Abstract
Based on the in vitro ability of opioid antagonists to activate a μ-opioid receptor mutant, S196A, we reasoned that when expressed in the appropriate sites in vivo, this mutant receptor could be used to elicit the analgesic effects of the opioids without the accompanying side effects, such as tolerance and dependence. To test this hypothesis, we introduced the S196A mutation into the mouse μ-opioid receptor by a knock-in strategy to test the ability of the opioid antagonist to produce analgesic effects. In these homozygous mice, we observed increased intrinsic efficacy of opioid analgesics with two antinociceptive tests: hot water tail-withdrawal and acetic acid-induced writhing tests. Opioid antagonists, such as naloxone and naltrexone, elicited antinociceptive effects similar to that of partial agonists. Most importantly, chronic treatment of the homozygous mice with naltrexone did not produce the expected tolerance response, whereas less physical dependence was observed than with chronic morphine treatment. Such in vivo properties suggest the possibility of using the S196A mutant of the μ-opioid receptor and opioid antagonists to minimize the spectrum of unwarranted side effects in pain management when opiate analgesics are used.
The control of acute pain and prevention and treatment of chronic pain have been under intensive study. From studies on the neurobiology of pain (1) due to the myriad molecules and neurotransmitters that are involved in peripheral nociceptive processing and in the function of spinal nociceptive integration (2), pharmacological approaches for pain management have been based on the activating or inactivating receptors involved in neurotransmission (3). For example, within the dorsal horn of the spinal cord, several peptides such as substance P, somatostatin, neuropeptide Y, galanin, and calcitonin gene-related peptide; excitatory amino acids such as glutamate and aspartate; inhibitory amino acids such as γ-aminobutyric acid; endogenous opioid peptides, adenosine, serotonin, norepinephrine, nitric oxide; and the arachidonic acid metabolites have all been implicated in the transmission and regulation of painful messages (4–6). Pharmacological agents or treatment paradigms have targeted the alteration of these receptors' activities. An excellent example is the development of neurokinin antagonists for pain management. Although animal studies indicated that selective ablation of spinal neurons containing the neurokinin-1 receptor could lead to a substantial reduction in allodynia and hyperalgesia induced by inflammation and nerve injury in rats (7), clinical studies with antagonists of substance P have not been successful in controlling pain resulting from migraines, rheumatoid arthritis, dental surgery, and posthepatic neuralgia (8).
Among all of the agents used in pain management, opioid analgesics are most efficacious in controlling moderate and severe postoperative pain. However, with the many well known adverse effects, such as respiratory depression, constipation, and nausea, and the problem of opioid-induced neurotoxicity (9–13), there are concerns surrounding the use of opioid analgesics. Decades of research have focused on designing an opioid analgesic agent that has the analgesic efficacy of morphine but is devoid of morphine's adverse effects. With the cloning of the multiple opioid receptors and subsequent knockout mice studies (14–16), it is unequivocal that the analgesic action of morphine is mediated via the μ-opioid receptor. Drug designs thus far have yielded partial agonists at the μ-opioid receptor such as buprenorphine, which does not alleviate but reduces adverse effects (18). Instead of continuing to evaluate agents that would elicit analgesic efficacy equal to morphine without the adverse effects, we have decided to explore the use of gene transfer in the development of an ideal analgesic paradigm. If an approach could be used to deliver a mutant opioid receptor with distant phenotype, activation of these mutant receptors at the specific nociceptive neurons might result in the painkilling effect of the administered drug without the adverse effects.
One such mutant receptor is the mutation of the Ser-196 in the fourth transmembrane domain of the μ-opioid receptor to either Leu or Ala (18). In Chinese hamster ovary cells stably expressing the S196A mutant, the opioid antagonist naloxone or naltrexone inhibited forskolin-stimulated adenylyl cyclase activity. Antagonists could also activate the G protein-coupled inwardly rectifying potassium channel (GIRK1) in Xenopus oocytes coexpressing the mutant opioid receptor and the GIRK1 channel (18). Hence, this S196A mutant of the μ-opioid receptor represents an opportunity to test our hypothesis. By introducing a modified receptor to specific pain transmission pathways, in combination with the use of opioid antagonists, pain can be controlled without the side effects that are associated with the activation of the endogenous opioid systems. Hence, a population of mice that express the S196A mutant receptors by a homologous recombination gene-targeting strategy was generated. The acute and chronic effects of various opioid ligands were tested on the mutant mice and compared with those in wild-type littermates.
Methods
Generation of Knock-In Mice.
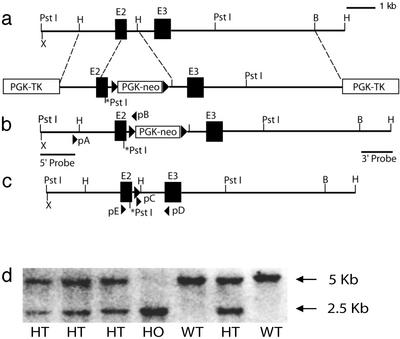
Mouse μ-opioid receptor (MOR) genomic clones were obtained from the 129/ola mouse genomic DNA library by screening using mouse μ-opioid receptor cDNA as the probe. Clone D3 containing exon 2 and flanking introns was used as the template to change the serine 196 codon of the μ-opioid receptor to the alanine codon by in vitro mutagenesis with two primers: 5′-AACTGGATCCTCTCTGCAGCCATTGGTCTG-3′ and 5′-CAGACCAATGGCTGCAGAGAGGATCCAGTT-3′. For selection purposes, a de novo PstI restriction site was created at the mutation site (Fig. 1). The mutated D3 clone was restriction endonuclease digested at the EcoNI site downstream from the splicing donor of intron 2 and end-blunted by the standard method. A LoxP-PGK-neo-LoxP fragment was extracted from neo-LoxP plasmid, end-blunted, and ligated to the intron 2 EcoNI site of the MOR gene. The resulting clone was released from the vector by HindIII and ligated to another MOR gene HindIII fragment containing exon 3 and flanking introns. A copy of the PGK-TK cassette was inserted as negative selection markers on the ends of both arms of the homologous sequences. This targeting construct, linearized with NotI, was used to transfect the SP1 embryonic stem cells. Cells that survived the 300 μg/ml G418 and 2 μM gancyclovir selection were verified to undergo homologous recombination by PCR, with primers pA and pB, and Southern blotting with both the 5′ and the 3′ probes.
Figure 1.
Replacement of μ-opioid receptor gene with the mutant encoding S196A. (a) The endogenous MOR gene and the targeting vector. (b) Mutant allele after the first round of homologous recombination, represented both in embryonic stem cells selected and in mutant mice retaining neo in m-intron 2. (c) The mutant allele after breeding of the homozygous mutant mice with EIIacre transgenic mice showing the deletion of neo cassette. (d) Screening of mutant mice by Southern blotting using the 5′ probe. Mouse genomic DNA was digested by PstI and hybridized to 32P-labeled 5′ probe. The 2.5-kb bands represent the mutant allele, and the 5-kb bands represent the wild-type allele.
Two independent clones, nos. 30 and 82, were used for the generation of knock-in mutant mice. Homozygous mutant mice were bred with EIIa-cre transgenic mice to delete the neo cassette within intron 2 of the MOR gene. After breeding with EIIa-cre transgenic mice, the F1 heterozygous mutant mice were bred to generate homozygous heterozygous mutant mice and wild-type littermates for use in experiments. The genotypes of the mice were determined by digesting mouse genomic DNA with PstI endonuclease and Southern blotting with the 5′ probe (Fig. 1d). All handling of animals and experimental procedures were performed as approved by the University of Minnesota Institutional Biosafety Committee. The expression of the μ-opioid receptor and the ligand-binding characteristics were determined by Scatchard analysis of [3H]DAMGO {[D-Ala-2,N-MePhe,Gly-5-ol]enkephalin, 50 Ci (1 Ci = 37 GBq)/mmol; Amersham Pharmacia} binding and competition binding using membranes prepared from mice killed at ≈7 weeks of age, as described (18).
Antinociception Testing.
All antinociceptive testing was done between 1:00 and 4:00 p.m. each day. Mice were placed in the room where measurements were made 2 h prior, for acclimatization. For tail-withdrawal testing, mouse tails were either immersed ≈2 cm into water heated to 53°C or placed over the radiant heat source of an analgesia meter (Columbus Instruments, Columbus, OH); radiant heat intensity was adjusted for a 3- to 5-s baseline latency. Cutoff times of 24 s for warm water and 12 s for radiant heat were used to minimize tail damage. Tail-withdrawal responses were recorded 30 min after s.c. drug injection, except for naloxone, for which a 12-min interval was used. Percent of maximum possible effect (%MPE) was calculated by the following formula: (measured latency − baseline latency) 100/(cut-off time − baseline latency). Each dose involved 8–12 mice of each genotype. ED50 values were derived from regression analyses of the linear portion of each dose–response curve or calculated by nonlinear regression. A two-way ANOVA (genotype and dose) was used to determine genetic differences in drug-induced analgesia. Student's t tests were used to calculate any differences between genotypes for the same dose groups.
Testing for inhibition of abdominal constriction was conducted as described (19). Briefly, mice were placed in individual 30-cm-diameter Plexiglas observation chambers for a 30-min acclimatization period. Twenty minutes after the s.c. injection (5 min for naloxone) of various opioid drugs, mice were injected i.p. with 10 ml/kg 0.6% (wt/vol) acetic acid and returned to the observation chambers; abdominal constriction responses were counted for 20 min after the i.p. injection. For control, mice from each genotype were injected with saline s.c. before acetic acid injection, and the mean count of abdominal constriction for the saline group was recorded. The %MPE for the writhing test was calculated by the following formula: (mean count of saline group − count of drug group) 100/(mean count of saline group).
Chronic Drug Treatment.
Mice were treated chronically with sustained-release morphine pellets (75 mg per pellet), naltrexone pellets (30 mg per pellet), or placebo pellets (obtained from the National Institute on Drug Abuse) implanted s.c. Seventy-two hours later, mice were placed individually into test chambers consisting of transparent round plastic boxes (30-cm diameter, 40-cm height) 30 min before naloxone-HCl injection. Naloxone doses of 0.03–100.0 mg/kg i.p. were given, and the number of rearings, jumps from platform (a jump was defined as any response by a mouse in which all four feet were off the ground at the same time), wet dog shakes, paw tremors, and tremors were counted over a 10-min period. Occurrence of diarrhea was noted as present or absent in six 5-min intervals and normalized according to a maximum of six possible episodes (0 = 0% 6 = 100%). The weight of each animal was determined before and 30 min after naloxone injection. Natural signs of withdrawal were observed 10–16 h after the removal of the morphine pellets after 3 days of implantation. Values were analyzed by two-way ANOVA (genotype and treatment) between subjects. Individual comparisons were made by the two-tailed Dunnett's test, after the main effect of ANOVA.
Results
To examine the function of the S196A mutant μ-opioid receptor in vivo, we designed a targeting vector that would replace the Ser-196 codon within the MOR gene with that of Ala (Fig. 1 a–c). A population of homozygous mutant mice with the neo cassette within the second intronic sequence was generated by a gene-targeting approach (Fig. 1d). These mice did not exhibit detectable [3H]DAMGO binding or the μ-opioid receptor. After deleting the neo cassette by breeding the homozygous knock-in mice with the EIIa-cre transgenic mice constitutively expressing cre-recombinase in their early embryonic stages (20), expression of the mutant receptor can be detected with RT-PCR, in situ hybridization studies, and radioactive ligand receptor-binding assays. However, Scatchard analyses of the binding data obtained with [3H]DAMGO revealed an ≈85% decrease in the amount of receptor expressed (Bmax = 17.5 ± 2.3 fmol/mg protein in the homozygous mutant mice vs. Bmax = 113 ± 4.3 fmol/mg protein in wild-type mice). The reduced expression of the receptor proteins was reflected in the drastic decrease of the correctly spliced MOR mRNA (data not shown). There was no significant difference in DAMGO affinity for the wild-type and mutant receptors, with Kd values equal to 2.6 ± 0.3 nM and 3.6 ± 0.7 nM, respectively. The relative affinities of several ligands for this mutant receptor were also tested and were determined to be similar to those observed with the wild-type mice. These data are in agreement with our reported data that the Ser-196 mutation did not alter the affinities of the agonists or agonists for the receptor in the in vitro models (18).
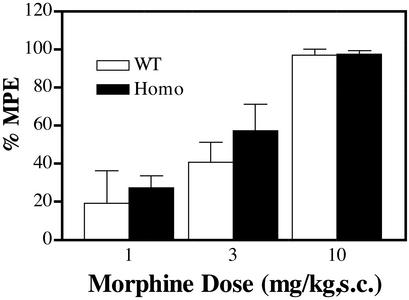
The ability of opioid agonists to elicit antinociceptive responses seems to be dependent on the μ-opioid receptor level. Several laboratories have reported decreases in morphine antinociception potency in the heterozygous MOR knockout mice, in which the expression level of the receptor was reduced by ≈50% (15, 16). To our surprise, when the two antinociceptive tests were carried out on the homozygous knock-in mice, there was no significant difference in either the ED50 values or the maximum effect of morphine antinociception between these two genotypes, even with ≈85% difference in receptor density (Fig. 2). Similarity in the maximal antinociceptive responses was anticipated because in the heterozygous MOR knockout mice or in mice treated with insurmountable doses of μ-opioid antagonist clocinnamox or β-funaltrexamine (14–16, 21), morphine could produce similar level antinociceptive responses with drastic decrease in morphine potency, which was not observed with the S196A knock-in mice. The potencies of morphine to inhibit the tail-withdrawal response or to reduce the acetic acid-induced writhing in the homozygous mice were similar to that of wild type (Fig. 2). Similar antinociception potency and efficacy were observed with other agonists such as methadone in the wild-type and the homozygous S196A knock-in mice. These data suggested that these S196A mutant receptors are more efficiently coupled to the effectors than the wild-type receptors.
Figure 2.
Opioid agonist antinociception in wild-type and homozygous mutant mice in tail-withdrawal test. Homozygous mutant mice and their wild-type littermates were treated with various doses of morphine. The latencies of tail withdrawal from radiant heat were recorded. Morphine responses: ED50 values are 4.4 ± 0.31 mg/kg for wild-type and 3.2 ± 0.7 mg/kg for homozygous mice. Similar results were observed by using warm water (53°C) as the heat source. Data are presented as the mean ± SEM with n = 8–12 mice in each group. There is no significant difference among genotypes for either drug; P > 0.05 by two-way ANOVA (genotype and dose).
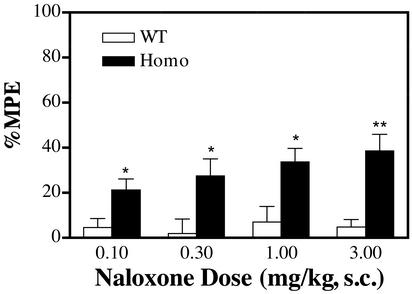
As suggested by our reported in vitro results, mutation of the μ-opioid receptor Ser-196 to either Leu or Ala would result in antagonist activation of the receptor mutant (18). With the mutant mice, there was a naloxone dose-dependent antinociceptive response that was not observed with wild-type littermates (Fig. 3). The inability of naloxone to elicit antinociceptive responses in wild-type animals distinguished the current studies from previous reports suggesting low doses of naloxone could produce analgesic responses due to its attenuation of the autoinhibition of enkephalin release (22). With both antinociceptive tests, the naloxone potency compared favorably with that of morphine. However, the maximal response of naloxone was only 40–50% of that observed with morphine. Similar observations were obtained with the other opioid antagonist, naltrexone.
Figure 3.
Opioid antagonists produced antinociceptive responses in homozygous mutant mice in tail-withdrawal test. Homozygous mutant mice (Homo) and their wild-type littermates (WT) were injected with various doses of naloxone, and the tail-withdrawal latencies from radiant heat were recorded 12 min after injection. The ED50 value for homozygous mice was determined to be 1.32 ± 0.08 mg/kg. Similar results were observed using warm water (53°C) as the heat source. *, P < 0.05; **, P < 0.01; Student's t test between wild-type and homozygous mice for the same dose groups, n = 10–12.
The increase in the intrinsic activities of opioid ligands by the S196A mutation was observed with the partial agonists' intrinsic activities. When the antinociceptive responses to opioid partial agonists such as nalorphine and nalbuphine were measured, there were significant increases in the maximal responses without significant changes in the potencies of these drugs observed with the homozygous mice. In the inhibition of the acetic acid-induced abdominal constriction test, 1 mg/kg nalorphine exhibited the same maximal antinociceptive response as morphine. Hence, the mutation of Ser-196 to Ala also resulted in the increase of intrinsic activities of the partial agonist.
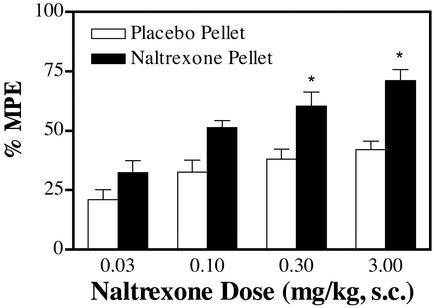
When the homozygous mice were chronically treated with morphine, rapid tolerance development was observed. When the mice were implanted with one 75-mg morphine pellet for 72 h, the ED50 value was changed from 2.0 ± 0.21 mg/kg in placebo pellets implanted in wild-type mice to 16 ± 1.6 mg/kg, or 8-fold tolerance, in wild-type mice implanted with the morphine pellets. On the other hand, when the homozygous mice were implanted with the morphine pellets and compared with those implanted with placebo pellets, the ED50 value increased from 3.0 ± 0.33 kg/mg to 110 ± 6.7 kg/mg, or 37-fold tolerance. However, when the mice were treated chronically with naltrexone pellet, tolerance to the drug was not observed. The ED50 values for naltrexone were determined to be 0.27 ± 0.35 mg/kg and 0.31 ± 0.26 mg/kg in placebo pellet-treated or naltrexone pellet-treated homozygous mice. This was in direct contrast with the reported studies in which chronic treatment of the mice expressing wild-type μ-opioid receptor with partial agonists such as nalorphine or nalbuphine did induce tolerance to the drugs (23). Furthermore, the maximal activity of naltrexone was even slightly increased after chronic treatment (Fig. 4). There was an increase in the maximal activities, which was accompanied by a >2-fold increase in the [3H]DAMGO binding. Whether such an increase in the opioid receptor level is due to the often-reported increase in the Bmax values after in vivo morphine or opioid antagonist treatment and whether the increase in receptor number is responsible for the increase in naltrexone activities remain to be demonstrated. Further, there was also a lack of morphine tolerance after chronic naltrexone treatment. When the ED50 values of morphine to inhibit the hot water tail-withdrawal were determined in the homozygous mice treated with either placebo or naltrexone pellets for 72 and 10 h after pellet removal, there was no significant alteration in ED50 values, 3.0 ± 0.33 mg/kg and 2.8 ± 0.26 mg/kg, respectively. The similarity in ED50 values of morphine in wild-type mice implanted with placebo and naltrexone pellets for 72 h suggested that the antinociceptive response to morphine was not affected by the residual naltrexone in the plasma.
Figure 4.
Absence of tolerance development after naltrexone pellet implantation. Homozygous mutant mice were implanted with a naltrexone pellet for 72 h, and naltrexone antinociceptive effects were measured by the tail-withdrawal test 10 h after pellet removal. The ED50 values for naltrexone were determined to be 0.27 ± 0.35 and 0.31 ± 0.26 mg/kg in placebo pellet-treated and naltrexone pellet-treated mice, respectively. *, P < 0.05; Student's t test as compared between the two treatments within the same dose group, n = 8–12.
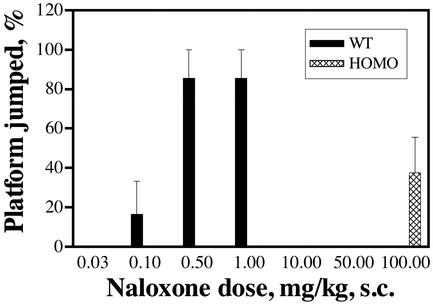
Another consequence of chronic opioid agonist treatment is the development of physical dependence. In mice, jumping behavior from a platform elicited by antagonists after chronic morphine treatment was shown to be a sensitive indicator of the degree of dependence (24). With a constant dose of morphine during chronic treatment, the frequency of jumping is directly related to the dose of naloxone administered. The alteration in the tolerance response after the S196A mutation could also affect the dependence development accordingly. This apparently is the case. As shown in Fig. 5, the wild-type mice exhibited the platform jumping behavior after 72 h of morphine pellet implantation at 0.1 mg/kg naloxone, with almost all of the mice jumping at 0.5–1.0 mg/kg. However, none of the homozygous mice exhibited platform-jumping behavior even at the 10–50 mg/kg dose of naloxone injected (Fig. 5). Only at the 100 mg/kg dose did naloxone induce <40% of the mice jumping from platform. The inability of naloxone to precipitate withdrawal after chronic morphine administration could be due to the absence of dependence in the homozygous mice. However, other withdrawal signs of physical dependence, such as weight loss, wet dog shakes, tremors, and diarrhea, could be observed in homozygous mice chronically treated with morphine, albeit less severe than those observed in wild-type mice treated with morphine (data not shown). The amount of body weight loss after naltrexone pellet implantation and removal in homozygous mice was similar to that observed in wild-type mice implanted with morphine pellet for 72 h and subsequently removed (data not shown). Naltrexone pellet implantation in wild-type mice did not result in a loss in body weight after pellet removal. Interestingly, although chronic naltrexone treatment did not produce tolerance development (Fig. 4), the chronic activation of the S196A mutant μ-opioid receptor by agonist, morphine, or the antagonist, naltrexone, would elicit dependence development, albeit much less severe.
Figure 5.
Attenuation of the opiate dependence in the homozygous mice. Mice were treated with morphine pellet for 3 days, and various doses of naloxone were administrated 72 h after pellet implantation. Platform jumping was then counted for 10 min, and the percentage of mice that showed jumping was determined. For the wild-type mice, a maximal naloxone dose of 1 mg/kg s.c. was used. For the homozygous mice, a maximal dose of 100 mg/kg s.c. was used.
Discussion
The ability to generate a single mutation of the μ-opioid receptor in vivo by the knock-in strategy allows us to examine the functional activities of a mutant receptor. Our previous report indicated that the unique mutation of the conserved Ser residue within the fourth transmembrane domain of the opioid receptor resulted in the ability of the opioid alkaloid antagonists to activate the mutant receptor. Our current in vivo antinociceptive measurements clearly indicated that in mice harboring the S196A mutation of the μ-opioid receptor, antagonists such as naloxone and naltrexone exhibited partial agonist properties.
In the course of the current studies, several properties of this S196A μ-opioid receptor mutant emerged. Due to the location of the 42-bp lox P site in intron 2 of the MOR gene, the correct splicing of the MOR message was impeded, resulting in ≈15% of the normal level of μ-opioid receptor expressed in the mutant mice. One would predict from other studies such as the receptor knockout mice (14–16) that the in vivo potency, if not the efficacy of morphine, would be reduced in these animals with lower receptor density. To our surprise, morphine and other opioid agonists tested exhibited equal efficacy in the S196A μ-opioid receptor mutant mice as in the wild-type mice. Furthermore, the efficacies of the opioid partial agonists tested were increased. Although the increases in efficacies were not applicable to all partial agonists, such as buprenorphine (data not shown), the similarity in the opioid agonists' activities in the wild-type and mutant mice suggests that the S196A mutant must have a higher intrinsic efficacy than the wild type.
In addition to the observed increase in the intrinsic efficacies of partial agonists and antagonists, mutation of the Ser-196 of μ-opioid receptor to Ala also resulted in an alteration in the responses to chronic drug treatment. Probably due to the relatively low receptor density, the degree of tolerance observed with the S196A knock-in mice after morphine pellet implantation was significantly greater than that observed with the wild-type animals. Such an increase in tolerance development was not accompanied by a parallel increase in dependence development. As shown in Fig. 5, the amount of naloxone needed to precipitate the platform-jumping behavior in the homozygous mice was >200-fold higher than that required for the wild-type animals. The apparent lack of physical dependence development in the homozygous mice could be caused by the partial agonistic properties of naloxone. However, the abilities of the partial agonist to precipitate morphine withdrawal signs were well documented both in animals (25) and in primates (26). Thus, the failure of naloxone to precipitate the withdrawal sign of platform jumping could not be due to the partial agonistic property of the drug in homozygous mice.
The most likely reason for the failure of naloxone to precipitate this withdrawal sign was due to the relatively low μ-opioid receptor level in the homozygous mice. Reduced physical dependence after chronic morphine treatment was reported with the CXBK mice, a strain of mice expressing low levels of μ-opioid receptor, and in mice treated with β-funaltrexamine to reduce the amount of μ-opioid receptor. In CXBK mice, a low dose of naloxone after chronic morphine treatment resulted in weight loss, diarrhea, and ptosis but not jumping and body shakes. In contrast, C57BL/6 mice treated similarly exhibited weight loss, diarrhea, ptosis, jumping, and body shakes (27). Similarly, β-funaltrexamine administered to rats s.c. 24 h before initiation and on day 3 of a 6-day period of morphine treatment reduced the development of physical dependence in a dose-dependent manner (28, 29). These studies, together with our current observations, suggest the degree of physical dependence is proportional to the amount of receptor expressed.
The failure of chronic naltrexone to produce tolerance is probably due to the inactivation of the endogenous ∂-opioid receptor by the antagonist. Previously, using ∂-opioid receptor-selective antagonist, naltrindole, or with antisense oligonucleotides to ∂-opioid receptor, different groups have reported that morphine tolerance and dependence were impeded (30, 31). The involvement of ∂-opioid receptors in the inhibition of the chronic morphine effect was conclusively demonstrated with the ∂-opioid receptor null animals in which morphine tolerance was completely blocked (32). Thus, the chronic naltrexone administration in the S196A homozygous mice is equivalent to the coadministration of a ∂-opioid receptor-selective antagonist and a μ-opioid receptor agonist to the wild-type mice. Naltrexone activates the S196A mutant μ-opioid receptor but inactivates the endogenous ∂-opioid receptor in the homozygous mice.
The ability of antagonist to elicit an antinociceptive effect, but not tolerance development, in the mutant mice represents an interesting opportunity to design pain treatment paradigms. Currently, strong narcotic analgesic is given systemically or intrathecally to patients with chronic pain. Adjuvants or drug combinations were administered to alleviate the side effects of the analgesics (3). With the ability of antagonists to activate the mutant receptor, it is possible to engineer and deliver the mutant receptor molecules at the sites of pain control. For example, in addition to the observed inability of naltrexone to elicit tolerance development, receptor domains, such as the phosphorylation sites and the arrestin-binding sites, could be eliminated to enhance the drug's activities during prolonged treatment. Receptor phosphorylation and arrestin binding have been implicated in morphine tolerance by studies using arrestin knockout mice (33). Hence, by knocking-in the mutation of the phosphorylation and arrestin-binding sites of the μ-opioid receptor in combination with the current S196A mutation, we should be able to generate animals in which naloxone or naltrexone would produce the acute antinociceptive responses without the chronic tolerance responses. Furthermore, with the identification of the upstream cis-elements of the opioid receptor genes that control the cell-specific expression of the receptors (34), it is possible to control the expression of delivered mutant opioid receptor at specific nociceptive neurons. Although there are many hurdles to overcome in the use of gene therapy in pain treatment paradigms, we propose that receptor engineering could represent a future direction in the development of ideal analgesics. By engineering a delivery vehicle that contains the cell-specific expression element controlling expression of the mutant μ-opioid receptor (i.e., the promoter), a treatment paradigm can be developed in which the mutant receptor expressed in patients by gene targeting can be activated by antagonists such as naloxone or naltrexone, whereas the endogenous opioid receptors remain inactive. By targeting specific regions involved in pain transmission, such as the substantia gelatinosa of the spinal cord, systemic administration of the antagonist will activate the mutant receptor at the injected sites, whereas the endogenous receptor systems remain inactive. Hence, pain relief can be achieved by such paradigms without eliciting the side effects of narcotic drugs.
Acknowledgments
We thank Drs. Li-Na Wei, Sheldon Sparber, Martin Wessendorf, and David Largaespada for scientific discussions. Our gratitude extends to Ms. Shan Maika for providing SP1 embryonic stem cells and for technical help. We thank Sandra Horn, Steve Schnell, and David Ou-Yang for excellent technical support. The Embryonic Stem Cell Core of Washington University kindly provided us with the neo-plasmid for targeting vector construction. This research was supported in part by National Institute on Drug Abuse Grants DA07339, DA00564, DA01583, DA11806, and DA70554 and the F. and A. Stark Fund of the Minnesota Medical Foundation.
Abbreviations
- MOR
mouse μ-opioid receptor
- DAMGO
[D-Ala-2,N-MePhe,Gly-5-ol]enkephalin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Besson J M. Lancet. 1999;353:1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- 2.Carlton S M, Coggeshall R E. Pain Forum. 1998;7:71–78. [Google Scholar]
- 3.MacPherson R D. Pharmacol Ther. 2000;88:163–185. doi: 10.1016/s0163-7258(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 4.Dray A. Can J Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- 5.Dickenson A H. Br J Anaesth. 1995;75:193–200. doi: 10.1093/bja/75.2.193. [DOI] [PubMed] [Google Scholar]
- 6.Urban L, Thompson S W N, Dray A. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Mantyh P W, Rogers S D, Honore P, Allen B J, Ghilardi J R, Li J, Daughters R S, Lappi D A, Wiley R G, Simone D A. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 8.Dray A, Rang H. Trends Neurosci. 1998;21:315–317. doi: 10.1016/s0166-2236(98)01291-0. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Neumann C M. In: Pain and Updated Review: Refresher Course Syllabus 9th World Congress on Pain. Max M, editor. Seattle: International Association for the Study of Pain; 1999. pp. 443–457. [Google Scholar]
- 10.Gibson T P. Am J Med. 1996;101:47S–53S. doi: 10.1016/s0002-9343(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 11.Jasinski D R. Acta Anaesthesiol Scand. 1997;41:184–186. doi: 10.1111/j.1399-6576.1997.tb04635.x. [DOI] [PubMed] [Google Scholar]
- 12.Le Bars M, Glowinski J, Bannwarth B. Therapie. 2000;55:343–347. [PubMed] [Google Scholar]
- 13.Pappagallo M, Heinberg L J. Semin Neurol. 1997;17:203–211. doi: 10.1055/s-2008-1040930. [DOI] [PubMed] [Google Scholar]
- 14.Matthes H W, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 15.Sora I, Takahashi N, Funada M, Ujike H, Revay R S, Donovan D M, Miner L L, Uhl G R. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loh H H, Liu H C, Cavalli A, Yang W, Chen Y F, Wei L N. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 17.Carr D B, Goudas L C. Lancet. 1999;353:2051–2058. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 18.Claude P A, Wotta D R, Zhang X H, Prather P L, McGinn T M, Erickson E J, Loh H H, Law P Y. Proc Natl Acad Sci USA. 1996;93:5715–5719. doi: 10.1073/pnas.93.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein M, Mogil J S, Japon M, Chan E C, Allen R G, Low M J. Proc Natl Acad Sci USA. 1966;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasko M, Pichel J G, Gorman J R, Sauer B, Okamoto Y, Lee E, Alt F W, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman D, Leander J D, Reel J K, Hynes M D. J Pharmacol Exp Ther. 1987;241:374–378. [PubMed] [Google Scholar]
- 22.Ueda H, Fukushima N, Kitao T, Ge M, Takagi H. Neurosci Lett. 1986;65:247–252. doi: 10.1016/0304-3940(86)90269-7. [DOI] [PubMed] [Google Scholar]
- 23.Martin W R, Gorodetzky C W. J Pharmacol Exp Ther. 1965;150:437–442. [PubMed] [Google Scholar]
- 24.Way E L, Loh H H, Shen F H. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- 25.Dum J E, Herz A. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aceto M D. Neuropeptides. 1984;5:15–18. doi: 10.1016/0143-4179(84)90015-5. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Hayashi Y, Misawa M. Life Sci. 1992;50:849–856. doi: 10.1016/0024-3205(92)90203-2. [DOI] [PubMed] [Google Scholar]
- 28.DeLander G E, Portoghese P S, Takemori A E. J Pharmacol Exp Ther. 1984;231:91–96. [PubMed] [Google Scholar]
- 29.Aceto M D, Dewey W L, Portoghese P S, Takemori A E. Eur J Pharmacol. 1986;123:387–393. doi: 10.1016/0014-2999(86)90713-2. [DOI] [PubMed] [Google Scholar]
- 30.Hepburn M J, Little P J, Gingras J, Kuhn C M. J Pharmacol Exp Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- 31.Abdelhamid E E, Sultana M, Portoghese P S, Takemori A E. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 32.Zhu Y, King M A, Schuller A G, Nitsche J F, Reidl M, Elde R P, Unterwald E, Pasternak G W, Pintar J E. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 33.Bohn L M, Gainetdinov R R, Lin F-T, Lefkowitz R J, Caron M G. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 34.Law P Y, Loh H H. J Pharmacol Exp Ther. 1999;289:607–624. [PubMed] [Google Scholar]