Abstract
The water channel AQP4 is concentrated in perivascular and subpial membrane domains of brain astrocytes. These membranes form the interface between the neuropil and extracerebral liquid spaces. AQP4 is anchored at these membranes by its carboxyl terminus to α-syntrophin, an adapter protein associated with dystrophin. To test functions of the perivascular AQP4 pool, we studied mice homozygous for targeted disruption of the gene encoding α-syntrophin (α-Syn−/−). These animals show a marked loss of AQP4 from perivascular and subpial membranes but no decrease in other membrane domains, as judged by quantitative immunogold electron microscopy. In the basal state, perivascular and subpial astroglial end-feet were swollen in brains of α-Syn−/− mice compared to WT mice, suggesting reduced clearance of water generated by brain metabolism. When stressed by transient cerebral ischemia, brain edema was attenuated in α-Syn−/− mice, indicative of reduced water influx. Surprisingly, AQP4 was strongly reduced but α-syntrophin was retained in perivascular astroglial end-feet in WT mice examined 23 h after transient cerebral ischemia. Thus α-syntrophin-dependent anchoring of AQP4 is sensitive to ischemia, and loss of AQP4 from this site may retard the dissipation of postischemic brain edema. These studies identify a specific, syntrophin-dependent AQP4 pool that is expressed at distinct membrane domains and which mediates bidirectional transport of water across the brain–blood interface. The anchoring of AQP4 to α-syntrophin may be a target for treatment of brain edema, but therapeutic manipulations of AQP4 must consider the bidirectional water flux through this molecule.
Cerebral edema is essentially a loss of water homeostasis entailing a net increase of water flux into the brain. The route of water influx in this life-threatening condition is unknown, and no efficient therapy exists. We have previously shown that the brain expresses a water channel molecule, AQP4, that is strongly enriched in those astrocyte membrane domains forming the interface between brain neuropil and extracerebral spaces filled with blood or cerebrospinal fluid (1–3). To determine whether the pools of AQP4 in these specialized membrane domains are responsible for the fast influx of water that occurs during the development of brain edema, one must specifically eliminate the perivascular and subpial pools of AQP4 while leaving other pools of AQP4 intact. This can be achieved by deletion of α-syntrophin (α-syn), an adapter protein in the dystrophin-associated protein complex that is required for anchoring AQP4 at these specialized membrane domains (4). Mice homozygous for targeted disruption of the gene encoding α-syntrophin (α-Syn−/−) exhibit a marked reduction of AQP4 in perivascular and subpial membranes but not in other locations in brain, because total brain AQP4 protein content is not reduced (4).
The first aim of the present study was to use α-Syn−/−mice to investigate whether a selective depletion of the perivascular AQP4 pool reduces the volume of postischemic brain edema. This would confirm that AQP4 is involved in water homeostasis (5) and would identify the specific AQP4 pool that admits entry of water during formation of brain edema. Furthermore, this would reveal whether the primary control of water exchange across the blood–brain barrier occurs in membranes of astroglial cells rather than endothelial cells.
If transport of water through AQP4 is bidirectional, lack of AQP4 at the brain–blood interface should hinder the efflux of water generated constitutively from brain energy metabolism. Thus, a second aim of this study was to explore whether α-Syn−/− mice exhibit swelling of perivascular end-feet when compared to their WT counterparts. An increase in the volume of end-feet would indicate an impeded efflux of water due to AQP4 deficiency in perivascular membranes.
A major problem in the treatment of stroke patients is the persistence of the postischemic brain edema. A third aim of this study was to test the hypothesis that slow dissipation of brain edema could result from ischemia-dependent reduction of AQP4 in perivascular end-feet in brains of WT mice. This hypothesis was pursued by high resolution immunogold analysis by using AQP4-selective Abs.
Materials and Methods
Animals.
Experimental protocols were approved by the Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines for the care and use of animals. Studies were conducted with male mice homozygous for targeted disruption of the gene encoding α-syntrophin (α-Syn−/−). WT C57B/6 mice were used as controls. α-Syn−/− mice were bred on a C57B/6 background to avoid effects of differing genetic strains (6).
Brain Ischemia.
Ten α-Syn−/− mice and 10 WT mice with body weights of 27–32 g were anesthetized with 1–1.2% halothane in oxygen-enriched air, and rectal temperatures were controlled at 37 ± 0.5°C with heating lamps during the entire surgical procedure. Mice were then subjected to unilateral middle cerebral artery occlusion in a randomized fashion (7–9). In brief, a 5-0 nylon monofilament was inserted via an external carotid artery stump to a point 6-mm distal to the internal carotid artery/pterygopalatine bifurcation, and the common carotid was temporarily occluded with a 6-0 nylon suture. Middle cerebral artery occlusion was verified at 30 min by allowing the animal to awaken and a neurological deficit scoring was performed: 0 = normal motor function; 1 = flexion of torso and of contralateral forelimb upon lifting the animal by the tail; 2 = circling to the contralateral side but normal posture at rest; 3 = leaning to the contralateral side at rest; 4 = no spontaneous motor activity. Mice with clear neurological deficits (neurological deficit score ≥2) were reanesthetized for withdrawal of the suture and reperfusion after 90 min of artery occlusion. After 23 h of reperfusion, brains were harvested for assessment of injury volume. Forebrains were sliced into five 2-mm thick coronal sections and stained with 1% triphenyltetrazolium chloride, and total infarction volume measured by digital imaging (Nikon DXM video camera) and image analysis software (sigmascan pro, Jandel, San Rafael, CA). Infarction volume was integrated across sections and over the entire ipsilateral hemisphere. Cerebral edema was analyzed by measuring the percentage of hemispheric enlargement (10) [(ischemic hemisphere area − nonischemic hemisphere area)/nonischemic hemisphere area × 100%].
A separate cohort of five α-Syn−/− mice and five WT mice were subjected to the middle cerebral artery occlusion–reperfusion protocol, followed by intracardiac perfusion with 4% paraformaldehyde in phosphate buffer containing 0.2% picric acid at pH 6.0, then pH 10.0. Brains were cut into 0.5- to 1.0-mm slices, cryoprotected, quick-frozen in liquid propane (−170°C), and subjected to freeze substitution (4, 11). Specimens were then embedded in methacrylate resin (Lowicryl HM20) and polymerized by UV light at <0°C. Ultrathin sections were incubated with Abs to AQP4 (Alpha Diagnostic, San Antonio, TX) or α-syntrophin (6), then with goat anti-rabbit IgG Ab coupled to colloidal gold, and examined with a Philips CM 10 electron microscope at 60 kV (4).
Quantification and Statistical Analysis.
Infarction volume and physiological data between groups were analyzed by two-way ANOVA with Fisher's post hoc test. For the quantification of AQP4 immunogold labeling and end-feet swelling, digital images of sections from four α-Syn−/− mice (120 images) and two WT mice (65 images) were acquired and quantified with a commercial image analysis program (Soft Imaging Systems, Münster, Germany) (12). Non-end-feet labeling density was measured as labeling per unit area excluding all perivascular processes. Swelling of end-feet was calculated by dividing area by length of the membrane facing the basal lamina. Data are presented as mean ± SEM.
Results
Immunoelectron Microscopy of Astroglial End-Feet.
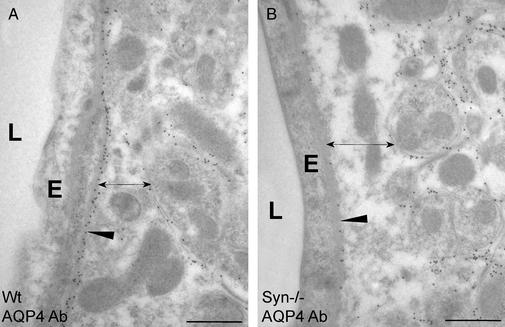
A major alteration in the expression of AQP4 protein was recently reported in brains of α-Syn−/− mice (4). We have confirmed these findings by high-resolution immunogold electron microscopy of brain tissues obtained from mice killed without manipulations. Abs specific for AQP4 produced strong immunolabeling of perivascular astroglial end-foot membranes in brain tissues from WT mice with less immunolabeling of other membranes (Fig. 1A). This contrasts markedly with brain tissues from α-Syn−/− mice that yielded reduced immunolabeling of perivascular astroglial end-foot membranes (Fig. 1B). The reduction in AQP4 immunogold signal occurred specifically in the end-foot membrane domain facing the endothelial basal lamina; other parts of the astrocyte plasma membrane, including the end-foot membrane domain facing the neuropil, showed no loss of AQP4 but rather an increase (Fig. 1B).
Figure 1.
Subcellular localization of AQP4 in perivascular astroglial cells in cerebellum under basal conditions. WT (A) and α-Syn−/− (B) mice were killed without other manipulations. Ultrathin sections of brain were incubated with anti-AQP4 and immunogold particles and analyzed by electron microscopy (see Materials and Methods). AQP4 in perivascular end-feet is reduced (arrowhead) and astroglial end-feet width is increased (double-headed arrow) in α-Syn−/−mice. E, capillary endothelial cell; L, lumen. (Bars = 0.5 μm.)
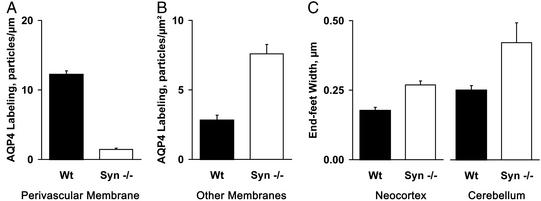
Quantification of the AQP4 immunolabeling demonstrated that the density of gold particles overlying perivascular astroglial end-foot membranes is >8-fold higher in neocortex from WT mice than from α-Syn−/− mice (Fig. 2A). Conversely, the density of gold particles overlying non-end-foot membranes in astroglia is almost 2.7 times higher in neocortex from α-Syn−/− mice than from WT mice (Fig. 2B). Targeted disruption of the gene encoding α-syntrophin did not affect AQP4 expression in ependymal cells (which retained an enrichment of AQP4 in basolateral membranes) or in astroglia surrounding osmosensory neurons (which showed a nonpolarized expression of AQP4 similar to WT mice). Some nontelencephalic brain regions appear to contain a perivascular AQP4 pool that is only partly dependent on α-syntrophin, pointing to regional differences in AQP4 anchoring (M.A.-M., unpublished data).
Figure 2.
Quantification of AQP4 immunogold labeling and width of perivascular astroglial end-feet under basal conditions. α-Syn−/− (n = 4) and WT (n = 2) mice were killed without other manipulations, and brain sections were prepared for immunogold electron microscopy and quantifications (see Materials and Methods). (A) AQP4 immunogold labeling density of perivascular astroglial end-feet in neocortex. Labeling density represents the number of gold particles divided by the length of the perivascular end-feet membranes (P < 0.01). (B) AQP4 immunogold labeling density of non-end-feet membranes in neocortex. Labeling density represents the number of gold particles divided by the area (P < 0.05). (C) Width of perivascular astroglial end-feet measured in neocortex or cerebellum (P < 0.01). The increase in volume is proportional to the difference in width of end-feet.
As AQP4 is the only water channel known to be expressed in perivascular astroglial end-feet, we hypothesized that lack of AQP4 may impede water flux between neuropil and blood. Light microscopy revealed no overt changes in astroglial cell volume (not shown), and examination of electron micrographs revealed no changes in volumes of endothelial cells, mitochondria, or organelles (Fig. 1). A selective increase in the volume of perivascular astroglial end-feet is apparent when cerebellum from α-Syn−/− mice is compared to WT mice by electron microscopy (Fig. 1 A and B, double-headed arrows). Morphometric analyses confirmed that end-feet in neocortex and cerebellum of α-Syn−/− mice are 1.5 and 1.7 times wider than in WT mice (Fig. 2C). Thus, AQP4 appears to facilitate the release of water from astroglia into brain capillaries during basal conditions, because swelling of perivascular astroglial end-feet reflects the absence of AQP4 from this site. These observations indicate that resistance to water crossing the brain–blood interface occurs at the level of astroglial end-foot membranes rather than in capillary endothelia.
Occlusion of Middle Cerebral Artery Followed by Reperfusion.
During brain ischemia, cells within the zone of infarction die from anoxia, whereas cells in surrounding tissues may succumb because of edema or oxidant injuries. We used a standard protocol in which the middle cerebral artery was occluded for 90 min while the mice were under anesthesia. On awakening, the neurological defect scores were determined, but α-Syn−/− mice and WT mice showed no differences (not shown). The occlusion was then removed and the mice were killed after 23 h of reperfusion.
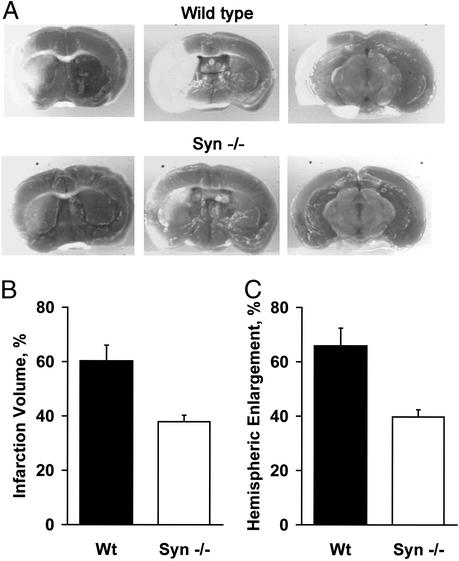
Serial brain sections were treated with triphenyltetrazolium to stain mitochondria in noninfarcted tissue (Fig. 3A). The volumes of brain infarctions in α-Syn−/− mice, 38 ± 2%, were consistently smaller than the corresponding infarctions in WT mice, 60 ± 6% (Fig. 3B). Brain edema, estimated by measuring hemispheric enlargement, was also consistently lower in the α-Syn−/− mice, 40%, compared to the WT counterparts, 66% (Fig. 3C).
Figure 3.
Difference in brain injuries sustained by α-Syn−/− and WT mice after ischemia reperfusion. After 90 min of middle cerebral artery occlusion and 23 h of reperfusion, α-Syn−/− (n = 8) and WT (n = 9) mice were killed, and the brains were sectioned. Slices were incubated with triphenyltetrazolium chloride, and infarction volume and hemispheric enlargement were determined (see Materials and Methods). (A) Corresponding serial brain sections reveal smaller infarct zone (unstained) in slices from α-Syn−/− mice compared to WT mice. (B) Quantification of infarction volumes determined by image analysis. (C) Determination of hemispheric enlargement. Brains of α-Syn−/− mice were partially protected (P < 0.05 for both parameters).
Immunoelectron Microscopy of Astroglial End-Feet After Ischemic Injury.
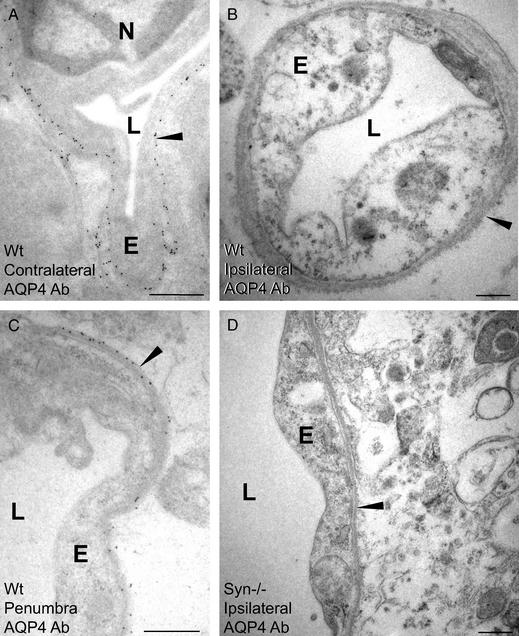
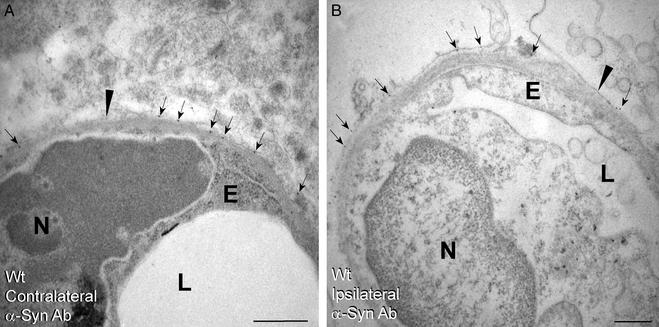
Brain sections from WT mice were analyzed for possible alterations in the pattern of AQP4 after the middle cerebral artery occlusion–reperfusion protocol. Sections of the contralateral hemisphere showed that the AQP4 distribution pattern was unchanged from the basal state (Fig. 4A). Surprisingly, sections of the ipsilateral hemisphere showed that AQP4 immunolabeling was nearly abolished in the ischemic core (Fig. 4B) and was reduced in the penumbra (Fig. 4C). Although AQP4 immunolabeling was reduced in all membrane domains, the reductions were most pronounced in the perivascular membranes. In contrast, incubation of these sections with Abs to α-syntrophin showed no alteration in the immunolabeling pattern in contralateral or ipsilateral hemispheres (Fig. 5). Thus, coupling of AQP4 to α-syntrophin in astroglial end-feet is apparently sensitive to ischemia and reperfusion. In addition to amelioration of infarct volume and hemispheric enlargement, brain sections from α-Syn−/− mice showed better preservation of ultrastructure and maintenance of endothelial cell volume (Fig. 4D). Corresponding sections from WT mice revealed severe perivascular edema and perturbation of cellular integrity, particularly in the ischemic core (Fig. 4B).
Figure 4.
Immunogold labeling of AQP4 in brain neocortex after ischemia-reperfusion. WT mice (A–C) and α-Syn−/− mice (D) were subjected to middle cerebral artery occlusion and reperfusion before intracardiac perfusion fixation and immunogold labeling of AQP4 for electron microscopy (see Materials and Methods). Note that AQP4 immunolabeling in WT brain is spared in perivascular membranes (arrowheads) of contralateral hemisphere (A), totally absent from the ipsilateral infarct core (B), and partially reduced in penumbra (C). Ultrastructural derangement including endothelial swelling is much more pronounced in ischemic core from brain of WT mice (B) than in brain of α-Syn−/− mice (D). (Bars = 0.5 μm.) N, nucleus; E, endothelial cell; L, lumen.
Figure 5.
Immunogold labeling of α-syntrophin in neocortex from WT mice after ischemia reperfusion. Perivascular membranes (arrowheads) from contralateral hemisphere (A) and ipsilateral hemisphere (B) infarct core showed equivalent α-syntrophin immunolabeling (arrows). (Bars = 0.5 μm.) N, nucleus; E, endothelial cell; L, lumen.
Discussion
The interface between neuropil and the vascular space through which water exchange occurs is composed of endothelia, basal lamina, and the perivascular end-feet of astrocytes. These end-feet express high concentrations of the water channel AQP4 (2, 12), which is densely packed in microcrystalline square arrays (3). We recently found that mice homozygous for targeted disruption of the gene encoding α-syntrophin show a marked loss of AQP4 in perivascular and subpial end-feet (4), whereas other astroglial AQP4 pools are maintained. Here we have quantified the reduced expression of AQP4 in perivascular astroglial end-feet in brains of α-Syn−/− mice and examined the effect of α-syntrophin ablation on brain water homeostasis.
We hypothesized that the specific population of AQP4 in perivascular astroglial end-feet is the major pathway for brain water influx and efflux. Accordingly, disruption of the gene encoding α-syntrophin was expected to slow the development of postischemic brain edema. Our quantitative assessment of edema volume confirmed this and also showed a reduction in infarct volume in brains of α-Syn−/− animals.
Previous studies have implicated AQP4 as an important component in the pathogenesis of brain edema (13–18). Recent studies of mice homozygous for targeted gene disruptions have reported partial protection against acute brain edema; however, these transgenic models are complex, because the mice have global protein deficiencies (19, 20). Interpretation of the study of dystrophin-null mice (mdx-βgeo) is potentially confounded, because the animals lack all splice forms of dystrophin, the major component of the dystrophin-associated protein complex (21). Interpretation of the study of Aqp4−/− mice (5) is also difficult, because AQP4 is missing from all sites in brain, airways, and kidney. Unfortunately, the Aqp4−/− mice are not available to us for follow-up studies.
The α-Syn−/− mice used in this study have a relatively subtle structural defect; α-syntrophin is missing, but the other four syntrophin isoforms are still expressed (6). Moreover, the basic structure of the dystrophin-associated protein complex remains intact in α-Syn−/− mice, and only the population of AQP4 in perivascular astroglial end-feet is mislocalized (4). Our study revealed partial protection of acute brain edema, similar to that reported for Aqp4−/− (5) and dystrophin-null mice (21). Thus, the population of AQP4 in perivascular astroglial end-feet is the likely pathway for rapid water influx during the onset of acute brain edema.
Water flux through AQP4 is a passive process driven by osmotic gradients (1, 22). Just as perivascular AQP4 provides a likely route of water influx during onset of edema, this population of AQP4 would be expected to mediate water efflux during the dissipation of brain edema. Surprisingly, WT mice were found to have a nearly complete loss of perivascular AQP4 pool after ischemia reperfusion. This loss was not due to a general breakdown of membrane integrity, because the perivascular population of α-syntrophin was unchanged. Rather, it seems that the link between α-syntrophin and AQP4 is severed after an ischemic insult, probably reflecting the fact that this link is subject to regulation by other molecules (4). Down-regulation of AQP4 may protect against water influx during the onset of acute brain edema but may also prolong the edematous state by interfering with brain water efflux during the resolution phase.
Both WT and α-Syn−/− mice show marked reductions of perivascular AQP4 at the time of peak edema. Thus, our hypothesis that the perivascular pool of AQP4 mediates water efflux as well as influx could not be tested by comparing the rate of edema resolution. Instead, we asked the simple question whether the α-Syn−/− mice showed overt changes in cell volume that could be interpreted as being secondary to loss of the normal route for constitutive water efflux. This water efflux is substantial because oxidation of one molecule of glucose to CO2 releases six molecules of water, but when linked to ATP synthesis via the tricarboxylic acid cycle, 36 additional molecules of water are released (23). Even more importantly, the uptake of metabolic substrates such as glucose and lactate may be coupled to water influx. We reasoned that if the perivascular AQP4 population mediates efflux of metabolically generated water, elimination of the population of AQP4 at this site would cause the perivascular end-feet to swell. This prediction was borne out by our quantitative analyses showing that basal state brains of α-Syn−/− mice had an increase in the volume of perivascular end-feet without changes in other cell compartments.
Although AQP4 is the only water channel known to be expressed in perivascular end-feet, the possibility must be considered that altered brain water homeostasis in α-Syn−/− mice is due to disrupted localization of proteins other than AQP4. Perivascular astroglial end-feet contain multiple transport proteins including transporters of monocarboxylates, glucose, and glutamate, all of which are known to cotransport water (24). Potential mislocalization of these cotransport proteins was evaluated in brains of α-Syn−/− mice, but no abnormalities were observed (M.A.-M., unpublished data).
After completion of our studies, a very interesting study reported that a peptide corresponding to the C terminus of N-methyl-d-aspartate receptor disrupts the association with postsynaptic density protein PSD-95 resulting in reduced brain injury after ischemia reperfusion (25). This raises the question of whether neuronal NO synthase and AQP4 are mislocalized in both studies. Preliminary studies have indicated that neuronal NO synthase is not mislocalized in brains of α-Syn−/− mice (M.A.-M., unpublished data). Nevertheless, it remains possible that the explanation for the notable improvement of brain edema after ischemia reperfusion may involve mislocalization of several proteins. Additional studies will be required to explore this possibility.
Our studies indicate that the population of AQP4 in perivascular astroglial end-feet plays a key role in exchange of water between the brain and vascular space during normal physiology, as predicted by Frigeri et al. (26), as well as during brain ischemia. Thus, water influx and efflux are controlled at a site distinct from the blood–brain barrier, known to reside at the level of the endothelial cells. Identification of the molecular and anatomic basis of brain water homeostasis may serve as a platform for development of new strategies for the treatment of brain edema. The bidirectionality of water flux through AQP4 must be taken into account if this therapeutic approach is to be successful.
Acknowledgments
We thank Søren Nielsen, John W. Griffin, and Valina L. Dawson for critical readings of the manuscript and David Kozono for his valuable assistance with graphics. This work was supported by grants from the National Institutes of Health, the Human Frontier Science Program, the Norwegian Research Council, and COST (European Cooperation in the Field of Scientific and Technical Research). A.B. is an Established Investigator Awardee of the American Heart Association.
References
- 1.Jung J S, Bhat R V, Preston G M, Guggino W B, Baraban J M, Agre P. Proc Natl Acad Sci USA. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S, Nagelhus E A, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen O P. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rash J E, Yasumura T, Hudson C S, Agre P, Nielsen S. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neely J D, Amiry-Moghaddam M, Ottersen O P, Froehner S C, Agre P, Adams M E. Proc Natl Acad Sci USA. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manley G T, Fujimura M, Ma T, Noshita N, Filiz F, Bollen A W, Chan P, Verkman A S. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 6.Adams M E, Kramarcy N, Krall S P, Rossi S G, Rotundo R L, Sealock R, Froehner S C. J Cell Biol. 2000;150:1385–1397. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto S, Xue R, Sugo N, Sawada M, Blizzard K K, Poitras M F, Johns D C, Dawson T M, Dawson V L, Crain B J, et al. Stroke (Dallas) 2002;33:1101–1106. doi: 10.1161/01.str.0000014203.65693.1e. [DOI] [PubMed] [Google Scholar]
- 8.Eliasson M J L, Sampei K, Mandir A S, Hurn P D, Traystman R J, Bao J, Pieper A, Wang Z Q, Dawson T M, Snyder S H, et al. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 9.Goyagi T, Goto S, Bhardwaj A, Dawson V L, Hurn P D, Kirsch J R. Stroke (Dallas) 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Reaume A G, Huang T T, Carlson E, Murakami K, Chen S F, Hoffman E K, Scott R W, Epstein C J, Chan P H. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen O P. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 12.Nagelhus E A, Veruki M L, Torp R, Haug F M, Laake J H, Nielsen S, Agre P, Ottersen O P. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, et al. Brain Res Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 14.Vajda Z, Promeneur D, Doczi T, Sulyok E, Frokiaer J, Ottersen O P, Nielsen S. Biochem Biophys Res Commun. 2000;270:495–503. doi: 10.1006/bbrc.2000.2472. [DOI] [PubMed] [Google Scholar]
- 15.Ke C, Poon W S, Ng H K, Pang J C, Chan Y. Neurosci Lett. 2001;301:21–24. doi: 10.1016/s0304-3940(01)01589-0. [DOI] [PubMed] [Google Scholar]
- 16.Vizuete M L, Venero J L, Vargas C, Ilundain A A, Echevarria M, Machado A, Cano J. Neurobiol Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- 17.Kiening K L, van Landeghem F K H, Schreiber S, Thomale U W, von Deimling A, Unterberg A W, Stover J F. Neurosci Lett. 2002;324:105–108. doi: 10.1016/s0304-3940(02)00180-5. [DOI] [PubMed] [Google Scholar]
- 18.Saadoun S, Papadopoulos M C, Davies D C, Krishna S, Bell B A. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma T H, Yang B X, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wertz K, Fuchtbauer E M. Dev Dyn. 1998;212:229–241. doi: 10.1002/(SICI)1097-0177(199806)212:2<229::AID-AJA7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Vajda Z, Pedersen M, Fuchtbauer E M, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely J D, Agre P, Frokiaer J, et al. Proc Natl Acad Sci USA. 2002;99:13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa H, Ma T H, Skach W, Matthay M A, Verkman A S. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 23.Lehninger A L. Biochemistry, the Molecular Base of Cell Structure and Function. New York: Worth; 1970. p. 386. [Google Scholar]
- 24.Loo D D F, Wright E M, Zeuthen T. J Physiol (London) 2002;542:53–60. doi: 10.1113/jphysiol.2002.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd J W, Wang Y-T, Salter M W, Tymianski M. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 26.Frigeri A, Nicchia G P, Nico B, Quondamatteo F, Herken R, Roncali L, Svelto M. FASEB J. 2001;15:90–98. doi: 10.1096/fj.00-0260com. [DOI] [PubMed] [Google Scholar]