Abstract
Social isolation (SI) of male mice lasting >4 weeks is associated with aggression toward intruders and a down-regulation of brain allopregnanolone (Allo) content. SI of female mice fails to down-regulate brain Allo content or to induce aggressiveness. Fluoxetine (Prozac in clinical use) is an S- and R-fluoxetine (FLX) mixture, which in mammals is metabolized into S- and R-norfluoxetine (NFLX). The S isomers of FLX and NFLX are more active than their respective R isomers in normalizing brain Allo down-regulation and in reducing the aggressiveness induced by SI. Thus, FLX stereospecifically reduces brain Allo down-regulation and the aggressiveness induced by SI, whereas serotonin (5-HT) uptake inhibition lacks stereospecificity. The doses of S-FLX and S-NFLX that reduce aggressiveness and Allo brain content down-regulation induced by SI are at least one order of magnitude lower than the doses that block 5-HT reuptake. Doses of imipramine that inhibit 5-HT uptake neither reduce aggressiveness nor normalize brain Allo down-regulation. We conclude that Allo brain content normalization is a better candidate than 5-HT reuptake inhibition to explain the reduction of aggressiveness elicited by S-FLX and S-NFLX.
Aggressive behavior and violence are included in the phenotypic expression of several central nervous system disorders (i.e., schizophrenia, premenstrual dysphoria, major manic-depressive illness, posttraumatic stress disorder, and epilepsy).
Human and animal studies suggest that often a genetic component contributes to aggressive behavior. For example, the altered transcription of genes encoding for various serotonin (5-HT) receptor subtypes or for the 5-HT transporter has been implicated in the pathophysiology of violence and aggression in humans and other mammals (1–3). However, a Mendelian inheritance of these 5-HT neurotransmission dysregulations could not be demonstrated (4). Probably, epigenetic factors contribute to aggressive behavior.
Social isolation (SI) of male mice for >4 weeks causes a syndrome characterized by anxiety, decreased susceptibility to barbiturates and other γ-aminobutyric acid (GABA)mimetic drugs, and frequent severe attacks against an intruder mouse (5, 6). Socially isolated (SI) male mice also show a marked decrease of Allo brain content (5, 7) and a down-regulation of serotonergic neurotransmission (8–10).
Valzelli and his coinvestigators (11) pioneered studies of SI-induced aggression in mice and concluded that this aggressiveness involves an impairment of 5-HT neurotransmission (11, 12). This conclusion was supported by mouse knockout studies of the 5-HT transporter (3), and by other studies in which mice received selective 5-HT reuptake inhibitors (SSRIs), which mitigate aggression in psychiatric patients (13, 14) and reduce SI-induced aggression in mice (5, 6).
In patients with major depression, the allopregnanolone (Allo) content of cerebrospinal fluid (CSF) is reduced, and this reduction is reversed by appropriate treatment with fluoxetine (FLX) or fluvoxamine (15). Interestingly, in depressed patients the extent of CSF Allo level increases elicited by FLX could be correlated with a decrease in Hamilton scores (15). FLX and other SSRIs in a dose- and time-related manner increased the reduced Allo levels in various brain structures of SI rodents (5, 16). In SI male mice, the down-regulation of Allo brain content, which is reversed by the dose of FLX that also reduces aggressive behavior, prolongs the pentobarbital (PTB)-induced loss of the righting reflex (5, 6) that is reduced by SI. The doses of FLX that modify the duration of the righting reflex loss induced by PTB and the brain content of Allo only slightly inhibit 5-HT reuptake.
These findings raise the following questions: (i) is the mitigation of SI-induced aggression elicited by SSRIs specifically related to their action on 5-HT reuptake? (ii) Is the anti-aggressive action of SSRIs dependent on their ability to normalize the down-regulation of brain Allo content elicited by SI? (iii) Is the SSRI-induced normalization of brain Allo content independent from 5-HT reuptake? To answer these and related ancillary questions, we have tested whether the treatment of male mice with various doses of FLX and norfluoxetine (NFLX) stereoisomers (shown in Table 1) stereospecifically and in a dose-dependent manner modifies aggression and Allo brain content. However, the inhibition of 5-HT reuptake is not stereospecific (17).
Table 1.
Ex vivo inhibition of 5-HT uptake in brain cortical slices of mice treated with S-FLX, R-FLX, or imipramine
| Treatment | Dose, μmol/kg i.p. | [14C]-5-HT uptake in brain slices, fmol/mg protein/min | Inhibition, % of control |
|---|---|---|---|
| Vehicle | 860 ± 158 | 100 | |
| S-FLX | 1.5 | 661 ± 127 | 77 |
| 9.0 | 525 ± 88* | 61 | |
| 18.0 | 421 ± 65* | 49 | |
| R-FLX | 1.5 | 705 ± 82 | 82 |
| 9.0 | 585 ± 101* | 68 | |
| 18.0 | 439 ± 93* | 51 | |
| Imipramine | 64.0 | 55 ± 8† | 7 |
Drugs were administered 30 min (R- and S-FLX) and 60 min (imipramine) before ex vivo [14C]-5-HT uptake measurements. Data represent the mean ± SE of three to six SI mice.
P < 0.05.
P < 0.01, when compared with vehicle-treated mice.
Materials and Methods
Animals and Drug Treatment.
Adult male or female Swiss–Webster mice (Harlan Breeders, Indianapolis), 22–25 g body weight, maintained under a 12-h dark/light cycle, and food and water ad libitum, were used for all experiments. Animals were housed either in groups of five to six per cage (24 × 17 × 12 cm) or individually (SI) in a cage of the same size for a time period varying from 1 day to 8 weeks preceding our behavioral and biochemical measurements (5). The vivarium temperature was kept near 24°C, and the humidity was kept near 65%. Group-housed (GH) and SI male or female mice were subjected to i.p. injections of R/S-FLX, R- or S-FLX, R- or S-NFLX, or imipramine. Vehicle or tested drugs were prepared in 1% DMSO solutions and given i.p., as 0.1 ml per 10 g of body weight.
R/S-FLX, R-FLX, S-FLX, R-NFLX, and S-NFLX were a generous gift from Eli Lilly. Imipramine was provided by Sigma. Heptafluorobutyric acid anhydride (HFBA) was purchased from Pierce. Unless otherwise specified, all organic solvents were of HPLC grade and were purchased from Fisher Scientific.
Resident–Intruder Test.
To test the aggressive behavior of resident male or female SI mice, an intruder mouse of the same gender was placed in a resident home cage (24 × 17 × 12) and resident–intruder interactions were videotaped for 10 min. The aggressive behavior of resident SI mice was characterized by an initial pattern of exploratory activity around the intruder, which was followed by rearing and tail rattle, accompanied in few seconds by wrestling and/or a violent biting attack. The total duration of these attacks and/or wrestling during the 10 min observation period was measured as described (18, 19).
Measurement of PTB-Induced Loss of the Righting Reflex.
The duration of the PTB-induced loss of the righting reflex in GH and SI male or female mice was measured as reported (20) after i.p. injections of PTB-Na (50 mg/kg; 0.5 mg per 0.1 ml).
Locomotion Measures.
A computerized AccuScan 12 Animal Activity Monitoring System (Columbus Instruments, Columbus, OH) assisted by versamax software (AccuScan Instruments, Columbus, OH) was used to quantitatively monitor locomotor activity in mice, as described (21). Each activity cage consisted of a Perspex box (20 × 20 × 20 cm) surrounded by horizontal and vertical infrared sensor beams. The interruptions per 15 min of the horizontal sensors were taken as a measure of horizontal activity, whereas those of vertical sensors measured rearing activity. Between 1 and 3 p.m., activity was recorded from GH and SI mice for 15 min beginning 30 min after a single i.p. injection of vehicle or various drugs.
Brain Neurosteroid Content.
Extraction, derivatization, and gas chromatography-mass spectrometry analyses of neurosteroids were performed with minor modifications as described (16). (i) Olfactory bulbs (OB) (this structure expresses the highest Allo levels in rodent brains) (7) or frontal cortices were homogenized in 10 vol of distilled water containing 2–5 fmol/ml [3H]progesterone and [3H]Allo (New England Nuclear) to monitor the HPLC retention profile and 1 pmol deuterium-labeled Allo (Allo-17,21,21,21-D4) and deuterium-labeled progesterone (progesterone-1,2,6,7-D4); (Cambridge Isotope Laboratories, Cambridge, MA) used as internal standards. The supernatants were extracted with ethyl acetate and after lyophilization were purified with HPLC, as described (22). (ii) The HPLC fractions containing Allo or progesterone were derivatized with HFBA and subjected to gas chromatography-mass fragmentographic analysis.
Mass fragmentographic analysis of derivatized Allo or progesterone was performed in the standard electron impact mode. The detection limit for Allo was ≈10 fmol; the standard curve was linear between 5 and 105 fmol. For Allo or progesterone quantification, the m/z ion-monitoring mode was 510 for HFBA-progesterone, 514 for HFBA-d-progesterone, 496 for HFBA-Allo, and 500 for HFBA-d-Allo.
Quantitative RT-PCR Analyses of 5α-Reductase Type I mRNA.
The mRNA was quantified with competitive RT-PCR as described by Grayson and Ikonomovic (23). Primers for 5α-reductase type I mRNA quantification were as follows: reverse 308–331, 5′-ACCATGACTCATTGCTCCCTGCTT-3′, forward 1–24, 5′-CATCATCAGTGGTACCTCGAGAAG-3′. Templates for 5α-reductase type I internal standards contained a restriction endonuclease site XbaI, which on digestion generated fragments of 135 and 182 bp (7). Each primer pair annealed to a single RNA template on multiple blast comparisons and yielded a single band of the correct molecular size after amplification of the RNA isolated from the mouse brain.
5-HT Uptake ex Vivo.
Ex vivo inhibition of [14C]-5-HT uptake was measured after administration of equimolar doses of R- and S-FLX to SI mice by using a modification of the method of Shaskan and Snyder (24). Mice were decapitated 30 min after treatment with each compound, and the brains were immediately excised and cut in cubic slices (0.3 × 0.3 mm, ≈6 mg protein). After a first washing step using Locke's solution (154 mM NaCl/5.6 mM KCl/3.6 mM NaHCO3/2.3 mM CaCl2/10 mM glucose/1 mM MgSO4/1 mM Hepes/10 μM pargyline/1 mM ascorbic acid, pH 7.4), brain slices were incubated at 37°C for 5 min in the presence of 50 nM [14C]-5-HT (60 mCi/mmol; 1 Ci = 37 GBq).
The uptake was terminated by filtration through GF/B glass fiber filters. The uptake of [14C]-5-HT detected in the presence of 1 μM FLX was considered to be nonspecific (because of the uptake of [14C]-5-HT by other monoaminergic uptake systems) (24) and was considered a background value, which was subtracted from the total uptake of [14C]-5-HT.
Statistical Analysis.
Data are given as means ± SEMs unless otherwise indicated. Comparisons between the control group and each of the treatment groups were performed by one-way ANOVA followed by Dunnett's test.
The relationship between brain Allo content and the severity of SI-induced aggressiveness in vehicle-, R- or S-FLX-, and R- or S-NFLX-treated mice was determined by Pearson's product moment correlation (25). The IC50 or EC50 values were calculated from dose-response curves analyzed by the “quantal dose-response: probits test” using the computer program of Tallarida and Murray (26) equipped with a statistical package. Statistical comparisons among the different IC50s were performed with the “cohort package software” (www.cohort.com). Differences were considered significant at P < 0.05.
Results
Allo Biosynthesis and Aggressive Behavior in SI Male Mice.
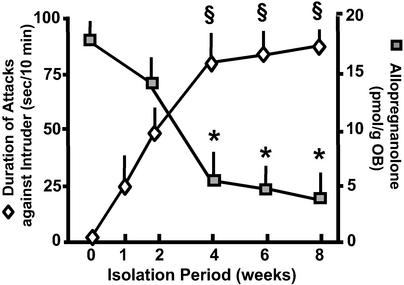
When adult (2.5 months old) male mice are SI for a period of 8 weeks, there is a time-related increase of aggressiveness during the first 4 weeks of SI when a plateau of aggressiveness is reached that lasts up to 8 weeks of SI (Fig. 1). SI-induced aggressive behavior intensity is inversely related to the extent of Allo brain content down-regulation. Allo content was measured in various brain structures of mice, but we report only the Allo content of OB because it contains the highest amount of Allo among various brain regions and because the dissection of this structure is easily replicated for biochemical assays. Although OB Allo content is already reduced after 2 weeks of SI, it is decreased by a greater extent (60–70%) after 4 weeks of SI (Fig. 1), during the plateau of aggression intensity.
Figure 1.
Time course of aggressiveness development and OB Allo content decrease during SI in male mice. Aggression of a resident mouse against an intruder was measured as the duration of attacks in 10 min. Allo was determined in the OB of the same mice killed immediately after termination of the resident–intruder test. Each value is the mean ± SEM of eight animals. *, P < 0.01 when OB Allo content at a given SI time period is compared with SI period 0-week; §, P < 0.01 when the duration of attacks at a given SI time period is compared with SI period 1-week.
The decline of frontal cortex Allo levels after 4 weeks is comparable to that found in the OB of the same mice (Allo pmol/g: 7.8 ± 1.5 in GH mice and 3.2 ± 0.9 in SI mice; mean ± SE; n = 6; P < 0.01). In GH and SI male mice, the body weight increases by a comparable extent despite SI (data not shown).
Comparison of Aggressive Behavior and Allo Steady-State Levels in SI Male and Female Mice.
Further evidence that the down-regulation of brain Allo during SI is an important contributor in establishing a high level of aggressiveness was provided by studies in SI female mice. Unlike their male counterparts, SI females neither develop aggression nor show a decrease in the duration of PTB-induced loss of righting reflex (Table 2) or in the OB content of Allo even after 6 weeks of SI (Table 2). As expected, brain progesterone content was higher in females than in males, and SI failed to change this difference. The levels of frontal cortex 5α-reductase type I mRNA expression were decreased in SI male mice but failed to change in SI female mice. Because 5α-reductase is a rate-limiting enzyme in Allo biosynthesis, the decrease of this enzyme in SI male but not in SI female mice suggests that this enzyme down-regulation may contribute to the selective reduction of Allo brain content in the SI male mice. In fact, this interpretation is supported by the fact that SI female mice maintain normal levels of Allo and 5α-reductase mRNA.
Table 2.
Duration of righting reflex loss (RRL) after PTB, duration of attacks against an intruder, 5α-reductase mRNA expression in frontal cortex, and progesterone and Allo content in OB of SI and GH male and female mice
| Mice | PTB RRL, min | Duration of attacks against an intruder, sec/10 min | 5α-Reductase mRNA, attomol/μg total RNA | Progesterone, pmol/g | Allo, pmol/g |
|---|---|---|---|---|---|
| Male | |||||
| GH | 78 ± 9.8 | None | 359 ± 23 | 19 ± 6.6 | 15 ± 4.2 |
| SI | 40 ± 7.5** | 80 ± 11 | 171 ± 11* | 16 ± 5.6 | 6.2 ± 1.5** |
| Female | |||||
| GH | 83 ± 13 | None | 420 ± 45 | 64 ± 14 | 13 ± 2.0 |
| SI | 74 ± 5.8 | None | 470 ± 39 | 55 ± 11 | 13 ± 1.7 |
Duration of attacks against a same-sex intruder was determined by resident–intruder test after 6 weeks of SI. PTB (50 mg/kg)-induced RRL was measured in separate groups of mice. Allo and progesterone levels and 5α-reductase mRNA expression were determined in SI mice killed immediately after termination of aggressive behavior test.
, P < 0.05;
, P < 0.01, when male GH mice are compared to SI male mice. Each value is the mean ± SEM of four to six animals.
FLX, NFLX, and Their Stereoisomers Reduce Aggression and Normalize Allo Brain Levels in SI Male Mice.
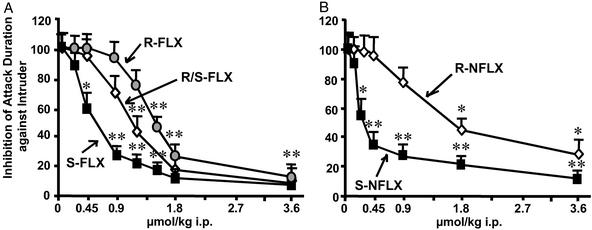
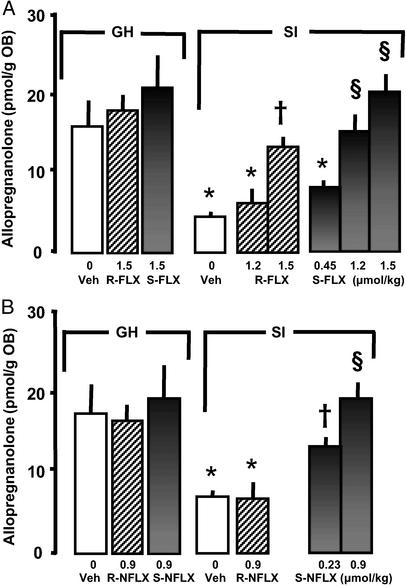
We were able to confirm (5, 6) that low doses of racemic (R/S) FLX (IC50: 1.1 μmol/kg, Table 3) reduce aggression in SI male mice (Fig. 2A). However, the S-FLX isomer was approximately two times more potent than the R isomer in reducing aggressive behavior (Fig. 2A and Table 3) and in normalizing the SI-induced down-regulation of OB Allo content (Fig. 3A). S-NFLX was at least three times more potent than S-FLX in antagonizing SI-induced aggressive behavior (IC50 = 0.20 μmol/kg; see Table 3 and Fig. 2), and in the dose range of 0.23 to 0.9 μmol/kg, normalized the SI-induced reduction of OB Allo content (Fig. 3B). R-NFLX is 7- to 8-fold less potent than S-NFLX in reducing SI-induced aggressive behavior (Fig. 2B and Table 3) and in doses of 0.9 μmol/kg failed to normalize OB Allo levels (Fig. 3B).
Table 3.
Inhibition of aggressiveness in SI male mice and in vitro inhibition of 5-HT uptake into rat synaptosomes by FLX and NFLX stereoisomers
| Drug tested | Inhibition of aggressive behavior, IC50, μmol/kg | Inhibition of 5-HT uptake,*Ki, nM |
|---|---|---|
| R/S-FLX | 1.08 ± 0.015† | 20 ± 2 |
| S-FLX | 0.71 ± 0.03‡ | 22 ± 3 |
| R-FLX | 1.3 ± 0.024 | 35 ± 5 |
| S-NFLX | 0.2 ± 0.08§ | 14 ± 4¶ |
| R-NFLX | 1.53 ± 0.2 | 309 ± 22 |
Data are from Wong et al. (17).
P < 0.05 when R/S-FLX is compared to S-FLX or R-FLX. IC50 values were calculated according to the method of Tallarida and Murray (26).
P < 0.01 when S-FLX is compared to R-FLX.
P < 0.001 when S-NFLX is compared to R-NFLX and S-FLX.
¶P < 0.001 when S-NFLX is compared to R-NFLX.
Figure 2.
Dose-related inhibition of aggressive behavior (% of vehicle-treated mice) elicited by FLX and NFLX stereoisomers in SI male mice. (A) R-, R/S-, and S-FLX. (B) R- and S-NFLX. Each value is the mean ± SEM of 8–12 mice. Drugs were given 30 min before the test. *, P < 0.05; **, P < 0.01, when FLX- or NFLX-treated mice were compared with vehicle-treated mice.
Figure 3.
Increase of Allo levels in the OB of SI male mice after treatment with FLX and NFLX stereoisomers. (A) S- and R-FLX. (B) S- and R-NFLX. Each value is the mean ± SEM of four to six mice. Drugs were given 30 min before decapitation. *, P < 0.05 when vehicle-, R- or S-FLX-, or R-NFLX-treated SI mice were compared with vehicle-treated (veh) GH mice. †, P < 0.05; §, P < 0.01 when R or S-FLX- or S-NFLX-treated SI mice were compared with vehicle-treated SI mice.
The administration of 0.9 μmol/kg R- or S-NFLX and 1.5 μmol/kg R- or S-FLX failed to change OB Allo content in GH mice (Fig. 3).
To ascertain whether the effects of S-NFLX on aggressive behavior are dependent on an impairment of locomotor activity, GH and SI mice were treated with 1.5 μmol/kg S-NFLX 30 min before testing. As expected, locomotor activity was higher in SI than GH mice [horizontal activity, SI = 3870 ± 297, GH = 2526 ± 257 (P < 0.05, n = 4), and vertical activity, SI = 398 ± 45, GH = 203 ± 42 (P < 0.05, n = 4)]. However, S-NFLX failed to alter the motor activity of either group of mice (horizontal activity: 4028 ± 362 vs. 3870 ± 297 in SI mice; 2244 ± 170 vs. 2526 ± 257 in GH mice; and vertical activity: 512 ± 121 vs. 398 ± 45 in SI mice; 302 ± 59 vs. 203 ± 42 in GH mice treated with S-NFLX and vehicle, respectively).
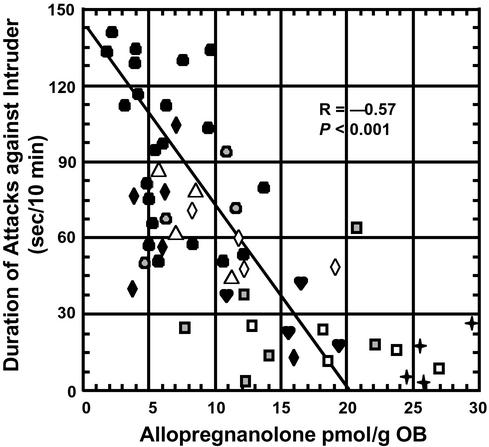
Fig. 4 shows that after SI there is a consistent correlation between the duration of the attacks against an intruder and the OB Allo content after treatment with either vehicle or different doses of FLX or NFLX stereoisomers. A Pearson correlation test showed a statistically significant negative correlation (r = −0.57; P < 0.001) between the concentration of Allo and the expression of aggressive behavior.
Figure 4.
Negative correlation between the duration of attacks and Allo content in the OB of SI male mice after i.p. administration of vehicle (●, n = 21), R-FLX (♦, 1.2 μmol/kg, n = 6; ♡, 1.5 μmol/kg, n = 4), S-FLX (▵, 0.45 μmol/kg n = 4;  , 1.2 μmol/kg, n = 6;
, 1.2 μmol/kg, n = 6;  , 1.5 μmol/kg, n = 4), R-NFLX (
, 1.5 μmol/kg, n = 4), R-NFLX ( , 0.9 μmol/kg, n = 4), and S-NFLX (⋄, 0.23 μmol/kg, n = 4; □, 0.9 μmol/kg, n = 5) (Pearson's product moment correlation).
, 0.9 μmol/kg, n = 4), and S-NFLX (⋄, 0.23 μmol/kg, n = 4; □, 0.9 μmol/kg, n = 5) (Pearson's product moment correlation).
To obtain further evidence that a decrease in Allo brain content may be operative in inducing aggression, Allo was injected into SI male mice. This neuroactive steroid dose-dependently inhibited the duration of attacks [% of vehicle-treated mice; 68 ± 11% with 4 μmol/kg; 26 ± 4%, (P < 0.01) with 8 μmol/kg; and 8.6 ± 2.9%, (P < 0.01) with 16 μmol/kg Allo, respectively].
Can the SSRI Potency of FLX and NFLX Stereoisomers Be Related to Degree of Aggressiveness and/or to Brain Allo Content?
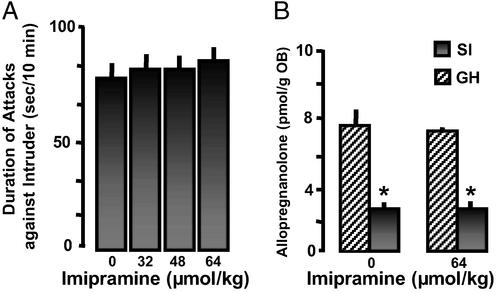
There is a clear dissociation between the ability of brain slices prepared from mice receiving FLX or NFLX to take up 5-HT (Table 1) and to either normalize SI-induced Allo brain down-regulation (Fig. 3) or to decrease aggressiveness (Fig. 2 and Table 3). In fact, the reduction of aggressive behavior and the normalization of Allo OB content elicited by FLX and NFLX are stereospecific, whereas the inhibition of 5-HT reuptake lacks stereospecificity. The ex vivo SSRI potency of S-FLX is at least one order of magnitude lower than EC50 to either reduce aggressive behavior or to normalize Allo OB content after SI (compare Tables 1 and 3 and Fig. 3). Imipramine, a tricyclic antidepressant that also blocks 5-HT reuptake administered in a dose range varying from 32 to 64 μmol/kg, i.p., resulted in a complete inhibition of 5-HT reuptake (Table 1). However, as shown in Fig. 5A, imipramine neither changed SI-induced aggression nor the SI-induced decrease of OB Allo content (Fig. 5B).
Figure 5.
Imipramine fails to block aggression or to normalize decreased Allo brain content in SI male mice. Each value is the mean ± SEM of eight mice. Imipramine was given 60 min before test. *, P < 0.01 when vehicle or imipramine-treated SI mice were compared with vehicle- or imipramine-treated GH mice.
Discussion
Allo Brain Content and Aggressive Behavior.
Our findings demonstrate that SI male mice develop aggressive behavior toward an intruder with an intensity that appears to be related to the extent of Allo brain content down-regulation. In addition, S-NFLX and S-FLX both normalize SI-induced reduction of Allo levels and abolish the attacks against an intruder. Their potency not only is several-fold higher than that of their respective R stereoisomers but also appears to be unrelated to their intrinsic SSRI potency.
Allo acts as a positive allosteric modulator of GABA action at GABA type A (GABAA) receptors (27–31) and the Allo concentration in rodent brain is sufficient to elicit a physiological positive allosteric modulation of GABA action at GABAA receptors (32, 33). In male mice during protracted (4–8 weeks) SI, Allo brain content is decreased by ≈60% (Fig. 1). SI also down-regulates the expression of GABAA receptor α subunits (Pinna et al., unpublished). Thus, the decrease of Allo brain content in SI mice is presumably part of the molecular mechanisms underlying the increased level of anxiety, the decreased response of GABAA receptors to agonists, such as PTB (5) and muscimol (32), or to positive allosteric modulators of GABA action at GABAA receptors, such as benzodiazepines (6), and the expression of SI-induced aggressive behavior. In fact, such behavioral alterations are abated by administering Allo doses that per se fail to affect motor activity or cause sedation in GH male mice. Additionally, a reduction of OB Allo content elicited by 17β-17-[bis(1 methylethyl)amino carbonyl] androstane-3,5-diene-3-carboxylic acid (SKF 105111) (a blocker of 5α-reductase type I) is associated with an increase in aggressiveness of GH mice (6).
These results taken together support the view that Allo mitigates the aggressive behavior elicited by SI because: (i) In male mice the intensity of aggressive behavior induced by SI is inversely correlated with the decrease of brain Allo content (Figs. 1 and 4); (ii) Allo dose-dependently reduces the SI-induced aggression against a male intruder; and (iii) SI female mice that fail to develop aggressive behavior toward an intruder also fail to express a decrease in brain 5α-reductase type I expression and of brain Allo content (Table 2).
Although the mechanisms whereby SI causes a down-regulation of 5α-reductase and Allo expression in male but not in female mice remain to be elucidated, the difference in the response to SI between male and female mice is consistent with the view that a decrease of Allo brain content is associated and perhaps maintained by a decrease of brain 5α-reductase type I expression.
S-NFLX, the Drug of Choice to Control Aggressive Behavior.
Because an impairment of 5-HT neurotransmission has been associated with aggressive behavior in SI male mice (3, 34), we studied whether the potency of FLX and NFLX in reducing aggression can be correlated with their inhibitory efficacy on 5-HT reuptake mechanisms. When the potency of FLX and NFLX stereoisomers to inhibit 5-HT reuptake is compared with their ability to reduce SI-induced intruder-aggression and to normalize the associated reduction of Allo brain content, it becomes apparent that their ability to normalize Allo and mitigate aggression occurs for doses that are at least one order of magnitude smaller than the doses required to block 5-HT reuptake. Moreover, R- and S-FLX and S-NFLX studied either ex vivo (Table 1) or in vitro (Table 3, also ref. 17) are virtually equipotent 5-HT reuptake inhibitors, indicating the absence of stereospecificity. In contrast, the actions of FLX and NFLX on Allo brain content and aggression are strictly stereospecific (see Table 3). Moreover, S-NFLX is about 3- to 4-fold more potent than S-FLX and 7- to 8-fold more potent than R-NFLX in reducing SI-induced aggressive behavior and in normalizing Allo brain content down-regulation. The EC50 doses for ex vivo SSRI activity are at least one order of magnitude higher than their EC50 to reduce SI-induced aggressive behavior or to normalize the associated down-regulation of Allo brain levels. FLX and NFLX efficacy in blocking norepinephrine and dopamine reuptake is two orders of magnitude lower than their inhibitory efficacy on 5-HT reuptake (17). Very likely, the induction of other monoamine uptake mechanisms cannot be related to either the antagonism of SI-induced aggressive behavior or to the normalization of Allo brain levels elicited by these drugs.
Studies with imipramine further support the hypothesis that the effects of FLX and NFLX on aggressive behavior are very likely independent of 5-HT reuptake inhibition. In fact, imipramine doses that ex vivo block 5-HT reuptake (Table 1) fail to reduce SI-induced aggressive behavior or to normalize brain Allo levels (Fig. 5).
Drug-induced changes in locomotor activity may contribute to the drug-induced down-regulation of aggressive behavior toward an intruder (3). However, this possibility seems remote because of the evidence that S-NFLX, up to an anti-aggressive dose of 1.5 μmol/kg, fails to reduce exploratory activity in both SI and GH mice. This observation suggests that the anti-aggressive action of S-NFLX may not be linked to a general reduction of motor activity and further, may support the possibility that a normalization of altered neurosteroid steady-states in one or more brain areas may play a major role in the reduction of aggressive behavior against an intruder. However, we have not yet studied this possibility.
Mechanisms of Action of S-FLX or S-NFLX on Neurosteroid Biosynthesis.
When an in vitro system containing purified recombinant 3α-hydroxysteroid-oxidoreductase (3α-HSOR) was used, it was found that micromolar concentrations of the racemic form of FLX directly stimulate the production of Allo by increasing the affinity of 3α-HSOR for the Allo precursor 5α-DHP (35). Pending a study of the action of the S or R stereoisomers of FLX and NFLX on 3α-HSOR, we can only hypothesize that the doses of FLX and NFLX used in our experiments may be too low (i.e., nanomolar range) to directly activate 3α-HSOR. Such a lack of a direct action of low doses of FLX and NFLX on 3α-HSOR is also supported by the lack of action of these FLX doses on Allo brain levels in GH mice. It is also important to mention that 3α-HSOR expression is not changed during SI (7). Thus, unless 3α-HSOR has changed its affinity for S-FLX and S-NFLX during SI, the activation of this enzyme by S-FLX and S-NFLX seems improbable as a cause for the normalization of Allo brain level observed in SI mice.
The decrease of Allo content in brain of SI mice is presumably caused by a decrease of 5α-reductase expression (7) (see also Table 2). Thus, the normalization of brain Allo by FLX and NFLX could be caused by their action on 5α-reductase. However, because the onset of S-NFLX- and S-FLX-induced modification of brain Allo levels is very rapid (less than 1/2 h), it seems unlikely that their action is mediated via a rapid induction of 5α-reductase expression.
Change in the affinity of 5α-reductase for the substrate or cofactors produced by S-FLX or S-NFLX administration is a possibility that we cannot exclude, but it seems improbable based on the report that racemic FLX in concentrations up to 50 μM failed to change the affinity of this enzyme for progesterone or for NADPH in vitro (35). Thus, the high potency and stereospecificity of FLX and NFLX in decreasing aggressive behavior and in normalizing Allo brain content during SI supports the notion that these compounds are active on some specific mechanisms of brain neurosteroidogenesis that are perturbed during SI. Although progesterone steady-state levels are not increased by FLX treatment (5), one cannot exclude the possibility that these drugs, in doses that do not interfere with 5-HT reuptake, may activate brain steroidogenesis, perhaps operating on a specific mechanism upstream from progesterone. Probably these mechanisms may include interactions with mitochondrial benzodiazepine (mBZD) receptors, with the polypeptide diazepam-binding inhibitor that binds to mBZD receptors and activates steroidogenesis (36), or with the expression of the steroidogenic acute regulatory protein (StAR) that transfer cholesterol to mBDZ receptors (37, 38).
We have previously established that depressed patients exhibit a down-regulation of Allo steady-state levels in the CSF that can be overcome by a treatment with FLX which decreases the Hamilton score (15). It would be important to establish whether aggressive behavior is a symptomatic component of a depressive syndrome in which the brain levels of 5α-reductase type I are decreased. Similar to depressed patients in whom FLX and fluvoxamine improve behavior and normalize Allo (15), mice with low levels of brain 5α-reductase and Allo respond to FLX with a normalization of Allo and a decrease in the signs of aggression (see ref. 6, and present study). In addition, these mice respond to FLX with a return to normal of the PTB-induced loss of the righting reflex, which suggests a normalization of the GABAA receptor function down-regulation (5).
The above-reported preliminary data encourage further investigation of SI effects on the expression of different GABAA receptor subunits in various brain structures.
Acknowledgments
We thank Dr. Dennis L. Murphy, National Institute of Mental Health, and Dr. Richard W. Olsen, University of California, Los Angeles, School of Medicine, for constructive criticisms and suggestions in the preparation of the manuscript. This study was supported by National Institute of Mental Health Grants MH 49486 and MH 56890 (to A.G.).
Abbreviations
- FLX
fluoxetine
- NFLX
norfluoxetine
- 5-HT
serotonin
- SI
social isolation
- SI
socially isolated
- GH
group housed
- Allo
allopregnanolone
- SSRIs
selective 5-HT reuptake inhibitors
- GABA
γ-aminobutyric acid
- HFBA
heptafluorobutyric acid anhydride
References
- 1.Brown G L, Linnoila M I. J Clin Psychiatry. 1990;51S:31–41. [PubMed] [Google Scholar]
- 2.Gingrich J A, Hen R. Psychopharmacology (Berlin) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 3.Holmes A, Murphy D L, Crawley J N. Psychopharmacology (Berlin) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- 4.Mathews C A, Freimer N B. In: Comprehensive Textbook of Psychiatry. 7th Ed. Sadock B J, Sadock B A, editors. Philadelphia: Lippincott William & Wilkins; 2000. pp. 184–199. [Google Scholar]
- 5.Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov D P, Watanabe H, Mienville J-M, Guidotti A, Costa E. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson A M, Costa E. Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 7.Dong E, Matsumoto K, Uzunova V, Sugaya I, Costa E, Guidotti A. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avis H H. Psychol Bull. 1974;81:47–63. doi: 10.1037/h0035522. [DOI] [PubMed] [Google Scholar]
- 9.Valzelli L, Bernasconi S. Neuropsychobiology. 1979;5:129–135. doi: 10.1159/000117674. [DOI] [PubMed] [Google Scholar]
- 10.Heidbreder C A, Weiss I C, Domeney A M, Pryce C, Homberg J, Hedou G, Feldon J, Moran M C, Nelson P. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 11.Valzelli L. In: Aggressive Behavior. Garattini S, Sigg S B, editors. Amsterdam: Excerpta Medica; 1969. pp. 70–76. [Google Scholar]
- 12.Rilke O, Freier D, Jahkel M, Oehler J. Pharmacol Biochem Behav. 1998;59:891–896. doi: 10.1016/s0091-3057(97)00509-1. [DOI] [PubMed] [Google Scholar]
- 13.Coccaro E F, Kavoussi R J. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M T, Dinan T G. Acta Psychiatr Scand. 2001;104:84–91. doi: 10.1034/j.1600-0447.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 15.Uzunova V, Sheline Y, Davis J M, Rasmusson A, Uzunov D P, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzunov D, Cooper T B, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong D T, Bymaster F P, Reid L R, Mayle D A, Krushinski J H, Robertson D W. Neuropsychopharmacology. 1993;8:337–344. doi: 10.1038/npp.1993.33. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Cai B, Satoh T, Ohta H, Watanabe H. Pharmacol Biochem Behav. 1991;39:167–170. doi: 10.1016/0091-3057(91)90416-y. [DOI] [PubMed] [Google Scholar]
- 19.Cai B, Matsumoto K, Ohta H, Watanabe H. Pharmacol Biochem Behav. 1993;44:519–525. doi: 10.1016/0091-3057(93)90161-l. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Ojima K, Watanabe H. Brain Res. 1996;708:1–6. doi: 10.1016/0006-8993(95)01277-x. [DOI] [PubMed] [Google Scholar]
- 21.Pinna G, Galici R, Schneider H H, Stephens D N, Turski L. Proc Natl Acad Sci USA. 1997;94:2719–2723. doi: 10.1073/pnas.94.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheney D L, Uzunov D, Costa E, Guidotti A. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayson D R, Ikonomovic S. In: In Vitro Neurochemical Techniques, Neuromethods. Boulton A A, Baker G B, Bateson A N, editors. Vol. 34. Clifton, NJ: Humana; 1998. pp. 127–151. [Google Scholar]
- 24.Shaskan E G, Snyder S H. J Pharmacol Exp Ther. 1970;175:404–418. [PubMed] [Google Scholar]
- 25.Sokal R R, Rohlf F J. In: Biometry. Wilson J, editor. New York: Freeman; 1981. pp. 565–572. [Google Scholar]
- 26.Tallarida R J, Murray R B. Manual of Pharmacologic Calculations with Computer Programs. 2nd Ed. New York: Springer; 1987. [Google Scholar]
- 27.Puia G, Santi M R, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 28.Puia G, Duĉiĉ I, Vicini S, Costa E. Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 29.Mihalek R M, Banerjee P K, Korpi E R, Mi Z P, Tretter V, Sage J R, Guidotti A, Li Z, Olsen R W, Homanics G E. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belelli D, Casula A, Ling A, Lambert J J. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 31.Spigelman I, Li Z, Banerjee P K, Mihalek R M, Homanics G E, Olsen R W. Epilepsia. 2002;43:3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville J-M, Costa E, Guidotti A. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 33.Puia G, Mienville J-M, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- 34.Miczek K A, Maxson S C, Fish E W, Faccidomo S. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 35.Griffin L D, Mellon S H. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa E, Guidotti A. Life Sci. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- 37.Artemenko I P, Zhao D, Hales D B, Hales K H, Jefcoate C R. J Biol Chem. 2001;276:46583–46596. doi: 10.1074/jbc.M107815200. [DOI] [PubMed] [Google Scholar]
- 38.Bose H, Lingappa V R, Miller W L. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]