Abstract
Huntington's disease (HD) is an inherited, progressive neurological disorder that is caused by a CAG/polyglutamine repeat expansion and for which there is no effective therapy. Recent evidence indicates that transcriptional dysregulation may contribute to the molecular pathogenesis of this disease. Supporting this view, administration of histone deacetylase (HDAC) inhibitors has been shown to rescue lethality and photoreceptor neurodegeneration in a Drosophila model of polyglutamine disease. To further explore the therapeutic potential of HDAC inhibitors, we have conducted preclinical trials with suberoylanilide hydroxamic acid (SAHA), a potent HDAC inhibitor, in the R6/2 HD mouse model. We show that SAHA crosses the blood–brain barrier and increases histone acetylation in the brain. We found that SAHA could be administered orally in drinking water when complexed with cyclodextrins. SAHA dramatically improved the motor impairment in R6/2 mice, clearly validating the pursuit of this class of compounds as HD therapeutics.
Huntington's disease (HD) is an inherited progressive neurological disorder caused by a CAG/polyglutamine (polyQ) expansion and is associated with selective cell death and the deposition of polyQ aggregates in the brain (1). Recent studies suggest that transcriptional dysregulation might play a role in HD pathogenesis (2–4) by decreasing several functions including histone acetyltransferase and Sp1 and TAFII130 activity (5–13). Although the molecular basis of transcriptional dysfunction in HD requires further dissection, transcriptional repression is the predominant result of dysregulation.
Histone deacetylases (HDACs) work in concert with histone acetyl transferases to modify chromatin and regulate transcription (14). HDAC inhibitors such as trichostatin A and suberoylanilide hydroxamic acid (SAHA) have been shown to act selectively on gene expression and are potent inducers of growth arrest, differentiation, and/or apoptotic cell death of transformed cells in vitro and in vivo (15–18). Recent studies in cell culture, yeast, and Drosophila models of polyQ disease have indicated that HDAC inhibitors might provide a useful class of agents to ameliorate the transcriptional changes in HD (9–11). For example, alleviation of transcriptional repression in Drosophila models either by genetic reduction of Sin3A corepressor activity or through administration of HDAC inhibitors was shown to rescue lethality and photoreceptor neurodegeneration (11).
Therapeutic trials in humans are challenging, because of the late onset, variability, and slow progression of HD. Therefore, before testing novel therapeutics in clinical trials, compounds must be rigorously and extensively evaluated in genetic HD mouse models, a wide range of which are now available (19). The R6/2 line (20, 21) has been used the most extensively for preclinical trials (22–25). The early onset of impairment (5–6 weeks), rapid progression of the disease (severely impaired at 12–14 weeks), and reproducibility of the phenotype makes it particularly suitable for this type of study.
Here we ask whether the beneficial effects of HDAC inhibitors in Drosophila models of polyQ disease can be reproduced in preclinical mouse trials. We show that SAHA can cross the blood–brain barrier and demonstrate bioactivity in brain tissue. SAHA has an effective concentration of ≈2.5 μM and is relatively insoluble in aqueous solution. We show that when complexed with 2-hydroxypropyl-β-cyclodextrin (HOP-β-CD), we are able to administer SAHA in drinking water and dramatically improve motor impairment in the R6/2 mouse model.
Methods
Drug Formulation.
SAHA (Aton Pharma) was solubilized in 5 molar equivalents of HOP-β-CD (ICN) in water. In the main trial, 0.67 g of SAHA was added to a solution of 18 g of HOP-β-CD in 1 liter of water, heated until fully dissolved, and rapidly cooled on ice to room temperature. This solution was administered to the mice in place of drinking water and replaced weekly. SAHA solutions of various concentrations were prepared by maintaining the molar ratio between SAHA and HOP-β-CD. Placebo was an equivalent concentration of HOP-β-CD without SAHA.
Animals.
Affected mice were hemizygous R6/2 females (20) [available from the Induced Mutant Resource, The Jackson Laboratory, code B6CBA-TgN (HDexon1)62] bred and reared in our colony by backcrossing R6/2 males to C57BL/6 × CBA F1 females. Transgenic animals were identified before weaning by PCR of tail-tip DNA, and CAG repeat size was determined (26). Control mice were WT female littermates. Mice were weaned into their treatment groups at 4 weeks of age. All animals had unlimited access to rodent breeding chow (Special Diet Services, Witham, U.K.) from a food hopper and placebo or drug solution. From age 12 weeks, all animals were additionally given mash consisting of powdered chow mixed with the drug solution as given in the drinking bottle. The mice were subject to a 12-h light (08:00–20:00), 12-h dark (20:00–08:00) cycle. Living conditions and baseline characteristics of study groupings were rigorously standardized.
Organotypic Slice Culture Assay.
Organotypic hippocampal slice cultures were established as described (27). SAHA in HOP-β-CD solution was added to the culture medium from day 1 until the termination of the experiment at concentrations from 0.025 to 250 μM, and HOP-β-CD and media controls were included. We established that HOP-β-CD has no effect on polyQ aggregation over a 5-log concentration range (data not shown), and therefore the HOP-β-CD concentration required to complex 250 μM was used as the vehicle in this experiment. The medium was changed twice weekly, and slices were fixed at weekly intervals from 2 weeks in culture. The huntingtin aggregate load was assessed by quantitative indirect immunofluorescence (27), and results were analyzed by using the general linear model ANOVA with false discovery rate correction (28).
Behavioral Analysis.
Rotarod impairment was assessed on a Ugo Basile 7650 accelerating Rotarod (Linton Instrumentation, Diss, U.K.), modified as described (29). Animals (R6/2: n = 13, WT: n = 12 per treatment group) were tested at 4 weeks of age to establish their baseline performance and again at 8, 10, and 12 weeks of age. Grip strength was measured once per fortnight (29), and animals were weighed weekly to the nearest 0.1 g. Statistical analysis of grip strength used ANOVA on the mean grip strength. Rotarod data were analyzed at each time point by using repeated measures in a linear mixed effects model (in S-plus).
Antibodies, Western Blotting, Histology, and Immunohistochemistry.
Antibodies were as follows: huntingtin exon 1 protein (S830) (30); ubiquitin (DAKO); acetylated histone H2A (Lys-5) and acetylated histone H2B (Serotec); and acetylated histone H3 and acetylated histone H4 (Upstate Biotechnology, Lake Placid, NY). Histones were isolated from whole brain, and Western blots for histone acetylation (31) were as published. Brains for histology and immunohistochemistry were frozen in isopentane and stored at −80°C until required, and 15-μm sections were cut by using a cryostat (Bright Instrument, Huntingdon, U.K.). Immunohistochemistry was performed as described (27). Nissl staining was carried out by standard protocols (32), with the exception that frozen sections were postfixed in 4% paraformaldehyde for 30 min and washed in TBS (50 mM Tris⋅HCl, pH 7.5/0.9% NaCl) before staining.
Real-Time RT-PCR Analysis.
RNA was prepared from whole brain by using the RNeasy mini kit (Qiagen, Crawley, U.K.) according to the manufacturer's recommendations. Real-time PCR was performed as described (33) by using the ABI Prism 7700 (Perkin–Elmer-Applied Biosystems). R6/2 transgene primers were: GCTGCACCGACCGTGAGT (forward), CAGGCTGCAGGGTTAC (reverse), and CAGCTCCCTGTCCCGGCGG (probe). Abl primers were: CAAATCCAAGAAGGGGCTCTCT (forward), TCGAGCTGCTTCGCTGAGA (reverse), and CCCTGCAGAGGCCAGTGGCATCT (probe).
Results
SAHA Crosses the Blood–Brain Barrier and Increases Histone Acetylation in Brain.
Our initial experiments sought to determine whether SAHA crosses the blood–brain barrier. SAHA is relatively insoluble in aqueous solutions, and in previous mouse efficacy studies (31), it had been administered at doses up to 100 mg/kg i.p. in 100% DMSO or orally in the diet (34). The parenteral mode of administration was likely to be impractical in an efficacy study that required daily dosing, as DMSO is not well tolerated on repeated injection. Therefore, alternative vehicles for SAHA delivery were investigated and we found that SAHA is capable of forming a complex with HOP-β-CD with greatly enhanced aqueous solubility.
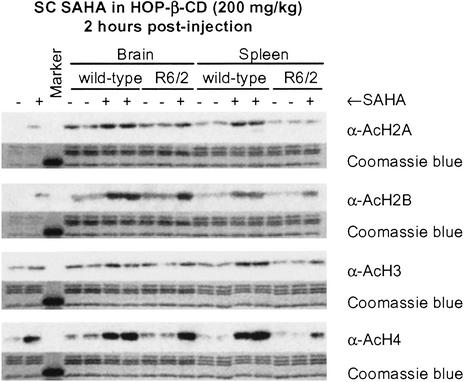
Increased levels of histone acetylation resulting from i.p. injection of 50 mg/kg SAHA are readily detected in tumor tissue (31). To determine whether comparable levels of acetylation are apparent in brain, either 100 mg/kg or 200 mg/kg SAHA in HOP-β-CD was administered to WT and R6/2 mice by a single s.c. injection, and brain and spleen samples were taken 2, 3, and 6 h after drug administration. For comparison, 100 mg/kg SAHA in either HOP-β-CD or DMSO was administered to WT and R6/2 by single i.p. injection. We found no difference in the degree of histone acetylation between untreated WT and R6/2 mice. Significant increases in histone acetylation could be detected only on s.c. administration of 200 mg/kg (Fig. 1). Administration of SAHA dramatically increased acetylation of histones H2B and H4 2 h postinjection (Fig. 1); this increase was maintained at 3 h and had diminished by 6 h (data not shown). With this regimen, increases in histone acetylation were similar in the brain and spleen and comparable between WT and R6/2 mice. Therefore, SAHA is able to cross the blood–brain barrier and mount a biological response.
Figure 1.
SAHA crosses the blood–brain barrier and increases histone acetylation. Histones H2B and H4 are dramatically hyperacetylated 2 h post-s.c. administration of the SAHA HOP-β-CD complex in both brain and spleen. There is no difference in acetylation between genotypes.
SAHA Can Be Administered in Drinking Water.
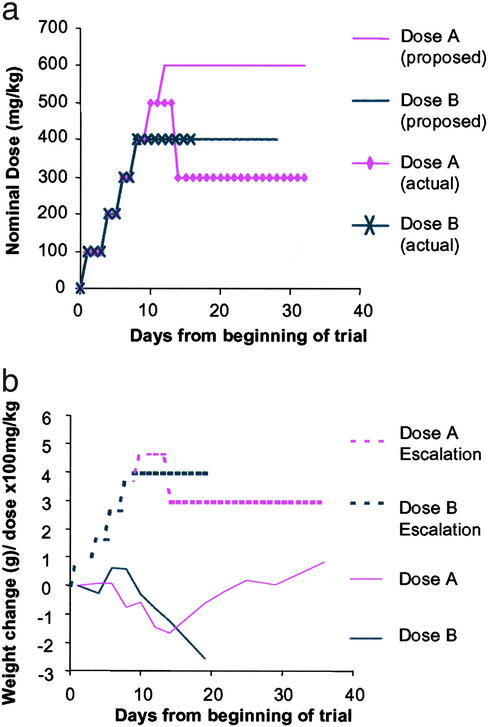
We had observed increases in histone acetylation in brain tissue after s.c. administration of 200 mg/kg SAHA in HOP-β-CD. However, at the concentration required to achieve SAHA dosages of 100–200 mg/kg by injection, the cyclodextrin vehicle was viscous and difficult to handle. Therefore, we aimed to administer 200 mg/kg SAHA in HOP-β-CD in drinking water. Assuming that a 20-g mouse drinks 3 ml/day (35), this corresponds to a dose of 1.33 g/liter. Using a dose escalation strategy (Fig. 2a, Table 1), we found that we could administer oral doses of up to 2 g/liter in WT mice for up to 3 weeks starting at 6–7 weeks of age without significant weight loss or other adverse effects (Fig. 2b). However, application of this dosing regime to R6/2 mice resulted in weight loss, and one mouse (of four) died within 1 week. R6/2 mice on the HOP-β-CD placebo concentration corresponding to the 2 g/liter SAHA dose suffered no adverse effects, and R6/2 mice receiving up to 1.33 g/liter SAHA also showed no adverse symptoms (data not shown).
Figure 2.
Dose escalation strategy for oral SAHA administration in WT mice. (a) To determine the maximum dose of SAHA, which when complexed with HOP-β-CD could be administered to WT mice in drinking water, a dose escalation strategy was used (Table 1). Our initial intention was to assess the tolerability of 2.67 g/liter (400 mg/kg) and 4 g/liter (600 mg/kg). (b) Doses of 2.67 g/liter SAHA and above caused dramatic weight loss. On lowering the dose from 3.33 g/liter to 2 g/liter (300 mg/kg), the formulation was well tolerated.
Table 1.
Strategy used to establish dosing regime for SAHA
| Dosage | Genotype | Treatment | Route | Duration | Outcome |
|---|---|---|---|---|---|
| 200 mg/kg | WT and R6/2 | SAHA and placebo | s.c. | 5 days | Irritation at injection site and death; study arm terminated |
| 0.67 g/liter | WT and R6/2 | SAHA and placebo | Oral | 3 weeks | Mice survived; no significant weight loss |
| 1.33 g/liter | WT and R6/2 | SAHA and placebo | Oral | 3 weeks | Mice survived; no significant weight loss |
| DE to 2 g/liter | WT | SAHA and placebo | Oral | 3 weeks | Mice survived; no significant weight loss |
| DE to 2 g/liter | R6/2 | SAHA and placebo | Oral | 1 week | Mice lost weight; death occurred; study arm terminated |
| DE to 2 g/liter | R6/2 | Placebo | Oral | 1 week | Mice survived; no significant weight loss; study arm terminated |
| DE to 2.67 g/liter | WT | SAHA and placebo | Oral | 3 weeks | Mice lost weight; study arm terminated |
Summary of tolerability studies used to determine optimum dosing regime of SAHA. DE, dose escalation.
We therefore initiated an eight-arm efficacy trial comprising two drug (0.67 g/liter and 1.33 g/liter SAHA in HOP-β-CD) and two placebo arms (the corresponding HOP-β-CD concentrations) for WT and R6/2 mice. Drug administration commenced at 30 days of age after an initial testing week, which was used to sort the mice into well-matched treatment groups (Fig. 3a). Similarly, the CAG repeat size of the R6/2 mice was well matched at 200 ± 4 (SD). Unexpectedly, given the results of our pilot studies, both WT and R6/2 mice on 1.33 g/liter SAHA failed to gain weight, and after 2 weeks, weighed >20% less than mice on the corresponding placebo (data not shown). In addition, 2/12 WT and 2/13 R6/2 mice in this study arm died at ≈6 weeks of age. Therefore, we terminated the 1.33 g/liter SAHA and placebo arms of the experiment and proceeded only with the 0.67 g/liter and corresponding placebo groups.
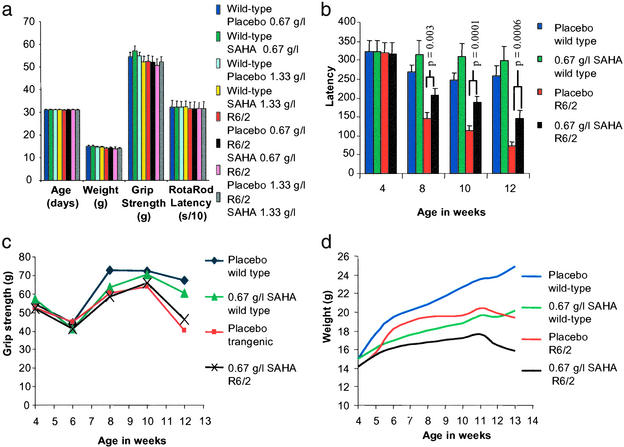
Figure 3.
Study design and outcome measures for the SAHA preclinical trial. (a) Comparison of the variation in age, weight, grip strength, and Rotarod performance between treatment groups and before SAHA or placebo administration. (b) R6/2 mice treated with 0.67 g/liter SAHA show a highly significant improvement in Rotarod performance. Latency to fall at 8 weeks of age was 207 versus 144 s (P = 0.003), at 10 weeks was 187 versus 113 s (P = 0.0001), and at 12 weeks was 144 versus 72 s (P = 0.0006). There was no significant difference in the performance of SAHA- and placebo-treated WT mice (P = 0.28 at 8 weeks, P = 0.14 at 10 weeks, P = 0.38 at 12 weeks). (c) Administration of SAHA does not improve mean grip strength in either WT or R6/2 mice. However, correction for weight revealed a relative improvement in grip strength in the R6/2 mice treated with SAHA (P = 0.012). (d) Both WT and R6/2 mice treated with SAHA failed to gain weight to the same extent as compared with their littermates taking the placebo control. Also, we found no treatment-related difference in the magnitude of weight loss of R6/2 mice compared with WT.
SAHA Improves Motor Impairment in R6/2 Mice.
The effect of SAHA administration on the R6/2 phenotype was assessed by Rotarod analysis of motor impairment, grip strength, and failure to gain weight. R6/2 mice treated with 0.67 g/liter SAHA showed a strong and consistent improvement in Rotarod performance as compared with those on placebo (Fig. 3b). The regression of mean latency times at weeks 8, 10, and 12 showed a highly significant difference between R6/2 mice on placebo and R6/2 mice on SAHA (P = 0.0009). There were no significant differences in performance of SAHA-treated WT mice compared with WT mice on placebo (Fig. 3b). The performance of both WT and R6/2 mice on placebo was consistent with findings from recent experiments by using the same protocol at all time points (R6/2: 8 weeks, 141–148 s; 12 weeks, 55–77 s) (WT: 8 weeks, 250–288 s; 12 weeks, 230–262 s). In contrast, the mean performance of R6/2 mice on SAHA from 8 weeks was well above the upper limit from all previous tests.
We found no significant difference in mean grip strength (Fig. 3c) between treated and placebo mice of either genotype at any age. WT mice in both treatment arms performed significantly better than R6/2 mice at 12 weeks of age (placebo: P < 10−5; SAHA, P < 0.02). SAHA did not prevent the failure of R6/2 mice to gain weight (Fig. 3d). R6/2 mice were ≈20% lighter than WT mice at 13 weeks in both treatment groups. However, both WT and R6/2 mice treated with SAHA failed to gain weight to the same extent as their littermates taking the placebo control (both ≈18% at 13 weeks compared with appropriate placebo group). If this additional weight loss is caused by marginal toxicity of SAHA, it seems that there is no interaction with genotype: WT and R6/2 mice are equally affected. By regression analysis, weight was a significant positive predictor of mean grip strength in R6/2 (P = 0.015), but not in WT (P = 0.229) mice at 12 weeks of age. Entering both weight and treatment received into a multiple regression model revealed an improvement in grip strength at 12 weeks in R6/2 (P = 0.012) but not in WT (P = 0.810) mice treated with SAHA.
Effects of SAHA on Gross and Cellular Brain Morphology.
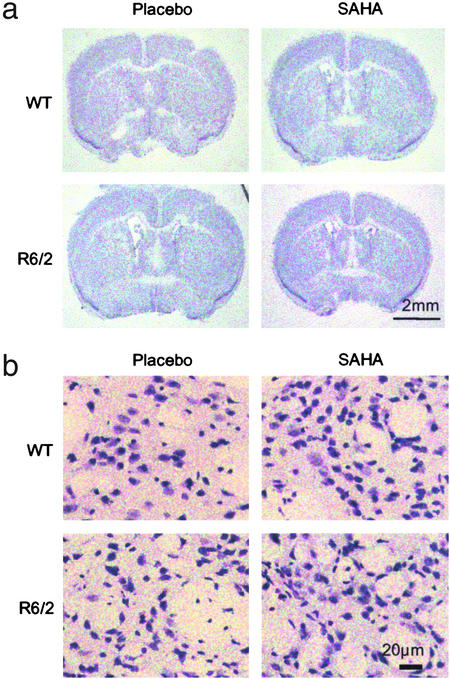
Examination of Nissl-stained brain sections revealed no overt difference in gross morphology between WT and R6/2 mice treated with either SAHA or placebo (Fig. 4a). Although the R6/2 mean brain weight was significantly less than WT (Student's t test: R6/2, 0.396 g; WT, 0.447 g; P = 0.01), there was no difference by regression analysis between mice treated with SAHA or placebo (P = 0.777). Similarly, there was no correlation with body weight within each genotype (P = 0.243).
Figure 4.
Effect of SAHA on gross and cellular brain morphology. Frozen brain sections were cut from mice at 13 weeks of age, between Bregma 0 and 0.5 mm in the region of 0.26 mm and stained for Nissl substance with cresyl violet. (a) There is no marked change in gross morphology between R6/2 and WT mice in either treatment group. (b) Cellular atrophy is apparent in Nissl-stained sections from R6/2 brains. Treatment of R6/2 mice with SAHA resulted in Nissl staining more closely resembling that seen in WT mice.
Examination of the Nissl-stained sections under higher power revealed cellular atrophy in the R6/2 striatum as compared with WT (Fig. 4b) as documented (22). Treatment of R6/2 mice with SAHA resulted in Nissl staining more closely resembling that in WT mice.
SAHA Does Not Inhibit PolyQ Aggregation.
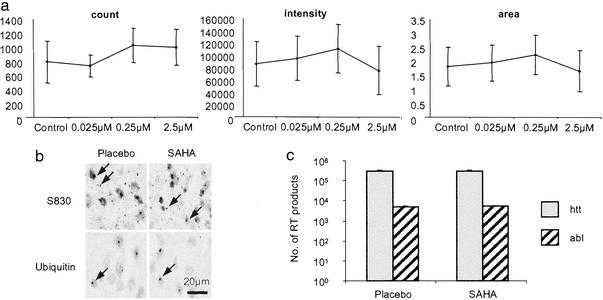
To rule out that SAHA might exert its effects through the inhibition of polyQ aggregation, its ability to act as an aggregation inhibitor was tested in an organotypic slice culture assay (27). We have developed this technique to bridge the gap between wholly in vitro aggregation assays and preclinical trials and allow us to quantify aggregate formation in a system in which aggregates form at the same rate and in the same sequence as they do in vivo. Hippocampal slices were established from R6/2 neonates at P7 and cultured in the presence of SAHA complexed with HOP-β-CD at concentrations ranging from 0.025 to 250 μM over a period of 4 weeks. Concentrations of 25 and 250 μM proved toxic. After 3 and 4 weeks there was no difference in the aggregate load between slices cultured in 0.025, 0.25, or 2.5 μM SAHA as compared with vehicle control (Fig. 5a). By a less quantitative immunohistochemical approach, no treatment-related differences in aggregate load were detected by using either antihuntingtin or antiubiquitin antibodies to immunoprobe sections of postmortem brains from R6/2 mice (Fig. 5b.).
Figure 5.
SAHA does not inhibit polyQ aggregation or decrease transgene protein levels. (a) Quantification of aggregate load in hippocampal slice cultures that have been incubated in the presence of SAHA or HOP-β-CD for 4 weeks. SAHA has no effect on aggregate count, aggregate fluorescence intensity, or aggregate area. Error bars = SD. (b) Immunodetection of polyQ aggregates (arrow) using antibodies raised against huntingtin (S830) and ubiquitin in postmortem brains from R6/2 mice at 13 weeks of age that had been administered SAHA or placebo for 8 weeks (age 13 weeks). (c) Real-time PCR to determine the level of expression of the R6/2 transgene and c-abl oncogene. R6/2 mice (10–11 weeks) had been treated with 0.67 g/liter SAHA (n = 7) or placebo (n = 7) for 17 days.
SAHA Does Not Down-Regulate the R6/2 Transgene.
To ensure that SAHA is not acting directly on the transgene promoter to down-regulate the R6/2 transgene, RNA was prepared from the brains of R6/2 mice that had been treated with either 0.67 g/liter SAHA or placebo for 17 days. Experiments were performed in triplicate, and the number of real-time PCR products was determined for the R6/2 transgene by using the c-abl oncogene as control (Fig. 5c). There is no difference in the level of expression of the R6/2 transgene (P = 0.92) or c-abl (P = 0.69) between SAHA- and placebo-treated mice.
Discussion
HDAC inhibitors have been shown to reduce polyQ toxicity in a Drosophila model of HD (11). We now show that the potent HDAC inhibitor, SAHA, dramatically improves Rotarod performance in the R6/2 HD mouse model. R6/2 mice treated with 0.67 g/liter SAHA demonstrated a significant improvement at 8 weeks of age after only 3 weeks of drug administration. At 12 weeks, the R6/2 mice taking SAHA were performing as well as the placebo group did at 8 weeks, indicating a delay by as much as 1 month in the decline in Rotarod performance. These results are yet more impressive given that our mice were housed in environmentally enriched conditions, which alone improved the Rotarod performance of R6/2 mice by ≈40% of the difference between R6/2 and WT (29). Therefore, drugs tested in our preclinical trials must cause significant additional improvement to be registered as effective. Our demonstration that SAHA treatment in part redresses the loss of striatal Nissl staining suggests that this may represent a neuropathological correlate of motor impairment.
SAHA is a small hydrophobic molecule and its relative insolubility in aqueous solution posed considerable difficulties. To combat this, we determined that SAHA is capable of forming a complex with HOP-β-CD with greatly enhanced aqueous solubility. Cyclodextrins are doughnut-shaped molecules consisting of six (α), seven (β), or eight (γ) glucose units linked by α-1,4 glycosidic bonds with their hydrophobic faces innermost. Small hydrophobic molecules can enter the central cavity to form a complex with the cyclodextrin. Because the hydrophilic surfaces of the cyclodextrins face outward, aqueous solubility is imparted on the complexes. Complexation with cyclodextrins can have additional benefits. These include a reduction in drug toxicity and irritation at the site of administration, masking of unpleasant tastes, and alteration of the pharmacokinetic profile of a drug, often increasing the half-life. HOP-β-CD is a nontoxic semisynthetic cyclodextrin, which is widely used in vitro and in vivo as a drug carrier in both enteral and parenteral formulations (36).
SAHA did not ameliorate the failure of R6/2 mice to gain weight, possibly reflecting its narrow therapeutic window with beneficial effects being masked by its toxicity. When SAHA administration was initiated at 30 days, doses ≥1.33 g/liter were toxic to both WT and R6/2 mice. Adverse effects were also seen in both WT and R6/2 mice at the effective dose of 0.67 g/liter, with mice failing to gain weight at the same rate as placebo-treated mice. However, despite this inherent toxicity, no R6/2 mice or WT mice died before the termination of the experiments at 13 weeks, and maintenance of grip strength in SAHA-treated R6/2 mice may indicate a sparing of muscle atrophy.
This study corroborates the use of HDAC inhibitors as therapeutic compounds for HD. Our demonstration that SAHA administration increases histone acetylation supports the hypothesis that it acts by redressing transcriptional repression. Although our results demonstrate that the identification of potential therapeutic compounds in Drosophila models can translate to preclinical mouse trials, we would caution against proceeding too rapidly to clinical trials. Demonstration of efficacy with SAHA is particularly encouraging as this drug is ≈103 fold more potent than the butyrate class of HDAC inhibitors [on a molar basis as an inhibitor of HDAC activity (15)] that are also under consideration. However, novel hydroxamic acid derivatives with enhanced activity as potential HD therapeutics also should be assessed.
Acknowledgments
We thank Nancy Wexler and the Hereditary Disease Foundation for discussions and inspiration and Caroline Whitehouse and David Grimwade for help with real-time PCR. This work was supported by grants from the Wellcome Trust (Grants 051897, 060360, and 066270), the Hereditary Disease Foundation, the Huntington's Disease Society of America, the Human Frontiers Science Program (Grant RG0132), the Medical Research Council (Grant G9800001), the DeWitt Wallace Fund for Memorial Sloan–Kettering Cancer Center, the National Institutes of Health (Grant CA-0974823), and the Kleberg Foundation. Memorial Sloan–Kettering Cancer Center and Columbia University jointly hold the patents on the hydroxamic hybrid polar compounds, including SAHA, which are exclusively licensed to Aton Pharma, Inc., of which P.A.M. and V.M.R. are founders. Both institutions and the founders have an equity position in Aton Pharma, Inc.
Abbreviations
- HD
Huntington's disease
- SAHA
suberoylanilide hydroxamic acid
- HDAC
histone deacetylase
- polyQ
polyglutamine
- HOP-β-CD
2-hydroxypropyl-β-cyclodextrin
References
- 1.Bates G P, Harper P S, Jones A L, editors. Huntington's Disease, Oxford Monographs on Medical Genetics. Oxford: Oxford Univ. Press; 2002. [Google Scholar]
- 2.Cha J H, Kosinski C M, Kerner J A, Alsdorf S A, Mangiarini L, Davies S W, Penney J B, Bates G P, Young A B. Proc Natl Acad Sci USA. 1998;95:6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luthi-Carter R, Strand A, Peters N L, Solano S M, Hollingsworth Z R, Menon A S, Frey A S, Spektor B S, Penney E B, Schilling G, et al. Hum Mol Genet. 2000;9:1259–1271. doi: 10.1093/hmg/9.9.1259. [DOI] [PubMed] [Google Scholar]
- 4.Luthi-Carter R, Hanson S A, Strand A D, Bergstrom D A, Chun W, Peters N L, Woods A M, Chan E Y, Kooperberg C, Krainc D, et al. Hum Mol Genet. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- 5.Kazantsev A, Preisinger E, Dranovsky A, Goldgaber D, Housman D. Proc Natl Acad Sci USA. 1999;96:11404–11409. doi: 10.1073/pnas.96.20.11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffan J S, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu Y Z, Gohler H, Wanker E E, Bates G P, Housman D E, Thompson L M. Proc Natl Acad Sci USA. 2000;97:6763–6768. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimohata T, Nakajima T, Yamada M, Uchida C, Onodera O, Naruse S, Kimura T, Koide R, Nozaki K, Sano Y, et al. Nat Genet. 2000;26:29–36. doi: 10.1038/79139. [DOI] [PubMed] [Google Scholar]
- 8.Nucifora F C, Jr, Sasaki M, Peters M F, Huang H, Cooper J K, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson V L, et al. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 9.McCampbell A, Taye A A, Whitty L, Penney E, Steffan J S, Fischbeck K H. Proc Natl Acad Sci USA. 2001;98:15179–15184. doi: 10.1073/pnas.261400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes R E, Lo R S, Davis C, Strand A D, Neal C L, Olson J M, Fields S. Proc Natl Acad Sci USA. 2001;98:13201–13206. doi: 10.1073/pnas.191498198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffan J S, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol B L, Kazantsev A, Schmidt E, Zhu Y Z, Greenwald M, et al. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 12.Li S H, Cheng A L, Zhou H, Lam S, Rao M, Li H, Li X J. Mol Cell Biol. 2002;22:1277–1287. doi: 10.1128/mcb.22.5.1277-1287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunah A W, Jeong H, Griffin A, Kim Y M, Standaert D G, Hersch S M, Mouradian M M, Young A B, Tanese N, Krainc D. Science. 2002;296:2238–2243. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- 14.Marks P, Rifkind R A, Richon V M, Breslow R, Miller T, Kelly W K. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 15.Richon V M, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind R A, Marks P A. Proc Natl Acad Sci USA. 1998;95:3003–3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richon V M, Sandhoff T W, Rifkind R A, Marks P A. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks P A, Richon V M, Rifkind R A. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 18.Marks P A, Rifkind R A, Richon V M, Breslow R. Clin Cancer Res. 2001;7:759–760. [PubMed] [Google Scholar]
- 19.Bates G P, Murphy K P. In: Huntington's Disease, Oxford Monographs on Medical Genetics. Bates G P, Harper P S, Jones A L, editors. Oxford: Oxford Univ. Press; 2002. pp. 387–426. [Google Scholar]
- 20.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S W, Bates G P. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 21.Davies S W, Turmaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante R J, Andreassen O A, Jenkins B G, Dedeoglu A, Kuemmerle S, Kubilus J K, Kaddurah-Daouk R, Hersch S M, Beal M F. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante R J, Andreassen O A, Dedeoglu A, Ferrante K L, Jenkins B G, Hersch S M, Beal M F. J Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Ona V O, Li M, Ferrante R J, Fink K B, Zhu S, Bian J, Guo L, Farrell L A, Hersch S M, et al. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 25.Karpuj M V, Becher M W, Springer J E, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 26.Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates G P. Nat Genet. 1997;15:197–200. doi: 10.1038/ng0297-197. [DOI] [PubMed] [Google Scholar]
- 27.Smith D L, Portier R, Woodman B, Hockly E, Mahal A, Klunk W E, Li X J, Wanker E, Murray K D, Bates G P. Neurobiol Dis. 2001;8:1017–1026. doi: 10.1006/nbdi.2001.0438. [DOI] [PubMed] [Google Scholar]
- 28.Hochberg Y, Benjamini Y. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 29.Hockly E, Cordery P M, Woodman B, Mahal A, Van Dellen A, Blakemore C, Lewis C M, Hannan A J, Bates G P. Ann Neurol. 2002;51:235–242. doi: 10.1002/ana.10094. [DOI] [PubMed] [Google Scholar]
- 30.Sathasivam K, Woodman B, Mahal A, Bertaux F, Wanker E E, Shima D T, Bates G P. Hum Mol Genet. 2001;10:2425–2435. doi: 10.1093/hmg/10.21.2425. [DOI] [PubMed] [Google Scholar]
- 31.Butler L M, Agus D B, Scher H I, Higgins B, Rose A, Cordon-Cardo C, Thaler H T, Rifkind R A, Marks P A, Richon V M. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 32.Bancroft J D, Stevens A. Theory and Practice of Histological Techniques. 3rd Ed. New York: Churchill Livingstone; 1990. [Google Scholar]
- 33.Grimwade D, Outram S V, Flora R, Ings S J, Pizzey A R, Morilla R, Craddock C F, Linch D C, Solomon E. Cancer Res. 2002;62:4730–4735. [PubMed] [Google Scholar]
- 34.Cohen L A, Amin S, Marks P A, Rifkind R A, Desai D, Richon V M. Anticancer Res. 1999;19:4999–5005. [PubMed] [Google Scholar]
- 35.Wolfensohn S, Lloyd M. Handbook of Laboratory Animal Management and Welfare. Oxford: Blackwell; 1998. [Google Scholar]
- 36.Uekama K, Hirayama F, Irie T. Chem Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]