Abstract
β-Adrenergic receptor stimulation increases heart rate and shortens ventricular action-potential duration, the latter effect due in part to a cAMP-dependent increase in the slow outward potassium current (IKs). Mutations in either KCNQ1 or KCNE1, the IKs subunits, are associated with variants (LQT-1 and LQT-5) of the congenital long QT syndrome. We now show that cAMP-mediated functional regulation of KCNQ1/KCNE1 channels, a consequence of cAMP-dependent protein kinase A phosphorylation of the KCNQ1 N terminus, requires coexpression of KCNQ1 with KCNE1, its auxiliary subunit. Further, at least two KCNE1 mutations linked to LQT-5 (D76N and W87R) cause functional disruption of cAMP-mediated KCNQ1/KCNE1-channel regulation despite the response of the substrate protein (KCNQ1) to protein kinase A phosphorylation. Transduction of protein phosphorylation into physiologically necessary channel function represents a previously uncharacterized role for the KCNE1 auxiliary subunit, which can be disrupted in LQT-5.
Control of the rate and force of contraction of the heart by the autonomic nervous system, which consists of the sympathetic and parasympathetic divisions, is a fundamental property of the cardiovascular system. Stimulation of the sympathetic nervous system (SNS) in response to exercise or emotional stress results in a rapid and dramatic increase in heart rate that, to ensure adequate diastolic filling time between beats, is accompanied by a concomitant reduction of the ventricular action-potential duration at the cellular level and the corresponding QT interval of the electrocardiogram. Dysfunctional regulation of cardiac electrical activity in the face of SNS activity can lead to arrhythmias (1).
SNS control of cardiac electrical activity is mediated by the activation of β-adrenergic receptors that regulate the activity of select ion-channel proteins via cAMP-dependent protein kinase A (PKA). cAMP-dependent modulation of a slowly activating potassium-channel current, IKs (the slow outward potassium current), increases repolarization current and is key in SNS control of the QT interval. The role of IKs in this process is of particular interest and importance, because the genes that encode the subunit components of the IKs channel, KCNQ1 and KCNE1 (2, 3), have been linked to the congenital long-QT syndrome. Long-QT syndrome, a rare disease in which the QT interval of the electrocardiogram is prolonged because of dysfunctional ventricular repolarization, is associated with syncope, seizures, and sudden death (4). Mutations in KCNQ1, which codes for the α subunit of the IKs channel, cause LQT-1, and mutations in KCNE1, the gene coding for the auxiliary β subunit of the IKs channel, cause LQT-5 (5). In mutation carriers, triggers of arrhythmias are gene-specific, and carriers of mutations in either KCNQ1 or KCNE1 are at greatest risk of experiencing a fatal cardiac arrhythmia in the face of elevated SNS activity (6). Thus unraveling the molecular links between the SNS and regulation of the KCNQ1/KCNE1 channel has direct implications for the mechanistic basis of triggers of arrhythmias in this disorder. Furthermore, KCNQ1 is expressed with KCNE3 in the small intestine, colon, and kidney (7) and KCNE2 in stomach (8). Understanding a putative role of the KCNE1 subunit in cAMP-dependent regulation of KCNE1/KCNQ1 channels is of general interest to the problem of the physiological roles of these channels, and their regulation, in a wide range of tissues.
The KCNQ1/KCNE1 channel forms a macromolecular signaling complex that is coordinated by the binding of the targeting protein Yotiao (9) to a leucine-zipper motif (10, 11) in the KCNQ1 C-terminal domain (12). PKA and protein phosphatase 1, which bind to Yotiao, thus are recruited directly to the channel microdomain and regulate it by phosphorylation of a single N-terminal KCNQ1 residue, Ser-27 (12). Disruption of the leucine zipper by mutation, which ablates cAMP-dependent regulation of the channel, occurs for at least one previously reported LQT-1 mutation (12, 13). In the present study, we investigated the role of the auxiliary subunit KCNE1 in cAMP-dependent regulation of the KCNQ1/KCNE1 channel. We find that KCNQ1 phosphorylation is independent of coassembly with this subunit, but transduction of the phosphorylated channel into the physiologically essential increase in reserve channel activity requires the presence of KCNE1. We show further that LQT-5 mutations of KCNE1 can disrupt this functional regulation without interfering with cAMP-mediated phosphorylation of the channel. Thus our study reveals both a previously uncharacterized role of the KCNE1 subunit and mechanism by which signals from the brain to the heart can be uncoupled in cardiovascular disease.
Materials and Methods
Cell Culture and Transfection.
Chinese hamster ovary (CHO; American Type Cell Culture) cells were cultured in Ham's F12 medium and transiently transfected by using Lipofectamine with Lipofectamine-PLUS reagents (Invitrogen) as reported by us (12). Cells were passaged the day before transfection at 20–30% confluence and cultured in an incubator with 5% CO2. Dynabeads M-450 anti-CD8 beads (Dynal, Oslo) were used to visually identify cells cotransfected with CD8 (0.4 μg) and channel-subunit cDNAs of interest.
Electrophysiology.
Cells were plated in culture dishes placed on the stage of an inverted microscope (IMT-2, Olympus, New Hyde Park, NY), and currents were recorded by using the whole-cell patch-clamp technique with Axopatch 200A amplifiers (Axon Instruments, Foster City, CA) with solutions described for CHO cells (12). Series resistance was 2–8 MΩ. Activation (isochronal) of KCNQ1/KCNE1 current was studied by analysis of deactivating tail currents recorded at −40 mV after a series of 2-s activating pulses (20-mV increments) from a holding potential of −65 mV. Pulse frequency was 0.067 Hz unless noted otherwise. In each figure we show representative current traces recorded by using the same protocol [2-s activation pulse to +60 mV followed by a return pulse (2 s) to −40 mV] for each combination of subunits and constructs. In addition, we also show activation curves for each construct and combination measured from deactivating current tails (as above) recorded after a broad range of activating pulse voltages. The activation curves show tail-current amplitude plotted vs. activating test-pulse voltage. After rupture of the cell membrane, internal solutions without cAMP (control) or with 200 μM cAMP plus 0.2 μM okadaic acid (OA; Calbiochem) were dialyzed at room temperature for 13 min before measurements were made. PCLAMP 8.0 software (Axon Instruments) was used to both generate the voltage-clamp protocols and acquire data. Statistical significance was assessed with Student's t test for simple comparisons; differences at P < 0.05 were considered to be significant. Deactivating tail currents were fit with functions containing single exponential time components by using ORIGIN 6.1 (OriginLab, Northampton, MA) or PCLAMP software.
Molecular Biology.
A phospho-specific antibody was raised to recognize the following phosphorylated KCNQ1 N-terminal domain sequence: (C)-GARRGpSAGL. The antibody was raised in rabbits; purification was first with the nonphosphorylated peptide and then with the phosphorylated peptide (Zymed). The antibody was tested against back-phosphorylation (14) experiments (see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org) as well as direct phosphorylation assays for KCNQ1. Specificity was determined by ELISA and Western blotting after phosphorylation with PKA. Affinity-purified phospho-specific antibody (Zymed) specifically recognized only the phosphorylated form of KCNQ1. CHO cells cultured in 50-ml flasks were transfected with cDNAs for KCNQ1, KCNE1, and Yotiao (0.4, 0.4, and 2 μg, respectively). Two days after transfection, cells were incubated with 0.3 mM 8-bromo-cAMP (Sigma) and 1 μM OA at 37°C for 15 min before being harvested by mechanical scrapping. Cells then were lysed in a lysis buffer (150 mM NaCl2/1 mM EDTA/10 mM Tris, pH 7.5/1% Triton X-100). Cell lysates were resolved by 4–20% SDS/PAGE. Phosphorylated KCNQ1 channels were detected by using the rabbit anti-phospho-KCNQ1 antibody (1:250) and visualized by chemiluminescence with the ECL-plus Western blotting detection system (Amersham Pharmacia). A goat anti-KCNQ1 antibody (1:2,000, Santa Cruz Biotechnology) was used to detect total KCNQ1 channels in the lysate. Western blot images were scanned and analyzed by using Scion IMAGE BETA 4.0.2 (Scion, Frederick, MD) according to manufacturer instructions. In experiments designed to detect phosphorylation of KCNQ1 Ser-27 (pKCNQ1), intensities of images using the phospho-specific KCNQ1 antibody were normalized to those obtained with the Santa Cruz Biotechnology antibody (detecting total KCNQ1 protein) for each experiment to minimize possible effects of loading errors. The corrected signal for pKCNQ1 in the presence of cAMP/OA was normalized to the signal for pKCNQ1 in the absence of cAMP/OA. These ratios and number of experiments are reported in Results.
Results
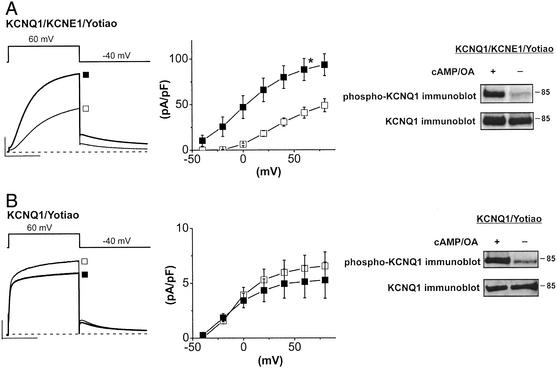
To investigate a possible role of KCNE1 in the PKA-mediated regulation of IKs channels, we studied the effects of cAMP and the phosphatase inhibitor OA on currents expressed in CHO cells. Dialysis of patch-clamped CHO cells expressing hKCNQ1, hKCNE1, and Yotiao with cAMP (0.2 mM) and OA (0.2 μM) increases the activity of KCNQ1/KCNE1 channels. As we have reported (12), this results in almost a 2-fold increase in channel activity that can be quantified by measuring the amplitude of deactivating current tails in the absence and presence of cAMP and OA (after +60-mV conditioning pulses: −cAMP/OA 40.5 ± 5.3 pA/pF, n = 6; +cAMP/OA 92.2 ± 11.9 pA/pF, n = 11; P < 0.05) (Fig. 1A). In addition to the effect on current amplitude, dialysis of cAMP/OA causes a negative shift in the voltage dependence of channel activation (V1/2 = 34.9 ± 1.8 mV, −cAMP/OA, n = 10; V1/2 = 4.1 ± 4.3 mV, +cAMP/OA, n = 10) (Fig. 1A; refs. 12, 15, and 16). However, in cells expressing only Yotiao and KCNQ1 without hKCNE1, there is no significant effect of intracellular cAMP and OA on KCNQ1-channel activity (after +60-mV conditioning pulses: −cAMP/OA, 6.3 ± 1.2 pA/pF, n = 5; +cAMP/OA, 5.6 ± 1.3 pA/pF, n = 7, not significant) (Fig. 1B).
Figure 1.
KCNE1 is required for functional transduction of PKA phosphorylation of KCNQ1. (A) cAMP-dependent regulation of the KCNQ1/KCNE1 channel by dialysis [cAMP (0.2 mM)/OA (0.2 μM)]. Shown are mean currents (Left) in the absence (open squares, n = 6) and presence (filled squares, n = 11, +60-mV pulse, −40-mV return) of cAMP/OA as well as plots of mean tail current ± SEM vs. activating pulse voltage (Center). *, P < 0.05, Student's t test. (Right) Immunoblot using anti-phospho-KCNQ1 (Upper) to detect phosphorylated protein and anti-KCNQ1 (Lower) to detect total KCNQ1 for cells incubated with cAMP/OA (+) or untreated cells (−) (Materials and Methods). CHO cells were transfected with KCNQ1, KCNE1, and Yotiao. (Scale, 100 pA/pF, 1 s.) (B) Requirement of coassembly of KCNE1 for cAMP-dependent regulation but not phosphorylation of KCNQ1 channels. KCNQ1 current was measured in CHO cells cotransfected with KCNQ1 and Yotiao in the absence (open squares, n = 5) and presence (filled squares, n = 7) of cAMP/OA. Records and plots are as described above. (Scale, 10 pA/pF, 1 s.) (Right) Immunoblot (as described above) of phosphorylated (Upper) and total (Lower) KCNQ1 measured in lysates of cells treated (+) or not treated (−) with cAMP/OA (Materials and Methods).
KCNE1 was not required for phosphorylation of KCNQ1 in these experiments. There was no measurable difference in phosphorylation of KCNQ1 in CHO cells transfected with KCNQ1 and Yotiao, plus or minus KCNE1, and then exposed to the membrane-permeant cAMP analog 8-bromo-cAMP (0.3 mM) plus OA (1 μM). Phosphorylation of KCNQ1 was detected by immunoblot with an antibody raised against an epitope that includes phosphorylated KCNQ1 Ser-27 (Materials and Methods; Fig. 1) as well as by back-phosphorylation of immunoprecipitated KCNQ1 (Fig. 5). Using image analysis of the intensity of immunoblots for KCNQ1 Ser-27 corrected for loading error by total KCNQ1 intensity (Materials and Methods), we found that exposure to cAMP + OA increased KCNQ1 phosphorylation 3.1- ± 0.2-fold (n = 4) with KCNE1 coexpression and 2.9- ± 0.2-fold (n = 3) without KCNE1 coexpression. KCNE1, which does not contain PKA consensus phosphorylation sites (17), is not a substrate for PKA phosphorylation (data not shown). Taken together, these results suggest that the transduction of PKA-dependent phosphorylation of KCNQ1 into an increase in channel activity requires the presence of the auxiliary subunit KCNE1.
Fig. 2 shows that variation in KCNE1/KCNQ1 stoichiometry can modulate the functional response of assembled channels. Dialysis of CHO cells transfected with Yotiao and fusion proteins in which one KCNE1 subunit was fused with two KCNQ1 subunits (1:2) with cAMP (0.2 mM) and OA (0.2 μM) caused only a 53% increase in expressed current amplitude (after +60-mV conditioning pulses: −cAMP/OA, 30.5 ± 4.7 pA/pF, n = 6; +cAMP/OA, 50.6 ± 5.2 pA/pF, n = 5; P < 0.05) (Fig. 2A). However, when cells were transfected with Yotiao, the same 1:2 KCNE1/KCNQ1–KCNQ1 fusion protein plus KCNE1, cell dialysis with cAMP (0.2 mM), and OA (0.2 μM) resulted in a 3-fold increase in current (after +60-mV conditioning pulses: −cAMP/OA, 21.7 ± 2.2 pA/pF, n = 6; +cAMP/OA, 76.7 ± 11.3 pA/pF, n = 10; P < 0.01) (Fig. 2B), which is not significantly different from the cAMP/OA-induced current increase in cells independently transfected with KCNE1 and KCNQ1 (Fig. 1A). These results confirm the importance of KCNE1 coexpression in the functional response of KCNE1/KCNQ1 channels to cAMP.
Figure 2.
KCNE1 modulates response of KCNE1–KCNQ1 fusion protein constructs to cAMP. (Insets) A fusion protein consisting of a previously reported KCNE1–KCNQ1 tandem multimer (29) fused to KCNQ1 without (A) and with (B) additional KCNE1. (A) Moderate increase in KCNE1–KCNQ1–KCNQ1 (1:2) fusion protein currents by dialysis of cAMP (0.2 mM) and OA (0.2 μM). CHO cells were transfected with the 1:2 fusion protein and Yotiao. Shown are mean currents (+60-mV pulse, −40-mV return, Lower Left) in the absence (open squares, n = 6) and presence (filled squares, n = 5) of cAMP/OA and plots of mean tail amplitude ± SEM vs. test-pulse voltage (Lower Right). *, P < 0.05, Student's t test (test-pulse voltages, +60 mV). (Scale, 100 pA/pF, 1 s.) (B) Additional KCNE1 cotransfection rescued cAMP-dependent regulation of KCNE1–KCNQ1–KCNQ1 (1:2) fusion protein (Upper) currents. Currents were recorded in CHO cells cotransfected with the 1:2 fusion protein, KCNE1, and Yotiao in the absence (open squares, n = 6) and presence (filled squares, n = 10) of cAMP/OA. Traces and plots are shown in A. **, P < 0.01, Student's t test (test-pulse voltages, +60 mV). (Scale, 100 pA/pF, 1 s.) (C) Summary of tail-current amplitudes after depolarization to +80 mV. Without dialysis of cAMP/OA (open bars), tail-current amplitudes recorded from the 1:2 fusion protein and the 1:2 fusion protein plus KCNE1 were not significantly different. When cAMP/OA was applied to CHO cells (gray bars), additional KCNE1 coexpression with the 1:2 fusion protein increased the mean tail-current amplitude significantly (P < 0.05, Student's t test). Number of experiments: 1:2 (without cAMP, n = 6; with cAMP/OA, n = 5) and 1:2 + KCNE1 (without cAMP, n = 6; with cAMP/OA, n = 10).
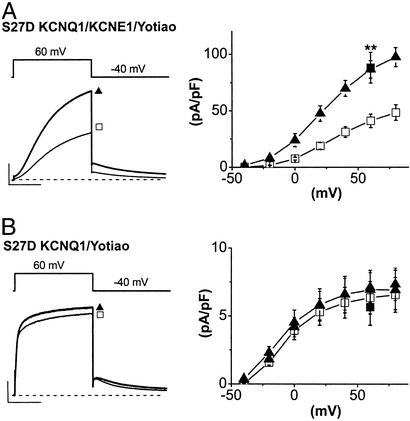
We next altered the charge of KCNQ1 residue 27 by substitution of aspartate for serine to, in part, simulate KCNQ1 phosphorylation and determine whether functional consequences of this altered charge also required coexpression of KCNQ1 and KCNE1. A similar approach has been used to identify functional consequences of PKA-dependent regulation of Kir 6.2 channels (18). We found indeed that the S27D mutation reconstituted most functional consequences of KCNQ1 phosphorylation when coexpressed with KCNE1. It enhanced current amplitude almost 2-fold despite the absence of exogenous cAMP (after +60-mV conditioning pulses: WT, 40.9 ± 6.0 pA/pF, n = 18; S27D, 86.5 ± 7.6 pA/pF, n = 6; P < 0.01) and caused a negative shift in channel activation of expressed current (V1/2 = 23.2 ± 4.2 mV, n = 6) (Fig. 3A). However, the S27D mutation neither significantly enhanced KCNQ1-channel activity nor altered activation voltage dependence without KCNE1 coexpression (Fig. 3B). In the absence of KCNE1, there was no significant difference between mean tail-current amplitudes (after +60-mV conditioning pulses) of WT and S27D mutant channels (WT, 6.3 ± 1.2 pA/pF, n = 5; S27D, 6.9 ± 1.4 pA/pF, n = 5; not significant). The addition of cAMP plus OA to these cells did not significantly further alter expressed channel activity (data not shown), supporting that phosphorylation of sites other than Ser-27 is not required for PKA-mediated regulation of KCNQ1/KCNE1 channels (12). These data suggest that most of the functional consequences of KCNQ1 phosphorylation are accounted for by the change in charge at residue 27 imposed by the S27D KCNQ1 mutation. Furthermore, the data indicate that physiologically relevant functional consequences of this change in charge require coexpression of KCNQ1 with the KCNE1 subunit. This result strongly suggests that PKA-dependent phosphorylation of KCNQ1 modulates channel activity via a change in charge at residue 27, and that coexpression with KCNE1 is required to transduce the altered residue to modified gating.
Figure 3.
Modulation of channel activity by aspartate substitution for KCNQ1 Ser-27 requires coassembly with KCNE1. (A) Enhancement of KCNQ1/KCNE1-channel current by S27D mutation of KCNQ1. Shown are mean currents (Left) and plots of tail amplitude vs. test-pulse voltage (Right) measured in CHO cells cotransfected with KCNQ1 (WT, open squares, n = 18; S27D, filled triangles, n = 6), KCNE1, and Yotiao. **, P < 0.01. Data from Fig. 1A (filled squares) (KCNQ1–KCNE1–Yotiao with cAMP/OA after depolarization to +60 mV) are superimposed for comparison. The mean currents shown (Left) were elicited by +60-mV pulse returning to −40 mV. (Scale, 100 pA/pF, 1 s.) (B) The S27D mutation does not increase current amplitude in the absence of KCNE1. Data are shown as described above except that current was measured in CHO cells cotransfected with KCNQ1 (WT, open squares, n = 5; S27D, filled triangles, n = 5) and Yotiao but without KCNE1. WT (KCNQ1 + Yotiao + cAMP/OA) tail amplitude after +60 mV from Fig. 1B (filled square) is superimposed for comparison. Mean current (Left) was elicited by a +60-mV pulse returning to −40 mV. (Scale, 10 pA/pF, 1 s.)
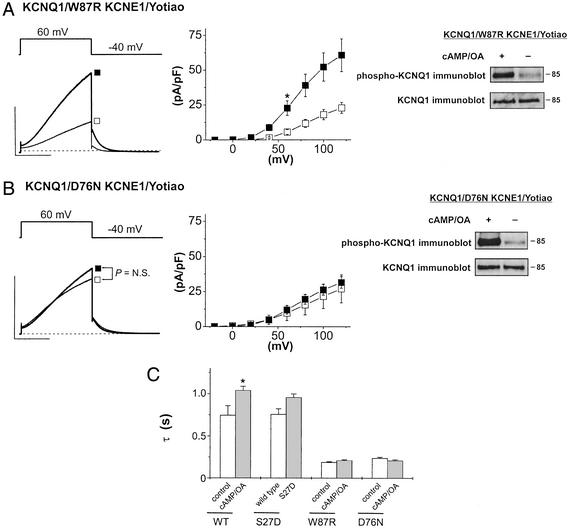
Because we have found that functional consequences of KCNQ1 phosphorylation require the presence of the KCNE1 subunit, we next tested for the possibility that KCNE1 mutations linked to LQT-5 might also alter functional regulation of expressed channels. We investigated the effects of two previously reported LQT-5 mutations in the KCNE1 C terminus W87R (19) and D76N (19) on both channel function and phosphorylation. Both mutants reduced basal channel activity by ≈50% when coexpressed with KCNQ1 in CHO cells compared with WT KCNE1 coexpressed with KCNQ1 (compare Figs. 1 and 4), but neither mutation interfered with KCNQ1 phosphorylation (Fig. 4 A and B). Image analysis of the intensity of immunoblots for KCNQ1 Ser-27 (Materials and Methods) revealed that cAMP + OA increased KCNQ1 phosphorylation 3.9- ± 1.6-fold (n = 3) with W87R coexpression and 3.6- ± 0.47-fold (n = 3) with D76N coexpression. The W87R mutation did not inhibit the cAMP-dependent increase in mean channel activity when expressed with KCNQ1 (2.7-fold increase in current tails after +60 mV: control, 5.7 ± 1.7 pA/pF, n = 7; cAMP/OA, 22.7 ± 5.2 pA/pF, n = 9; P < 0.05) (Fig. 4A). However, expression of the D76N mutation with KCNQ1 eliminated cAMP-mediated enhancement (after +60-mV conditioning pulses: control, 10.9 ± 7.0 pA/pF, n = 7; cAMP/OA, 12.0 ± 3.0 pA/pF, n = 8; not significant) (Fig. 4B).
Figure 4.
Inherited mutations of KCNE1 disrupt PKA-dependent functional regulation but not phosphorylation of KCNE1/KCNQ1 channels. (A) W87R KCNE1. Shown are KCNQ1/KCNE1 currents (Left) plot of tail current vs. pulse voltage (Middle) measured in CHO cells cotransfected with KCNQ1, W87R KCNE1, and Yotiao without (open squares, n = 7) or with (filled squares, n = 9) cAMP/OA in the recording pipette (*, P < 0.05). Mean currents shown were elicited by +60-mV pulses returning to −40 mV. (Scale, 50 pA/pF, 1 s.) (Right) Immunoblot as described for Fig. 1 for phosphorylated (Upper) and total (Lower) KCNQ1 from cells transfected with KCNQ1, W87R KCNE1, and Yotiao and treated (+) or not treated (−) with cAMP/OA (Materials and Methods). (B) D76N KCNE1. As described above, KCNQ1/KCNE1 current was measured in CHO cells cotransfected with KCNQ1, D76N KCNE1, and Yotiao without (control, open squares, n = 7) or with (filled squares, n = 8) cAMP/OA in the recording pipette. Mean currents were elicited by +60-mV pulses returning to −40 mV, and the plot shows tail-current amplitude vs. test-pulse voltage. (Scale, 50 pA/pF, 1 s.) (Right) Immunoblot of phosphorylated (Upper) and total (Lower) KCNQ1 from cells transfected with KCNQ1, D76N KCNE1, and Yotiao and treated (+) or not treated (−) with cAMP/OA (Materials and Methods). (C) Disruption cAMP-dependent slowing of channel deactivation by both LQT-5 mutations. The plot shows mean ± SEM of time constants determined from the decay of current tails at −40 mV after depolarization to +60 mV (WT and S27D) or +100 mV (W87R and D76N). Cells were transfected as follows (Yotiao was included in all experiments): WT (KCNQ1 + KCNE1); S27D (S27D + KCNE1); W87R (KCNQ1 + W87R); and D76N (KCNQ1 + D76N). Comparisons are for currents measured without (open bar) and with (filled bar) cAMP/OA in the recording pipette except for S27D, for which the open bar represents WT channels and the filled bar represents S27D channels, both without cAMP/OA. Number of experiments: WT (−, n = 6; +, n = 11), S27D (WT, n = 7; S27D, n = 9), W87R (−, n = 7; +, n = 9), and D76N (−, n = 7; +, n = 8). *, P < 0.05.
Channel deactivation at −40 mV after voltage depolarization was well fit by single exponential functions (Materials and Methods). Time constants extracted from this analysis are summarized in Fig. 4C. PKA-dependent phosphorylation of KCNQ1 significantly increased the time constant of channel deactivation of KCNQ1/KCNE1 channels as did the KCNQ1 mutant S27D when coexpressed with KCNE1 (compare open and filled bars, Fig. 4). Both the W87R and D76N mutations coexpressed with KCNQ1 sped channel deactivation relative to WT KCNE1/KCNQ1 channels (Fig. 4C, open bars), and PKA-dependent phosphorylation [dialysis of cells with cAMP (0.2 mM) + OA (0.2 μM)] had no significant effect on tail kinetics (Fig. 4). Thus, transduction of PKA-dependent phosphorylation of KCNQ1 into altered channel kinetics requires coassembly with the KCNE1 subunit, and inherited KCNE1 mutations can disrupt this key functional property.
Discussion
Regulation of the cardiac ventricular action-potential duration by the SNS is due in part to β-adrenergic receptor-mediated stimulation of the activity of KCNQ1/KCNE1 channels, which provides a reserve of outward current that acts to speed the repolarization process (20). This stimulation, a consequence of PKA phosphorylation of a single residue (Ser-27) of the KCNQ1 subunit, is coordinated by a C-terminal domain macromolecular signaling complex (12). The importance of the KCNQ1/KCNE1 channel and its regulation is clear, because carriers of mutations in either KCNQ1 (LQT-1) or KCNE1 (LQT-5), which reduce the activity of KCNQ1/KCNE1 channels in general (2, 3, 5, 21, 22), or of mutations that specifically disrupt the signaling complex (13, 23), are at greatest risk of triggered arrhythmias in the face of increased SNS stimulation (6, 24, 25). The arrhythmia risk most likely is due to mutation-induced imbalance in SNS control of key ion-channel targets, which include KCNQ1/KCNE1 channels, L-type calcium channels (20), and the ryanodine receptor, an intracellular calcium-release channel (26). Because it is now understood that these channels are controlled by local signaling complexes (12, 26, 27), inherited mutations can uncouple any combination of these substrates from SNS-mediated modulation.
The discovery that the KCNQ1/KCNE1 channel is a macromolecular complex that includes the assembly of signaling molecules coordinated by the targeting protein Yotiao was a first step in unraveling roles that disease-linked mutations may have in disruption of critical signaling pathways linking the brain with the heart (12). Phosphorylation of the KCNQ1/KCNE1 channel, mediated by the SNS, causes at least two important functional changes in channel activity: an increase in current density during and after activating depolarizing pulses and, importantly, a slowing of the deactivation of channels after the termination of activating pulses (15). Because KCNQ1/KCNE1 channels activate with a slow time course during depolarization (in vivo the depolarizing event is the ventricular action potential), during each action potential the accumulation of open KCNQ1/KCNE1 channels ultimately will be one of the key determinants of ventricular action potential, and hence QT interval, duration. SNS-mediated increase in channel activity will provide more channel activity per action potential and hence will tip the balance of currents in favor of repolarization earlier than in the absence of SNS stimulation, leading to action-potential duration shortening. Additionally, because β-adrenergic receptor stimulation slows the deactivation of this channel activity between beats, the accumulation of open KCNQ1/KCNE1 channels will grow faster on a beat-by-beat basis with rather than without SNS stimulation (28).
Our data show that these functional effects of KCNQ1 phosphorylation require coexpression with WT KCNE1 subunits, and that the S27D KCNQ1 mutation mimics most of the effects of channel phosphorylation. In the absence of KCNE1, there is no significant effect of KCNQ1 phosphorylation on expressed channel activity. Notably, without coexpression of KCNE1, phosphorylation of KCNQ1 does not cause a hyperpolarization of channel activation, suggesting that KCNE1-altered gating may be a requirement to transduce the phosphorylation into modulated channel activity. Furthermore, even point mutations of KCNE1 can severely disrupt the important physiologically relevant functional consequences KCNQ1 phosphorylation as evidenced by the D76N and W87R mutations. Both the D76N and W87R mutations reduce basal current density when expressed with WT KCNQ1 subunits. This effect would be expected to reduce repolarizing current, prolong cellular action potentials, and contribute to prolonged QT intervals in mutation carriers even in the absence of SNS stimulation. However, in addition, the mutations would be expected to eliminate or reduce physiologically important reserve K+-channel activity in the face of SNS stimulation. The W87R mutation speeds channel-deactivation kinetics, which then are not slowed by cAMP. Thus, the data suggest that the W87R mutation eliminates the important cAMP-dependent accumulation of channel activity that is the normal response to the SNS. The D76N mutation ablates functional regulation of the channels by cAMP. The result is an expected delay in the onset of repolarization that is more pronounced in the face of SNS stimulation. In the case of both mutations studied, our data, obtained in a mammalian cell line, suggest that mutation carriers would be expected to have inadequate reserve K+-channel activity to offset SNS-mediated stimulation of L-type Ca2+ channels and thus not adequately control action-potential duration in the face of elevated sympathetic tone.
The present work demonstrates the important functional role played by the KCNE1 subunit in linking SNS signaling to functional changes in a key ion channel in the human heart: the KCNQ1/KCNE1 channel. Disease-linked mutations in KCNE1 do not uncouple the assembled channel complex from SNS-derived molecular signals as do mutations of the KCNQ1 C-terminal leucine-zipper motif (12) but instead can alter, in physiologically significant manners, the functional response of the phosphorylated channel complex to these signals. The uncoupling of functional consequences of channel phosphorylation by inherited mutation of an auxiliary subunit thus is a previously uncharacterized mechanism that can contribute to risk of cardiac events in the face of SNS activity in carriers of some KCNE1 mutations. Because KCNQ1 is expressed in multiple tissues with other members of the KCNE gene family, our results also suggest that tissue-specific functional regulation of KCNQ1-channel complexes very likely could be determined by KCNE family members with which KCNQ1 associates (7, 8).
Supplementary Material
Acknowledgments
We thank Dr. S. O. Marx for help with design of anti-phospho KCNQ1; Drs. S. Reiken and S. Feinmark for help with phosphorylation and back- phosphorylation assays; and Drs. C. E. Clancy and A. R. Marks for helpful discussion. J.K. was supported by American Heart Association Postdoctoral Fellowship 0120282T. This work was supported by National Institutes of Health Grants HL P01-67849 and HL R01-32557 (to R.S.K.).
Abbreviations
- SNS
sympathetic nervous system
- PKA
protein kinase A
- IKs
the slow outward potassium current
- CHO
Chinese hamster ovary
- OA
okadaic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wit A L, Hoffman B F, Rosen M R. Am Heart J. 1975;90:795–803. doi: 10.1016/0002-8703(75)90471-8. [DOI] [PubMed] [Google Scholar]
- 2.Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 3.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 4.Keating M T, Sanguinetti M C. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 5.Splawski I, Shen J, Timothy K W, Lehmann M H, Priori S, Robinson J L, Moss A J, Schwartz P J, Towbin J A, Vincent G M, et al. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz P J, Priori S G, Spazzolini C, Moss A J, Vincent G M, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating M T, et al. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder B C, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch T J. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 8.Attali B. Trends Pharmacol Sci. 2002;23:249–251. doi: 10.1016/s0165-6147(02)02029-1. [DOI] [PubMed] [Google Scholar]
- 9.Lin J W, Wyszynski M, Madhavan R, Sealock R, Kim J U, Sheng M. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landschulz W H, Johnson P F, McKnight S L. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 11.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 12.Marx S O, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks A R, Kass R S. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 13.Piippo K, Swan H, Pasternack M, Chapman H, Paavonen K, Viitasalo M, Toivonen L, Kontula K. J Am Coll Cardiol. 2001;37:562–568. doi: 10.1016/s0735-1097(00)01124-4. [DOI] [PubMed] [Google Scholar]
- 14.Marx S O, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks A R. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.Walsh K B, Kass R S. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 16.Walsh K B, Kass R S. Am J Physiol. 1991;261:C1081–C1090. doi: 10.1152/ajpcell.1991.261.6.C1081. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal E M, Kaczmarek L K. J Neurosci. 1992;12:290–296. doi: 10.1523/JNEUROSCI.12-01-00290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y F, Jan Y N, Jan L Y. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi L, Shen Z, Dennis A T, Priori S G, Napolitano C, Ronchetti E, Bryskin R, Schwartz P J, Brown A M. Hum Mol Genet. 1999;8:1499–1507. doi: 10.1093/hmg/8.8.1499. [DOI] [PubMed] [Google Scholar]
- 20.Kass R S, Wiegers S E. J Physiol (London) 1982;322:541–558. doi: 10.1113/jphysiol.1982.sp014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roden D M, Spooner P M. J Cardiovasc Electrophysiol. 1999;10:1664–1683. doi: 10.1111/j.1540-8167.1999.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 22.Splawski I, Tristani-Firouzi M, Lehmann M H, Sanguinetti M C, Keating M T. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 23.Paavonen K J, Swan H, Piippo K, Hokkanen L, Laitinen P, Viitasalo M, Toivonen L, Kontula K. Heart. 2001;86:39–44. doi: 10.1136/heart.86.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss A J, Zareba W, Hall W J, Schwartz P J, Crampton R S, Benhorin J, Vincent G M, Locati E H, Priori S G, Napolitano C, et al. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu W, Antzelevitch C. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 26.Marx S O, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang Y M, Rosemblit N, Marks A R. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulme J T, Ahn M, Hauschka S D, Scheuer T, Catterall W A. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan P C, Shaw R M, Rudy Y. Circulation. 1999;99:2466–2474. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Xia J, Kass R S. J Biol Chem. 1998;273:34069–34074. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.