Abstract
Photoperiodism is a day-length-dependent seasonal change of physiological or developmental activities that is widely found in plants and animals. Photoperiodic flowering in plants is regulated by photosensory receptors including the red/far-red light-receptor phytochromes and the blue/UV-A light-receptor cryptochromes. However, the molecular mechanisms underlying the specific roles of individual photoreceptors have remained poorly understood. Here, we report a study of the day-length-dependent response of cryptochrome 2 (cry2) and phytochrome A (phyA) and their role as day-length sensors in Arabidopsis. The protein abundance of cry2 and phyA showed a diurnal rhythm in plants grown in short-day but not in plants grown in long-day. The short-day-specific diurnal rhythm of cry2 is determined primarily by blue light-dependent cry2 turnover. Consistent with a proposition that cry2 and phyA are the major day-length sensors in Arabidopsis, we show that phyA mediates far-red light promotion of flowering with modes of action similar to that of cry2. Based on these results and a finding that the photoperiodic responsiveness of plants depends on light quality, a model is proposed to explain how individual phytochromes and cryptochromes work together to confer photoperiodic responsiveness in Arabidopsis.
Photoperiodic flowering in plants was the first photoperiodism phenomenon documented (1). The flowering of long-day (LD) or short-day (SD) plants occurs or is accelerated in the LD or SD condition, respectively. Arabidopsis is a facultative LD plant for which flowering-time regulation has been extensively studied (2–5). Although the detailed mechanism underlying photoperiodism is not well understood, extensive plant physiological studies support a hypothesis referred to as the external coincidence model (6–8). According to this hypothesis, the light signal must interact at the appropriate time of the day (or “coincide”) with the photoperiodic response rhythm (PRR) of a cellular activity to confer photoperiodic responsiveness. It has been found that mRNA expression of flowering-time genes in Arabidopsis, including CO, GI, and FT, exhibited circadian rhythms, which have different phase shapes in plants grown in LD compared with plants grown in SD (9–12). Therefore, the day-length-dependent circadian expression of one or more flowering-time genes may represent the PRR.
Arabidopsis relies on at least nine photosensory receptors, including five phytochromes (phyA–phyE), two cryptochromes (cry1 and cry2), and two phototropins (phot1 and phot2), to regulate most of its light responses (13–16). Among these photoreceptors, phytochromes and cryptochromes are known to regulate flowering time (5). It has also been found that phyA and cry2 protein abundance is regulated by light (17, 18) and that cry2 expression changes in response to photoperiod (19). These studies indicate that cry2 and phyA may act as major day-length sensors. Indeed, it has been found that the coincidence of light perception by cry2 and phyA with the peak circadian expression of the CO gene is critical for the induction of the expression of the flowering-time gene FT and photoperiodic flowering (20).
To understand further how different photoreceptors regulate photoperiodism, we investigated the expression of cry2 and phyA in response to photoperiods. We also analyzed how phyA regulates flowering, and how the actions of different photoreceptors may integrate into the mechanisms underlying photoperiodic flowering in Arabidopsis.
Materials and Methods
Plant Materials.
All mutants used are in the Columbia (Col-4) background. In addition to the mutants described (21), multiple photoreceptor mutants were prepared by genetic screens or crosses. The alleles of the mutants involved are: cry2-1 (22), cry1-304 (21), hy4-B104 (23), phyB-9 (24), and phyA-211 (25). The cry2-1/phyA-403 double mutant and cry2-1 cry1-304 phyA-412 triple mutant were isolated from ethyl methanesulfonate-mutagenized cry2-1 or fast-neutron mutagenized cry2-1/cry1-304 double-mutant seeds, respectively, and backcrossed twice to the wild type (Col-4). The flowering times and hypocotyl lengths were measured as described (21). The “days to flower” were the days between the date plants were placed under light to the date the first flower bud appeared. Immunoblot analyses were as described (18).
Light Sources and Spectra Measurements.
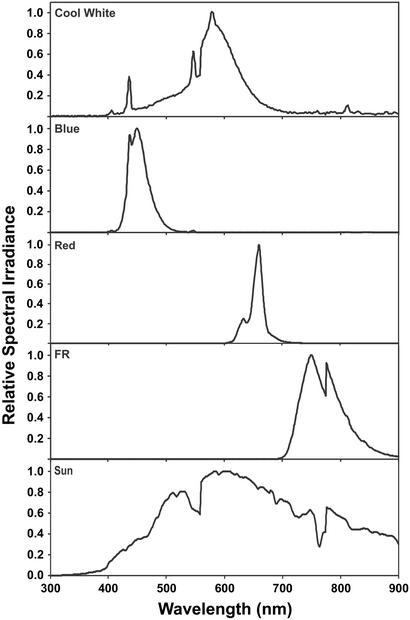
Plants were illuminated with fluorescent cool white light, unless otherwise specified. Lights used were cool white fluorescent bulbs (F48T12/CW/HO, General Electric), Bili Blue fluorescent bulbs (F48T12/B-450/HO, Interlectric, Warren, PA) filtered through a blue acrylic filter (2424 Blue, Polycast Technology, Stamford, CT), red fluorescent bulbs (F48T12/R-660/HO, Interlectric) filtered through a red acrylic filter (2423 Red, Polycast Technology), and infrared fluorescent bulbs (F48T12/IR-750/HO, Interlectric) filtered through an acrylic far-red (FR) filter (FRF 700, Westlake Plastics, Lenni, PA). Light spectra and fluence rates were measured by using an LI-1800 spectroradiometer (Li-Cor, Lincoln, NE) fitted with a remote cosine receptor with a perception range of 180°. The wavelengths of the transmission peaks for the filtered blue, red, and FR light were 449, 660, and 750 nm, respectively. The spectra of laboratory and natural light are shown in Fig. 1.
Figure 1.
Spectra of experimental and natural light. The spectra of experimental light (Cool White, Blue, Red, FR) and direct sunlight (Sun, University of California, Los Angeles campus on June 10, 2000, at 15:30 PST) are shown as the relative spectral irradiance in the wavelength range of 300–900 nm.
Results
The Photoperiod-Dependent Daily Oscillation of cry2 and phyA.
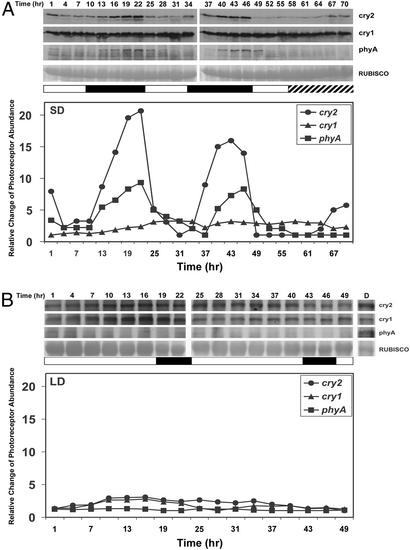
Among the five phytochromes and two cryptochromes in Arabidopsis, phyA and cry2 are the only photoreceptors for which the protein level is known to be regulated by light-induced proteolysis (17, 18, 26–28). As demonstrated in Fig. 2, the abundance of cry2 and phyA protein exhibited a daily oscillation in plants grown in SD; the abundance of these two photoreceptors decreased in the daytime but increased in the night (Fig. 2A). In contrast, little daily fluctuation of cry2 and phyA protein abundance was detected in plants grown in the LD condition (Fig. 2B). The abundance of cry1 protein, which, apparently, is not regulated by light (18), showed little oscillation in either SD or LD (Fig. 2). We conclude that the cry2 and phyA protein abundance changes at different times of the day and that the daily oscillation of cry2 and phyA depends on day length.
Figure 2.
Immunoblots showing photoperiod-dependent daily oscillation of cry2 and phyA protein abundance. Seven-day-old wild-type (Col-4) seedlings grown in SD (A; ≈130 μmol·s−1·m−2) or LD (B; ≈65 μmol·s−1·m−2) photoperiod were sampled at the times indicated, starting 1 h after dawn. Each immunoblot was probed with anti-CRY2, stripped, and reprobed with anti-CRY1 and then with anti-PHYA, as described (18). The ribulose-1, 5-biphosphate carboxylase/oxygenase (RUBISCO) large subunit shown as the Ponceau S-stained band is included as a loading control. White and black bars depict the light and dark period, respectively. Hatched bars represent the subjective dark period illuminated with light. Levels of CRY2 (●), CRY1 (▴), and PHYA (■) were first normalized against the total protein measured from the Ponceau S-stained membrane, and the normalized values were divided by the lowest value of same immunoblot (Upper) and shown as the relative change of photoreceptor abundance (Lower).
The cry2 Oscillation Depends on Blue Light.
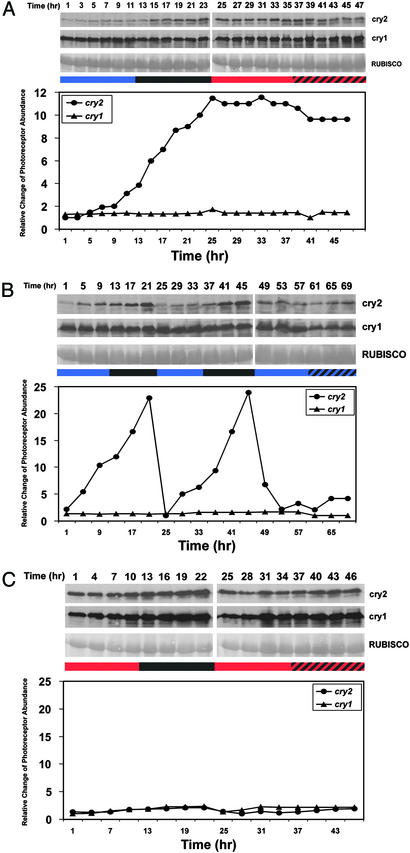
Given that cry2 and phyA are degraded in light, one may expect that the daily oscillation of these two photoreceptors is more likely a diurnal rhythm that depends on light than a circadian rhythm that is controlled by the circadian clock. Indeed, the oscillation of both cry2 and phyA observed in plants grown in SD diminished drastically when plants were transferred from SD to constant light, although cry2 abundance continued to rise slightly in the subjective night under the constant light condition (Fig. 2A). To distinguish further the role of light and the circadian clock in the regulation of cry2 protein oscillation, we tested how different wavelengths of light may affect cry2 expression. Arabidopsis seedlings grown in 12-h blue light/12-h dark (12 hBL/12 hD) photoperiods were transferred to either constant red light (Fig. 3A) or constant blue light (Fig. 3B), and the cry2 protein was analyzed before and after the transfer. The cry2 level showed a clear diurnal rhythm when plants were grown in 12 hBL/12 hD photoperiods (Fig. 3 A and B) but not in plants grown in 12-h white light/12-h dark (not shown) or 12-h red light/12-h dark photoperiods (Fig. 3C). The rhythmic change of cry2 abundance found in 12 hBL/12 hD photoperiods disappeared completely when plants were transferred to constant red light (Fig. 3A). The oscillation of cry2 protein abundance also disappeared almost completely in constant blue light, although a very small increase of cry2 abundance in the subjective night is discernable (Fig. 3B). The cry2 protein levels remained constantly high in plants transferred to continuous red light (Fig. 3A) or constantly low in plants transferred to continuous blue light (Fig. 3B). These results confirmed that the diurnal rhythm of cry2 depends on blue light. Taking together the observations that (i) cry2 shows a robust oscillation in 9-h white light/15-h dark (SD) or 12 hBL/12 hD, but not in 18-h white light/6-h dark (LD) or 12-h red light/12-h dark photoperiods, (ii) the cry2 oscillation is barely detectable in plants transferred from photoperiods to the constant light conditions, and (iii) cry2 is degraded in blue light but not in red light (18), we conclude that the diurnal rhythm of the cry2 oscillation in SD is determined primarily by blue light-dependent cry2 degradation.
Figure 3.
Effects of different wavelengths of light on cry2 protein expression. (A) Seven-day-old wild-type (Col-4) seedlings were grown in 12 hBL/12 hD photoperiod illuminated with blue light (≈40 μmol·s−1·m−2) and then transferred to continuous red light (≈40 μmol·s−1·m−2). (B) Seedlings were grown in 12 hBL/12 hD photoperiod illuminated with blue light (≈35 μmol·s−1·m−2) and then transferred to continuous blue light with the same fluence rate. (C) Seedlings were grown in 12-h red light/12-h dark photoperiod illuminated with red light (≈60 μmol·s−1·m−2) and then transferred to continuous red light with the same fluence rate. Samples were collected at the times indicated. Immunoblots (Upper) and the relative change of the photoreceptor abundance (Lower) are as described in Fig. 2. The colored bars depict the light periods with respective light spectra, the black bar depicts the dark period, and the hatched bar depicts the subjective dark period illuminated with continuous light.
The Circadian Clock and the Daily Oscillation of cry2.
As mentioned above, cry2 abundance continued to increase slightly in the subjective night, in plants transferred from SD or 12 hBL/12 hD photoperiod to constant light (Figs. 2A and 3B). Moreover, in plants grown in 12 hBL/12 hD photoperiods, the cry2 level increases well before the subjective night (Fig. 3 A and B). In the 12 hBL/12 hD photoperiod, cry2 protein levels slowly increased in the light phase, which is consistent with the “anticipation” phenomenon often associated with a circadian rhythm. This observation is consistent with the role of the circadian clock in the regulation of cry2 expression. However, such an anticipation seems specific to blue light, because it was not found in plants grown in 12-h red light/12-h dark (Fig. 3C) or 9-h white light/15-h dark photoperiods (Fig. 2A). This observation seems to argue that the circadian clock may not be directly related to the cry2 oscillation, because the circadian clock functions in both blue light and red light. It is conceivable that cry2 may mediate a blue light regulation of a specific output pathway of the circadian clock that controls CRY2 mRNA expression (29, 30). Alternatively, there may be a feedback regulation of cry2 turnover, and phytochromes may suppress the feedback regulation.
phyA Mediates the FR Light Promotion of Flowering.
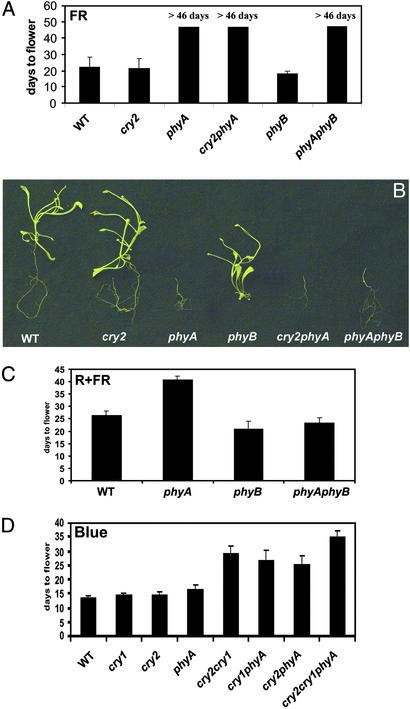
Because phyA is well known for mediating FR inhibition of hypocotyl elongation (13), we investigated whether phyA may also mediate FR promotion of flowering. We grew plants in nutrient medium under illumination with continuous FR light (Fig. 1, FR) and compared the flowering time of different genotypes (Fig. 4 A and B). Because photosynthetic pigments absorb mainly red light and blue light, FR light does not support photosynthesis. However, we reasoned that because Arabidopsis plants grown in nutrient medium can flower in darkness (31), plants grown in nutrient medium should also be able to flower in FR light. Indeed, wild-type, as well as cry2- and phyB-mutant, plants started to flower ≈20 days after planting (Fig. 4 A and B). In contrast, the phyA mutant and double mutants containing the phyA lesion did not flower under this condition for as long as 46 days, when the growth medium became dehydrated and the experiment was terminated (Fig. 4 A and B).
Figure 4.
Effects of different wavelengths of light on the flowering time of various Arabidopsis photoreceptor mutants. (A) The flowering times of the indicated genotypes grown on nutrient medium (Murashige and Skoog + 1% sucrose, 1% phytoagar) under continuous FR light (≈56 μmol·s−1·m−2) were measured as described. The means of three independent experiments with individual samples containing 17–101 plants and the corresponding standard errors are shown. (B) Twenty-four-day-old plants of the indicated genotypes grown under continuous FR light as described in A. (C and D) Plants of indicated genotypes were grown on soil under continuous R+FR (C; LD; ≈75 μmol·s−1·m−2 with an R:FR ratio of 2.5:1.0) or continuous blue light (D; ≈50 μmol·s−1·m−2). The means of three independent experiments with individual samples containing 13–64 plants and the corresponding standard errors are shown.
That the phyA mutant failed to flower in FR light may be because phyA mediates the promotion of flowering in FR light, or it may be a manifestation of a general developmental defect resulting from the phyA mutation. Because floral initiation is determined at the young seedling stage (21, 32), it is also possible that the lack of phyA-dependent process in seedlings affects both flowering-time control and other developmental processes. We analyzed the flowering time for plants grown in compound soil under red+FR (R+FR) light that supports photosynthesis. The phyA mutant flowered significantly later (>40 days) than the wild type (≈26 days) in the R+FR light condition, whereas phyA flowered only slightly later than the wild type when grown in continuous red light (data not shown). This result supports the notion that phyA mediates FR promotion of flowering. The observation that the phyA mutant failed to flower in FR indicates phyA can act independently from other photoreceptors in FR. On the other hand, when grown in R+FR light, the phyAphyB double mutant flowered more like the phyB-mutant parent than the phyA-mutant parent (Fig. 4C), suggesting that phyA function is at least partially dependent on phyB. Therefore, under natural light conditions, phyA would mediate an FR-dependent promotion of flowering in two different ways: a suppression of phyB function and a promotion of flowering independent of phyB.
cry1, cry2, and phyA Act Redundantly in Mediating the Direct Blue Light Promotion of Flowering.
Unlike other phytochromes, phyA has been known to mediate blue light inhibition of hypocotyl elongation (33–36). To test whether phyA may regulate flowering time in response to blue light, we compared the flowering times of the phyA mutant and the cryptochrome mutants grown in continuous blue light (Fig. 4D). The monogenic cry1, cry2, or phyA mutants flowered at about the same time as the wild type; however, a double mutant impaired in any two of the three photoreceptors or the cry1cry2phyA triple mutant flowered significantly later than the wild type when grown in continuous blue light (Fig. 4D). These results indicate that the three photoreceptors function redundantly in mediating the direct blue light promotion of flowering.
Three Photoreceptor Pathways Regulate Flowering Time in Arabidopsis.
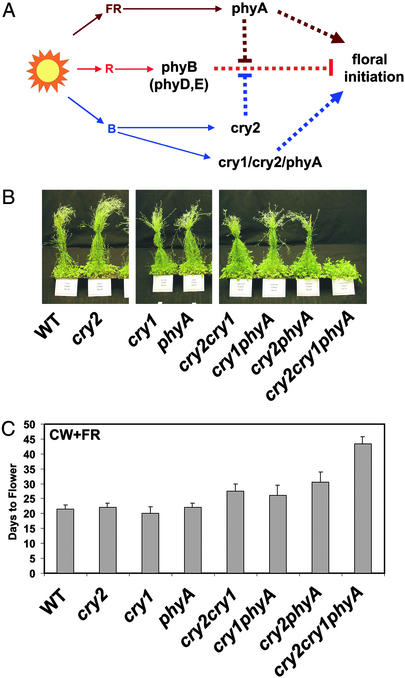
Based on these observations and the previous studies (21, 37), we propose that there are three basic photoreceptor pathways, referred to as the R, B, and FR pathways (Fig. 5A). These pathways perceive the red, blue, or FR regions of the spectrum from sunlight, respectively, to regulate flowering time in Arabidopsis. The R pathway is regulated by phyB, which acts in a partially redundant manner with phyD and phyE (38, 39) to mediate a red light inhibition of flowering. The B pathway is mediated primarily by cryptochromes, but also by phyA. In the B pathway, cry2 plays a major role in mediating blue light suppression of the R pathway, whereas cry1, cry2, and phyA act redundantly in mediating the direct blue light promotion of flowering. The FR pathway is regulated by phyA, which acts in two modes, a suppression of the R pathway and the direct FR promotion of flowering.
Figure 5.
Effects of continuous light on Arabidopsis flowering time. (A) A model depicting functions of photoreceptors regulating floral initiation in Arabidopsis grown in continuous lights. The arrows represent a stimulatory effect, and the lines terminated with a bar represent an inhibitory effect on flowering. (B) Thirty-six-day-old plants of the indicated genotypes grown under continuous cool white-plus-FR light (CW+FR; ≈93 μmol·s−1·m−2 with an R:B:FR ratio of ≈2.8:1.1:1.0). (C) Flowering time of the genotypes indicated grown on soil under CW+FR as described in B. The means of three independent experiments with individual samples containing 20–50 plants and the corresponding standard errors are shown.
According to the model in Fig. 5A, the B and FR pathways are functionally redundant in natural light rich in both blue and FR spectra (Fig. 1, Sun). Such a functional redundancy may explain a puzzling observation on the cry2 mutant. It was reported that the cry2 mutant flowered significantly later than the wild type when grown in fluorescent cool white (CW) light, which contains little FR (Fig. 1, Cool White; refs. 22, 37 and 40). In contrast, the cry2 mutant has also been found to flower at about the same time as the wild type when grown in light rich in FR (12, 37). One explanation for this discrepancy is that the cry2 action depends on the presumed active form of phytochrome (Pfr) that decreases in plants grown in light rich in FR (37). An alternative interpretation, as predicted by the model shown in Fig. 5A, is that the FR pathway mediated by phyA is functionally redundant with the B pathway mediated mainly by cry2. Therefore, when cry2-mutant plants are grown under light rich in FR, the FR pathway is robust enough to compensate for the impaired B pathway. We investigated this question by comparing the flowering time of plants grown in continuous CW+FR light (Fig. 5 B and C). As expected, none of the monogenic photoreceptor mutants showed a significant flowering-time alteration in this light condition. Mutations in any two of the three photoreceptor genes, including CRY1, CRY2, and PHYA, resulted in a slight delay in flowering. Significantly, the cry1cry2phyA triple mutant showed the most dramatic delay in flowering: the triple mutant took about twice as long to flower as the wild type when grown in continuous CW+FR light (Fig. 5 B and C). This result is consistent with the notion that the cry2-dependent B pathway and the phyA-dependent FR pathway act redundantly with a similar mode of action to promote floral initiation.
The Photoperiodic Sensitivity of Arabidopsis Depends on Light Quality.
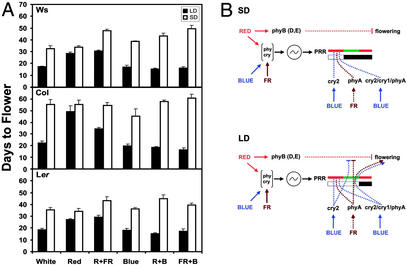
The model proposed in Fig. 5A appears to explain most, if not all, of the phenotypic changes observed in photoreceptor mutants analyzed in various continuous light conditions. However, this hypothesis provides little explanation for the role of photoreceptors in photoperiodic flowering. To understand photoreceptor control of photoperiodism further, we analyzed how light quality affects photoperiodic flowering of Arabidopsis. We compared the flowering time of three of the most widely used Arabidopsis accessions (Col, Ler, WS) grown in LD or SD illuminated with light of specific wavelengths. As shown in Fig. 6A, all three Arabidopsis accessions tested showed the least photoperiodic sensitivity in red light: the difference in flowering time between SD and LD was the smallest under red light illumination. In contrast, plants grown in blue light are highly responsive to photoperiods (Fig. 6A). To explain the light quality-dependent photoperiodic sensitivity observed, we reinterpreted the three photoreceptor-signaling pathways depicted in Fig. 5A in the context of the external coincidence hypothesis. According to the new model shown in Fig. 6B, the signal transduction of the B and FR pathways is regulated (or gated) by the PRR. The B and FR pathways promote floral initiation only when they “coincide” with the “sensitive” phase of the PRR (Fig. 6B, green bar). The effect of B and FR pathways on floral initiation diminishes in the “insensitive” phase of the PRR (Fig. 6B, red bar). In the absence of blue light and FR light, neither the B pathway nor the FR pathway is active, so plants grown in red light are insensitive to photoperiods, and they flower at about the same time in LD and in SD (Fig. 6A). In the absence of the R pathway, the B and FR pathways are still gated by the PRR that is controlled by the circadian clock. Moreover, cry2 and phyA, which are the major photoreceptors mediating the B and FR pathways, respectively, can discriminate day length by changing their expression pattern. Therefore, plants grown in photoperiods illuminated with blue light (or any spectra permissive to B or FR pathways) are still sensitive to photoperiods. It is noted that the red light used in our experiments, for which the tail of its radiation spectrum spreads beyond 700 nm (Fig. 1), may contain residual FR. This may explain why there is a residual photoperiodic sensitivity in plants grown in R photoperiods, and why plants grown in B+R photoperiods showed a photoperiod sensitivity similar to that grown in B+FR (Fig. 6A).
Figure 6.
Effects of different wavelengths of light on photoperiodic flowering of Arabidopsis. (A) Flowering time of wild-type Arabidopsis plants of the Wassilewskija (Ws), Columbia-4 (Col), and Landsberg erecta (Ler) ecotypes grown under LD, SD photoperiods illuminated with cool white light photoperiods (White, LD: 72 μmol·s−1·m−2; SD: 145 μmol·s−1·m−2), red light photoperiods (Red, LD: 55 μmol·s−1·m−2; SD: 113 μmol·s−1·m−2), red-plus-FR light photoperiods (R+FR, LD: 34 μmol·s−1·m−2; SD: 70 μmol·s−1·m−2; R:FR ratio of ≈2.5:1.0), blue light photoperiods (Blue, LD: 38 μmol·s−1·m−2; SD: 80 μmol·s−1·m−2), red-plus-blue light photoperiods (R+B, LD: 66 μmol·s−1·m−2; SD: 137 μmol·s−1·m−2; R:B ratio of ≈2.7:1.0), or FR-plus-blue light photoperiods (FR+B, LD: 38 μmol·s−1·m−2; SD: 78 μmol·s−1·m−2; FR:B ratio of ≈1.2:1.0). Fluence rates were adjusted so that plants grown in LD or SD received a similar amount of total irradiance per 24 h. The means of three independent experiments with individual samples containing 21–58 plants and the corresponding standard errors are shown. (B) An external coincidence model depicting photoreceptor functions in photoperiodic flowering of Arabidopsis. The arrows and the lines terminated with a bar represent a positive effect and a negative effect, respectively. The white bars depict the light (day) period and the black bars depict the dark (night) period. The waveform enclosed in a circle depicts the circadian oscillator that regulates the PRR depicted by the bars colored red-green-red. The green portion of the PRR represents the hypothesized “sensitive” phase that coincides with signaling of the B and FR pathways during LD photoperiods.
Discussion
cry2 and phyA as Day-Length Sensors in Arabidopsis.
In this report, we showed a daily oscillation of cry2 and phyA protein abundance in plants grown in SD but not in LD. The cry2 and phyA oscillation is largely a light-dependent process with the circadian clock playing a relatively minor role. The major mechanism underlying cry2 and phyA oscillation is most likely the light-induced proteolysis of the photoreceptors. The day-length-dependent diurnal rhythm of protein abundance of cry2 and phyA provides a direct mechanism for day-length perception in Arabidopsis. Given that the activity of a photoreceptor must depend on light and thus be influenced by the day length, any photoreceptor could potentially act as a day-length sensor. However, the total activity of cry2 and phyA should change much more dramatically in response to the changing day length than other photoreceptors, because the relative abundance of cry2 and phyA changes in different photoperiods. More importantly, the diurnal rhythm of cry2 and phyA expression provides a mechanism for the “coincidental” interaction between light signaling and the PRR such as the circadian expression of flowering-time genes. Therefore, cry2 and phyA are more likely to act as the major day-length sensors.
phyA Mediates FR Promotion of Flowering.
phyA is well known for mediating FR inhibition of hypocotyl elongation (33, 41, 42). The phyA mutant was found to flower later than the wild type when grown in an LD regime including a day extension of incandescent light rich in FR (43). We show here that phyA mutants grown in continuous FR failed to flower (Fig. 4 A and B). Moreover, phyA mutants flowered significantly later than the wild type in continuous R+FR light (Fig. 4C). These results indicate that phyA mediates the FR promotion of flowering.
Phytochromes are photointerconvertible molecules that interconvert between the FR-absorbing Pfr and the R-absorbing phytochrome (Pr) form. The Pfr is usually considered the physiologically active form, whereas Pr is the inactive form. This interpretation imposes an apparent difficulty in explaining how phyA may mediate an FR response in light-grown adult plants in which the phyA abundance is significantly lower than that in etiolated seedlings. However, it has been recently reported that the active form of phyA may be neither Pfr nor Pr (44). Instead, a short-lived intermediate generated during photoconversion from Pfr to Pr may be the active form. This hypothesis explains not only some well studied FR-dependent high-irradiance responses, such as FR inhibition of hypocotyl elongation (44), but also the phyA-mediated FR promotion of flowering reported here.
Roles of Individual Photoreceptors in Photoperiodic Flowering.
Based on studies carried out mostly in continuous light, it was proposed that there are three photoreceptor signaling pathways: the R, B, and FR pathways regulating floral initiation in Arabidopsis (Fig. 5A). The R pathway mediates red light inhibition of floral initiation, whereas the B and FR pathways mediate the B- or the FR-dependent suppression of the R pathway as well as the B- or the FR-dependent direct promotion of flowering. To interpret how photoreceptors regulate photoperiodic flowering, we propose a modified external coincidence model (Fig. 6B). According to this model, the signal transduction of the B and FR pathways can promote floral initiation only when they coincide with the sensitive phase of the PRR. For an LD plant like Arabidopsis, the coincidence of the B and FR pathways and the sensitive phase of PRR is more likely to occur in LD than in SD; therefore, LD plants flower earlier in LD than in SD. This hypothesis seems to explain not only results of genetic studies of photoreceptor mutants carried out in continuous light, but also the effect of light quality on photoperiodic responsiveness and Arabidopsis photoperiodic flowering in general.
Acknowledgments
This work was supported in part by United States Department of Agriculture Grant 99-35304-8085, National Institutes of Health Grant GM56265, National Science Foundation Grant MCB-0091391, and the University of California, Los Angeles, Faculty Grant Program. T.M. was supported in part by a predoctoral University of California, Los Angeles–National Science Foundation/Integrative Graduate Education and Research Training Bioinformatics Training Award DGE-9987641.
Abbreviations
- LD
long-day
- SD
short-day
- PRR
photoperiodic response rhythm
- 12 hBL/12 hD
12-h blue light/12-h dark
- FR
far-red
- cry
cryptochrome
- phy
phytochrome
References
- 1.Garner W W, Allard H A. J Agric Res. 1920;18:553–606. [Google Scholar]
- 2.Koornneef M, Alonso-Blanco C, Peeters A J M, Soppe W. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:345–370. doi: 10.1146/annurev.arplant.49.1.345. [DOI] [PubMed] [Google Scholar]
- 3.Levy Y Y, Dean C. Plant Cell. 1998;10:1973–1990. doi: 10.1105/tpc.10.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pineiro M, Coupland G. Plant Physiol. 1998;117:1–8. doi: 10.1104/pp.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C. Plant Physiol. 2000;123:39–50. doi: 10.1104/pp.123.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunning E. Ber Dtsch Bot Ges. 1936;54:590–607. [Google Scholar]
- 7.Thomas B, Vince-Prue D. Photoperiodism in Plants. New York: Academic; 1997. [Google Scholar]
- 8.Lumsden P J, Millar A J. Biological Rhythms and Photoperiodism in Plants. Oxford: BIOS Scientific; 1998. [Google Scholar]
- 9.Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kardailsky I, Shukla V K, Ahn J H, Dagenais N, Christensen S K, Nguyen J T, Chory J, Harrison M J, Weigel D. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 12.Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 13.Quail P H, Boylan M T, Parks B M, Short T W, Xu Y, Wagner D. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- 14.Briggs W R, Huala E. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Cashmore A R, Jarillo J A, Wu Y J, Liu D. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 16.Lin C. Plant Cell. 2002;2002S:S207–S225. doi: 10.1105/tpc.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vierstra R D. In: Photomorphogenesis in Plants. 2nd Ed. Kendrick R E, Kronenberg G H M, editors. Dordrecht, The Netherlands: Kluwer; 1994. pp. 141–162. [Google Scholar]
- 18.Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore A R. Proc Natl Acad Sci USA. 1998;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Din El-Assal S, Alonso-Blanco C, Peeters A J, Raz V, Koornneef M. Nat Genet. 2001;29:435–440. doi: 10.1038/ng767. [DOI] [PubMed] [Google Scholar]
- 20.Yanovsky M J, Kay S A. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- 21.Mockler T C, Guo H, Yang H, Duong H, Lin C. Development (Cambridge, UK) 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- 22.Guo H, Yang H, Mockler T C, Lin C. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- 23.Bruggemann E P, Doan B, Handwerger K, Storz G. Genetics. 1998;149:1575–1585. doi: 10.1093/genetics/149.3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed J W, Nagpal P, Poole D S, Furuya M, Chory J. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed J W, Nagatani A, Elich T D, Fagan M, Chory J. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanklin J, Jabben M, Vierstra R D. Proc Natl Acad Sci USA. 1987;84:359–363. doi: 10.1073/pnas.84.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad M, Jarillo J A, Cashmore A R. Plant Cell. 1998;10:197–208. doi: 10.1105/tpc.10.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo H, Duong H, Ma N, Lin C. Plant J. 1999;19:279–287. doi: 10.1046/j.1365-313x.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 29.Harmer S L, Hogenesch J B, Straume M, Chang H S, Han B, Zhu T, Wang X, Kreps J A, Kay S A. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- 30.Toth R, Kevei E E, Hall A, Millar A J, Nagy F, Kozma-Bognar L. Plant Physiol. 2001;127:1607–1616. doi: 10.1104/pp.010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki T, Komeda Y. Plant J. 1993;4:801–811. [Google Scholar]
- 32.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 33.Whitelam G C, Johnson E, Peng J, Carol P, Anderson M L, Cowl J S, Harberd N P. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casal J J, Mazzella M A. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neff M M, Chory J. Plant Physiol. 1998;118:27–35. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poppe C, Sweere U, Drumm-Herrel H, Schafer E. Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 37.Mas P, Devlin P F, Panda S, Kay S A. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- 38.Devlin P F, Patel S R, Whitelam G C. Plant Cell. 1998;10:1479–1488. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devlin P F, Robson P R, Patel S R, Goosey L, Sharrock R A, Whitelam G C. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koornneef M, Hanhart C J, van der Veen J H. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- 41.Nagatani A, Reed J W, Chory J. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parks B M, Quail P H. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson E, Bradley M, Harberd N P, Whitelam G C. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinomura T, Uchida K, Furuya M. Plant Physiol. 2000;122:147–156. doi: 10.1104/pp.122.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]