Abstract
Several experimental and clinical studies have shown that oxidized low-density lipoprotein and oxidation-sensitive mechanisms are central in the pathogenesis of vascular dysfunction and atherogenesis. Here, we have used p66Shc−/− and WT mice to investigate the effects of high-fat diet on both systemic and tissue oxidative stress and the development of early vascular lesions. To date, the p66Shc−/− mouse is the unique genetic model of increased resistance to oxidative stress and prolonged life span in mammals. Computer-assisted image analysis revealed that chronic 21% high-fat treatment increased the aortic cumulative early lesion area by ≈21% in WT mice and only by 3% in p66Shc−/− mice. Early lesions from p66Shc−/− mice had less content of macrophage-derived foam cells and apoptotic vascular cells, in comparison to the WT. Furthermore, in p66Shc−/− mice, but not WT mice, we found a significant reduction of systemic and tissue oxidative stress (assessed by isoprostanes, plasma low-density lipoprotein oxidizability, and the formation of arterial oxidation-specific epitopes). These results support the concept that p66Shc−/− may play a pivotal role in controlling systemic oxidative stress and vascular diseases. Therefore, p66Shc might represent a molecular target for therapies against vascular diseases.
Keywords: atherosclerosis‖oxygen radicals‖transgenic mouse
The p66Shc protein is one of the three isoforms encoded by the mammalian Shc locus. The Shc overlapping proteins (p66Shc, p52Shc, and p46Shc) share a C-terminal Src homology 2 (SH2) domain, a central collagen-homologous (CH) region, and an N-terminal phosphotyrosine binding domain and differ for N termini of different lengths. P66Shc, in particular, is characterized by an additional CH region at the N terminus. Shc proteins are cytoplasmic substrates of activated tyrosine kinases and have been implicated in the transmission of activation signals from tyrosine kinases to Ras proteins (1–3). In 1999, it became clear that Shc proteins might serve broad cellular functions. Homozygous mutation of p66Shc in mice was shown to induce increased resistance to oxidative stress and lifespan extension (4). Recently, it has been demonstrated that the p66Shc longevity gene increases intracellular reactive oxygen species (ROS), thereby affecting the rate of oxidative damage to nucleic acids; in advance p66Shc function on ROS, metabolism is necessary for appropriate p53-dependent apoptosis (5).
Cells within the arterial wall produce several species of free radicals, and, as is well known, an important functional index of healthy status is represented by vascular function (reviewed in refs. 6 and 7). A multitude of clinical studies and reports with experimental animal models have shown that oxidized low-density lipoprotein (oxLDL) and redox-sensitive pathways are key modulators of vascular dysfunction and atherogenesis (reviewed in refs. 8–10). Interestingly, oxLDL and its byproducts may induce early proatherogenic events not only in adults but also in arteries of the fetus and child (11, 12). Motivated by this background, we have used p66Shc−/− mice to address the effects of a very high-fat diet (HFD) on the development of early vascular lesions, systemic oxidative stress, and plasma LDL oxidizability. Both WT and p66Shc−/− mice have developed plasma hypercholesterolemia on HFD, but we have found a significant reduction of systemic oxidative stress, plasma LDL oxidizability, arterial oxidation-specific epitopes, and early atherosclerotic lesions in p66Shc−/− mice as compared to WT. Therefore, p66Shc may play a pivotal role in controlling oxidative stress and vascular dysfunction in vivo.
Materials and Methods
Animals and Diet.
p66Shc−/− mice were generated in our laboratory as described (4). Male p66Shc−/− and WT mice (SV/129) were fed a regular chow diet (5% fat; TRM 2018, Harlan Teklad, Madison, WI) and maintained in a temperature-controlled room with a 12 h light/12 h dark cycle. Because the SV/129 mouse strain when fed to 15% HFD is less prone to develop atherosclerotic lesions than the C57BL/6J mouse strain (13–15), at 16 weeks of age (weight range 28–29 g) mice were fed a 21% HFD containing 0.15% cholesterol and 19.5% casein, without cholic acid (Custom Formula, Harlan Teklad) and maintained for 12–13 weeks before analyzing plasma lipids and lesion areas. This diet induces early lesions in the aortas of susceptible mice (13–15). All experimental procedures complied with the Guide for the Care and Use of Laboratory Animals, the Guidelines of the Italian National Institutes of Health, and the position of the American Heart Association and the Council of the American Physiological Society. All experiments were approved by the Institutional Animal Care and Research Advisory Committees of the European Institute of Oncology and the Federazione Italiana Ricerca sul Cancro Institute for Molecular Oncology.
Lipid Plasma Determinations, LDL Susceptibility to Oxidation, Lag Time, and Analysis of Thiobarbituric Acid-Reactive Substances (TBARS).
Plasma cholesterol and triglyceride levels were determined by an automated enzymatic procedure (Roche Molecular Biochemicals). To investigate the susceptibility of LDL to oxidation, we determined the levels of TBARS and conjugated dienes (16, 17). LDL particle (d = 1.006–1.063 g/ml) was isolated from 2-ml samples of pooled plasma by sequential-density ultracentrifugation in a KBr gradient (16). LDLs were determined by the Lowry method (18) and LDL oxidation was induced by 1 μM copper sulfate at 37°C for 12 h, as described (16, 17). The lipid-peroxide content of oxLDL was determined as TBARS, as described (16, 17). Briefly, oxLDLs were incubated with 5% trichloroacetic acid and 1.3% thiobarbituric acid (TBA) at 90°C for 60 min and then cooled in ice-cold water. After centrifugation, 200 μl of the supernatant was recovered and differences between A530 and A630 were determined. Absorbance units were converted to malondialdehyde equivalents per mg of LDL with the use of a standard curve obtained with 1,1,3,3-tetramethoxypropane. Conjugation of dienes in LDL was carried out as reported (16, 17). LDL-conjugated dienes were measured every 10 min, by absorbance at 234 nm, for 4 h at 23°C. The lag time was determined as the intercept of the baseline and the slope of the absorbance curve during the propagation phase.
Evaluation of Systemic Oxidative Stress by Measurement of 8-Isoprostane by Enzyme Immunoassay.
Plasma levels of the isoprostane 8-epi-PGF2α (PGF2α, prostaglandin F2α), an index of systemic oxidative stress, were determined using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) as described (19).
Morphometric Assessment, Immunohistochemistry of Lesion Components, Terminal Deoxynucleotidyltransferase (TdT)-Mediated dUTP End Labeling (TUNEL) Assay, and Acetylcholine-Dependent Relaxation.
Methods for the quantification of early lesions in the aorta were as reported (15, 16, 19). Briefly, animals were killed by cervical dislocation and the aorta was dissected, cleaned of adherent fat and fascia, cut open, washed with cold sterile PBS with 2 mM EDTA, and placed in ice-cold PBS containing 50 μM butylated hydroxytoluene, 0.001% aprotinin, 50 mM EDTA, and 0.008% chloramphenicol. Each arterial segment was then divided into two parts. One of these was immersed in ornithine transcarbamoylase (OTC), flash frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. From each artery, 30 7-μm-thick cryosections were stained with oil red O and counterstained with hematoxylin. Morphometric, computer-assisted image analysis on 24-bit color image determination of lipid-rich lesions was performed as described (11, 12, 15, 16, 19). The second part of each arterial segment was fixed in buffered 10% formalin and embedded in paraffin, and serial sections (5 μm thick) were prepared for immunohistochemical analysis of different lesion components as described (15, 16, 19). The following mAbs were used: NA59 and E06 against LDL oxidation-specific epitopes were kindly donated by W. Palinski and J. L. Witztum (University of California at San Diego; refs. 15, 16, and 19); F4/80 to mouse monocyte/macrophage-derived foam cells (Serotec; refs. 16 and 19); and NP1533975 to the native form of apolipoprotein B on LDL (Boehringer Mannheim; refs. 15, 16, and 19). Immunohistochemistry was performed using an avidin–biotin–peroxidase method as described (11, 12, 15, 16, 19). Briefly, endogenous peroxidase was quenched by a 10-min incubation with 0.3% hydrogen peroxide in PBS containing 0.5 mM butylated hydroxytoluene at 48°C. Slides were washed twice with PBS and incubated for 60 min with the primary antibody. Slides were then thoroughly rinsed, incubated for 30 min with biotinylated horse anti-mouse or anti-rat IgG (1:500 dilution; Dako), washed, and incubated for 30 min with avidin–biotin–peroxidase (Dako). Antibodies bound were visualized by 20-min incubation with 3,3-diaminobenzidine tetrahydrochloride. Immunohistochemical procedures were performed using an automated tissue staining machine (TM500, BioTek Solutions, Baltimore). Apoptotic cells were detected in situ on aortic cross sections by using a modified TUNEL assay as described (20). Briefly, sections were dewaxed, rehydrated, and incubated in 20 μg/ml proteinase K. After 1 h, endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 5 min. Then, fragmented DNA were nick end-labeled with a mixture of TdT (21.5 units per section; Sigma) in a TdT buffer for 90 min at 37°C. The reaction was stopped by a 15-min incubation in 0.5 M EDTA. Detection was made by streptavidin-conjugated peroxidase, followed by a 15-min incubation in aminoethyl carbazole. The sections were then counterstained with hematoxylin and calculated as TUNEL-positive cells per total number of nuclei. In negative control experiments, TdT was omitted from the labeling mixture and no staining was detected. Finally, 3-mm abdominal aortic rings of a subset of mice (n = 5 for each group) were used to assess the preliminary vascular relaxation rate in the presence of 1 μM acetylcholine, as described (21, 22).
Statistical Analysis.
Results were analyzed by one-way ANOVA followed by Bonferroni's corrected t test. Data were analyzed in a double-blinded way regarding type of mice (p66Shc−/− and WT mice) and diet [HFD vs. normal diet (ND)] by two independent investigators. Moreover, all arterial sections were read twice. Data shown are mean ± SEM. P < 0.05 was defined as significant.
Results
A 21% HFD Increases Serum Lipids in WT and p66Shc−/− Mice.
To examine the role of p66Shc in the early atherogenic process, male p66Shc−/− and WT mice were chronically fed 21% HFD. They were then killed, and their blood was collected and evaluated by serum lipid profile. Serum levels of total cholesterol and triglyceride did not differ significantly between p66Shc−/− and WT mice fed a normocholesterolemic diet. Administration of HFD induced significant and comparable modifications of the serum lipid profile in both groups. Plasma cholesterol in WT mice was 68 ± 6 mg/dl for the ND-treated group and 196 ± 18 mg/dl for the HFD-treated group (P < 0.001 vs. ND). In the p66Shc−/− mice, plasma cholesterol was 64 ± 8 mg/dl for the ND-treated group and 177 ± 25 mg/dl for the HFD-treated group (P < 0.001 vs. ND; P was not significant vs. WT mice on HFD). The plasma triglyceride levels in WT mice were 72 ± 5 mg/dl for the ND-treated group and 137 ± 31 mg/dl for the HFD-treated group (P < 0.01 vs. ND). In p66Shc−/− mice, plasma triglyceride levels were 65 ± 6 mg/dl for the ND-treated group and 128 ± 35 mg/dl for the HFD-treated group (P < 0.01 vs. ND; P was not significant vs. WT on HFD). Thus, the 21% HFD provoked an ≈3-fold increase of serum cholesterol in both the WT and p66Shc−/− mice, comparable to that observed in the SV/129 strain of mice when subjected to a 15% fat diet (13, 23).
p66Shc−/− Mice Are More Resistant to HFD-Induced Atherogenesis.
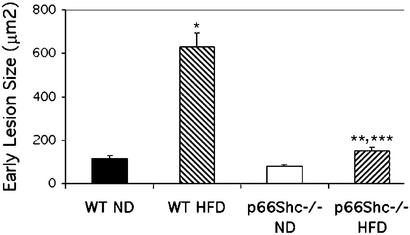
Both strains, WT and p66Shc−/−, developed only very early lesions in the range of 20–250 μm2. Computer-assisted image analysis of aortic sections revealed that high-fat treatment increased the aortic cumulative early lesion area (mainly in the aortic origin) by 21% (P < 0.0001) in WT mice (n = 10 in two experiments) and only by 3% in p66Shc−/− mice (n = 10 in two experiments) (Fig. 1). Thus, p66Shc−/− mice had markedly reduced development of these early lesions in our experimental conditions. It is noteworthy that the extent of early lesions seen in the present study is comparable to those reported previously in the SV/129 strain fed with 15% HFD and measured by classical en face staining (13, 14). To preliminarily investigate whether the reduced formation of early lesions in the p66Shc−/− mice has a prompt functional consequence in the arterial tone, we also analyzed the extent of acetylcholine-induced relaxation in abdominal aortic rings from WT and p66Shc−/− mice treated with HFD. Results showed an improvement of vascular relaxation to −6.0 log M acetylcholine by ≈30% in p66Shc−/− mice (n = 5), as compared with WT (n = 5) mice (P < 0.02).
Figure 1.
Computer-assisted image analysis of aortic atherosclerotic lesion areas in p66Shc−/− and WT mice after chronic treatment with 21% HFD. *, P < 0.00001 vs. WT (ND); **, P < 0.05 vs. p66Shc−/− (ND); ***, P < 0.0001 vs. WT (HFD). Results are expressed as the mean ± SEM of lesions from 10 animals in each group.
Reduced Expression of Oxidation Epitopes in p66Shc−/− Arterial Walls.
Based on the above data, we then characterized the early lesions by immunohistochemistry. Staining with specific mAbs revealed a marked decrease in the accumulation of intimal macrophage-derived foam cells (−35 ± 8% of mAb F4/80-positive sections; P < 0.01), arterial oxidation-specific epitopes of oxLDL (−45 ± 9% of mAb NA59; P < 0.01), and native LDL (−28 ± 5% of mAb NP153975-positive sections; P < 0.05) in p66Shc−/− mice treated with 21% HFD, as compared with matched controls. Fig. 2 shows a typical immunostaining pattern of arterial oxidation-specific epitopes, using the E06 mAb, which recognizes epitopes similar to those recognized by mAb NA59.
Figure 2.
(A and B) Immunostaining for oxidation-specific epitopes expressed as mAb E06-positive arterial sections: A, p66Shc−/− (HFD); B, WT (HFD). (Magnification, ×320.) Arrows indicate the degree of staining. (C) Graph of cumulative immunostaining results. *, P < 0.0001 vs. WT (ND); **, P = 0.056 (not significant) vs. p66Shc−/− (ND); ***, P < 0.0001 vs. WT (HFD).
Reduced Vascular Apoptosis in p66Shc−/− Mice.
An increasing body of evidence from both animal models and human tissues suggests that apoptosis is involved in atherogenic lesion progression (10, 24). Many proatherogenic factors, including oxLDL, angiotensin II, and oxidative stress, can promote apoptosis of vascular cells. Because p66Shc−/− fibroblasts (5) and endothelial cells (unpublished data) are highly resistant to oxidative stress-induced apoptosis, we have investigated the frequency of apoptotic cells in aortic sections from WT and p66Shc−/− mice by the TdT assay. The percentage of apoptotic cells detected by TdT was significantly reduced in arterial cross-sections from p66Shc−/− mice treated with 21% HFD in comparison to data obtained in WT mice (2.1 ± 0.8 vs. 7.4 ± 5.2; P < 0.001; Fig. 3).
Figure 3.
TdT-mediated dUTP end labeling (TUNEL)-positive cells in WT (A) and the lower extent in p66Shc−/− (B), both fed on HFD. (Magnification, ×640.) Arrows indicate positive cells.
Reduced Systemic Oxidative Stress and LDL Oxidizability in p66Shc−/− Mice.
Data available on oxidation-sensitive mechanisms in the arterial wall were coupled to evaluation of systemic oxidative stress and LDL oxidizability in our experimental conditions. Table 1 shows that p66Shc−/− mice have reduced systemic oxidative stress (isoprostanes) and susceptibility of LDL to ex vivo oxidation compared with WT mice (as shown by significant reduction of TBARS, as well as the prolongation of lag time).
Table 1.
Parameters of susceptibility to ex vivo peroxidation of LDL and systemic oxidative stress derived from experimental groups of the study protocol
| Mice | LDL lag time, min | LDLTBARS, nmol/mg protein | Plasma isoprostane 8-epi-PGF2α, pg/ml |
|---|---|---|---|
| ND WT | 145 ± 23 (n = 8) | 14.3 ± 2.9 (n = 8) | 101 ± 16 (n = 6) |
| HFD WT | 113 ± 14 (n = 8) | 24.4 ± 4.2 (n = 8) | 122 ± 19 (n = 6) |
| ND p66Shc−/− | 152 ± 23 (n = 8) | 13.1 ± 2.8 (n = 8) | 93 ± 12 (n = 6) |
| HFD p66Shc−/− | 138 ± 13 (n = 8)* | 18.2 ± 2.9 (n = 8)* | 105 ± 15 (n = 6)* |
Formation of TBARS at 12 h after exposure of LDL to 1 μM copper sulfate. Lag time represents an index of LDL oxidizability; increased values of lag time reflect increased resistance of LDL to oxidative modification (17, 19).
P < 0.05 vs. 21% HFD-treated WT mice by ANOVA followed by t test and Bonferroni's correction.
Discussion
Mutation of p66Shc longevity gene in mice increases both resistance to oxidative stress and life span, indicating that p66Shc is a genetic determinant of redox status and aging in mammals (4). Here we report, in the p66Shc−/− mice, the reduction of systemic oxidative stress, as well as plasma LDL oxidation, arterial oxidation epitopes, and early atherogenic lesions. These results provide a genetic model for investigating the involvement of radical-dependent pathogenic mechanisms in vessel diseases.
Hypercholesterolemia increases reactive oxygen species (ROS) and reactive nitrogen species production, resulting in oxidation and peroxidation of lipids, proteins, and lipoproteins (10, 24–26). Isoprostanes are produced from polyunsaturated fatty acids through radical-catalyzed mechanisms and represent a reliable in vivo marker of oxidative stress (10, 19, 26). Similarly, radicals promote oxidative modifications of LDLs, which accumulate within the arterial wall (10, 24–26). Experimental and clinical data suggest a role for both isoprostanes and oxLDL in atherogenesis, and antioxidants reduce atherosclerosis in the hypercholesterolemic animal models (10, 19, 24–26). In hypercholesterolemic p66Shc−/− mice, we found reduced levels of isoprostanes and oxLDLs and a marked reduction of oxidation-specific epitopes and foam cells in the arterial wall, showing that loss of p66Shc expression protects against oxidative stress and early lesion formation. Because levels of intracellular oxidants are reduced in p66Shc−/− cells (5), p66Shc might favor oxidation-sensitive mechanisms in the hypercholesterolemic mice.
OxLDL induces apoptosis of vascular cells, and this phenomenon may enhance the progression of the atherosclerotic lesions, as well as plaque instability (10, 27). Here, we found a significant reduction of apoptosis in the early lesions of p66Shc−/− mice. Although the frequency of apoptotic cells was low in early lesions, this might be related to reduced levels of trapped oxLDL in p66Shc−/− arterial wall. As a concomitant mechanism of resistance to vessel disease, p66Shc−/− vascular cells may be more resistant than WT cells to oxidative stress-induced apoptosis. Notably, cultured p66Shc−/− fibroblasts (5) and endothelial cells (unpublished data) are more resistant to peroxide or UV-induced apoptosis.
From a clinical point of view, recent clinical studies have shown that early coronary angiography after non-Q wave myocardial infarction frequently observes that an angiographic culprit advanced atherosclerotic lesions cannot be identified in more than one-third of patients (28, 29). This phenomenon can be explained by the fact that also early atherosclerotic lesions can promote the “soil” for coronary heart disease. Moreover, the intravascular ultrasound (IVUS) findings show that atherosclerotic disease is characteristically diffuse and involves the entire arterial tree, with progression including formation of multiple, potentially rupture-prone plaques that are not associated with arterial stenosis (30). These findings together with the evidence of early lesions in human fetuses and children (11, 12) suggest the need for aggressive and early systemic intervention that targets modifiable risk factors to reduce coronary heart disease morbidity and mortality.
In conclusion, absence of p66Shc expression might contribute to the protection from HFD-induced early atherogenesis through multiple mechanisms, including reduction of systemic oxidative stress, plasma LDL oxidizability, tissue oxidation-specific epitopes and vascular cell apoptosis. Understanding of the pathophysiological role of p66Shc might, therefore, contribute dissecting the relevance and hierarchy of those early events in atherogenesis. Furthermore, p66Shc might represent a molecular target for the designing of newly developed therapies against vascular diseases and other disease conditions.
Acknowledgments
This paper is dedicated to the memory of Dr. Russel Ross, who passed away in March of 1999. This work was supported by National Institutes of Health Grant HL57665 and an Associazione Italiana Ricerca sul Cancro grant.
Abbreviations
- HFD
very high-fat diet
- ND
normal diet
- LDL
low-density lipoprotein
- oxLDL
oxidized LDL
- TBARS
thiobarbituric acid-reactive substances
- TdT
terminal deoxynucleotidyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci P G. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 2.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 3.Luzi L, Gonfalonieri S, Di Fiore P P, Pelicci P G. Curr Opin Genet Dev. 2000;10:668–674. doi: 10.1016/s0959-437x(00)00146-5. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolci P P, Lanfrancone L, Pelicci P G. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 5.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura I M, Raker V A, Maccarana M, et al. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 6.Luscher T F, Noll G. J Hypertens. 1996;14:S111–S119. doi: 10.1097/00004872-199609002-00020. [DOI] [PubMed] [Google Scholar]
- 7.Napoli C, Ignarro L J. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 8.Witztum J L, Steinberg D. J Clin Invest. 1991;88:1785–1791. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg D. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 10.Napoli C, de Nigris F, Palinski W. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 11.Napoli C, D'Armiento F P, Mancini F P, Witztum J L, Palumbo G, Palinski W. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoli C, Glass C K, Witztum J L, Deutch R, D'Armiento F P, Palinski W. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 13.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 14.Paigen B, Ishida B Y, Verstuyft J, Winters R B, Albee D. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- 15.Palinski W, Napoli C, Reaven P D. In: Contemporary Cardiology: Vascular Disease and Injury–Preclinical Research. Simon D I, Rogers C, editors. Totowa, NJ: Humana; 2000. pp. 149–174. [Google Scholar]
- 16.de Nigris F, D'Armiento F P, Somma P, Casini A, Sarlo F, Andreini I, Mansueto G, DeRosa G, Bonaduce D, Condorelli M, Napoli C. Int J Cardiol. 2001;81:107–115. doi: 10.1016/s0167-5273(01)00542-3. [DOI] [PubMed] [Google Scholar]
- 17.Napoli C, Postiglione A, Triggiani M, Corso G, Palumbo G, Ambrosio G, Carbone V, Ruocco A, Montefusco S, Malorni A, et al. Atherosclerosis. 1995;11:263–275. doi: 10.1016/0021-9150(95)05612-2. [DOI] [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr L. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Napoli C, Ackah E, De Nigris F, Del Soldato P, D'Armiento F P, Crimi E, Condorelli M, Sessa W C. Proc Natl Acad Sci USA. 2002;99:12467–12470. doi: 10.1073/pnas.192244499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Condorelli G, Aycock J K, Frati G, Napoli C. FASEB J. 2001;15:2162–2170. doi: 10.1096/fj.01-0032com. [DOI] [PubMed] [Google Scholar]
- 21.Napoli C, Paternò R, Faraci F M, Taguchi H, Postiglione A, Heistad D D. Stroke. 1997;28:2266–2272. doi: 10.1161/01.str.28.11.2266. [DOI] [PubMed] [Google Scholar]
- 22.Liu L H, Paul R J, Sutliff R L, Miller M L, Lorenz J N, Pun R Y, Duffy J J, Doetschman T, Rimura Y, MacLennan D H, et al. J Biol Chem. 1997;272:30538–30545. doi: 10.1074/jbc.272.48.30538. [DOI] [PubMed] [Google Scholar]
- 23.Jolley C D, Dietschy J M, Turley S D. Am J Physiol. 1999;276:G1117–G1124. doi: 10.1152/ajpgi.1999.276.5.G1117. [DOI] [PubMed] [Google Scholar]
- 24.Napoli C, Witztum J L, Calara F B, de Nigris F, Palinski W. Circ Res. 2000;87:946–952. doi: 10.1161/01.res.87.10.946. [DOI] [PubMed] [Google Scholar]
- 25.Napoli C, de Nigris F, Welch J S, Calara F B, Stuart R, Glass C K, Palinski W. Circulation. 2002;105:1360–1367. doi: 10.1161/hc1102.106792. [DOI] [PubMed] [Google Scholar]
- 26.Boullier A, Bird D A, Chang M K, Dennis E A, Friedman P, Gillotte-Taylor K, Horkko S, Palinski W, Quehenberger O, Shaw P, et al. Ann NY Acad Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- 27.Schreyer S A, Vick C M, LeBoeuf R C. J Biol Chem. 2002;277:12364–12368. doi: 10.1074/jbc.M111727200. [DOI] [PubMed] [Google Scholar]
- 28.Waxman S. Cardiol Clin. 1999;17:295–305. doi: 10.1016/s0733-8651(05)70076-x. [DOI] [PubMed] [Google Scholar]
- 29.Kerensky R A, Wade M, Deedwania P, Boden W E, Pepine C J. J Am Coll Cardiol. 2002;39:1456–1463. doi: 10.1016/s0735-1097(02)01770-9. [DOI] [PubMed] [Google Scholar]
- 30.Nissen S E. Am J Med. 2002;112,Suppl. 8A:27S–33S. doi: 10.1016/s0002-9343(02)01087-2. [DOI] [PubMed] [Google Scholar]