Abstract
We show here that cells within human adult bone marrow can contribute to cells in the adult human brain. Cerebellar tissues from female patients with hematologic malignancies, who had received chemotherapy, radiation, and a bone marrow transplant, were analyzed. Brain samples were obtained at autopsy from female patients who received male (sex-mismatched) or female (sex-matched, control) bone marrow transplants. Cerebella were evaluated in 10-μm-thick, formaldehyde-fixed, paraffin-embedded sections that encompassed up to ≈50% of a human Purkinje nucleus. A total of 5,860 Purkinje cells from sex-mismatched females and 3,202 Purkinje cells from sex-matched females were screened for Y chromosomes by epifluorescence. Confocal laser scanning microscopy allowed definitive identification of the sex chromosomes within the morphologically distinct Purkinje cells. In the brains of females who received male bone marrow, four Purkinje neurons were found that contained an X and a Y chromosome and two other Purkinje neurons contained more than a diploid number of sex chromosomes. No Y chromosomes were detected in the brains of sex-matched controls. The total frequency of male bone marrow contribution to female Purkinje cells approximated 0.1%. This study demonstrates that although during human development Purkinje neurons are no longer generated after birth, cells within the bone marrow can contribute to these CNS neurons even in adulthood. The underlying mechanism may be caused either by generation de novo of Purkinje neurons from bone marrow-derived cells or by fusion of marrow-derived cells with existing recipient Purkinje neurons.
Keywords: stem cell‖plasticity‖cell fusion‖cell fate change
In humans, bone marrow has been reported to contribute to human epithelium and liver, but not to the brain (1, 2). Here we investigated whether bone marrow-derived cells could cross the blood–brain barrier and contribute to neurons in the CNS. Previous studies in mice have shown that bone marrow-derived cells can contribute to neuronal cell types in the CNS, including a class of highly specialized neurons in the brain, the Purkinje neurons (3–5).
Purkinje neurons are generated only during early brain development. In humans, generation of Purkinje neurons starts at 16 weeks of gestation and is complete by the end of the 23rd week (6). Most of the maturation of the characteristic dendritic trees of human Purkinje neurons is finalized during the first year of life (7). By contrast to other neurons in the adult brain, there is no evidence for the generation of new Purkinje neurons after birth, even in cases of severe Purkinje cell loss caused by trauma or genetic disease (8, 9).
The human brain contains ≈15 million Purkinje cells, which are among the largest neurons in the CNS (10). A typical Purkinje neuron has >50-fold the volume of neighboring neurons in the brain, and its complex dendritic extensions receive inputs from as many as one million granule cells. Purkinje cells play vital roles in maintaining balance and regulating movement. A loss of Purkinje cells results in deficits in these functions in several disorders: ataxia-telangiectasia, the most common cause of progressive ataxia in infancy; Menkes' Kinky Hair syndrome; the alcoholic cerebellar degenerations, particularly Wernicke–Korsakoff syndrome; and various prion diseases including scrapie, Creutzfeldt–Jakob, and Kuru (11). Thus, renewal or rescue of Purkinje neurons could have significant therapeutic implications.
Here we investigate whether cells from the bone marrow could contribute to Purkinje neurons in human brains later in life. This study was made possible by access to a large repository of postmortem tissues from transplanted individuals, some of whom were females who had received male bone marrow. Even in the highly fixed and extensively digested material obtained from these human autopsy specimens, Purkinje cells could be distinguished because of their unique size, location, and morphology and subsequently analyzed for chromosome composition by using X- and Y-specific DNA probes. The distinctive properties of Purkinje neurons allowed for unambiguous identification of the cells without the requirement of using antibodies against neuron-specific antigens, a procedure precluded by the prior protein digestion needed for in situ hydridization. The results presented here show clearly that male donor-derived bone marrow cells contribute to the human Purkinje neuron population in the brains of the engrafted females.
Methods
Tissue Specimens.
At death, brains were removed and fixed intact in neutral buffered formalin (3.7–4.0% formaldehyde) for 10–14 days. Tissue blocks were then embedded in paraffin. For this study, 10-μm sections were cut from cerebellar tissue of each transplant patient and from untransplanted male and female control brains and mounted onto glass slides. Only half of a Purkinje cell nucleus could be included in these 10-μm sections; thicker sections could not be used because Y chromosomes could not be identified by epifluorescence before in-depth confocal analysis.
In Situ Hybridization.
Paraffin was removed from sections with three changes of xylene (10 min each), rehydrated through graded alcohols (3 min each), and washed twice with double distilled water (ddH2O). Sections were placed in 0.2 M HCl at room temperature for 15 min and rinsed twice in ddH2O and once in Tris-EDTA. Sections were then digested in Proteinase K (3.8 μg/ml Tris-EDTA) at 37°C for 37 min and rinsed twice with ddH2O and once with 2× SSC. Slides were placed in preheated pretreatment solution (sodium isothiocyanate, Vysis, Downers Grove, IL) at 82°C for 37 min followed by three rinses at room temperature with 2× SSC. Sections were digested in protease I (pepsin) 4 mg/ml in protease I buffer (Vysis) for between 10 and 37 min (the time differing depending on fixation of sample), followed by three rinses in 2× SSC. Sections were denatured for 5 min at 73°C in 49 ml of formamide (fresh or frozen aliquots)/7 ml of 20× SSC/14 ml of ddH2O, then dehydrated through a graded series of ethanols. A CEP XY DNA probe (Vysis) was applied to each section, sealed under a glass coverslip, and incubated overnight at 42°C. The Vysis probes detect the alpha satellite sequences in the centromere region of the X chromosome (DXZ1 locus) and the satellite III heterochromatin DNA at the Yq12 region of the Y chromosome (DYZ1 locus) (see Vysis). The next day, coverslips were removed in 2× SSC, rinsed for 2 min in 2× SSC/0.1% Nonidet P-40 at 73°C, allowed to air dry, and mounted in DAPI II mountant (Vysis) to which To-Pro-3 iodide (Molecular Probes) was added at a dilution of 1:3,000.
Microscopy.
Cerebellar sections were viewed at ×63 by using a Zeiss LSM510 laser scanning confocal microscope equipped with epifluorescence. The margin between the granular cell layer and the acellular molecular layer was scanned for Purkinje cell bodies and the presence of a Y chromosome by using epifluorescence. The green Y chromosomes were evident as a lime-green dot in the midst of the yellow-green autofluorescent cytoplasm. A total of 5,860 Purkinje cells from sex-mismatched bone marrow transplants and 3,202 Purkinje cells from sex-matched transplants were counted and assessed for the presence of a Y chromosome (green, visible by epifluorescence), allowing a determination of the overall frequency of Y chromosome-containing cells. As part of the blind study, images of every 20th Purkinje neuron were scanned on the confocal microscope by acquiring 1-μm serial optical sections through the portion of the nucleus present in the section (<50%). The stacks of images were then used for further analysis of the sex chromosome content of Purkinje cells in general and the frequency of zero, one, and two sex-chromosomes within nuclear sections of the size analyzed here. From all of the control and test Purkinje cells serially scanned and reconstructed, a total of 214 nuclei were used to assess the average number of sex chromosomes in randomly sampled Purkinje neurons.
Results
Characterization of Probes for in Situ Hybridization.
Purkinje neurons were selected for this analysis because they can be readily identified based on their unique morphology and location. However, methods for visualizing their chromosome content by in situ hybridization presented major challenges. The extensive formalin fixation (1–2 weeks) 5–12 years ago made it necessary to vigorously digest the tissue with proteases to allow access of the DNA probes while preserving Purkinje morphology. In addition, the digestion protocol had to be optimized for each sample. The thickness of the sections analyzed encompassed less than half of the Purkinje cell nucleus, but was necessary to allow the identification of chromosomes by epifluorescence in thousands of cells before confocal analysis. Thicker sections were attempted but were impractical because they precluded prior screening by epifluorescence and required in-depth confocal imaging of each cell to determine whether a Y chromosome was present.
As shown in Fig. 1, in control male and female cerebellar sections processed for in situ hybridization, human X and Y chromosomes can be readily visualized with the specific, labeled probes. Large, yellow, pear-shaped Purkinje neurons are easily recognized between the cell-sparse molecular layer containing stellate and basket neurons (Fig. 1 Left) and the inner granular layer (Fig. 1 Right), composed primarily of small granule neurons and a few Golgi neurons (Fig. 1 A and D). The characteristically large size of the Purkinje cells and thick dendritic projections that extend into the molecular layer are readily apparent. Nuclei of Purkinje cells, visualized as blue when stained with To-Pro-3, have typical diffuse chromatin and a distinctive large nucleolus, whereas the nuclei of the neurons in the surrounding granular layer have very little cytoplasm, small nuclei with densely packed chromatin and no obvious nucleolus. Thus, these cell types are easily distinguished by histology after in situ hybridization without the need of antibody staining, an assay precluded by the digestion procedure.
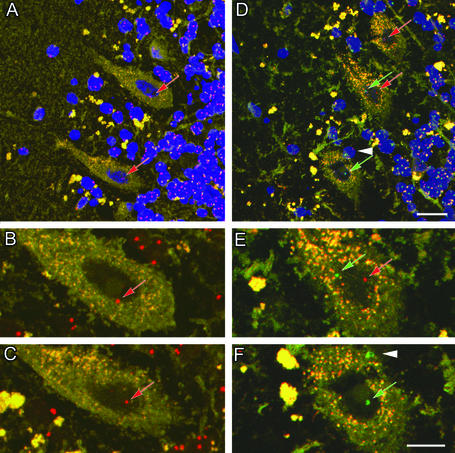
Figure 1.
Controls. Identification of Purkinje neurons and specificity of the human X and Y chromosome DNA probes. Sections from control female and male cerebella were processed with a mixture of the X (red) and Y (green) probes. The nucleus was counterstained with To-Pro-3 (blue) and imaged by using a scanning confocal microscope at 1-μm optical sections. (A–C) Labeling in a female control cerebellum. (A) Two Purkinje neurons are clearly defined with a large nucleus surrounded by a large cytoplasmic region. Each cell has one X chromosome labeled (red arrows). (B and C) Enlargements of the Purkinje neurons in A without the blue nuclear label to facilitate visualization of the chromosome. Note that many of the granular neurons have two X chromosomes in contrast to the large Purkinje cells. (D–F) Male control cerebellum with three Purkinje neurons labeled. Note that one neuron has one X chromosome (red arrow), another has an X and a Y chromosome (red and green arrows), whereas the third has only one Y chromosome (green arrow). (E and F) Enlargements of D without the blue nuclear label. The white arrowhead in D and F shows a Y chromosome from a closely abutting cell. Note that many of the granular neurons contain one X (red) and one Y (green) chromosome. (Scale bars: 20 μm, A and D; 10 μm, B, C, E, and F.)
In situ hybridization revealed that X and Y probes yielded red and green signals that clearly distinguished the two sex chromosomes by confocal microscopy. The Vysis X chromosome probe is conjugated to Spectrum orange that fluoresces at a peak of 588 nm (red), whereas the Y chromosome probe is conjugated to Spectrum green that fluoresces at 524 nm (green). Fortuitously, the autofluorescence in the green and red channels superimposed to yield a yellow color that allowed distinction of the Purkinje cell body cytoplasm. In cerebellar sections from control female brains, Y chromosome labeling was never detected. Fig. 1 shows labeling with both X and Y chromosome probes of sections from controls, a normal female brain (A–C) and a normal male brain (D–F). In Fig. 1A two female Purkinje cells and in Fig. 1D three male Purkinje cells are shown between the cell-sparse molecular layer (left) and the granular layer (right). Enlargements of the Purkinje cells in Fig. 1A are shown in Fig. 1 B and C, and enlargements of Fig. 1D are in Fig. 1 E and F, but without nuclear staining to enhance the visualization of the chromosomes. Note that two sex chromosomes are not always seen in every control Purkinje nucleus because of the thin sections required (Fig. 1 B, C, and F). In contrast, the nuclei of most of the smaller granule neurons exhibit staining of two chromosomes, as the entire nucleus is usually contained in the section. However, in 10-μm sections two or more granule neuron nuclei may be superimposed, giving the impression of more than two sex chromosomes per cell. It was possible to verify that each granule neuron nucleus contained only two sex chromosomes by examining individual serial optical sections within the stack. Occasionally, the X chromosome (Fig. 1B) or the Y chromosome (Fig. 1E) appears to be outside or proximal to the Purkinje nucleus, but this is caused by the projection of stacked serial confocal images. This finding was confirmed by examining individual 1-μm optical sections within the stack that are sufficiently thin to permit precise cellular localization of the chromosome (not shown). On the other hand, in some cases, a chromosome belongs to an abutting cell, which is evident from the cytoplasm separating the two cells (compare Fig. 1 D and F, white arrowhead). In the granular cell layer many cells can be seen with one X and one Y chromosome. Because these cells are small and densely packed with little cytoplasm, it is often difficult to distinguish the borders between adjacent cells, a problem not encountered with Purkinje neurons because of their large size and abundant cytoplasm.
Characterization of Bone Marrow Transplant Recipients and Donors.
Cerebellar tissue samples obtained at autopsy were analyzed from female patients with hematologic malignancies. Initially, chemotherapy was accompanied in most patients by total body irradiation to reduce the malignant cell population and decrease rejection of donor cells. In a few cases marrow cells from male donors were then infused into female patients, whereas most received sex-matched bone marrow. Immunosuppressive agents were given to decrease graft-versus-host reactivity (GVHD; Table 1). The four subjects of the study were selected based on the following criteria: sex (male donor and female recipient), availability of brain tissue, survival for 3–15 months posttransplant, and death unrelated to CNS complications. Five female patients transplanted from female donors were chosen as controls by using the same criteria. Cerebellar tissue sections were cut and coded in Seattle to ensure patient anonymity and “blinded” analysis at Stanford.
Table 1.
Characteristics of transplant recipients and donors
| Recipient number* | Sex of donor | Age of donor | Relation to recipient | Age of recipient | Recipient diagnosis† | Conditioning treatment‡ | Marrow cell dose§ | GVHD prophylaxis¶ | Days survival posttransplant | Cause of death‖ |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex-matched | ||||||||||
| 15 | F | 45.4 | Unrelated | 9.7 | ALL | CY, 1440 | 162.7 | C, M | 16 | GVHD liver failure |
| 21 | F | 47.6 | Sister | 62 | RAEB | BU, CY | 123.4 | C, M | 41 | Organizing pneumonia |
| 46 | F | 54.5 | Sister | 52.7 | RAEB-T | BU, CY, 1200 | 125.1 | C | 23 | Disseminated aspergillosis |
| 54 | F | 31.2 | Sister | 35.1 | CML | BU, CY | 67 | C, M | 89 | Diffuse alveolar damage |
| 98 | F | 4.1 | Child | 36 | RAEB-T | BU, CY, ATG | 92.4 | C, M, STER | 12 | Disseminated Candida krusei |
| Sex-mismatched | ||||||||||
| 28 | M | 40.6 | Brother | 37.2 | AML | CY, 1200 | 286 | C, STER | 398 | |
| Second transplant | M | 41.6 | Brother | 38.2 | AML | BU, CY | 270 | McAb | 24 | Pseudomonas bronchopneumonia |
| 37** | M | 42.8 | Brother | 50 | CML | CY, 1320 | 119 | C, M | 158 | Disseminated Clostridium perfringens |
| 68 | M | 36.8 | Unrelated | 34.6 | CML | CY, 1200 | 101 | C, M | 102 | GVHD and diffuse alveolar damage |
| 71 | M | 26.9 | Unrelated | 24.7 | CML | CY, 1200 | 157.9 | C, M, HUAT | 308 | Pneumocystis carinii pneumonia |
These numbers are a random code assigned for this study only. Patient identity is only known to a single investigator (R.C.H.).
Diagnosis: ALL, acute lymphoblastic leukemia; RAEB, refractive anemia with excess of blasts (T, in transformation); CML, chronic myelogenous leukemia; AML, acute myelogenous leukemiac.
Treatment: CY, cyclophosphamide; BU, busulfan; ATG, anti-thymocyte globulin; number (e.g., 1440) is centigray (cGy) of total body irradiation.
The total marrow cell dose (×108).
GVHD prophylaxis treatment: C, cyclosporine; M, methotrexate; STER, prednisolone; McAb, monoclonal antibody; HUAT, humanized anti-T cell antibody.
No sex-mismatched recipients died of CNS complication or had significant CNS pathology at the time of death.
Recipient 37 may have had increased Y chromosome contribution to Purkinje cells relative to other recipients because of better reconstitution by donor bone marrow or undetected increase in blood–brain barrier permeability.
Evidence for Y Chromosome in Cells of Blood Vessels and Cerebellar Parenchyma.
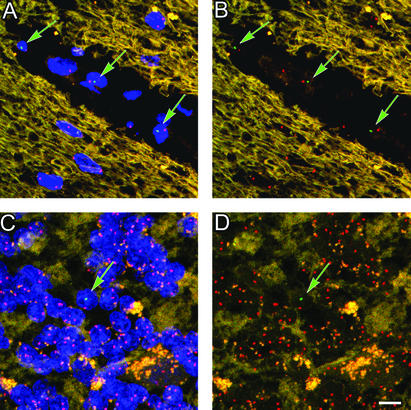
Examination of cells within blood vessels and parenchyma of cerebella underscored the high degree of specificity of the Y chromosome probe. In sections from all of the sex-mismatched transplant patients, Y and X chromosomes were found in numerous cells, presumably blood cells, within the lumina of cerebellar vessels (Fig. 2 A and B). Variation among patients may have resulted from differing degrees of hematopoietic reconstitution that was not determined years ago when these patients died and could no longer be ascertained. In sex-mismatched transplant patients, an occasional male donor-derived cell (Y chromosome in nucleus) was found in the granular cell layer (Fig. 2 C and D), whereas a Y chromosome was never found in the granular cell layer of female patients who received a bone marrow transplant from a female donor. Cells in the parenchyma are likely to be macrophages and microglia that are well known to be derived from bone marrow (12–14). Because of the inability to perform immunohistochemistry on these highly digested tissues, the specific identity of these cells could not be discerned, because unlike Purkinje cells, their morphology was not distinct.
Figure 2.
Male donor-derived cells in the circulation as well as the parenchyma of the cerebellum. (A) Donor-derived male blood cells are present in blood vessels in female recipients (field is representative of 20 examples imaged). (B) Same image as in A with the blue nuclear labeling removed. (C) Occasionally donor-derived cells can be found in the granular and molecular layers of the cerebellum possibly en route to the Purkinje layer (field is representative of >30 images captured). (D) Same image as in C with the blue nuclear labeling removed. (Scale bar: 10 μm.)
Evidence for Y Chromosome in Purkinje Cells of Female Brains.
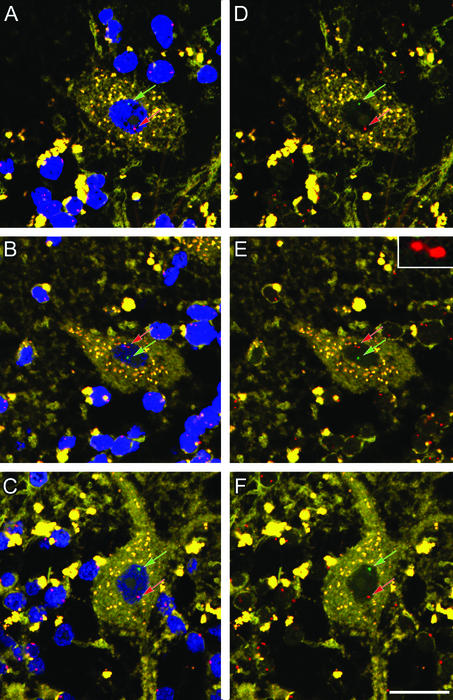
Male chromosomes were readily detected by epifluorescence in the relatively thin sections of Purkinje neurons from female brains. Following along the border of the dendritic layer, each Purkinje cell was examined for the presence of a green-labeled Y chromosome, and those with Y chromosomes were then imaged at high resolution with the confocal scanning laser microscope. Y chromosomes were found in four of the total 5,860 Purkinje nuclei examined by epifluorescence in sex-mismatched transplant patients (Figs. 3 and 4 A and B). No Y chromosomes were found in Purkinje nuclei from sex-matched transplant patients (controls). In rare cases, the X chromosome assumed a dumbbell configuration, as seen in Fig. 3E (see Inset). The distance between the two red spots in 14 different X chromosomes that had dumbbell shapes averaged 1.1 ± 0.3 μm and the greatest distance between two such spots was 1.9 μm. Dumbbells were not caused by radiation and bone marrow transplantation as they are routinely observed in normal cells, as discussed in the Vysis protocol booklet, in which criteria are provided to distinguish a single chromosome with a dumbbell shape from two distinct chromosomes. Analysis of distances between the two red spots allowed distinction of whether such signals derived from one (Fig. 3 B and E) or two (Fig. 4) chromosomes (see below).
Figure 3.
Evidence of male Y chromosome in Purkinje neurons. (A–C) Three examples of male bone marrow-derived nuclei in Purkinje cell. Each neuron has one X (red arrow) and one Y (green arrow) chromosome. These Purkinje neurons appear to be well integrated into the surrounding cerebellum with a mature morphology including dendrites. (D–F) Same images as A–C with the blue nuclear counterstain removed to highlight the red and green probes. The single X chromosome imaged in B and E has a dumbbell shape, a phenomenon observed infrequently. This chromosome is enlarged in the Inset (E) to demonstrate that it is a single chromosome. Note the wisp of red-labeled chromatin connecting the two lobes that are 1.18 μm apart, whereas the Y chromosome is 3.52 μm from the X. (Scale bar: 20 μm.)
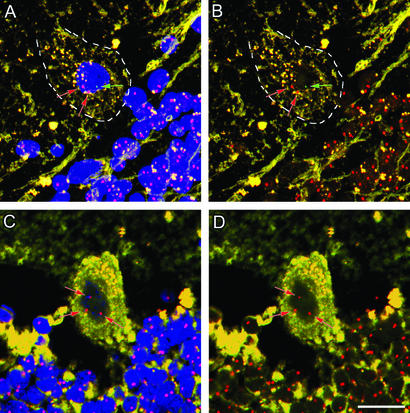
Figure 4.
Evidence for fusion between donor-derived bone marrow cells and host Purkinje neurons. Two examples of triple sex chromosomes in Purkinje neurons. (A) Male to female transplant. There are two X (red) and one Y (green) chromosomes in this cell. (B) Same image as A without the nuclear counterstain. The two X chromosomes are 4.22 μm apart, whereas the Y chromosome is 4.44 μm and 6.08 μm separated from the two X chromosomes. The distance between each chromosome indicates that these are each unique chromosomes. (C) Male to female transplant. There are three distinct X chromosomes (red arrows) in this cell. These chromosomes are 4.16 μm, 6.12 μm, and 4.61 μm apart. (Scale bar: 10 μm.)
In two of the Purkinje cells analyzed, three sex chromosomes were observed within the same Purkinje nucleus (Fig. 4). In one case, a Y chromosome was detected together with two X chromosomes in a serial stack of optical confocal images (Fig. 4A). In another case, one of the randomly scanned Purkinje cells was found to contain three X chromosomes (Fig. 4B). No dumbbells were evident. Indeed, the closest chromosomes in the cells with three chromosomes were >4.0 μm apart. Thus, it is highly unlikely that the probe bound parts of a single chromosome. Notably, the finding of these two cells with more than a diploid sex chromosome composition raised the possibility that the contribution of donor-derived bone marrow cells to the Purkinje neuron population might occur by fusion of these two cell types.
Mechanism: Change in Cell Fate or Cell Fusion?
Although the possibility remains that the Purkinje cells with one X and one Y chromosome arose de novo from cells within the bone marrow, an argument based on sampling can be made in support of cell fusion. Each of the cells in Fig. 3 contain only two sex chromosomes, which might suggest that they resulted directly from a male stem cell present in the bone marrow that changed to become a Purkinje neuron in the brain. On the other hand, because less than half of a Purkinje cell nucleus was encompassed in the sections analyzed, it is quite possible that our analyses did not include all sex chromosomes present in a given Purkinje cell. Nonetheless, whenever a Y chromosome was detected, an X chromosome was also present. To address the possibility that the sex chromosomes were underrepresented in our sample, the probability of observing zero, one, or two chromosomes in a section containing less than half of a Purkinje cell nucleus was determined. A total of 214 randomly scanned cells were selected and analyzed for the number of sex chromosomes they contained by reconstructing a series of optical sections obtained from confocal images. The results revealed the following frequencies of X chromosomes in optical sections: 32% contained zero, 46% contained one, and 21% contained two chromosomes. Thus, in diploid cells only one-fifth of randomly sampled 10-μm sections of Purkinje nuclei exhibited the full complement of sex chromosomes. As a result, the finding of an X and a Y chromosome in the same partial Purkinje cell nucleus may well underestimate the total number of sex chromosomes in that cell. In addition, the low frequency of a diploid chromosome content suggests that detection of three chromosomes would occur in less than one-fifth of all cells analyzed and that the probability of detecting four chromosomes would be exceedingly low. Taken together, this analysis and our data support the hypothesis that cell fusion may have occurred in some cases.
Discussion
The data presented show that adult human bone marrow cells can contribute to mature Purkinje neurons in adult women with hematologic malignancies. Even though this is not a frequent event (0.1% of the cells examined), it is surprising that it occurs at all because the generation or repair of these cells after birth had not been documented (2, 6–9). Because there are 15 million Purkinje cells in the human adult brain, by extrapolation, the total number of cells affected by a bone marrow transplant could be quite substantial.
Although the frequencies of bone marrow contribution in the two other human studies reported were higher, it should be noted that the nature of the tissues examined was quite different (1, 2). Most notably, cells in the liver and epithelium are known to have high regenerative capabilities in response to damage. Such actively dividing cells are particularly susceptible to the effects of radiation, creating a potential selective pressure for renewal by other cell types. By contrast, Purkinje neurons do not divide and are not known to be able to regenerate lost cells by division (6, 7). Thus the highly regenerative milieu that may be conducive to the incorporation of bone marrow-derived cells in these other two tissues is lacking in the cerebellum. Because contribution of bone marrow to CNS neurons can occur, even in humans, an understanding of how this occurs would not only be of fundamental interest, but also potentially important for enhancing the frequency of this event.
It is intriguing to hypothesize that the bone marrow-derived cells that are now being detected in diverse tissues may constitute a systemic back-up for repair or regeneration of injured cells when the tissue itself cannot meet the need. Two mechanisms can be envisioned. Cells from bone marrow could fuse with damaged cells, providing them with an undamaged nucleus. Alternatively, donor cells could undergo a cell fate change and replenish damaged tissues by changing their phenotype to that typical of the tissue region to which they migrated.
One might imagine that for elaborate, large, highly specialized cells such as Purkinje neurons that are known to develop early in life and establish millions of connections, it might be particularly difficult to recreate such structures and interactions in the complex environment of an adult brain. In the case of this cell type, fusion with a blood cell might “rescue” the Purkinje cell and prevent it from destruction, serving as a means of salvaging damaged cells.
Fusion has been proposed as a mechanism of bone marrow contribution to tissues (15). Recent reports showed that under strong selective pressure in tissue culture, embryonic stem cells fused with either bone marrow cells or neural stem cells (16, 17). Despite early studies that suggested they were tetraploid (18), Purkinje cells have now clearly been shown to be diploid (19–21). Consequently, analyses of sex chromosome composition could determine not only whether bone marrow contributed to Purkinje cells, but also provide insight into the underlying mechanism. For example, an XY karyotype might suggest a cell fate change, whereas more than two sex chromosomes per cell would implicate fusion of bone marrow-derived cells with a preexisting neuron. That cell fusion gave rise to the observed results is supported by the finding of three sex chromosomes in two of the Purkinje cells sampled (Fig. 4). Previous reports showed that after transplantation of GFP-labeled bone marrow cells into mice, some Purkinje cells in the rodent brain were green (3); however, chromosome contribution was not analyzed. Here we report that not only is it clear that male chromosomes derived from bone marrow are detectable in female Purkinje cells in human brains, but the frequency with which two sex chromosomes was observed (Fig. 3), as well as the finding of more than two sex chromosomes per Purkinje cell (Fig. 4), suggest that the chromosome composition may well be caused by fusion of the bone marrow-derived cells with these particular neurons in the brain.
However, the possibility that in response to damage induced by radiation and chemotherapy, de novo Purkinje cell formation might occur also remains. De novo formation of differentiated cells from bone marrow already has been shown in highly regenerative tissues such as epithelium, liver, and muscle (1, 2, 22). Until recently, damaged CNS neurons in adults were not thought to be replaced, but recent evidence suggests that neural stem cells within the brain can achieve this function. Even hippocampal neurons that have complex architecture have been shown to arise from neural stem cells (23). Thus, different cell types may enlist bone marrow-derived cells for repair or for regeneration by different mechanisms, by fusion, or by a cell fate change, respectively. Regardless of the mechanism, this retrospective analysis of existing tissues suggests a biological role for bone marrow-derived cells in the brain, not only in mice but also in humans.
Acknowledgments
This research was supported by National Institutes of Health grants (to R.C.H. and J.M.W.); the Life and Health Insurance Medical Research Fund and a National Institutes of Health predoctoral training grant (to T.R.B.); and National Institutes of Health Grants AG09521, AG20961, HD18179, and HL65572, the Ellison Medical Foundation, and the McKnight Endowment Fund for Neurosciences (to H.M.B.).
Abbreviation
- GVHD
graft-versus-host reactivity
References
- 1.Korbling M, Katz R L, Khanna A, Ruifrok A C, Rondon G, Albitar M, Champlin R E, Estrov Z. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Nat Med. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 3.Priller J, Persons D A, Klett F F, Kempermann G, Kreutzberg G W, Dirnagl U. J Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mezey E, Chandross K J, Harta G, Maki R A, McKercher S R. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 5.Brazelton T R, Rossi F M, Keshet G I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 6.Miyata M, Miyata H, Mikoshiba K, Ohama E. Acta Neuropathol. 1999;98:226–232. doi: 10.1007/s004010051073. [DOI] [PubMed] [Google Scholar]
- 7.Zecevic N, Rakic P. J Comp Neurol. 1976;167:27–47. doi: 10.1002/cne.901670103. [DOI] [PubMed] [Google Scholar]
- 8.Gatti R A. Clin Immunol Immunopathol. 1991;61:S10–S15. doi: 10.1016/s0090-1229(05)80032-7. [DOI] [PubMed] [Google Scholar]
- 9.Okeda R, Gei S, Chen I, Okaniwa M, Shinomiya M, Matsubara O. Acta Neuropathol. 1991;81:450–457. doi: 10.1007/BF00293467. [DOI] [PubMed] [Google Scholar]
- 10.Palay S L, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin: Springer; 1974. [Google Scholar]
- 11.Graham D I, Lantos P L, editors. Greenfield's Neuropathology. 6th ed. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 12.Hickey W F, Kimura H. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy D W, Abkowitz J L. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 14.Krivit W, Sung J H, Shapiro E G, Lockman L A. Cell Transplant. 1995;4:385–392. doi: 10.1177/096368979500400409. [DOI] [PubMed] [Google Scholar]
- 15.Blau H M. Nature. 2002;419:437. doi: 10.1038/419437a. [DOI] [PubMed] [Google Scholar]
- 16.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz D M, Nakano Y, Meyer E M, Morel L, Petersen B E, Scott E W. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 17.Ying Q L, Nichols J, Evans E P, Smith A G. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 18.Lapham L W. Science. 1968;159:310–312. doi: 10.1126/science.159.3812.310. [DOI] [PubMed] [Google Scholar]
- 19.Mann D M, Yates P O, Barton C M. J Comp Neurol. 1978;180:345–347. doi: 10.1002/cne.901800210. [DOI] [PubMed] [Google Scholar]
- 20.Manuelidis L, Manuelidis E E. Exp Neurol. 1974;43:192–206. doi: 10.1016/0014-4886(74)90140-x. [DOI] [PubMed] [Google Scholar]
- 21.Mares V, Lodin Z, Sacha J. Brain Res. 1973;53:273–289. doi: 10.1016/0006-8993(73)90214-x. [DOI] [PubMed] [Google Scholar]
- 22.LaBarge M A, Blau H M. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Gast D, Gage F H. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]