Abstract
Proteinaceous aggregates containing α-synuclein represent a feature of neurodegenerative disorders such as Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Despite extensive research, the mechanisms underlying α-synuclein aggregation remain elusive. Previously, tissue transglutaminase (tTGase) was found to contribute to the generation of aggregates by cross-linking pathogenic substrate proteins in Huntington's and Alzheimer's diseases. In this article, the role of tTGase in the formation of α-synuclein aggregates was investigated. Purified tTGase catalyzed α-synuclein cross-linking, leading to the formation of high molecular weight aggregates in vitro, and overexpression of tTGase resulted in the formation of detergent-insoluble α-synuclein aggregates in cellular models. Immunocytochemical studies demonstrated the presence of α-synuclein-positive cytoplasmic inclusions in 8% of tTGase-expressing cells. The formation of these aggregates was significantly augmented by the calcium ionophore A23187 and prevented by the inhibitor cystamine. Immunohistochemical studies on postmortem brain tissue confirmed the presence of transglutaminase-catalyzed ɛ(γ-glutamyl)lysine cross-links in the halo of Lewy bodies in Parkinson's disease and dementia with Lewy bodies, colocalizing with α-synuclein. These findings, taken together, suggest that tTGase activity leads to α-synuclein aggregation to form Lewy bodies and perhaps contributes to neurodegeneration.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of proteinaceous cytoplasmic inclusions known as Lewy bodies (1, 2). These inclusions are also present in dementia with Lewy bodies (DLB) (3). Although the molecular pathogenesis of these disorders remains poorly understood, several lines of evidence point to a key role for α-synuclein, which is a major constituent of Lewy bodies and whose mutations have been associated with rare autosomal dominant forms of PD (4–7). α-Synuclein is a small protein of 140 aa consisting of three modular domains including an N-terminal lipid-binding amphipathic α-helix, a central amyloid-binding domain encoding the non-Aβ component of Alzheimer plaques, and a C-terminal acidic tail (8). α-Synuclein exists in either a natively unfolded conformation (9) or as an α-helix in the presence of phospholipid vesicles (10), suggesting a dynamic regulation of its function depending on the local cellular environment. Because of its unfolded structure, α-synuclein is prone to self-aggregate and to cause the aggregation of other proteins, a property that may underlie its involvement in Lewy body formation and its contribution to the pathogenesis of PD (11–13). In vitro, α-synuclein is capable of self-aggregating into fibrils in a time-, temperature-, pH-, and concentration-dependent manner (12, 14). Other factors such as mutations, C-terminal truncation, metal ions, and oxidative stress have also been shown to increase α-synuclein aggregation in vitro (15–17). Additionally, factors thought to play a role in PD increase α-synuclein aggregation in several cellular models. These include pathogenic α-synuclein mutations, oxidative stress, proteasomal impairment, mitochondrial defects, and interaction with other proteins, such as parkin and synphilin-1 (18–23). Thus, the identification of triggers for α-synuclein aggregation in vivo is critical because such factors are likely to be involved in the pathogenesis of PD and other α-synucleinopathies.
Transglutaminases (TGase) are a family of proteins that catalyze a calcium-dependent transamidating reaction that results in cross-linking of proteins via ɛ(γ-glutamyl) lysine bonds (24). Transglutaminase 2 (tTGase) is unique in this family, in that it has GTPase and ATPase activities in addition to its transamidating activity (25, 26). It is expressed in the mammalian nervous system and human brain, localizing predominantly in neurons (27, 28). Recent studies demonstrated possible involvement of tTGase in several neurodegenerative processes by catalyzing the formation of protein aggregates. In Alzheimer's disease (AD), tTGase catalyzes the cross-linking of neurofilament, amyloid precursor protein, tau, and non-Aβ component (29–32). In addition, TGase activity and expression are elevated in affected areas of AD brains compared with normal controls (33). Studies have also stressed the role of tTGase in polyglutamine expansion neurodegenerative disorders such as Huntington's disease (HD). Polyglutamine residues are excellent targets for this enzyme, whereas TGase activity and expression are elevated in HD-affected brain regions (28, 34, 35).
In the present article, we demonstrate that tTGase catalyzes the formation of α-synuclein aggregates in vitro as well as in cellular models. Moreover, we show the presence of ɛ(γ-glutamyl) lysine bonds, which is indicative of TGase activity, in Lewy bodies in PD and DLB. Our findings suggest that this enzyme is involved in the formation of Lewy bodies by cross-linking α-synuclein and possibly in the pathogenesis of α-synucleinopathies.
Materials and Methods
cDNA Cloning and Materials.
α-Synuclein cDNA was cloned by PCR from human fetal brain cDNA library (Stratagene) (36) and subcloned into pcDNA3.1 (Invitrogen) and pTYB11 (New England Biolabs). tTGase cDNA was amplified by PCR from human liver cDNA library by using primers 5′-aagaattcAACAGGCGTGACGCCAGTTCTAAACTTGAAACAAAACAA-3′ and 5′-aagaattcGGAATTGTGTATTGCAAACATGGAGTGGAG-3′ (lowercase letters indicate additional nucleotides designed to facilitate cloning). The PCR product was inserted into pSG5 expression vector (Stratagene), and its sequence matched perfectly with the known cDNA sequence of human tTGase (37). A catalytically inactive mutant of tTGase (C277S) was kindly provided by G.V.W. Johnson, University of Alabama at Birmingham (33, 38). Mouse monoclonal tTGase antibody (CUB 7402) was purchased from DAKO. We also used a rabbit polyclonal tTGase antibody as described (27). Guinea pig liver tTGase, cystamine (CTM), and A23187 were purchased from Sigma. Rabbit polyclonal α-synuclein antibodies were obtained from Sigma and Chemicon. Monoclonal anti-α-synuclein antibodies, SYN-1 and LB509, were from Transduction Laboratories (Lexington, KY) and Zymed, respectively. Monoclonal antibody recognizing ɛ(γ-glutamyl) lysine isodipeptide bonds (81D1C2) was purchased from Covalab (Lyon, France). Recombinant human α-synuclein protein was expressed in pTYB11 and purified by the IMPACT T7 system (New England Biolabs) according to the supplier's instructions.
Cell Culture and Transfection.
COS-7 and human embryonic kidney (HEK) 293T cell lines were maintained in DMEM containing 10% FBS. Transfections were performed by using FuGene 6 reagent (Roche Molecular Biochemicals) for a 6-h incubation period, and then treatments were initiated.
In Vitro Cross-Linking Reaction of α-Synuclein by tTGase.
The reaction was performed at 37°C for 2 h in a buffer containing 50 mM Tris⋅HCl (pH 7.5), 2 mM leupeptin, and 1 mM purified α-synuclein. Depending on reaction conditions specified in Results, purified guinea pig liver tTGase (10 nM), DTT (0.1 mM), CaCl2 (5 mM), and/or EDTA (0.1 mM, 5 mM) were added. The reaction was stopped by the addition of 20 mM EDTA, and products were analyzed by SDS/PAGE followed by Western blot.
Immunoprecipitation and Western Blot.
Cells were lysed in a buffer containing PBS with 1% Triton X-100 and a mixture of protease inhibitors (Roche Molecular Biochemicals). After homogenizing with 20 strokes by using a Dounce homogenizer, cells were centrifuged at 20,000 × g at 4°C for 30 min. The soluble and insoluble fractions were used in Western blot analysis using α-synuclein antibody (SYN-1) or tTGase antibody (CUB 7402). Triton X-100 insoluble pellets were dissolved in a buffer (PBS plus 1% Triton X-100/1% SDS) containing a mixture of protease inhibitors with sonication. After centrifugation, supernatant was diluted in 10 vol of the same buffer lacking SDS and used for immunoprecipitation with SYN-1 antibody. Immunoprecipitates were analyzed by Western blots with anti-isodipeptide antibody (81D1C2) or anti-α-synuclein antibody SYN-1 with the ECL detection system (NEN). To study the interaction between α-synuclein and tTGase, cotransfected cells were lysed, and soluble fraction was subjected to immunoprecipitation with rabbit polyclonal tTGase antibody. Immunoprecipitates or total cell lysates were analyzed by Western blots with SYN-1 antibody.
Immunocytochemistry.
HEK 293T cells transiently transfected as described in Results were cultured in the presence or absence of indicated chemicals in collagen-coated Biocoat slides (Becton Dickinson) for 48 h. Cells were fixed in 4% paraformaldehyde in PBS for 20 min, washed with PBS three times, and permeabilized with 0.5% Triton X-100 in PBS for 10 min. After washing the cells again with PBS, they were blocked with 1% BSA in PBS for 20 min. Cells were incubated with rabbit polyclonal α-synuclein antibody (Sigma) and mouse monoclonal tTGase antibody (CUB 7402) diluted in 1% BSA at 4°C for 2 h. Cells were washed five times for 5 min each with PBS. Anti-rabbit IgG-rhodamine-conjugated and anti-mouse IgG-FITC-conjugated antibodies were diluted in 1% BSA and incubated at 4°C for 1 h. Cells were washed five times with PBS and analyzed under a fluorescence microscope (Zeiss, Axiophot). For quantification of inclusions, 10 microscopic fields were randomly selected, and the percentage of inclusion-positive cells was counted among transfected cells.
Immunohistochemistry on Human Brain Tissues.
Postmortem brain tissues from patients with PD and DLB were fixed in formaldehyde for 2 weeks and embedded in paraffin. Six-micrometer sections from substantia nigra were immunostained individually with rabbit anti-α-synuclein polyclonal antibody (1:500, Chemicon) and mouse monoclonal isodipeptide antibody (81D1C2, 1:50) by using the avidin-biotin-peroxidase method as described (39) with the Envision Plus kit (DAKO). Double immunohistochemistry with both antibodies was performed by using the Histostain-DS kit (Zymed) following the manufacturer's protocol. Colocalization of both signals in this kit is visualized under the light microscope as black color.
Results
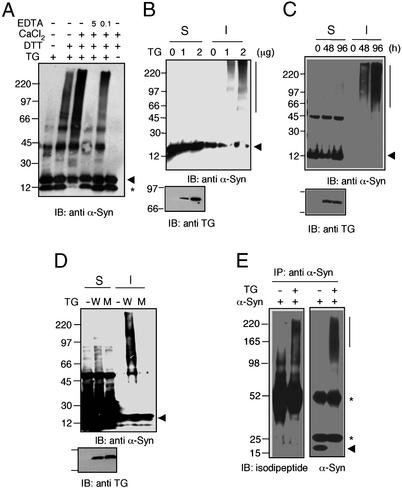
To study the in vitro cross-linking of α-synuclein, purified recombinant human α-synuclein protein was incubated with guinea pig liver tTGase at 37°C, and the reaction products were analyzed by SDS/PAGE, followed by Western blotting with anti-α-synuclein antibody (Fig. 1A). Reduced form of tTGase in the presence of DTT induced the formation of high molecular weight aggregates of α-synuclein. Generation of these aggregates was significantly enhanced by the addition of CaCl2, a known activator of tTGase (40). On the other hand, chelation with EDTA dose-dependently blocked aggregate formation. These results suggest that α-synuclein is a target for tTGase in vitro.
Figure 1.
α-Synuclein is cross-linked and aggregated by tTGase. (A) Purified recombinant human α-synuclein was incubated with guinea pig tTGase for 2 h at 37°C under the indicated conditions and subjected to Western blot with SYN-1 antibody. Arrowhead indicates α-synuclein monomer. Asterisk denotes a cleavage product of α-synuclein that is generated during protein purification. (B) COS-7 cells were transiently transfected with 1 μg of α-synuclein expression plasmid along with the indicated amounts of tTGase-expressing plasmid. Cells were lysed at 48 h posttransfection and separated into Triton X-100 soluble (S) and insoluble (I) fractions. The same amount of proteins in both fractions was subjected to SDS/PAGE, followed by immunoblot analysis with α-synuclein antibody SYN-1 and tTGase antibody CUB7402. (C) COS-7 cells were transfected with the same amount (1 μg) of α-synuclein and tTGase expression plasmids and lysed at 48 and 96 h posttransfection. (D) COS-7 cells were transfected with α-synuclein and WT tTGase (W) or the inactive mutant tTGase (C277S) (M). (E) After transfecting COS-7 cells with the indicated expression plasmids, α-synuclein was immunoprecipitated from the Triton X-100-insoluble fraction, followed by Western blot analysis with antiisodipeptide antibody (81D1C2) or SYN-1. Note the decrease in monomer α-synuclein (arrowhead) as it is polymerized by tTGase. Asterisks indicate IgG heavy and light chains.
To examine tTGase-induced aggregation of α-synuclein in vivo, COS-7 cells were transfected with the α-synuclein expression plasmid in the absence or presence of a tTGase expression plasmid. The presence of tTGase dose-dependently induced the formation of α-synuclein high molecular weight aggregate bands running at apparent molecular masses >60 kDa in the detergent-insoluble fraction (Fig. 1B), detected by Western blot analysis with SYN-1 antibody. Similar results were obtained by using another α-synuclein antibody, LB509 (data not shown). The amount of these aggregates was much more abundant at 96 h posttransfection than at 48 h (Fig. 1C). In addition, expression of a catalytically inactive mutant (C277S) of tTGase failed to generate α-synuclein aggregates (Fig. 1D), indicating that their formation requires the catalytic activity of tTGase. Because ɛ(γ-glutamyl)lysine cross-links are the footprints of tTGase activity, we determined the presence of isodipeptide bonds in these aggregates by immunoprecipitating α-synuclein from the detergent-insoluble fraction. The complex immunoprecipitated from cells transfected with both α-synuclein and tTGase, but not from cells transfected with only α-synuclein, was detected by an antibody recognizing the isodipeptide bonds produced by tTGase activity (Fig. 1E). The latter data indicate that these aggregates are formed as a result of tTGase activity. The same findings were reproduced in HEK 293T cells transiently transfected with α-synuclein and tTGase (data not shown). Collectively, these observations suggest that α-synuclein is a cellular substrate of tTGase.
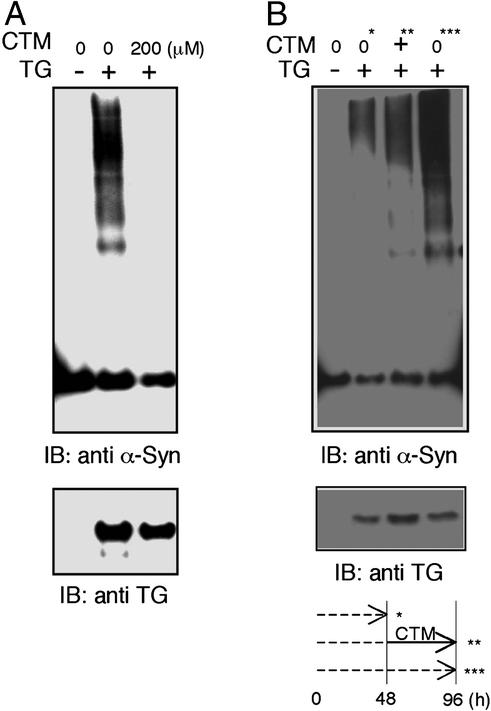
CTM is known to inhibit tTGase activity through a disulfide exchange reaction and serves as a competitor for tTGase by blocking access of the glutamine residue in substrate proteins to the active site of the enzyme (41, 42). Therefore, we examined whether CTM is able to inhibit tTGase-induced α-synuclein aggregation in a cellular model. Incubation of α-synuclein- and tTGase-transfected COS-7 cells with 200 μM CTM for 48 h dramatically inhibited the formation of these aggregates (Fig. 2A). To examine if CTM can clear preformed aggregates, cells were harvested at 48 h (Fig. 2B, 0*) and 96 h (Fig. 2B, 0***) posttransfection without CTM treatment, and also harvested at 96 h (Fig. 2B, +∗∗) after CTM treatment during the final 48 h of incubation (Fig. 2B). Delayed treatment with CTM did not diminish the level of aggregates formed during the initial 48 h (Fig. 2B, compare lanes marked ∗ and ∗∗). Rather, CTM inhibited further de novo aggregation occurring during the final 48 h (Fig. 2B, compare lanes ∗∗ and ∗∗∗). This result suggests that CTM can prevent the formation of tTGase-induced α-synuclein aggregates but cannot resolve preexisting complexes.
Figure 2.
CTM prevents the formation of α-synuclein aggregates in a cellular model. COS-7 cells were transfected with α-synuclein in the presence or absence of tTGase as indicated. Triton X-100-insoluble fractions were subjected to Western blot analysis with SYN-1 and CUB7402. (A) Transfected cells were treated with 200 μM CTM or vehicle for 48 h. (B) Transfected cells were harvested at 48 h (0*) or 96 h (0***) posttransfection without CTM treatment, or harvested at 96 h (+**) posttransfection after 200 μM CTM treatment during the final 48 h. The experimental paradigm below shows incubation time (dotted line) and CTM treatment (solid line).
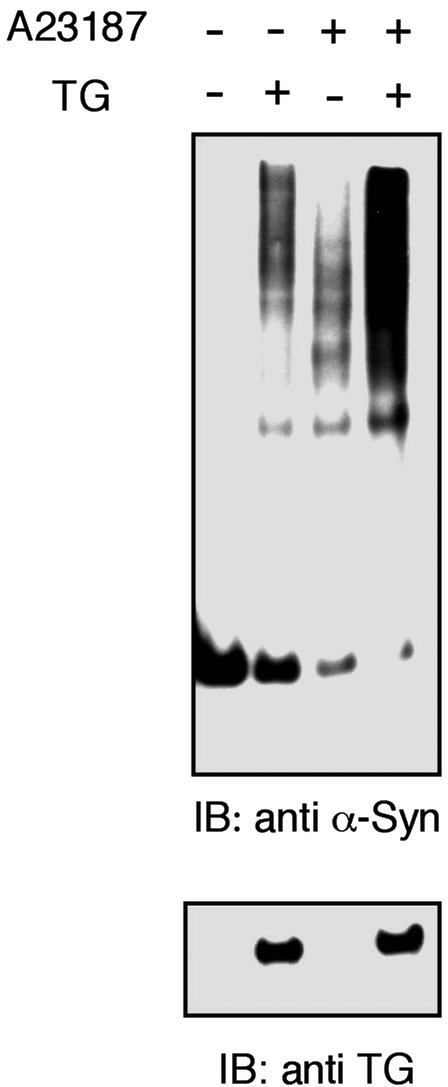
Considering the fact that the cross-linking activity of tTGase depends on calcium (40), we studied whether this cation is able to affect α-synuclein aggregation. For this experiment, we treated α-synuclein- and tTGase-transfected COS-7 cells with the calcium ionophore, A23187. The calcium mobilizer resulted in a significant increase in the formation of α-synuclein aggregates (Fig. 3). Notably, we observed some high molecular weight bands of α-synuclein in A23187-treated cells transfected with only α-synuclein, likely caused by activation of endogenous tTGase despite its low expression in COS-7 cells or the tendency of α-synuclein to form oligomers in the presence of calcium, as reported (43).
Figure 3.
Calcium ionophore treatment increases α-synuclein aggregation in a cellular model. COS-7 cells were transfected with α-synuclein in the presence or absence of tTGase expression vector as indicated. A23187 (0.1 μg/ml) was added to the culture medium for 48 h as indicated.
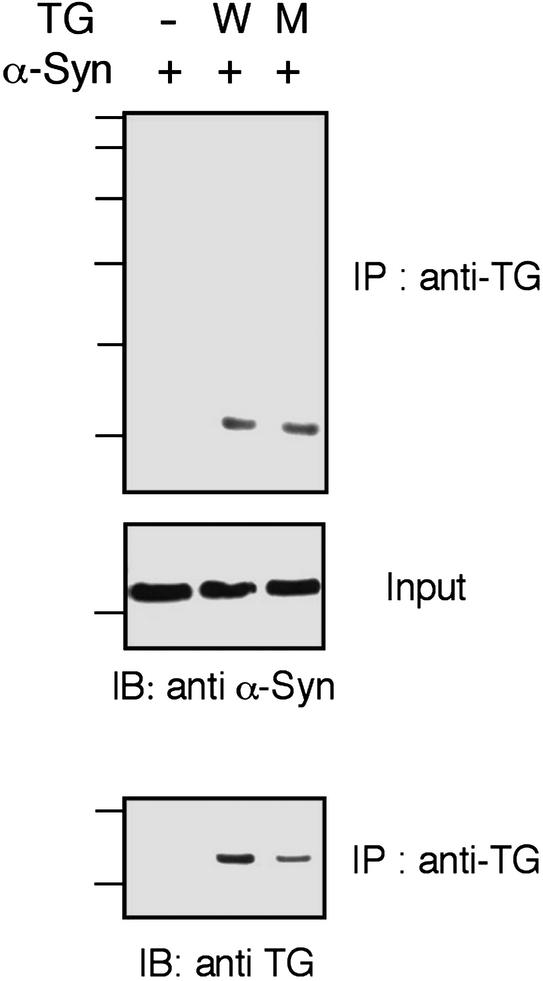
To determine whether α-synuclein and tTGase interact, coimmunoprecipitation was performed with COS-7 cells transiently transfected with α-synuclein and tTGase. Immunoprecipitation of tTGase also pulled down α-synuclein, indicating intermolecular interaction (Fig. 4). The inactive mutant (C277S) of tTGase also interacted with α-synuclein, suggesting that this interaction does not require the catalytic activity of the enzyme.
Figure 4.
α-Synuclein and tTGase interact in cells. COS-7 cells were transfected with α-synuclein plus either WT (W) or inactive mutant (M) tTGase. After the immunoprecipitation step with tTGase antibody (CUB 7402) from the detergent-soluble fraction, Western blot analysis was done with α-synuclein antibody (SYN-1) and tTGase antibody (CUB 7402). Western blot with SYN-1 antibody was also done on total cell lysate (Input).
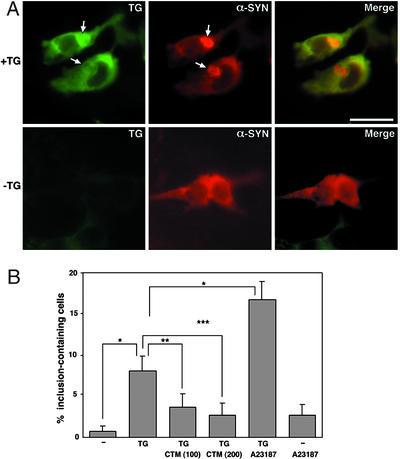
tTGase-induced α-synuclein aggregates could also be seen by immunocytochemistry in HEK293T cells transiently transfected with this enzyme and substrate. About 8% of cells expressing both proteins had microscopically visible aggregates, whereas only 0.7% of cells expressing only α-synuclein had inclusions. The very low level of α-synuclein aggregation into inclusions in the absence of tTGase overexpression likely represents the tendency of α-synuclein to aggregate (11) because endogenous tTGase levels in these cells are quite low (Figs. 1–4). These aggregates were localized in the cytosol, especially in the perinuclear region, and costained for tTGase (Fig. 5A). Consistent with our immunoblot analysis showing inhibition of aggregate formation (Fig. 2), CTM resulted in decreased inclusion formation in cells coexpressing both proteins in a dose-dependent manner (Fig. 5B). A23187, on the other hand, significantly increased aggregate formation (Fig. 5B), as seen in immunoblot analysis (Fig. 3).
Figure 5.
α-Synuclein aggregates are detected by immunocytochemistry. (A) HEK293T cells were transfected with α-synuclein in the absence or presence of tTGase expression vector, and cells were formalin fixed 48 h posttransfection. α-Synuclein stains with red fluorescence, whereas tTGase stains green. (Upper) Distinct α-synuclein aggregation (arrows) in cells overexpressing tTGase. (Lower) Cells with diffuse cytoplasmic localization of α-synuclein and no detectable tTGase. (Scale bar: 20 μm.) (B) Cells transiently transfected with α-synuclein in the absence or presence of tTGase were treated with CTM (100 or 200 μM) or A23187 (0.1 μg/ml) for 48 h and stained for α-synuclein and tTGase. Aggregate-containing cells among transfected cells were counted in 10 randomly selected fields. Each microscopic field had 10–20 transfected cells. The data represent means ± SEM. Significance levels determined by factorial ANOVA and the Bonferroni post hoc test are shown. *, P < 0.002; **, P < 0.06; ***, P < 0.02.
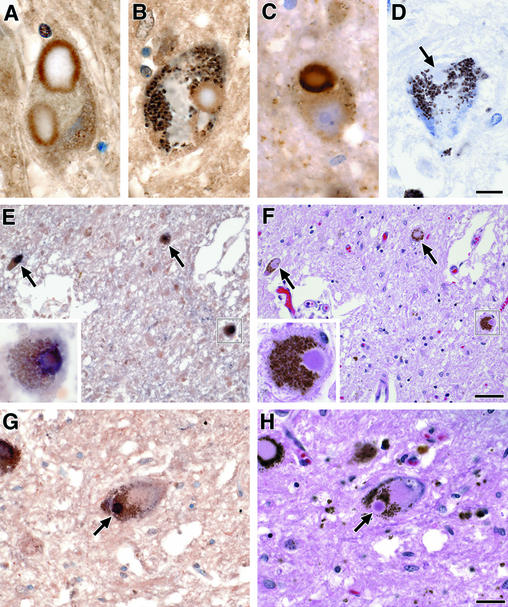
To detect evidence for TGase activity in α-synucleinopathies, nigral sections from brains of patients affected with PD and DLB were subjected to immunohistochemical stains by using specific antibodies to isodipeptide (81D1C2) and α-synuclein. Lewy bodies from both disease conditions stained with 81D1C2 in the halo (Fig. 6 A and B), similar to the well-known staining pattern of α-synuclein (Fig. 6C). Omission of the primary isodipeptide antibody did not give an immunohistochemical signal (Fig. 6D). To confirm the colocalization of α-synuclein and isodipeptide cross-links, brain sections from PD and DLB were costained simultaneously with both primary antibodies, and adjacent sections were stained with hematoxylin and eosin. Colocalization of both signals was seen in the halo of Lewy bodies (Figs. 6 E–H). These postmortem studies indicate the presence of isodipeptide cross-linked α-synuclein in Lewy bodies from PD and DLB brains.
Figure 6.
TGase-catalyzed isodipeptide bonds colocalize with α-synuclein in Lewy bodies. (A) Immunohistochemistry using isodipeptide primary antibody (81D1C2) in the substantia nigra of a patient with PD. (B) Similar stain as in A from a case with DLB. (C) Immunohistochemistry using rabbit polyclonal antibody for α-synuclein (Chemicon) in the nigra of a PD patient. (D) Negative control for the isodipeptide stain where the primary antibody is omitted. The arrow points to an unstained Lewy body. (E) Double staining for isodipeptide and α-synuclein on a nigral section from a patient with PD. Colocalization of these two stains appears as black. The microscopic field contains three neurons with Lewy bodies costained with both antibodies. The boxed neuron is shown at higher power magnification (Inset). (F) The section adjacent to E is stained with hematoxylin and eosin. (G) Similar double stain as in E from the nigra of a patient with DLB. (H) The section adjacent to G is stained with hematoxylin and eosin. The arrow points to the same Lewy body costained with the isodipeptide and α-synuclein antibodies. (Scale bar in D is 10 μm for A–D, scale bar in F is 50 μm for E and F, and scale bar in H is 25 μm for G and H.)
Discussion
In the present article, we demonstrate that α-synuclein is a substrate for tTGase both in vitro and in cellular models. The formation of α-synuclein aggregates is significantly increased by tTGase activity. Moreover, TGase-catalyzed cross-links colocalize with α-synuclein in Lewy bodies of PD and DLB. Based on these observations, we suggest that tTGase plays a role in forming α-synuclein-containing inclusions. Considering the potential deleterious cellular effects of protein aggregates (18, 44), tTGase also could contribute to neuronal death in PD, DLB, and perhaps other α-synucleinopathies.
The first indication for tTGase involvement in the pathogenesis of neurodegenerative disorders came from its catalysis of neurofilament cross-linking in AD (29). Subsequently, several reports demonstrated that components of extracellular senile plaques and intracellular neurofibrillary tangles are substrates for tTGase, thus suggesting participation of tTGase in the formation of plaques and tangles in AD brains (31, 45–47). In Huntington's disease, evidence for tTGase involvement is based on the finding of elevated enzymatic activity in affected regions of diseased brains, on studies demonstrating that the enzyme cross-links Huntington, and on the observation that such aggregates are formed to a greater extent with expansion of the polyglutamine repeats (35, 48). The role of tTGase in PD, however, had been less well understood. Previously, the non-Aβ component of amyloid plaques, which constitutes the midportion of α-synuclein, was found to be cross-linked to itself or to βA4 peptide by tTGase in vitro, and the tTGase-reactive amino acid residues in non-Aβ component were identified to be Gln-79 and Lys-80 (32). Whether these two residues are involved in cross-linking full-length α-synuclein in cellular models remains to be determined. Our present observations confirm TG-catalyzed cross-linking of full-length α-synuclein in cellular models and postmortem brain tissue.
tTGase-induced protein cross-linking can be regulated by factors implicated in the pathogenesis of PD. First, the interaction with calcium is critical for the transamidating activity of tTGase, as calcium induces a conformational change of the enzyme, exposing the active site to substrates (49, 50). Consistent with this action, α-synuclein aggregation is significantly increased by the calcium ionophore, A23187. Elevated concentrations of calcium resulting from complex I inhibition in the mitochondrial electron transport chain has been implicated in the pathogenesis of PD (51, 52), particularly because neuronal loss in PD is greatest in nigrosomes, where the calcium-binding protein calbindin is poorly expressed (53). Under such conditions, tTGase activity is expected to increase. Second, GTP binds to tTGase, resulting in a conformational change and reduced affinity to calcium, leading to inhibition of its transamidating activity (26). The mitochondrial toxin 3-nitropropionic acid has been shown to decrease GTP and ATP levels, resulting in significant enhancement of TGase activity in a cellular model (54). Therefore, it is probable that the impaired mitochondrial function in the Parkinsonian nigra (55, 56) can promote TGase activity, causing further protein aggregation. Consistent with this notion, TGase activity recently was reported to be higher in a small number of PD brains than in normal controls (57). Third, oxidative stress, which is one of the major factors for PD pathogenesis, can modify specific proteins as to make them better substrates for TGase. For example, exposure of crystallin to oxidizing free radicals enhances its amine donor capacity, thus increasing its susceptibility to cross-linking by TGase (58). A similar scenario can potentially contribute to α-synuclein aggregation in response to reactive oxygen species in the PD brain. Fourth, increased expression of tTGase itself can be another factor contributing to protein aggregation and subsequent pathogenetic events. The elevated expression of TGase in neurodegenerative disorders including AD, Huntington's disease, and PD (27, 28, 57) may accelerate not only protein aggregation but neuronal apoptosis as well (38, 59). Although there is no evidence to date for the involvement of tTGase in apoptotic events occurring in PD, exploring such a link would be of interest.
Administration of the TGase inhibitor CTM to Huntington's disease transgenic mice has been found to extend survival and to improve body weight and motor performance (60, 61). In our cellular model, the formation of α-synuclein aggregates was significantly reduced by CTM. Interestingly, in addition to its role as a tTGase inhibitor, CTM blocks caspase 3 activity and increases cellular glutathionine levels, suggesting that it may prolong neuronal survival (62). Based on these observations, CTM or a related compound with a similar mechanism of action would be a promising pharmacological tool to be tested in PD models.
In addition to biochemical studies in cellular models, we present evidence for TGase-mediated α-synuclein aggregate formation in human brain tissues from cases with PD and DLB. TGase forms a covalent ɛ(γ-glutamyl)lysine bond between a γ-carboxamide group of a glutamine and the ɛ-amino group of a lysine intermolecularly or intramolecularly. The presence of this isodipeptide bond, therefore, can be a marker for prior TGase action. The identification of this epitope in the halo of Lewy bodies and its colocalization with α-synuclein suggest that TGase contributes to the formation of Lewy bodies by cross-linking α-synuclein, rendering it resistant to biochemical degradation.
The foregoing observations point to a well-characterized enzymatic reaction as a trigger for α-synuclein aggregation in brains affected by α-synucleinopathies and, therefore, identify a therapeutic target that can be tested.
Acknowledgments
We are grateful to Dr. G.V.W. Johnson for providing the cDNA coding mutant tTGase (C277S). We also thank Mr. Ricardo Dreyfus for assistance with photomicroscopy.
Abbreviations
- AD
Alzheimer's disease
- CTM
cystamine
- DLB
dementia with Lewy bodies
- PD
Parkinson's disease
- TGase
transglutaminase
- tTGase
tissue TGase
- HEK
human embryonic kidney
References
- 1.Jenner P, Olanow C W. Ann Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 2.Pollanen M S, Dickson D W, Bergeron C. J Neuropathol Exp Neurol. 1993;52:183–191. doi: 10.1097/00005072-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Tortosa E, Newell K, Irizarry M C, Sanders J L, Hyman B T. Acta Neuropathol. 2000;99:352–357. doi: 10.1007/s004010051135. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M G, Crowther R A, Jakes R, Hasegawa M, Goedert M. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 6.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J T, Schols L, Riess O. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 7.Mouradian M M. Neurology. 2002;58:179–185. doi: 10.1212/wnl.58.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Riess O, Jakes R, Kruger R. Mol Med Today. 1998;4:438–444. doi: 10.1016/s1357-4310(98)01343-4. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb P H, Zhen W, Poon A W, Conway K A, Lansbury P T., Jr Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 10.Davidson W S, Jonas A, Clayton D F, George J M. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 11.Conway K A, Harper J D, Lansbury P T. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 12.Giasson B I, Uryu K, Trojanowski J Q, Lee V M. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 13.Paik S R, Lee J H, Kim D H, Chang C S, Kim Y S. FEBS Lett. 1998;421:73–76. doi: 10.1016/s0014-5793(97)01537-8. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Hsu L J, Sisk A, Xia Y, Takeda A, Sundsmo M, Masliah E. Brain Res. 1998;799:301–306. doi: 10.1016/s0006-8993(98)00514-9. [DOI] [PubMed] [Google Scholar]
- 15.El-Agnaf O M, Jakes R, Curran M D, Wallace A. FEBS Lett. 1998;440:67–70. doi: 10.1016/s0014-5793(98)01419-7. [DOI] [PubMed] [Google Scholar]
- 16.Crowther R A, Jakes R, Spillantini M G, Goedert M. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, Hsu L J, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. NeuroReport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 18.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee J M, Farer M, Wolozin B. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinou E, Chen Q, Weisse M, Giasson B I, Norris E H, Rueter S M, Trojanowski J Q, Lee V M, Ischiropoulos H. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rideout H J, Larsen K E, Sulzer D, Stefanis L. J Neurochem. 2001;78:899–908. doi: 10.1046/j.1471-4159.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee H J, Shin S Y, Choi C, Lee Y H, Lee S J. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 22.Junn E, Lee S S, Suhr U T, Mouradian M M. J Biol Chem. 2002;277:47870–47877. doi: 10.1074/jbc.M203159200. [DOI] [PubMed] [Google Scholar]
- 23.Engelender S, Kaminsky Z, Guo X, Sharp A H, Amaravi R K, Kleiderlein J J, Margolis R L, Troncoso J C, Lanahan A A, Worley P F, et al. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg C S, Birckbichler P J, Rice R H. FASEB J. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 25.Achyuthan K E, Greenberg C S. J Biol Chem. 1987;262:1901–1906. [PubMed] [Google Scholar]
- 26.Lai T S, Slaughter T F, Peoples K A, Hettasch J M, Greenberg C S. J Biol Chem. 1998;273:1776–1781. doi: 10.1074/jbc.273.3.1776. [DOI] [PubMed] [Google Scholar]
- 27.Kim S Y, Grant P, Lee J H, Pant H C, Steinert P M. J Biol Chem. 1999;274:30715–30721. doi: 10.1074/jbc.274.43.30715. [DOI] [PubMed] [Google Scholar]
- 28.Lesort M, Chun W, Johnson G V, Ferrante R J. J Neurochem. 1999;73:2018–2027. [PubMed] [Google Scholar]
- 29.Selkoe D J, Abraham C, Ihara Y. Proc Natl Acad Sci USA. 1982;79:6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho G J, Gregory E J, Smirnova I V, Zoubine M N, Festoff B W. FEBS Lett. 1994;349:151–154. doi: 10.1016/0014-5793(94)00663-6. [DOI] [PubMed] [Google Scholar]
- 31.Miller M L, Johnson G V. J Neurochem. 1995;65:1760–1770. doi: 10.1046/j.1471-4159.1995.65041760.x. [DOI] [PubMed] [Google Scholar]
- 32.Jensen P H, Sorensen E S, Petersen T E, Gliemann J, Rasmussen L K. Biochem J. 1995;310:91–94. doi: 10.1042/bj3100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson G V, Cox T M, Lockhart J P, Zinnerman M D, Miller M L, Powers R E. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- 34.Kahlem P, Green H, Djian P. Mol Cell. 1998;1:595–601. doi: 10.1016/s1097-2765(00)80059-3. [DOI] [PubMed] [Google Scholar]
- 35.Karpuj M V, Garren H, Slunt H, Price D L, Gusella J, Becher M W, Steinman L. Proc Natl Acad Sci USA. 1999;96:7388–7393. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett M C, Bishop J F, Leng Y, Chock P B, Chase T N, Mouradian M M. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 37.Gentile V, Saydak M, Chiocca E A, Akande O, Birckbichler P J, Lee K N, Stein J P, Davies P J. J Biol Chem. 1991;266:478–483. [PubMed] [Google Scholar]
- 38.Tucholski J, Johnson G V. J Neurochem. 2002;81:780–791. doi: 10.1046/j.1471-4159.2002.00859.x. [DOI] [PubMed] [Google Scholar]
- 39. Lee, S. S., Kim, Y. M., Junn, E., Lee, G., Park, K. H., Tanaka, M., Ronchetti, R. D., Quezado, M. M. & Mouradian, M. M. (2002) Neurobiol. Aging, in press. [DOI] [PubMed]
- 40.Peterson L L, Zettergren J G, Wuepper K D. J Invest Dermatol. 1983;81:95–100. doi: 10.1111/1523-1747.ep12540777. [DOI] [PubMed] [Google Scholar]
- 41.Birckbichler P J, Orr G R, Patterson M K, Jr, Conway E, Carter H A. Proc Natl Acad Sci USA. 1981;78:5005–5008. doi: 10.1073/pnas.78.8.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorand L, Parameswaran K N, Stenberg P, Tong Y S, Velasco P T, Jonsson N A, Mikiver L, Moses P. Biochemistry. 1979;18:1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen M S, Vorum H, Lindersson E, Jensen P H. J Biol Chem. 2001;276:22680–22684. doi: 10.1074/jbc.M101181200. [DOI] [PubMed] [Google Scholar]
- 44.Wanker E E. Mol Med Today. 2000;6:387–391. doi: 10.1016/s1357-4310(00)01761-5. [DOI] [PubMed] [Google Scholar]
- 45.Dudek S M, Johnson G V. Brain Res. 1994;651:129–133. doi: 10.1016/0006-8993(94)90688-2. [DOI] [PubMed] [Google Scholar]
- 46.Citron B A, SantaCruz K S, Davies P J, Festoff B W. J Biol Chem. 2001;276:3295–3301. doi: 10.1074/jbc.M004776200. [DOI] [PubMed] [Google Scholar]
- 47.Singer S M, Zainelli G M, Norlund M A, Lee J M, Muma N A. Neurochem Int. 2002;40:17–30. doi: 10.1016/s0197-0186(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 48.Karpuj M V, Becher M W, Steinman L. Neurochem Int. 2002;40:31–36. doi: 10.1016/s0197-0186(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 49.Ambrus A, Banyai I, Weiss M S, Hilgenfeld R, Keresztessy Z, Muszbek L, Fesus L. J Biomol Struct Dyn. 2001;19:59–74. doi: 10.1080/07391102.2001.10506720. [DOI] [PubMed] [Google Scholar]
- 50.Mariani P, Carsughi F, Spinozzi F, Romanzetti S, Meier G, Casadio R, Bergamini C M. Biophys J. 2000;78:3240–3251. doi: 10.1016/S0006-3495(00)76860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirsch E C, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. J Neural Transm. 1997;50,Suppl.:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- 52.Cassarino D S, Bennett J P., Jr Brain Res Rev. 1999;29:1–25. doi: 10.1016/s0165-0173(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 53.Damier P, Hirsch E C, Agid Y, Graybiel A M. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 54.Lesort M, Tucholski J, Zhang J, Johnson G V. J Neurochem. 2000;75:1951–1961. doi: 10.1046/j.1471-4159.2000.0751951.x. [DOI] [PubMed] [Google Scholar]
- 55.Parker W D, Jr, Boyson S J, Parks J K. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 56.Schapira A H. Movement Disorders. 1994;9:125–138. doi: 10.1002/mds.870090202. [DOI] [PubMed] [Google Scholar]
- 57.Citron B A, Suo Z, SantaCruz K, Davies P J, Qin F, Festoff B W. Neurochem Int. 2002;40:69–78. doi: 10.1016/s0197-0186(01)00062-6. [DOI] [PubMed] [Google Scholar]
- 58.Groenen P J, Seccia M, Smulders R H, Gravela E, Cheeseman K H, Bloemendal H, de Jong W W. Biochem J. 1993;295:399–404. doi: 10.1042/bj2950399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fesus L. Cell Mol Neurobiol. 1998;18:683–694. doi: 10.1023/A:1020638020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karpuj M V, Becher M W, Springer J E, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 61.Dedeoglu A, Kubilus J K, Jeitner T M, Matson S A, Bogdanov M, Kowall N W, Matson W R, Cooper A J, Ratan R R, Beal M F, et al. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lesort, M., Lee, M., Tucholski, J. & Johnson, G. V. (2002) J. Biol. Chem., in press. [DOI] [PubMed]