuropathol. 1989;78:438–443. doi: 10.1007/BF00688181. [DOI] [PubMed] [Google Scholar]
- 32.McNay E C, Fries T M, Gold P E. Proc Natl Acad Sci USA. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNay E C, Gold P E. J Gerontol A Biol Sci Med Sci. 2001;56:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 34.Duckrow R B, Beard D C, Brennan R W. Stroke. 1987;18:52–58. doi: 10.1161/01.str.18.1.52. [DOI] [PubMed] [Google Scholar]
- 35.Pardridge W M, Triguero D, Farrell C R. Diabetes. 1990;39:1040–1044. doi: 10.2337/diab.39.9.1040. [DOI] [PubMed] [Google Scholar]
- 36.Biessels G-J, van der Heide L P, Kamal A, Bleys R L, Gispen W H. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- 37.Plat L, Byrne M M, Sturis J, Polonsky K S, Mockel J, Fery F, Van Cauter E. Am J Physiol. 1996;271:E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]