Abstract
Transgenic PDAPP mice, which express a disease-linked isoform of the human amyloid precursor protein, exhibit CNS pathology that is similar to Alzheimer's disease. In an age-dependent fashion, the mice develop plaques containing β-amyloid peptide (Aβ) and exhibit neuronal dystrophy and synaptic loss. It has been shown in previous studies that pathology can be prevented and even reversed by immunization of the mice with the Aβ peptide. Similar protection could be achieved by passive administration of some but not all monoclonal antibodies against Aβ. In the current studies we sought to define the optimal antibody response for reducing neuropathology. Immune sera with reactivity against different Aβ epitopes and monoclonal antibodies with different isotypes were examined for efficacy both ex vivo and in vivo. The studies showed that: (i) of the purified or elicited antibodies tested, only antibodies against the N-terminal regions of Aβ were able to invoke plaque clearance; (ii) plaque binding correlated with a clearance response and neuronal protection, whereas the ability of antibodies to capture soluble Aβ was not necessarily correlated with efficacy; (iii) the isotype of the antibody dramatically influenced the degree of plaque clearance and neuronal protection; (iv) high affinity of the antibody for Fc receptors on microglial cells seemed more important than high affinity for Aβ itself; and (v) complement activation was not required for plaque clearance. These results indicate that antibody Fc-mediated plaque clearance is a highly efficient and effective process for protection against neuropathology in an animal model of Alzheimer's disease.
Immunization of the transgenic PDAPP mice with β-amyloid peptide (Aβ)-derived immunogens results in an antibody response that facilitates the clearance of plaques within the central nervous system (CNS) (1–4). Although a number of mechanisms are likely to operate in this clearance response (5, 6), our previous findings strongly indicate that antibody-mediated, Fc-dependent phagocytosis by microglial cells and/or macrophages is important to the process (7). Importantly, a T cell response was not required for amyloid plaque clearance. When peripherally administered, antibodies against Aβ entered the CNS of PDAPP transgenic mice, decorated amyloid plaques, and induced plaque clearance. Comparing different antibodies in an ex vivo assay with sections of PDAPP or Alzheimer's disease (AD) brain, there was a strong correlation between those that produced ex vivo efficacy and those that were efficacious in vivo. Fc receptors on microglial cells were found to be key for the clearance response in this assay. However, it has been reported that antibody efficacy can also be obtained in vivo by mechanisms that are independent of Fc interactions (8). Studies have indicated that an antibody directed against the midportion of Aβ, which cannot recognize amyloid plaques, appears to bind to soluble Aβ and reduce plaque deposition (6). In addition, it has been reported recently that short-term treatment with this antibody improved performance in an object-recognition task without affecting amyloid burden (9).
To understand the parameters of an antibody response that are required for neuronal protection, several questions should be considered. Is neuronal protection associated with plaque clearance, or is it necessary for antibodies to capture soluble aggregates of Aβ to protect neurons against the directly toxic effects of the peptide? Does a clearance response depend on Fc receptor-mediated phagocytosis of Aβ after antibody binding or on complement receptor-mediated phagocytosis after antibody binding and complement activation? Alternatively, is a clearance response independent of antibody Fc receptor function?
In the current study we approached these questions by examining the influence of different antibody epitopes and isotypes on plaque clearance and neuronal protection. The studies took advantage of the fact that some epitopes of Aβ are preferentially available for antibody binding within plaques, whereas others are only available for antibody capture of the soluble peptide. In addition, the isotype of an antibody is important for either Fc- or complement-mediated phagocytosis of Aβ by microglial cells, because antibody isotype defines its affinity for Fc receptors as well as its ability to activate complement. If plaque clearance and/or neuronal protection do not depend on Fc-mediated processes, then the isotype of an antibody against Aβ should have little impact on efficacy. These studies provide insight for the design of antibodies with therapeutic potential.
Materials and Methods
Aβ Fragments.
Peptides corresponding to Aβ1–5, Aβ3–9, Aβ5–11, and Aβ15–24 and the reverse sequence Aβ5–1 were synthesized contiguous to a 17-aa T cell epitope derived from ovalbumin (amino acids 323–339, ISQAVHAAHAEINEAGR) on a branched peptide framework (triple-lysine core with four peptide arms) to produce a multiantigen peptide as described (10). Polyclonal antibodies against Aβ1–42 (pAb 1–42) were raised and the Ig fraction was isolated as described (7). pAb-EL16, pAb-EL17, and pAb-EL20 were obtained from the sera of PDAPP mice immunized with peptides corresponding to Aβ1–7, Aβ15–24, and Aβ3–9, respectively, which had been synthesized on a branched framework as described above. pAb-EL26 was obtained from the sera of mice immunized with Aβ(7–1)-42. The peptides were synthesized by AnaSpec (San Jose, CA).
Monoclonal Antibodies (mAbs).
The production of mAbs 10D5 and 6C6, which were raised against synthetic Aβ1–28 coupled to a carrier protein, has been described (11). mAbs 12B4, 2C1, 12A11, and 3A3 were raised against synthetic Aβ1–42 by using similar methodology except that hybridoma supernatants were screened by an RIA. All antibodies were purified by HPLC and were free of endotoxin (<1 endotoxin unit/mg protein) as determined by the Limulus amoebocyte gel-clot assay (Associates of Cape Cod). The mAbs 3D6 and 21F12 were obtained as described (7), and 22D12 and 266 were raised against synthetic Aβ13–28 (12).
Epitope Mapping.
Epitope mapping of the mAbs and pAbs was performed by using an ELISA that measured antibody binding to overlapping peptides (10 amino acid peptides offset by 1 residue) covering the entire Aβ1–42 sequence. The first 32 peptides were biotinylated at the C terminus, and the last 10 peptides were biotinylated at the N terminus. The biotinylated peptides were synthesized by Mimotopes (Clayton, Victoria, Australia) and captured on streptavidin-coated wells of a 96-well plate (Pierce).
Passive and Active Immunization Procedures.
mAbs in PBS were given via passive administration (i.p. injection) at a dose of 10 mg/kg weekly for 6 months. For active immunization, 100 μg of Aβ fragment was administered by i.p. injection in complete Freund's adjuvant followed by boosts with 100 μg of peptide in incomplete Freund's adjuvant at 2 and 4 weeks, and monthly thereafter.
Antibody Binding to Aggregated and Soluble Aβ1–42.
Serum titers (determined by serial dilution) and mAbs binding to aggregated synthetic Aβ1–42 were performed by ELISA as described (1). Soluble Aβ1–42 refers to the synthetic Aβ1–42 peptide sonicated in dimethyl sulfoxide. Serial dilutions of sera or mAb at 20 μg/ml were incubated with 50,000 cpm [125]Aβ1–42 (≈190 μCi/μmol; labeling with Iodogen reagent, Pierce) overnight at room temperature. Fifty microliters of a slurry containing 75 mg/ml protein A Sepharose (Amersham Pharmacia) and 200 μg of rabbit anti-mouse IgG (H+L) (Jackson ImmunoResearch) was incubated with the diluted antibodies for 1 h at room temperature, washed twice, and counted on a Wallac gamma counter (Perkin–Elmer). All steps were performed in RIA buffer consisting of 10 mM Tris, 0.5 M NaCl, 1 mg/ml gelatin, and 0.5% Nonidet P-40, pH 8.0.
Ex Vivo Assay.
Cryostat sections (10 μm in thickness) of PDAPP mouse brain were thaw-mounted onto round polylysine-coated coverslips and placed in the wells of 24-well tissue-culture plates. Microglial cells and antibodies were added to the wells and cultured for 24 h as described (7). After incubation, cultures were extracted with an 8 M urea buffer and frozen quickly. Total Aβ level in the cultures was determined by ELISA as described (13).
Statistical analyses were performed by using PRISM 3.0 software (GraphPad, San Diego).
Results and Discussion
Aβ Epitope: Epitopes Within the N Terminus of Aβ Are Important for Plaque Clearance and Reduction of Neuritic Pathology.
We have shown previously that not all antibodies against Aβ can trigger plaque clearance in vivo. Efficacy can be predicted by the ability of antibodies to both bind plaques within unfixed sections of PDAPP or AD brains and trigger plaque clearance in an ex vivo assay (7). In the current study, a number of mAbs and pAbs directed against different epitopes of Aβ were examined for plaque reactivity and ex vivo efficacy. Only antibodies against epitopes within the N-terminal 11 aa of Aβ were found to be active in either regard (Table 1). These findings also illustrate that, of the five antibodies previously examined in vivo, those against N-terminal epitopes were effective in reducing plaque burden (pAb1–42, 3D6, and 10D5), whereas those against C-terminal epitopes were inactive (16C11 and 21F12) (7).
Table 1.
Antibodies directed against epitopes within the N-terminal 11 aa of Aβ1–42 bind amyloid plaques and trigger phagocytosis in an ex vivo assay
| Antibody | Epitope | Binds plaques (PDAPP) | Triggers ex vivo phagocytosis |
|---|---|---|---|
| 3D6 | 1–5 | ++ | ++ |
| pAb-EL16 | 1–7 | ++ | ++ |
| pAb1–42 | 1–11 | ++ | ++ |
| 10D5 | 3–7 | ++ | ++ |
| pAb-EL21 | 5–11 | + | + |
| pAb-EL26 | 11–26 | − | − |
| 22D12 | 18–21 | − | − |
| 266 | 16–24 | − | − |
| pAb-EL17 | 15–24 | − | − |
| 16C11 | 33–42 | − | − |
| 21F12 | 34–42 | − | − |
Both measures were scored on three-step visual rating system based on fluorescence intensity for the plaques and by degree of Aβ uptake as described (7).
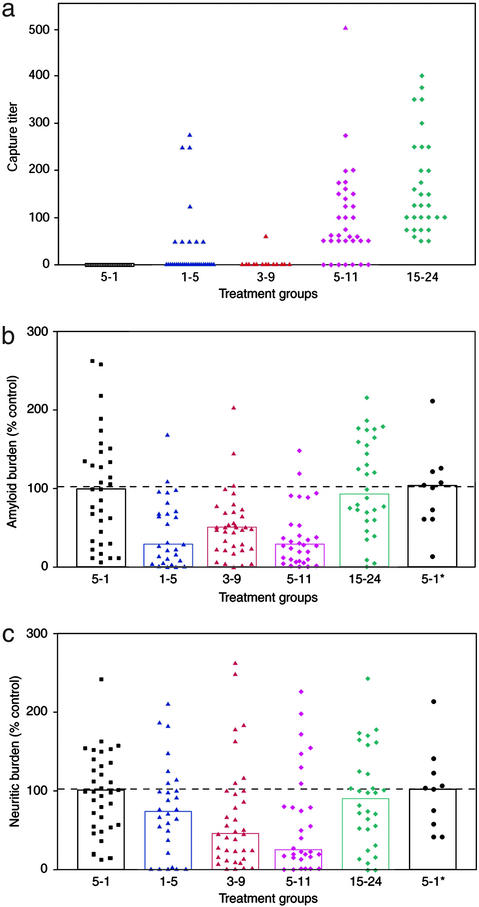
To extend these findings and further characterize epitopes within the N terminus of Aβ, a series of peptides were compared for their ability to trigger an efficacious antibody response in vivo. Twelve- to 13-month-old PDAPP mice were immunized with one of three N-terminal peptide fragments (Aβ1–5, Aβ3–9, or Aβ5–11) or a fragment derived from an internal region of the peptide (Aβ15–24) (Fig. 1a). The internal peptide Aβ15–24 encompasses the epitope of antibody 266, which exhibits high affinity for soluble Aβ (12), but as shown above it does not recognize plaques in sections of unfixed AD or PDAPP tissue (Table 1). Thus, it was of interest to determine whether a polyclonal response directed against this peptide could produce antibodies capable of plaque recognition or whether reactivity with soluble Aβ alone was sufficient to provide efficacy. In these studies, a peptide with reverse sequence (Aβ5–1) served as a negative control. The peptides were synthesized contiguous to a 17-aa T cell epitope derived from ovalbumin and presented in an identical multivalent configuration (see Materials and Methods). All the peptides (except Aβ5–1 reverse mer) produced sera that recognized aggregated synthetic Aβ1–42 by ELISA (Fig. 1b). In contrast, only sera against the N-terminal peptides were able to recognize Aβ within plaques; antisera against Aβ15–24 did not bind plaques despite strong reactivity with the synthetic aggregated peptide (Fig. 1c). There also were differences between the serum groups in their ability to capture soluble Aβ (Fig. 2a). Less than 30% of the sera from mice immunized with Aβ1–5 or Aβ3–9 captured the soluble peptide (27% and 5%, respectively). In contrast, sera from approximately half of the animals immunized with Aβ5–11 and all of those immunized with Aβ15–24 captured soluble Aβ1–42.
Figure 1.
Antibodies produced by immunization with N-terminal fragments of Aβ bind to amyloid plaques. (a) Peptides encompassing various domains of Aβ1–42 (synthesized contiguous to T cell epitope derived from ovalbumin) were used to immunize PDAPP mice. A reverse mer, Aβ5–1, was used as a negative control. (b) ELISA titers against aggregated Aβ1–42 were significantly higher over the length of the study in the Aβ5–11 and Aβ15–24 groups than in the Aβ1–5 group (1:14,457, P < 0.01, and 1:12,257, P < 0.05 vs. 1:3,647, respectively; ANOVA followed by post hoc Tukey's test). (c) Unfixed cryostat sections from untreated PDAPP mouse brain were exposed to the sera of mice immunized with Aβ5–1, Aβ3–9, Aβ5–11, or Aβ15–24 (titers normalized to 1:1,000 for staining). Antibodies to Aβ15–24 did not bind to amyloid plaques. (Scale bar, 500 μm.)
Figure 2.
Capture of soluble Aβ1–42 by antibodies is not associated with reduced amyloid burden or neuritic pathology. (a) Sera from mice immunized with fragments of Aβ were examined for their ability to capture radiolabeled soluble Aβ1–42 in an RIA. Sera from all animals immunized with Aβ15–24 were able to capture soluble Aβ1–42 (one serum sample had a titer higher than 1:1,350, and a precise titer was not determined) compared with 27% of those in the Aβ1–5 group and 3% of the Aβ3–9 group. Amyloid burden (b) and neuritic pathology (c) were evaluated with image analysis by a blinded microscopist. Values are expressed as a percentage of the mean of the Aβ5–1 group (negative control reverse mer peptide). The Aβ5–11 group was evaluated at a separate sitting from the other groups but in conjunction with the same negative control group as an internal reference (second Aβ5–1* set, on the right). Amyloid burden was reduced significantly in the Aβ1–5, Aβ3–9, and Aβ5–11 groups (P < 0.001). The bars represent median values, and the dashed horizontal line indicates the control level. Neuritic burden was reduced significantly in the Aβ3–9 and Aβ5–11 groups (P < 0.05). Neither endpoint was altered significantly by immunization with the Aβ15–24 group. Statistical analysis was performed with square-root transformation (to normalize nonparametric distributions) and analyzed with ANOVA. A Dunnett's test then was used to compare the multiple groups Aβ1–5, Aβ3–9, and Aβ15–24 with their Aβ5–1 control and Mann–Whitney for the Aβ5–11 group with its corresponding Aβ5–1* control.
Because the degree of Aβ deposition can vary greatly as PDAPP mice age, the in vivo study was designed with at least 30 animals per group. Efficacy data are shown for individual mice and expressed as the percentage of either amyloid burden or neuritic dystrophy relative to the mean of the control (set at 100%). Immunization with each of the three N-terminal peptides significantly reduced amyloid burden (46–61%, P < 0.002) (Fig. 2b). Furthermore, Aβ3–9 and Aβ5–11 significantly reduced neuritic pathology (34% and 41%, respectively; P < 0.05) (Fig. 2c). In contrast, immunization with Aβ15–24 provided no protection against either amyloid burden or neuritic pathology. These results further support the association between plaque binding and antibody efficacy. They also indicate that capture of soluble Aβ is not required for reduction of neuritic pathology, because the antibody response against Aβ3–9 provided strong plaque reactivity and the highest level of protection against neuronal dystrophy yet exhibited the weakest capacity for recognition of soluble peptide. These results, however, do not eliminate the possibility that antibodies specific for Aβ capture could provide efficacy at higher titers or over longer periods of time, as has been reported by DeMattos et al. (6) using the high-affinity capture antibody 266.
Antibody Isotype: IgG2a Antibodies Against Aβ Are More Efficient than IgG1 or IgG2b Antibodies in Reducing Neuropathology.
Murine phagocytotic effector cells such as microglia within the CNS express three different classes of IgG-specific Fc receptors (Fcγ receptors): a high-affinity receptor, FcγRI, and two low-affinity receptors, FcγRII and FcγRIII (14). FcγRII is a single-chain receptor with two major isoforms that apparently lack phagocytic capacity (15). FcγRI and FcγRIII are heterooligomeric complexes in which the specific ligand-binding α chains are associated with a common γ chain. The precise contribution of FcγRI and FcγRIII to the phagocytosis of opsonized particles has not been defined; however, it has been shown that both receptors, and in particular FcγRI, exhibit a higher affinity for murine IgG2a than for IgG1 or IgG2b (16). Furthermore, IgG2a has proven to be more effective in a number of in vivo clearance responses than the other antibody isotypes (17–20). Thus, if Fc-mediated phagocytosis of Aβ peptide is an important mechanism for antibody-mediated plaque clearance, then IgG2a antibodies would be expected to reduce plaque burden more efficiently than the other antibody isotypes.
To address this issue, experiments were conducted with six mAbs: two of each IgG isotype and all directed against the same epitope of Aβ (Aβ3–7). The epitope was defined further by amino acid substitution analysis; each antibody required the same three residues within Aβ3–7 for binding, and each could tolerate substitution within these residues by similar amino acids (data not shown). All the antibodies exhibited high avidity for aggregated Aβ1–42 (<1 nM); however, the IgG1 antibodies showed ≈10-fold greater binding avidity than the IgG2a antibodies (≈50 vs. 500 pM) (Table 2). In contrast, only two of the antibodies could appreciably capture soluble Aβ1–42 at antibody concentrations of 20 μg/ml: one of the IgG2b antibodies (12A11) and, to a lesser extent, one of the IgG2a antibodies (12B4). As a measure of their ability to trigger Fc-mediated plaque clearance, the six antibodies were compared in the ex vivo assay with primary mouse microglial cells and sections of brain tissue from PDAPP mice. Irrelevant IgG1, IgG2a, and IgG2b antibodies, having no reactivity toward Aβ or other components of the assay, were used as isotype-matched negative controls. To quantify the degree of plaque clearance/Aβ degradation that occurred by the end of the assay, Aβ was extracted from the cultures of microglia and brain sections (n = 3) with 8 M urea for analysis by ELISA (see Materials and Methods). As shown in Fig. 3, the two IgG2a antibodies against Aβ reduced peptide levels in the cultures more efficiently (73% and 69%, P < 0.001) than the IgG1 (28% and 35%, not significant) or IgG2b (48% and 59%, P < 0.05 and 0.001, respectively) antibodies. Because previous studies showed that ex vivo plaque clearance depends on Fc-receptor activity (7) and can occur in the presence of heat-inactivated serum, it is unlikely that complement played a significant role in mediating the effects observed in the ex vivo assay.
Table 2.
mAbs against Aβ3–7 have different avidity for aggregated and soluble synthetic Aβ1–42
| Antibody | Epitope | Isotype | ED50 on aggregated Aβ1–42, pM | % Capture of soluble Aβ1–42 |
|---|---|---|---|---|
| 6C6 | Aβ3–7 | IgG1 | 40 | 1 |
| 10D5 | Aβ3–7 | IgG1 | 53 | 1 |
| 2C1 | Aβ3–7 | IgG2a | 333 | 1 |
| 12B4 | Aβ3–7 | IgG2a | 667 | 8 |
| 3A3 | Aβ3–7 | IgG2b | 287 | 1 |
| 12A11 | Aβ3–7 | IgG2b | 233 | 30 |
As a comparison, the antibody 266 at 10 μg/ml would capture 70% of Aβ1–42.
Figure 3.
Comparing antibodies against Aβ3–7 in the ex vivo assay, IgG2a antibodies clear β amyloid plaque more efficiently that either the IgG1 or IgG2b isotypes. Murine primary microglial cells were cultured with unfixed cryostat sections of PDAPP mouse brain in the presence of antibodies of different isotypes directed against Aβ3–7. Irrelevant IgG1, IgG2a, and IgG2b antibodies were used as the respective isotype-matched negative controls. After 24 h of incubation, the total level of Aβ remaining in the cultures was measured by ELISA. The two anti-Aβ IgG2a antibodies reduced Aβ levels in the cultures (69% for 2C1 and 73% for 12B4; P < 0.001) more efficiently than the IgG2b isotype antibodies (48% for 12A11, P < 0.05, and 59% for 3A3, P < 0.001). The anti-Aβ IgG1 antibodies did not significantly reduce Aβ levels. Data were analyzed with ANOVA followed by a post hoc Dunnett's test.
The antibodies then were investigated for in vivo efficacy. Antibody (10 mg/kg) or PBS control was administered by weekly i.p. injection for 6 months as described (7). Antibodies within all groups maintained similar serum titers against aggregated Aβ1–42 (1:3,500) with the exception of the IgG1 antibody 10D5, which displayed 3- to 4-fold higher titers (≈1:13,000). At the end of the study, total levels of cortical Aβ were determined by ELISA. Although each of the antibodies significantly reduced total Aβ levels compared with the PBS control (P < 0.001) (Fig. 4a), there was a trend toward greater levels of reduction by the two IgG2a antibodies (61% and 69% reduction) than by the IgG1 (38% and 52%) or the IgG2b (44% and 31%) antibodies. These results suggest that the higher affinity of IgG2a for Fc receptors is an important parameter for clearance. Furthermore, because murine IgG1 antibodies cannot fix complement but provided levels of Aβ clearance comparable to the complement-fixing IgG2b antibodies, complement does not seem to play a critical role during the clearance process in vivo.
Figure 4.
Anti-Aβ IgG2a antibodies reduced AD-like neuropathology more efficiently than other isotypes in vivo. PDAPP mice received weekly i.p. injections of antibodies starting at 12 months of age for 6 months. (a) Total Aβ levels shown for individual mice sorted by treatment group (n = 30). The bars represent median values, and the dashed horizontal line indicates the control level. Although Aβ levels were reduced significantly in all antibody groups (P < 0.001 vs. PBS; ANOVA followed by post hoc Dunnett's test), the IgG2a groups exhibited the highest degree of clearance. (b) The percentage of frontal cortex occupied by neuritic dystrophy was determined by image analysis. The different groups within this experiment were analyzed in two sets by using the same PBS group as an internal standard (PBS-1 and PBS-2). PBS-1 was the control for the 6C6, 10D5, 12B4, and 12A11 groups, and PBS-2 was the control for the 2C1 and 3A3 groups. To compare the groups, values for individual animals are expressed as a percentage of the mean of their respective PBS control group (set at 100%). The bars represent median values, and the dashed horizontal line indicates the control level. Neuritic dystrophy was reduced significantly only by the IgG2a isotype antibodies (12B4, P < 0.05, and 2C1, P < 0.001; ANOVA followed by post hoc Dunnett's test). (c) Dystrophic neurites were labeled with the amyloid precursor protein-specific antibody 8E5, and were found in association with plaques. Relative to PBS control, the neuritic pathology was reduced significantly in animals treated with 12B4 but not 10D5. (Scale bar, 250 μm.)
The level of neuritic dystrophy then was examined in sections of brain tissue from the mice to determine the association between plaque clearance and neuronal protection. Again, data are shown for individual animals and expressed as the percentage of neuritic dystrophy relative to the mean of the control (set at 100%). Although all the antibodies triggered plaque clearance, only the IgG2a antibodies provided significant reduction in neuritic dystrophy (12B4, P < 0.05, and 2C1, P < 0.001) (Fig. 4 b and c). Interestingly, the antibody 10D5 (IgG1) was less effective than either of the IgG2a antibodies even though it exhibited higher avidity for aggregated Aβ1–42 (Table 2) as well as amyloid plaques (data not shown) and maintained significantly higher serum titers than the other antibodies. Thus, antibody isotype and affinity for Fc receptors seem to be important attributes for both clearance of Aβ and protection against neuritic dystrophy and may be more important than the relative avidity of antibodies for Aβ1–42 (Table 2). Also, these studies confirmed the observation obtained in the peptide immunization study described above that antibodies do not need to strongly capture soluble Aβ1–42 to provide protection against neuritic dystrophy. The antibody 12A11 (IgG2b) captured soluble monomeric Aβ1–42 more efficiently than either of the IgG2a antibodies (Table 1) but was not as effective. Also, both IgG2a antibodies provided similar protection even though only one could capture the soluble peptide detectably. The results of the current study are not necessarily inconsistent with other investigations and hypotheses surrounding anti-Aβ-based immunotherapies. Duration of treatment, route of administration, and specific antibody properties all likely have important effects on the observed outcomes. For example, Solomon et al. (5) suggested that anti-Aβ antibodies may directly inhibit or reverse amyloid fibril formation. Bacskai et al. (8) provided support for the possible direct dissolution mode of action by showing removal of plaque in vivo by F(ab′)2 fragments of an anti-Aβ antibody after direct application to the brain. It should be noted, however, that in vitro dissolution was reported to be restricted to antibodies against Aβ3–6 (21), whereas the antibody used in the in vivo study was against Aβ1–5. In addition, we show that antibodies against fragments Aβ1–5, Aβ3–9, and Aβ5–11 are all capable of reducing plaque burden. Thus the mechanism of plaque reduction does not seem to have the same restricted epitope as reported for in vitro fibril dissolution. Although the direct application of high-dose antibody to brain was capable of clearing plaque without Fc-mediated phagocytosis, the present data demonstrate that the efficiency in reducing plaque burden and neuritic dystrophy is best when the antibody isotype maximally supports phagocytosis and that efficacy can be achieved by antibodies of several epitope specificities that all are capable of binding plaque in vivo or ex vivo.
Another reported mechanism of efficacy is through capture of soluble Aβ (6). The use of antibody 266, a high-affinity capture antibody, at concentrations sufficient to produce detectable cerebrospinal fluid levels reduced plaque burden after chronic treatment. It is likely that the capture titer achieved by immunization with the Aβ15–24 fragment, although still substantially greater than the other immunogens tested in this study, is less than that achieved by 266 dosing, and this may impact the outcome. Thus it may be possible to achieve the same endpoint through multiple mechanisms including a chronic capture of Aβ species, dissolution of plaques, or phagocytosis of existing aggregates.
In summary, although antibodies against Aβ may exhibit efficacy in a number of ways, protection against AD-like neuropathology can be obtained by antibodies that bind to plaques and trigger Fc-mediated clearance. IgG2a antibodies, which exhibit higher affinity than other isotypes for phagocytic Fc receptors (in particular FcγRI), provided the highest level of plaque clearance and were the only anti-Aβ antibodies to provide neuronal protection under the conditions tested. Plaque clearance seemed independent of complement activation, because IgG1 antibodies, which cannot fix complement, were as effective as the complement-fixing IgG2b antibodies. These results are consistent with the role of high-affinity Fc receptors in other clearance systems, where they have been shown to be particularly effective for inducing clearance in conditions with low antibody concentrations (as would be anticipated in the CNS). The density of target-bound IgG that is required for complement activation has been reported to be higher than that required for Fc receptor-mediated phagocytosis (17). Accordingly, complement involvement in plaque clearance may be more pronounced at higher doses of antibody, where there would be an increased density of antibody bound to plaques. Interestingly, in contrast to other macrophage-activation paradigms, it has been shown that activation of phagocytic cells through Fc receptors results in production of the antiinflammatory cytokine IL-10 and inhibition of proinflammatory IL-12 (22). Thus, antibodies against Aβ may allow resolution of an otherwise chronic, unresolving inflammatory response associated with plaques in AD by both clearing Aβ and altering the inflammatory environment.
In addition, the current studies demonstrate that antibody epitopes within the N terminus of Aβ are important for plaque clearance and neuronal protection via an Fc-mediated mechanism. Passive administration of mAbs against defined Aβ epitopes reduced plaque burden and neuritic pathology to the same degree as active immunization. Although the 5- to 7-aa residue epitopes used for immunization in our studies are themselves too short to elicit T cell help for antibody production, we showed that these epitopes can be synthesized in conjunction with an exogenous T cell epitope to produce an efficacious antibody response after administration. Such an approach will avoid generation of T cell immunity against Aβ as a self-antigen and may preclude the potential issues with encephalitis that were observed recently with a subset of patients in the clinic after immunization with whole Aβ1–42 (23, 24). Likewise, T cell immunity will not be elicited with passive administration of antibodies against Aβ. Thus, both immunoconjugates containing defined epitopes of Aβ and mAbs against appropriate Aβ epitopes offer excellent alternatives to whole-peptide immunization for the treatment of AD.
Acknowledgments
We thank Chuck Davies for advice in the statistical analysis and Dr. Manuel Buttini for helpful editorial comments.
Abbreviations
- AD
Alzheimer's disease
- Aβ
β-amyloid peptide
- pAb
polyclonal Ab
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 2.Janus C, Pearson J, McLaurin J A, Mathews P M, Jiang Y, Schmidt S D, Chishti M A, Horne P, Heslin D, French J, et al. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 3.Morgan D, Diamond D M, Gottschall P E, Ugen K E, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, et al. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdsson E M, Scholtzova H, Mehta P D, Frangione B, Wisniewski T. Am J Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon B, Koppel R, Frenkel D, Hanan-Aharon E. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMattos R B, Bales K R, Cummins D J, Dodart J C, Paul S M, Holtzman D M. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bard F, Cannon C, Barbour R, Burke R L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 8.Bacskai B J, Kajdasz S T, McLellan M E, Games D, Seubert P, Schenk D, Hyman B T. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodart J C, Bales K R, Gannon K S, Greene S J, DeMattos R B, Mathis C, DeLong C A, Wu S, Wu X, Holtzman D M, Paul S. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 10.Tam J P. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson J P, Esch F S, Keim P S, Sambamurti K, Lieberburg I, Robakis N K. Neurosci Lett. 1991;128:126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- 12.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, et al. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravetch J V, Kinet J-P. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 15.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 16.Fossati-Jimack L, Ioan-Facsinay A, Reininger L, Chicheportiche Y, Watanabe N, Saito T, Hofhuis F M A, Engelbert Gessner J, Schiller C, Schmidt R E, et al. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azeredo da Silveira S, Kikuchi S, Fossati-Jimack L, Moll T, Saito T, Verbeek J S, Botto M, Walport M J, Carroll M, Izui S. J Exp Med. 2002;195:665–672. doi: 10.1084/jem.20012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arulanandam B P, O'Toole M, Metzger D W. J Infect Dis. 1999;180:940–949. doi: 10.1086/314996. [DOI] [PubMed] [Google Scholar]
- 19.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilson J A, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn A L, Hart M K. Science. 2000;287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 21.Frenkel D, Balass M, Solomon B. J Neuroimmunol. 1998;88:85–90. doi: 10.1016/s0165-5728(98)00098-8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson C F, Mosser D M. J Immunol. 2002;168:3697–3701. doi: 10.4049/jimmunol.168.8.3697. [DOI] [PubMed] [Google Scholar]
- 23.Senior K. Lancet Neurol. 2002;1:3. doi: 10.1016/s1474-4422(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 24.Schenk D. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]