Abstract
Thrombospondin 2 (TSP2)-null mice, generated by disruption of the Thbs2 gene, display a variety of connective tissue abnormalities, including fragile skin and the presence of abnormally large collagen fibrils with irregular contours in skin and tendon. In this study we demonstrate that TSP2-null skin fibroblasts show a defect in attachment to a number of matrix proteins, and a reduction in cell spreading. To investigate the molecular mechanisms responsible for these abnormal cell–matrix interactions, we compared the levels of matrix metalloproteinases (MMPs) in wild-type and mutant fibroblasts. Isolation and analysis of gelatinases from conditioned media by gelatin-agarose affinity chromatography and gelatinolytic assays demonstrated that TSP2-null fibroblasts produce a 2-fold increase in gelatinase A (MMP2) compared with wild-type cells. The adhesive defect was corrected by treatment of TSP2-null fibroblasts with soluble TSP2, with the MMP inhibitors BB94 and tissue inhibitor of metalloproteinase-2, and with a neutralizing antibody to MMP2. Moreover, stable transfection of TSP2-null fibroblasts with mouse TSP2 cDNA corrected both the adhesive defect and the altered expression of MMP2. Finally, MMP2 was shown to interact with TSP2 in a direct-binding plate assay. We conclude that TSP2 plays an important role in cell–matrix interactions, and that a deficiency in the protein results in increased levels of MMP2 that contribute to the adhesive defect in TSP2-null fibroblasts and could play a role in the complex phenotype of TSP2-null mice.
INTRODUCTION
Thrombospondin 2 (TSP2) is a secreted extracellular matrix glycoprotein whose functions are diverse and poorly understood (Bornstein and Sage, 1994; Adams et al., 1995; Kyriakides et al., 1998a). TSP2, together with its closest relative TSP1, and tenascin-C, osteopontin, and secreted protein acidic and rich in cysteine (SPARC) have been termed matricellular proteins to reflect a growing awareness that these functionally related proteins play a role as adaptors and modulators of cell–matrix interactions (Bornstein, 1995). Matricellular proteins do not subserve a primary structural role, in the sense that most collagens, laminins, and elastin are structural proteins. Rather, the complex nature of their functions derives from their ability to interact with multiple cell-surface receptors, cytokines, growth factors, proteases, and structural proteins. The contextual nature of their function thus reflects the composition of the matrix, the availability of cytokines and proteases, and the expression of integrins and other receptors in a given cellular environment. The expression of matricellular proteins is most prominent during development and growth, and in response to injury. In cells cultured in serum-containing medium, this response to injury has been termed “culture shock” (Sage, 1986).
TSP2 has been shown to bind heparan sulfate proteoglycans, low-density lipoprotein receptor-related protein (LRP), and the integrin αvβ3 (Chen et al., 1994, 1996). These molecules are also receptors for TSP1 and it is thought that TSP2, in view of its overall structural similarity to TSP1, may also serve as a ligand for other known TSP1 receptors (Bornstein, 1995). TSP2 has also been shown to inhibit the angiogenic activity of basic fibroblast growth factor in a corneal assay (Volpert et al., 1995), mitogenesis and formation of focal adhesions in bovine aortic endothelial cells (Murphy-Ullrich et al., 1993; Panetti et al., 1997), and the spreading of bovine adrenocortical cells (Pellerin et al., 1994). However, the functional properties of TSP2 in vivo remain elusive.
In an effort to understand the biological role of this matricellular protein, we generated TSP2-null mice by disruption of the Thbs2 gene in murine embryonic stem cells, followed by blastocyst injection and appropriate breeding of mutant animals (Kyriakides et al., 1998a). Mice that lack TSP2 develop a pleiotropic phenotype characterized by morphological changes in connective tissues, increased endosteal bone growth, an increase in vascular density, and a bleeding diathesis. The skin of mutant mice is fragile and has reduced tensile strength. Histological analysis of skin showed that collagen fibers are disorganized and lack the normal predominant parallel orientation to the epidermal surface, and examination of these fibers at the electron microscopic level revealed the presence of abnormally large collagen fibrils with irregular contours in tissues from mutant animals. Although the presence of TSP2 as a constituent of collagen fibers could not be documented in the developing or adult mouse (Kyriakides et al., 1998b), TSP2 was found to colocalize with collagen fibers in the tissue responses to injury that accompanied the foreign body reaction (Kyriakides et al., 1999a) and wound healing (Kyriakides et al., 1999b).
TSP2-null dermal fibroblasts were found to aggregate on bacteriological plastic or glass surfaces, and were more sensitive to release by trypsin from tissue culture plastic than were control cells (Kyriakides et al., 1998a). These findings suggested that TSP2-null cells have a defect in cell–matrix interactions, and/or increased cell–cell interactions, and ran counter to expectations because a peptide derived from TSP2 had been shown to destabilize focal adhesions in endothelial cells in vitro (Murphy-Ullrich et al., 1993). To understand better the role of TSP2 in cell–matrix and/or cell–cell interactions, and to elucidate the molecular mechanisms responsible for the abnormalities in TSP2-null mice, we have characterized the fibroblast adhesive defect in greater detail. In the present study we show that matrix metalloproteinase 2 (MMP2, gelatinase A) is increased in TSP2-null mouse dermal fibroblasts. Replacement of TSP2 by stable transfection of TSP2-null cells with TSP2 cDNA corrected the adhesive defect and restored normal MMP2 activity. Treatment of TSP2-null fibroblasts with recombinant TSP2, with the MMP inhibitors BB94 (Batimastat; [4-(N-hydroxyamino)-2R-isobutyl-3S-thienylthiomethyl-succinyl]-l-phenylalanine-N-methylamide) and tissue inhibitor of metalloproteinase-2 (TIMP-2), and with a neutralizing antibody to MMP2 also corrected the adhesive defect. Finally, TSP2 was shown to bind MMP2 directly. These observations provide a biochemical rationale for the development of an adhesive defect in cells that lack a protein with counteradhesive properties (Sage and Bornstein, 1991). The implications of these findings for the mode of action of TSP2, its role in cell–matrix interactions, and the phenotype of the TSP2-null mouse are discussed.
MATERIALS AND METHODS
Cell Culture and Cytochemistry
Skin fibroblasts were isolated by explant culture of biopsies taken from the backs of adult (2- to 3-mo-old) mice as described previously (Kyriakides et al., 1998a). Briefly, after removal of hair, specimens were cut into 1-mm3 fragments and allowed to adhere to the surface of 100-mm tissue culture dishes. Fragments derived from a 2-cm2 segment of skin were plated in a 100-mm dish in DMEM, supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin. Cells were passaged when the explant-derived cells had become confluent. After 2–3 passages, the cell population appeared, by light microscopy, to be composed entirely of fibroblasts. For cytochemical analysis, dermal fibroblasts were plated on chamber slides for 24 h in the presence of serum. The cells were then fixed with 2% paraformaldehyde in phosphate-buffered saline for 30 min at room temperature and actin cytoskeletons were visualized by staining with phalloidin. Immunocytochemistry with anti-TSP2 antibodies was performed as previously described (Kyriakides et al., 1998b).
Cell Attachment Assay
Confluent fibroblasts in culture were trypsinized, washed three times, and resuspended in serum-free medium containing 0.1% bovine serum albumin (BSA). The cell suspension was adjusted to a final concentration of 1 × 105 cells/ml, plated in wells (100 μl/well) of a 96-well tissue culture plate, and subsequently incubated at 37°C for 60 min. The wells were either untreated or coated overnight at 4°C with 100 μl/well of fibronectin, vitronectin, type I collagen, or TSP2 (Kyriakides et al., 1998b), each at 5 μg/ml in phosphate-buffered saline, except for type I collagen, which was coated in 0.01 N HCl. Laminin 5-coated multiple-well tissue culture plates were a gift from Dr. William Carter (Fred Hutchinson Cancer Research Center, Seattle, WA). Nonspecific cell-binding sites were blocked by the addition of 100 μl/well of 1% BSA in DMEM, and the plates were incubated at 4°C for 60 min before cells were added. Unattached cells in the 96-well tissue culture plates were removed by washing the plates gently with DMEM three times. The attached cells were determined by the CellTiter96 assay (Promega, Madison, WI) following the protocol of the manufacturer. Color yields, after a 60-min incubation at 37°C, were measured by the absorbance at 490 nm in a microplate reader with a SOFTmax PRO software (Molecular Devices, Sunnyvale, CA). In this assay an absorbance of 0.5 represents ∼5 × 104 attached cells. Alternatively, attached cells were fixed with 10% formalin in saline, stained with 1% methylene blue, and absorbance was measured at 650 nm as described previously (Kyriakides et al., 1998a).
Zymography
Fibroblast monolayer cultures, grown in DMEM supplemented with 10% fetal bovine serum, were switched to serum-free media and incubated for 18–20 h. The conditioned media were concentrated with a Centricon-10 (Amicon, Danvers, MA) and subjected to SDS-PAGE under nonreducing conditions in 7.5% acrylamide gels containing 0.1% gelatin. Protein concentrations were determined by the Lowry method and equal amounts of proteins were applied to the gel. After electrophoresis, the gels were washed with gentle shaking for 2 h at room temperature in 50 mM Tris-HCl buffer (pH 7.5) containing 100 mM NaCl and 2.5% Triton-X 100 to remove SDS. The gels were then incubated at 37°C with shaking for 18–20 h, in the same buffer containing 10 mM CaCl2, and subsequently stained with Coomassie blue. Zones of proteolysis appeared as clear bands against a blue background and were quantified with ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Gelatin-Agarose Affinity Chromatography and Western Blotting
Serum-free conditioned media or lysates of mouse skin fibroblasts were subjected to gelatin-agarose (Sigma, St. Louis, MO) affinity chromatography to isolate matrix metalloproteinases (MMPs). Radioimmunoprecipitation buffer composed of 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS was used to extract cellular proteins from fibroblasts. The extracts were dialyzed against the gelatin-agarose affinity chromatography starting buffer composed of 50 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 5 mM CaCl2, 0.05% Brij-35, and 0.02% NaN3. After a sample was applied to the gelatin-agarose affinity column, the column was washed thoroughly with starting buffer. Bound fractions were eluted with 7.5% dimethyl sulfoxide in starting buffer, pooled, dialyzed against 50 mM Tris-HCl, pH 7.5, 5 mM CaCl2, and 0.01% Brij-35, and concentrated with a Centricon-10 filter. The isolated proteins were then subjected to SDS-PAGE. Separated proteins were transferred electrophoretically (Towbin et al., 1979) from polyacrylamide gels to nitrocellulose membranes in a mini trans-blot cell (Bio-Rad, Hercules, CA) for 1 h at 100 V, followed by Western blot analysis with anti-MMP2 and anti-MMP9 polyclonal antibodies (Chemicon International, Temecula, CA). The resulting antigen–antibody complexes were detected by incubation with alkaline phosphatase-conjugated antibody and substrate (Bio-Rad). The separated proteins were also examined by zymography for gelatinase activity, as described above.
Gelatinolytic Assay
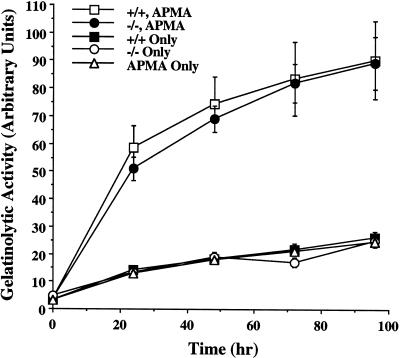
The activity of isolated MMP2 was determined by a gelatinolytic assay with soluble gelatin as a substrate (Lafuma et al., 1994). The gelatin was radiolabeled with [3H]acetic acid according to Cawston and Barrett (1979). Two micrograms of MMP2, isolated from cell culture-conditioned media, was incubated at 37°C for 2 h, with or without activation by 1 mM 4-aminophenylmercuric acetate (APMA) in 100 μl containing 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM CaCl2, and 0.01% Brij-35. Fifty micrograms of radiolabeled gelatin was heat denatured at 60°C for 15 min, cooled to 37°C, and added to the incubation mixture in a final volume of 200 μl. The reaction was allowed to proceed for 24, 48, 72, and 96 h at 37°C in the presence of 0.03% toluene to prevent bacterial contamination. Undegraded gelatin was precipitated at 4°C with a mixture of trichloracetic acid and tannic acid to a final concentration of 4 and 0.8%, respectively. The reaction mixture was centrifuged at 10,000 × g for 15 min at 4°C. Aliquots of the resulting supernatants were counted for radioactivity in a Beckman (Fullerton, CA) liquid scintillation counter.
RNA Analysis
Total RNA was extracted from confluent dermal fibroblast cultures with acid guanidinium thiocyanate-phenol-chloroform (Chomczynski and Sacchi, 1987). The absence of RNA degradation was checked by agarose gel electrophoresis with ethidium bromide staining. For quantitative assessment, 10–15 μg of total RNA was subjected to Northern hybridization analysis according to Ausubel et al. (1987). The cDNA probes pSP65 (for MMP2) and HS-026 (for MMP9) were provided by Dr. Zena Werb (University of California, San Francisco, CA) and Dr. Helene Sage (Hope Heart Institute, Seattle, WA), respectively. DECA probe specific for mouse β-actin was purchased from Ambion (Austin, TX). The cDNA inserts used as hybridization probes were separated from plasmid DNA on agarose gels, purified with silica-gel membranes (Qiagen, Valencia, CA), and radiolabeled with [α-32P]dCTP by multiprime DNA-labeling systems (Amersham Pharmacia Biotech, Piscataway, NJ). For reverse transcription-polymerase chain reaction (RT-PCR) analysis, 5 μg of total RNA was used for reverse transcription with Moloney murine leukemia virus reverse transcriptase and random primers (Stratagene, La Jolla, CA). Specific cDNA was amplified with Taq polymerase (Promega) and primers for TSP2. The forward and reverse primers, TS2G-A (5′-CTGGTGACCACGTCAAGGACACTTCAT-3′) and TS2G-B (5′-ATGCACCTTTGGCCACGTACATCCTGC-3′), result in the synthesis of a 539-bp exon 3 fragment of TSP2. RT-PCR products were separated on 2% agarose gels and were visualized by staining with ethidium bromide.
Treatment of Fibroblasts with Recombinant TSP2, MMP Inhibitors, and a Neutralizing Antibody to MMP2
Mouse recombinant TSP2 was prepared in insect cells as previously described (Kyriakides et al., 1998b). The MMP inhibitor BB94 was a kind gift from Dr. Alexander Clowes (University of Washington, Seattle, WA). Recombinant human TIMP-2 was purchased from Chemicon International. An affinity-purified rabbit anti-MMP2 neutralizing antibody, which had been shown to block specifically the proteolytic activity of MMP2 (Ray and Stetler-Stevenson, 1995; O'Reilly et al., 1999), was a kind gift from Dr. William G. Stetler-Stevenson (National Institutes of Health, Bethesda, MD). Normal rabbit IgG was purchased from Vector Laboratories (Burlingame, CA), and was used as a control. Dermal fibroblasts were incubated in cell culture media containing TSP2 at concentrations up to 80 nM, BB94 at concentrations up to 40 μM, TIMP-2 at 1 or 5 μg/ml, or anti-MMP2 at 2 μg/ml for 48 h, before analysis for attachment on fibronectin as described above.
Interaction of TSP2 with MMP2
Recombinant mouse full-length TSP2 was produced in insect cells and purified as previously described (Kyriakides et al., 1998b). Human TSP1 was purchased from Hematologic Technologies (Essex Junction, VT). Human MMP2 and rabbit anti-MMP2 polyclonal antibody were purchased from Chemicon International. Another preparation of human MMP2 was a kind gift of Dr. Christopher Overall (University of British Columbia, Vancouver, British Columbia, Canada). For the direct-binding enzyme-linked immunosorbent assay, 96-well microtiter plates (Linbro; Flow Laboratories, McLean, VA) were coated with TSP1, TSP2, fibronectin, and BSA (10 μg/ml, 50 μl/well) in 0.1 M Tris-HCl, pH 7.2, at 4°C overnight. The plates were blocked with 1% BSA in the same buffer containing 5 mM CaCl2 for 1 h at room temperature. MMP2 (4 μg/ml) in blocking solution was then added to wells for 2 h. Subsequently, rabbit anti-MMP2 antibody (1 μg/ml) was added for another 2 h, followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma). Color was developed with p-nitrophenyl phosphate substrate (1 mg/ml) in 10 mM diethanolamine, pH 9.5, containing 0.5 mM MgCl2. Between each incubation the wells were washed with Tris-HCl buffer to remove unbound protein. OD405 was measured in a microplate reader with SOFTmax PRO software (Molecular Devices).
Generation of TSP2-deficient Immortomice
Male Immortomice, purchased from Charles River Laboratories (Wilmington, MA), were mated with female TSP2-null mice. Heterozygous offspring were then bred to produce homozygous TSP2-null mice carrying the conditionally immortalizing simian virus 40 (SV40) large T antigen, H-2Kb-tsA58, as a transgene (Jat et al., 1991). The mice were analyzed for the TSP2 mutation by Southern blot analysis of tail DNA (Kyriakides et al., 1998a). The presence of the SV40 transgene was determined by PCR with forward primer 5′-AGCGCTTGTGTCGCCATTGTATTC-3′ and reverse primer 5′-GTCACACCACAGAAGTAAGGTTCC-3′, following the instructions of Charles River Laboratories. Amplification resulted in a ∼1000-bp DNA fragment.
Construction of Expression Vectors and DNA Transfection
Sense and antisense TSP2 expression plasmids were generated by ligation of a 3.5-kb pair EcoRI fragment of mouse TSP2 (mTSP2) cDNA into the mammalian expression vector pZeoSV (Invitrogen, San Diego, CA). The size and orientation of inserts were confirmed by restriction digestion with XhoI. For DNA transfection, dermal fibroblasts derived from TSP2-deficient Immortomice were used and maintained under fully permissive conditions, defined as growth at 33°C in the presence of 100 U of interferon-γ (Life Technologies, Gaithersburg, MD) per milliliter (Jat et al., 1991). Cells were transfected by Lipofectin with the PerFect lipid transfection kit (Invitrogen), and were grown in the presence of Zeocin, 0.2 mg/ml, to obtain stably transfected cell populations. After 2–3 wk, resistant cells were expanded as populations and screened by RT-PCR and Western blotting of total cell lysates to confirm the expression of TSP2. Two independent transfections were performed; resistant populations from the two experiments behaved identically. For adhesion and other assays, immortalized fibroblasts and transfected cells were cultured at 37°C in the absence of interferon-γ for 2–3 days before experiments.
RESULTS
TSP2-null Dermal Fibroblasts Exhibit an Adhesive Defect on a Variety of Substrates
We have previously reported that dermal fibroblasts, derived from Thbs2 −/− mice, aggregate on bacteriological plastic or glass surfaces and show an attachment defect in the presence of serum (Kyriakides et al., 1998a). In the current study, cell attachment assays were performed in the absence of serum, and the behavior of dermal fibroblasts, derived from both wild-type and TSP2-null animals, was compared. On tissue culture plastic, attachment of TSP2-null cells was significantly decreased compared with the wild-type cells (Figure 1A). Coating of the surface with fibronectin or laminin 5 increased the attachment of both wild-type and TSP2-null cells, but did not correct the adhesive defect of TSP2-null cells (Figure 1A), nor did coating with TSP2 itself (Figure 1B) or with vitronectin or type I collagen (our unpublished results).
Figure 1.
(A) Comparison of attachment of skin fibroblasts from TSP2 +/+ and TSP2 −/− mice. Fibroblasts were suspended in serum-free medium and allowed to attach to noncoated (NC), or to fibronectin (FN, 5 μg/ml)- or laminin 5 (L5, 5 μg/ml)-coated tissue culture plastic surfaces for 60 min at 37°C. TSP2-null fibroblasts showed a defect in adhesion to tissue culture plastic. Coating of tissue culture plates with FN or L5 did not correct the attachment defect. (B) Comparison of attachment of dermal fibroblasts on plastic and TSP2-coated surfaces. TSP2 does not correct the attachment defect. Conditions are described in A. All differences are significant (P < 0.005); n = 4.
TSP2-null fibroblasts also show a spreading defect and an abnormal actin cytoskeletal morphology that is indicative of compromised cell–matrix interactions and is consistent with the defect in attachment. When wild-type and TSP2-null fibroblasts were plated for 1 h on fibronectin-coated chamber slides in the absence of serum, control cells attached and displayed a spread morphology, whereas TSP2-null cells remained rounded (Figure 2, top). When cells were plated in the presence of serum for 24 h and stained with phalloidin, control cells appeared well-spread with clearly defined stress fibers, whereas mutant cells showed less spreading and a peripheral deposition of actin (Figure 2, bottom). Thus, the adhesive defect in mutant cells consists of abnormalities in both cell attachment and spreading.
Figure 2.
Spreading and cytoskeletal morphology of skin fibroblasts in vitro. Top, phase contrast micrograph of control (left) and TSP2-null (right) fibroblasts plated for 1 h on fibronectin-coated chamber slides in the absence of serum. Control cells attach and display spread morphology (arrowheads), whereas TSP2-null cells remain rounded. Bar, 25 μm. Bottom, fibroblasts from control (left) and TSP2-null (right) mice were plated on chamber slides for 24 h in the presence of serum. The cytoskeleton was visualized by staining with phalloidin. Control cells appeared well-spread, with clearly defined stress fibers, whereas mutant cells showed less spreading and a peripheral deposition of actin. Bar, 100 μm.
TSP2-null Dermal Fibroblasts Produce Increased Levels of MMP2
In an attempt to determine the cause of the adhesive defect in TSP2-null fibroblasts, we analyzed serum-free conditioned media and cell lysates from dermal fibroblast cultures by zymography. Proteins were fractionated by SDS-PAGE under nonreducing conditions in acrylamide gels containing 0.1% gelatin. A protease with a molecular mass of 72 kDa, corresponding to that of pro-MMP2, was found in both the conditioned media and cell lysates from dermal fibroblasts (Figure 3). This gelatinase was significantly increased in TSP2-null cells compared with wild-type cells. The doublet of MMP2 is not unusual (Haas et al., 1998) and is probably due to the presence of N-linked oligosaccharides because two potential N-linked glycosylation sites are present in MMP2 at Asn546 and Asn613 (Collier et al., 1988).
Figure 3.
Zymography of conditioned media and cell lysates from skin fibroblasts. Serum-free conditioned media or lysates from equal numbers of fibroblasts were applied to SDS-PAGE/0.1% gelatin under nonreducing conditions. A 72-kDa gelatinase (pro-MMP2) was found in both conditioned media and cell lysates. The gelatinolytic activity was significantly increased in media and lysates from TSP2−/− fibroblasts compared with those from TSP2+/+ cells. Mr standards are displayed on the left. The experiments are representative of five different experiments for the conditioned media and two for the cell lysates.
To characterize further the gelatinases produced by dermal fibroblasts, we used gelatin-agarose affinity chromatography to isolate gelatinases from protein extracts of normal mouse dermal fibroblasts. The bound fractions from the gelatin-agarose column were eluted and were subjected to Western blotting and zymography. By Ponceau S staining, only one major band with molecular mass of 72 kDa was found in the bound fractions (Figure 4, lane 1). This protein was immunoreactive with anti-MMP2, but not with anti-MMP9 antibodies (Figure 4, lanes 2 and 3). The gelatinolytic activity of the isolated protein was demonstrated by zymography (Figure 4, lane 4). The identity of the gelatinase was further confirmed by Northern blot analysis of mouse dermal fibroblast RNA, which showed the presence of mRNA for MMP2, but no signal for MMP9 (our unpublished results).
Figure 4.
Characterization of gelatinases in skin fibroblasts. Lysates of control skin fibroblasts were subjected to gelatin-agarose affinity chromatography, and the column-bound fractions were applied to SDS-PAGE followed by transfer to nitrocellulose membranes (lanes 1–3) or by gelatin zymography (lane 4). A 72-kDa protein is shown by Ponceau S staining (lane 1). The isolated protein showed gelatinolytic activity (lane 4) and reacted with anti-MMP2 antibody (lane 2), but not with anti-MMP9 antibody (lane 3). In a separate experiment (our unpublished results) we have verified that the anti-MMP9 antibody recognizes MMP9. Mr standards are displayed on the left.
To quantify the MMP2 produced by dermal fibroblasts, we subjected serum-free conditioned media from cell cultures to gelatin-agarose affinity chromatography. Protein concentrations of the affinity-purified material were determined by the Lowry method. A 2-fold increase in yield of MMP2 was obtained from the conditioned media of TSP2-null cells compared with that from wild-type cells (Table 1). However, no differences in the specific activity of the secreted activated MMP2 from TSP2-null and wild-type cells were found in gelatinolytic assays with soluble radiolabeled gelatin as a substrate in vitro (Figure 5). Thus, when equal amounts of purified protein were used in the assay, their gelatinolytic activities, upon activation by APMA, were equal. In addition, these assays revealed that the gelatinase secreted by dermal fibroblasts was largely in a latent form in both wild-type and mutant cells (Figure 5). Interestingly, no significant differences in the levels of MMP2 mRNA were observed when RNAs from wild-type and TSP2-null dermal fibroblasts were compared by Northern analysis (our unpublished results). Thus, the changes in TSP2-null fibroblasts that lead to increased levels of MMP2 presumably occur at the posttranscriptional level. This conclusion is consistent with a predominantly posttranscriptional mode of regulation of MMP2 activity in other circumstances (Yu et al., 1998). It is of interest that no differences in MMP2 activity were found in two independent experiments in which extracts of skin from wild-type and TSP2-null mice were examined by zymography (our unpublished results). This apparent discrepancy is addressed in the DISCUSSION.
Table 1.
Isolation of pro-MMP2 from conditioned media of mouse skin fibroblast cultures
| Cells | Conditioned media (ml) | Isolated MMP2 (μg) | Yield (μg/l) | Average (μg/l) |
|---|---|---|---|---|
| TSP2+/+; Exp. 1 | 320 | 23.4 | 73.1 | |
| Exp. 2 | 360 | 34.4 | 95.5 | 84.3 |
| TSP2 −/−; Exp. 1 | 440 | 58.2 | 132 | |
| Exp. 2 | 530 | 109 | 206 | 170 |
| Exp. 3 | 360 | 62 | 172 |
Serum-free conditioned media from adult skin fibroblast cultures were subjected to gelatin-agarose affinity chromatography. The bound fractions were eluted from the column with 7.5% dimethyl sulfoxide. This material was shown to consist almost entirely of pro-MMP2 by a Coomassie blue-stained gel and by Ponceau S staining of proteins that were transferred to a nitrocellulose membrane. A 2-fold increase in yield of pro-MMP2 was obtained from the conditioned media of TSP2-null cells compared with those from wild-type cells.
Figure 5.
Quantitative analysis of MMP2 activity with a gelatinolytic assay. Pro-MMP2, isolated from cell culture-conditioned media of TSP2-null (−/−) and wild-type (+/+) dermal fibroblasts, was incubated with 3H-labeled gelatin at 37°C with or without activation by 1 mM APMA. Degraded gelatin was measured by scintillation counting of the supernatant from trichloracetic acid and tannic acid precipitates. The isolated pro-MMP2s showed activity only after activation by APMA. When equal amounts of protein were applied, no difference in activity was observed between TSP2-null and wild-type cells. The results of six different experiments, in cpm, were normalized and expressed as arbitrary units.
Treatment of TSP2-null Fibroblasts with MMP Inhibitors Rescues the Adhesive Defect
To determine whether an increase in MMP2 is functionally related to the attachment defect in TSP2-null fibroblasts, we treated the cells with two different MMP inhibitors or with an anti-MMP2 antibody and attachment of the treated cells was quantified. BB94 is a general MMP inhibitor with an IC50 value of 4 nM for MMP2 in a cell-free assay (Murphy et al., 1993). However, effective concentrations in cell culture are considerably higher. Addition of BB94 to cell cultures at concentrations three orders of magnitude higher than the IC50 has been shown to be nontoxic (Bian et al., 1996). To inhibit MMP2 activity of dermal fibroblasts, we added BB94 to cell culture media and cells were incubated for 48 h before the attachment assay. Cells continued to grow during this incubation period and there was no evidence of toxicity by light microscopy. Although treatment of control fibroblasts with BB94 at a concentration of 40 μM increased their attachment by only 9%, the defective attachment of TSP2-null fibroblasts was corrected in a dose-dependent manner by concentrations of BB94 between 5 and 20 μM (Figure 6A). When fibroblasts were treated with recombinant human TIMP-2 at a concentration of 1 μg/ml, the attachment of control cells was increased by 43% and that of TSP2-null cells by 102% (Figure 6B). When TIMP-2 was used at a concentration of 5 μg/ml, the attachment of wild-type cells was increased by 2.5-fold and that of TSP2-null cells was increased 4.4-fold, thus nearly equaling the attachment level of wild-type cells (our unpublished results). Finally, fibroblasts were treated with an affinity-purified neutralizing rabbit polyclonal anti-MMP2 antibody. As shown in Figure 6C, the attachment of wild-type cells was increased by 51% and that of TSP2-null cells by 127%, almost reaching the level of wild-type cells. The increased attachment of wild-type cells in the presence of protease inhibitors suggests that MMPs are normally involved in modulating cell–matrix interactions during processes such as cell adhesion and migration, and supports the observations of Ray and Stetler-Stevenson (1995) with melanoma cells (see DISCUSSION).
Figure 6.
Rescue of the attachment defect in TSP2-null fibroblasts by treatment with MMP inhibitors. MMP inhibitors BB94 and TIMP-2 were present in cell culture media for 48 h before the attachment assay and in the serum-free media during the attachment to fibronectin-coated tissue culture surfaces. (A) Attachment defect of TSP2-null fibroblasts was corrected by treatment with BB94 in a dose-dependent manner (P < 0.01 for concentrations between 0 and 20 μM). (B) Treatment of fibroblasts with TIMP-2 at concentration of 1 μg/ml restored the attachment of TSP2-null cells to levels nearly equal to those of normal cells. (C) Treatment with a neutralizing antibody to MMP2 also restored attachment of TSP2-null cells to levels approaching those of wild-type cells. A duplicate experiment showed identical results.
Coincubation of TSP2-null Fibroblasts with Exogenous TSP2 Rescues the Adhesive Defect
The failure of immobilized TSP2 to rescue the adhesive defect of TSP2-null fibroblasts (Figure 1B) suggested that TSP2 does not function directly as an attachment factor. Initial attempts to rescue the adhesive defect in these cells by short-term culture in medium supplemented with recombinant TSP2 were unsuccessful (our unpublished results). However, when cells were incubated with TSP2 in solution for 48 h before the attachment assay, attachment was restored to normal levels in a dose-dependent manner (Figure 7). The finding that treatment of control fibroblasts with TSP2 also increased their attachment is consistent with the effects of inhibitors of MMP2 activity (see above) and supports our hypothesis that TSP2 functions to modulate MMP2 activity in the pericellular environment (see DISCUSSION).
Figure 7.
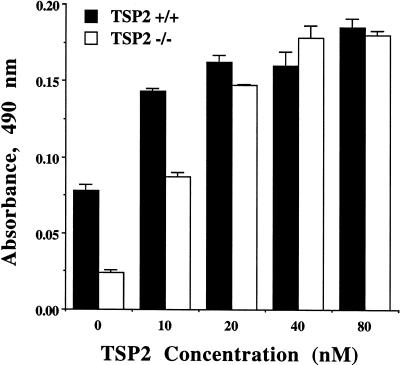
Rescue of the attachment defect in TSP2-null fibroblasts by treatment with exogenous TSP2. Recombinant TSP2 was added to cell culture media, and cells were incubated for 48 h before the attachment assay. Treatment of fibroblasts with TSP2 rescued the attachment defect of TSP2-null cells in a dose-dependent manner (P < 0.01 for all concentrations) and restored the attachment of TSP2-null cells to a normal level at a concentration of 40 nM.
Transfection of TSP2-null Fibroblasts with mTSP2 cDNA Restores Normal Attachment and Reduces MMP2 Levels
Although supplementation of TSP2-null fibroblasts with exogenous TSP2 restored normal attachment, these experiments were not well suited to the determination of whether MMP2 levels were also normalized. Numerous attempts to establish populations of TSP2-null fibroblasts that were stably transfected with TSP2 cDNA were unsuccessful because such cells quickly became senescent or spontaneously transformed. We therefore crossed TSP2-null mice and wild-type controls with mice carrying the conditionally immortalizing SV40 large T antigen, H-2Kb-tsA58, as a transgene. Dermal fibroblasts from the resulting crosses could be stably transfected and grown indefinitely at the permissive temperature of 33°C, and a normal phenotype and growth characteristics could be restored by growth at 37°C (see MATERIALS AND METHODS). Agarose gel electrophoresis of RT-PCR products from immortalized TSP2-null dermal fibroblasts, stably transfected with either sense pZeoSVmTSP2 or antisense pZeoSV2PSTm, showed a mTSP2-specific 0.54-kb fragment for both cell lines (our unpublished results). However, immunocytochemical analysis revealed that only sense-transfected cells expressed TSP2 protein (Figure 8). In this experiment, cells cultured in the presence of serum for 3 d, thus allowing for spreading of even the antisense-transfected cells. The attachment of nontransfected, immortalized TSP2-null fibroblasts to uncoated plastic or fibronectin-coated dishes resembled that of nonimmortalized TSP2-null cells and was reduced relative to wild-type cells (Figure 9A). The level of attachment of TSP2-null cells to fibronectin, relative to that of wild-type cells, is somewhat more than that seen in Figure 1A but is within the range seen for nonimmortalized cells. Thus, at least with regard to attachment, we can conclude that immortalization with SV40 large T antigen, followed by culture of cells under nonpermissive conditions, does not change the properties of these cells. Additional cell attachment assays showed that transfection with sense pZeoSVmTSP2 rescued the adhesive defect of immortalized TSP2-null fibroblasts, whereas the attachment of cells transfected with antisense pZeoSV2PSTm was comparable to that of nontransfected TSP2-null fibroblasts (Figure 9B). Importantly, MMP2 activity was restored to nearly normal levels in TSP2-transfected cells (Figure 9C). Thus, the increased gelatinolytic activity observed in the conditioned media of nontransfected TSP2-null cells (lane 1) was reduced by transfection of immortalized TSP2-null cells with TSP2 cDNA (lane 2) to a level approaching that in conditioned media from nontransfected immortalized wild-type cells (lane 3). Quantification of the gelatinolytic activity in conditioned media of TSP2-transfected TSP2-null cells in zymograms such as that in Figure 9C indicated that 76% of the difference between the activities of immortalized wild-type and TSP2-null cells had been restored by transfection.
Figure 8.
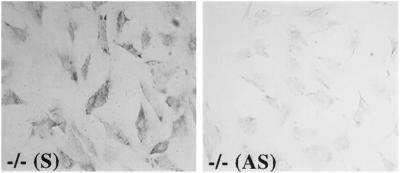
Stable transfection of skin fibroblasts with TSP2 expression vectors. Immortalized TSP2-null dermal fibroblasts were transfected with mTSP2 expression vectors and Lipofectin. Cells transfected with sense pZeoSVmTSP2 expressed TSP2 protein as revealed by immunocytochemistry with anti-TSP2 antibodies (left), whereas cells transfected with antisense vector, pZeoSV2PSTm, were negative (right). Bar, 100 μm.
Figure 9.
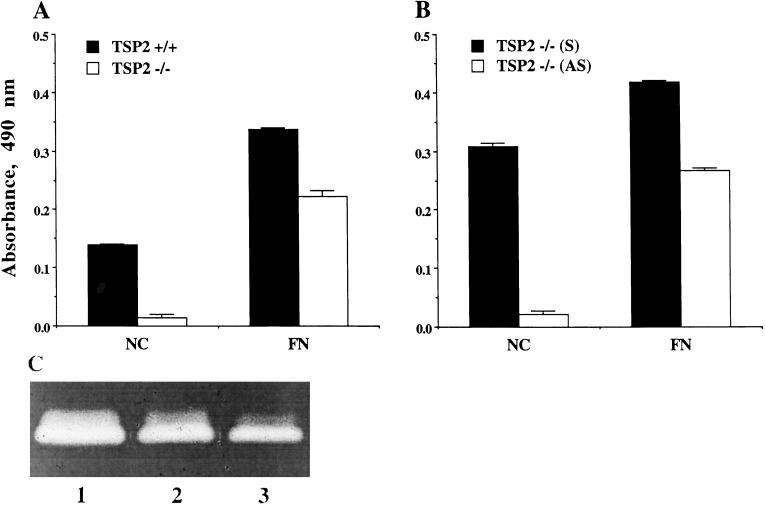
Rescue of the adhesive defect and restoration of normal MMP2 activity in TSP2-null dermal fibroblasts by transfection with mTSP2 cDNA. Immortalized dermal fibroblasts derived from TSP2-null mice were transfected with sense and antisense mTSP2 cDNA and compared for attachment to fibronectin (FN)-coated and noncoated (NC) tissue culture surfaces. (A) Compared with wild-type cells, nontransfected immortalized TSP2-null fibroblasts showed a marked defect in adhesion to tissue culture plastic, and coating with FN did not completely correct the adhesive defect. (B) Adhesive defect of TSP2-null fibroblasts was corrected by transfection with sense mTSP2 cDNA (S), but not with antisense mTSP2 cDNA (AS) expression vectors (P < 0.001); n = 4. (C) Equal amounts of serum-free conditioned media from nontransfected TSP2-null cells (lane 1), from immortalized TSP2-null dermal fibroblasts transfected with sense TSP2 cDNA (lane 2), and from immortalized wild-type cells (lane 3) were applied to SDS-PAGE/0.1% gelatin gels under nonreducing conditions and analyzed by zymography. This experiment is representative of two experiments with similar results.
TSP2 Interacts Directly with MMP2
Although the presence or absence of TSP2 could conceivably affect MMP2 activity indirectly in numerous ways, we chose to examine the possibility that TSP2 binds MMP2 because both TSP1 and TSP2 have been shown to be direct-binding inhibitors of a number of proteases (see DISCUSSION). As shown in Figure 10, the binding of MMP2 to either TSP1 or TSP2, determined in a sandwich direct-binding solid phase assay, is highly significant in comparison with the lack of binding to either fibronectin or BSA. Similar results were obtained with two different MMP2 preparations. It should be noted that binding of MMP2 to TSP2 in this assay is considerably less than its binding to gelatin (from 20 to 50%, depending on the MMP2 preparation; our unpublished results). However, this difference is consistent with a role for TSP2 as a modulator of MMP2 activity rather than as a substrate for the enzyme (see DISCUSSION). Both pro-MMP2 and the activated enzyme bound equally well to TSP2 (our unpublished results).
Figure 10.
TSP2 binds MMP2 directly. The interaction of MMP2 with substrate-bound proteins was determined by a direct-binding solid phase assay. MMP2 bound equally to TSP2 and TSP1 (P = 0.482), and the binding of MMP2 to either TSP2 or TSP1 was significantly higher in comparison with their binding to either fibronectin or BSA (P < 0.001). This experiment is representative of four experiments with two different preparations of MMP2, which yielded very similar results.
DISCUSSION
A prediction that is based on the matricellular concept, and one that has thus far turned out to be true, is that mice that are deficient for a matricellular protein are surprisingly normal on superficial examination. In contrast, mice with a targeted disruption of a gene encoding a structural protein usually display a lethal or severe phenotype. But, as has been apparent from a more careful analysis, mice lacking TSP2 (Kyriakides et al., 1998a), TSP1 (Lawler et al., 1998; Crawford et al., 1998), and SPARC (Gilmour et al., 1998; Norose et al., 1998) have many abnormalities, which reflect the diverse biological roles of these proteins. In adult animals these abnormalities are often revealed when challenges result in injuries to tissues (Sage and Bornstein, 1991). The culture of fibroblasts in serum-containing medium, as studied in this work, serves as an in vitro model for such an injury.
One of the features of TSP2-null fibroblasts in culture that initially led us to examine their adhesive properties was their marked tendency to aggregate under conditions that compromise attachment to a substratum, e.g., when plated on glass or on bacteriological plastic in the absence of serum (Kyriakides et al., 1998a). Thus, whereas control cells remained attached as single rounded cells, mutant cells aggregated into small clumps that often coalesced into sheets. Because there is a coordinated interplay and reciprocity between cell–cell interactions, mediated by cadherins, and cell-substratum adhesion, mediated by integrins (Monier-Gavelle and Duband, 1997; Levenberg et al., 1998), we analyzed control and TSP2-null fibroblasts for differences in cadherin levels. However, Western analysis with an anti-pancadherin antibody revealed no differences between protein extracts of TSP2-null and wild-type fibroblasts (our unpublished results). Although this result does not exclude the possibility that a significant increase in a minor but functionally important cadherin activity exists in TSP2-null cells, the attachment assays and associated findings described in this article point strongly to a primary defect in cell adhesion and a secondary increase in cell–cell interactions.
Deficiency in TSP2 Leads to a Functionally Important Increase in MMP2 Levels In Vitro
It seems likely that the increased MMP2 levels found in TSP2-null fibroblasts play a major role in the adhesive defect observed in cultured cells. In support of this conclusion, inhibition of MMP2 by BB94 or TIMP-2, or by a neutralizing antibody to MMP2, fully corrects the attachment defect in TSP2-null fibroblasts (Figure 6). Furthermore, restoration of TSP2 synthesis by stable transfection of TSP2-null fibroblasts with a TSP2 cDNA fully corrects the attachment defect in these cells and largely corrects the elevated MMP2 activity as well (Figure 9). The failure of recombinant TSP2 to correct the adhesive defect in TSP2-null fibroblasts, when applied directly to a substratum (Figure 1B) argues against a role for TSP2 as a direct adhesive factor. Such a role is in any event unlikely in view of the findings that control mouse dermal fibroblasts attach poorly and do not spread on TSP2 (our unpublished observations), and that TSP2 shares with TSP1 the property of destabilizing focal adhesions in endothelial cells (Murphy-Ullrich et al., 1993). On the other hand, the ability of a stably transfected TSP2 cDNA gene to correct the adhesive defect, and the associated increase in MMP2 levels in TSP2-null cells, demonstrate that these defects are a direct consequence of the TSP2-deficient state, and are not distantly related effects of a complex phenotype. It should be noted that although zymography (Figure 3) and gelatinolytic assays (Figure 5) indicated that the majority of MMP2 in wild-type and TSP2-null cells and conditioned medium was in the inactive pro-MMP2 form, the functional reversal observed with inhibitors of MMP2 argues for the existence of increased MMP2 activity in TSP2-null cells.
Possible Mechanisms of Action of MMP2 and Inhibition by TSP2
Increased MMP2 activity could reduce cell attachment by increasing the proteolytic cleavage of the ectodomains of cell-surface adhesion receptors and/or by attacking the matrix proteins with which these receptors interact. The former possibility is consistent with the observation that TSP2-null cells have a reduced number of receptors for fibronectin in culture (our unpublished results). Ray and Stetler-Stevenson (1995) have shown that inhibition of endogenous MMP2 activity by TIMP-2 in human melanoma cells increases cellular attachment and spreading. These authors also propose that proteolysis at the cell surface, rather than degradation of the extracellular matrix, is primarily responsible for the changes in cell adhesion caused by increased MMP2 activity. In a complementary experiment, Miyake et al. (1999) stably transfected mouse renal carcinoma cells with cDNAs for MMP2 or TIMP-2, or with a combination of the two cDNAs. The level of cell adhesion increased with increased TIMP-2 expression and correlated inversely with MMP2 expression.
It is of interest that MMP2 protein is increased in the conditioned media of cultured TSP2-null fibroblasts in the absence of a concomitant increase in MMP2 mRNA. In view of the demonstration that MMP2 interacts directly with TSP2 in vitro (Figure 10), we propose that TSP2 binds MMP2 extracellularly in vivo. Strong support for the interaction of MMP2 and TSP2 comes from a recent brief report in which a fragment of MMP2 was identified when the type I repeats of either TSP1 or TSP2 were used as bait in the yeast two-hybrid system. The interaction was verified by coimmunoprecipitation and Western blotting of the two proteins (Bein and Simons, 1999). It has been shown that TSP1, which is structurally similar to TSP2, can function as a direct-binding competitive inhibitor of neutrophil cathepsin G and elastase, and there is some indication that TSP2 can function similarly (Hogg, 1994). However, our preliminary experiments indicate that TSP2 does not inhibit active MMP2 directly, nor does it inhibit activation of pro-MMP2 by APMA. Both TSP1 and TSP2 are bound and internalized by the LRP receptor that may serve to regulate extracellular levels of these proteins (Chen et al., 1996). It is therefore possible that endocytosis of MMP2–TSP2 complexes, with subsequent degradation in lysosomes, also serves to reduce pericellular levels of pro- and/or active MMP2. Such a mechanism would also be consistent with our observation that MMP2 is increased in the culture medium of TSP2-null cells (Figure 3 and Table 1). LRP is known to mediate the catabolism of a number of proteinase–inhibitor complexes (Strickland et al., 1995), and a related two-step mechanism involving an unknown 170-kDa receptor and LRP has been implicated in the internalization of collagenase 3 (MMP 13) by rat fibroblasts (Barmina et al., 1999). The finding that prolonged incubation of TSP2-null cells with exogenous TSP2 restores normal adhesion (Figure 7) supports the direct binding of MMP2 by TSP2 as one mechanism by which proteolytic activity in the extracellular environment is regulated.
Although the intracellular mechanisms that mediate the reduced fibroblast adhesion that is associated with increased MMP2 levels are not known, the recent experiments of Carragher et al. (1999) suggest a plausible sequence of events. These authors have shown that the culture of human smooth muscle cells on polymerized collagen gels for 6 to 24 h induces the synthesis of both MMP1 and MMP2. This increase in extracellular proteolytic activity is correlated with cleavage of pp125FAK, paxillin, and talin, and with a reduction in focal adhesions. It was also shown that the extracellular changes are mediated by α2β1 integrin and result from the proteolytic activity of calpain I, which is known to be associated with focal adhesions. Furthermore, the cleavage of pp125FAK was partially suppressed by TIMP-1 and TIMP-2. A similar scenario might apply to dermal fibroblasts but involve a different integrin(s) because α2β1 integrin levels appear to be far lower in mouse fibroblasts than in human smooth muscle cells (our unpublished observations).
Significance of Increased MMP2 Levels in Cell Culture for the Phenotype of the TSP2-null Mouse
The increase in MMP2 seen in our studies of dermal fibroblasts in vitro could also be of significance in vivo, and could contribute to features of the TSP2-null mouse such as abnormal collagen fibril structure in skin, and increased angiogenesis. It is known that MMPs play important roles in the development of the extracellular matrix and in new blood vessel formation (Yu et al., 1998; Werb et al., 1999). However, two independent experiments in which extracts of control and TSP2-null adult skin were assayed for MMP2 activity by zymography showed no differences (our unpublished results). We propose the following explanation, which takes into account an important aspect of the biology of TSP2, for the apparent discrepancy between the in vitro and in vivo results. As shown by Kyriakides et al. (1998b) fibroblasts in normal adult mouse dermis synthesize only very low levels of TSP2 by antibody staining. However, during normal skin wound healing TSP2 is readily detectable in granulation tissue (Kyriakides et al., 1999b). This injury-related expression of TSP2 is a feature of matricellular proteins. Thus, one would not necessarily expect fibroblasts in uninjured adult TSP2-null mouse skin to have more MMP2 activity than fibroblasts in wild-type skin. We postulate that the phenotypic differences between TSP2-null and wild-type mice can result from small incremental differences in MMP2 activity during development and growth. Increased MMP2 levels in vivo could release angiogenic factors such as basic fibroblast growth factor and vascular endothelial growth factor from extracellular stores, as suggested for MMP9 (Vu et al., 1998), thus increasing their bioavailability and providing a partial explanation for the increased vascularity observed in the TSP2-null mouse (Kyriakides et al., 1998a). The ability of TSP2 to bind and inhibit MMP2 indirectly could also contribute to its antitumorigenic and antiangiogenic activities (Itoh et al., 1998; Streit et al., 1999)
Although it is possible that the presence of abnormal skin collagen fibrils and defective dermal fibroblast adhesion represent separate and unrelated consequences of the TSP2-deficient state in mice, a more parsimonious assumption is that the two phenomena are related. How then might alterations in the interactions of fibroblasts with matrix proteins lead to abnormalities in collagen fibrillogenesis? It has been shown by transmission electron microscopy that fibroblasts compartmentalize the adjacent extracellular space by extending long cellular processes into it. Growing collagen fibrils subsequently assemble in the resulting deep cellular crevasses or channels, in close association with the cell surface (Birk and Trelstad, 1986; Ploetz et al., 1991; Birk and Linsenmayer, 1994). It seems likely that cells interact with the fibril during this process and that such interactions are compromised in the TSP2-null mouse. Preliminary evidence for this hypothesis has come from electron microscopic examination of flexor limb tendons of 4- and 8-d postpartum mice. The apposition of growing collagen fibrils to the fibroblast cell surface in tendons from TSP2-null mice is less close than that in tendons from control mice of the same age, and the cytoplasmic processes that compartmentalize extracellular space and delimit fibril bundles are less regular. Fibril packing is also less regular in mutant tendons (Birk, Kyriakides, and Bornstein, unpublished observations). It is possible that these differences result from increased MMP2 activity in mutant fibroblasts.
Concluding Remarks
In conclusion, our studies demonstrate that TSP2 plays a complex role in regulating fibroblast function, and does not act directly as an adhesive or structural protein. Rather, TSP2 binds MMP2 and this binding is associated with a reduction in proteolytic activity in the pericellular environment, as inferred from studies with MMP2 inhibitors. We postulate that TSP2 functions by directing the protease to a scavenger receptor as a TSP2–enzyme complex. The substantial increase in MMP2 levels that accompany the adhesive defect in TSP2-null fibroblasts in vitro could have a counterpart in vivo and contribute to aspects of the phenotype of the TSP2-null mouse, including defective collagen fibrillogenesis and increased angiogenesis. Future studies will be aimed at defining further the biochemical basis for the increased MMP2 levels in mutant fibroblasts and at characterizing the cell-surface receptors and changes in intracellular signaling that are involved in the phenotypic differences in the TSP2-null mouse.
ACKNOWLEDGMENTS
We thank Drs. William Carter and Susana Gil for providing laminin 5-coated tissue culture plates, Dr. Zena Werb for the mouse MMP2 cDNA probe, Dr. Helene Sage for the mouse MMP9 cDNA probe, Dr. William Stetler-Stevenson for the neutralizing anti-MMP2 antibody, Dr. Christopher Overall for MMP2, and Dr. Alexander Clowes for BB94. We also thank Drs. Helene Sage and Lucas Armstrong for a critical review of the manuscript and helpful discussions, Jennifer Tullis for assistance with animal husbandry, and Qian Zhang for technical assistance. This work was supported by National Institutes of Health grants HL-18645 and AR-45418.
Abbreviations used:
- APMA
4-aminophenyl mercuric acetate
- LRP
low density lipoprotein receptor related protein
- MMP2
matrix metalloproteinase 2
- TIMP-2
tissue inhibitor of metalloproteinase-2
- TSP2
thrombospondin 2
REFERENCES
- Adams JC, Tucker RP, Lawler J. Molecular biology intelligence unit: The thrombospondin gene family. Austin, TX: Springer-Verlag, R.G. Landes Company; 1995. [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. John Wiley & Sons; 1994. pp. 4.9.1–4.9.16. [Google Scholar]
- Barmina OY, Walling HW, Fiacco GJ, Frije JMP, Lopez-Otin C, Jeffrey JJ, Partridge NC. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J Biol Chem. 1999;274:30087–30093. doi: 10.1074/jbc.274.42.30087. [DOI] [PubMed] [Google Scholar]
- Bein K, Simons M. Binding of thrombospondin 1 and 2 type I repeats to and regulation of metalloproteinase 2. Mol Biol Cell. 1999;10(suppl):73a. [Google Scholar]
- Bian J, Wang Y, Smith MR, Kim H, Jacobs C, Jackman J, Kung H-F, Colburn NH, Sun Y. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis. 1996;17:1805–1811. doi: 10.1093/carcin/17.9.1805. [DOI] [PubMed] [Google Scholar]
- Birk DE, Linsenmayer TF. Collagen fibril assembly, deposition, and organization into tissue-specific matrices. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. San Diego, CA: Academic Press; 1994. pp. 91–128. [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Thrombospondins. Methods Enzymol. 1994;245:62–85. doi: 10.1016/0076-6879(94)45006-4. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, paxillin, and talin. J Cell Biol. 1999;147:619–629. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston TE, Barrett AJ. A rapid and reproducible assay of collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979;99:340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Chen H, Sottile J, O'Rourke KM, Dixit VM, Mosher DF. Properties of recombinant mouse thrombospondin 2 expressed in Spodoptera cells. J Biol Chem. 1994;269:32226–32232. [PubMed] [Google Scholar]
- Chen H, Strickland DK, Mosher DF. Metabolism of thrombospondin 2. J Biol Chem. 1996;271:15993–15999. doi: 10.1074/jbc.271.27.15993. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He C, Bauer EA, Goldberg GI. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579–6587. [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich J, Ribeiro SMF, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β in vivo. Cell. 1998;92:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Lyon GJ, Carlton MBL, Sanes JR, Cunningham JM, Anderson JR, Hogan BLM, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloprotinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- Hogg PJ. Thrombospondin l as an enzyme inhibitor. Thromb Haemostasis. 1994;72:787–792. [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka Y, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Sioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci USA. 1999a;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Tam JWY, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999b;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu Y-H, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998a;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu Y-H, Yang Z, Bornstein P. The distribution of the matricellular protein thrombospondin 2 in tissues of embryonic and adult mice. J Histochem Cytochem. 1998b;46:1007–1015. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- Lafuma C, Azzi El Nabout R, Crechet F, Hovnanian A, Martin M. Expression of 72-kDa gelatinase (MMP-2), collagenase (MMP-1), and tissue metalloproteinase inhibitor (TIMP) in primary pig skin fibroblast cultures derived from radiation-induced skin fibrosis. J Invest Dermatol. 1994;102:945–950. doi: 10.1111/1523-1747.ep12384118. [DOI] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Katz BZ, Yamada KM, Geiger B. Long-range and selective autoregulation of cell-cell or cell-matrix adhesions by cadherin or integrin ligands. J Cell Sci. 1998;111:347–357. doi: 10.1242/jcs.111.3.347. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Gohji K, Yamanaka K, Hara S, Arakawa S, Nakajima M, Kamidono S. Relative expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in mouse renal cell carcinoma cells regulates their metastatic potential. Clin Cancer Res. 1999;5:2824–2829. [PubMed] [Google Scholar]
- Monier-Gavelle F, Duband J-L. Cross talk between adhesion molecules: Control of N-cadherin activity by intracellular signals elicited by β1 and β2 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Höök M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- O'Reilly MS, Wiederschain DW, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- Panetti TS, Chen H, Misenheimer TM, Getzler SB, Mosher DF. Endothelial cell mitogenesis induced by LPA: inhibition by thrombospondin-1 and thrombospondin-2. J Lab Clin Med. 1997;129:208–216. doi: 10.1016/s0022-2143(97)90141-4. [DOI] [PubMed] [Google Scholar]
- Pellerin S, Lafeuillade B, Chambaz EM, Feige JJ. Distinct effects of thrombospondin-1 and CISP/thrombospondin-2 on adenocortical cell spreading. Mol Cell Endocrinol. 1994;106:181–186. doi: 10.1016/0303-7207(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Ploetz C, Zycband EI, Birk DE. Collagen fibril assembly and deposition in the developing dermis: segmental deposition in extracellular compartments. J Struct Biol. 1991;106:73–81. doi: 10.1016/1047-8477(91)90064-4. [DOI] [PubMed] [Google Scholar]
- Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14:908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H. Culture shock: Selective uptake and rapid release of a novel serum protein by endothelial cells in vitro. J Biol Chem. 1986;261:7082–7092. [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions: SPARC, tenascin and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Tolsma SS, Pellerin S, Feige J, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. Apmis. 1999;107:11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Yu AE, Murphy AN, Stetler-Stevenson WG. 72 kDa Gelatinase (Gelatinase A): Structure, activation, regulation, and substrate specificity. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. San Diego, CA: Academic Press; 1998. pp. 85–113. [Google Scholar]