Abstract
Notch and NFκB pathways are key regulators of numerous cellular events such as proliferation, differentiation, or apoptosis. In both pathways, association of effector proteins with nuclear corepressors is responsible for their negative regulation. We have previously described that expression of a p65-NFκB mutant that lacks the transactivation domain (p65ΔTA) induces cytoplasmic translocation of N-CoR leading to a positive regulation of different promoters. Now, we show that cytoplasmic sequestration of p65 by IκBα is sufficient to both translocate nuclear corepressors SMRT/N-CoR to the cytoplasm and upregulate transcription of Notch-dependent genes. Moreover, p65 and IκBα are able to directly bind SMRT, and this interaction can be inhibited in a dose-dependent manner by the CREB binding protein (CBP) coactivator and after TNF-α treatment, suggesting that p65 acetylation is modulating this interaction. In agreement with this, TNF-α treatment results in downregulation of the Hes1 gene. Finally, we present evidence on how this mechanism may influence cell differentiation in the 32D myeloid progenitor system.
INTRODUCTION
Highly ordered chromatin structure represents a physical obstacle for transcriptional machinery and transcription factors to bind DNA (reviewed in Xu et al., 1999). Histone deacetylation and the enzymes responsible for this activity (HDACs), present in repressor complexes are mainly responsible for maintaining the repressive structure. Nuclear corepressors (NCoRs) such as N-CoR (nuclear receptor corepressor) or SMRT (silence mediator for retinoic acid and thyroid receptors) are thought to passively recruit HDACs to the repressor complexes (reviewed in Pazin and Kadonaga, 1997). Conversely, gene transcriptional activation correlates with the assembly of complexes that contain coactivators such as CBP/p300 with intrinsic histone acetyl transferase (HAT) activity, which promote nucleosome disruption and chromatin relaxation (reviewed in Xu et al., 1999). As cells are frequently exposed to the simultaneous activation of different signals a fundamental question is, how are different transduction pathways integrated to communicate the final message?
Notch and NFκB pathways play important roles in regulating cell proliferation (Kontgen et al., 1995; Carlesso et al., 1999; Baonza and Garcia-Bellido, 2000), cell differentiation (Egan et al., 1998; Feng and Porter, 1999; Milner and Bigas, 1999; Kaliman et al., 1999; Guttridge et al., 2000; Kaisho et al., 2001), and apoptosis (review in Barkett and Gilmore, 1999; Jehn et al., 1999; Shelly et al., 1999; Ohishi et al., 2000). Some connections between Notch and NFκB have previously been described (Guan et al., 1996; Bash et al., 1999; Wang et al., 2001). Moreover, both pathways can exert antagonistic or synergistic effects depending on the cellular context. For example, in T-cell development, constitutive activation of Notch (Robey et al., 1996), and suppression of NFκB activity by expression of IκBα32–36 (Boothby et al., 1997) result in opposite phenotypes for the acquisition of the CD8+CD4- T-cell fate. In fact, in a previous step, both Notch and NFκB activities are necessary for the survival of double positive CD4+CD8+ cells when the appropriate TCR signal is received (Deftos et al., 2000; Hettmann and Leiden, 2000).
NFκB is a family of transcription factors, including p50, p52, p65, RelB, and c-Rel, that associate as homo- or heterodimers to form the transcriptional regulatory complexes (reviewed in Karin and Ben-Neriah, 2000). Regulation of p65 is extremely complex because it can be modified by phosphorylation (Zhong et al., 1998; Carter et al., 1999; Madrid et al., 2001; Sizemore et al., 2002) and acetylation (Chen et al., 2001). These posttranslational modifications affect association of p65 with other molecules such as CBP (Zhong et al., 1998; Madrid et al., 2001) or the inhibitory molecules IκBs (Chen et al., 2001), which mainly regulate its subcellular localization. In fact, NFκB-IκBα complexes shuttle continuously between the nucleus and the cytoplasm, resulting in a predominant cytoplasmic localization in the absence of NFκB signaling (Carlotti et al., 2000; Huang et al., 2000; Huang and Miyamoto, 2001). The IκB proteins bind to NFκB dimers, masking their nuclear localization sequences and causing their cytoplasmic retention. After NFκB positive stimuli, IκB kinase complex phosphorylates IκBs in two conserved serines (Ser-32 and Ser-36 in IκBα), leading to ubiquitination and subsequent proteasomal degradation (reviewed in Karin and Ben-Neriah, 2000).
Notch-mediated cell–cell interactions have been shown to play an important role in regulating cell fate decisions. After ligand binding, Notch receptors undergo a proteolytic process that releases the intracellular domain (Notch-IC; reviewed in Egan et al., 1998; Milner and Bigas, 1999). The activated Notch-IC translocates to the nucleus and interacts with RBPjκ/CBF1 (Jarriault et al., 1998). RBPjκ is a transcription factor whose activity resembles that of nuclear hormone receptors. In the absence of Notch-IC, it recruits corepressor complexes and silences transcription of Notch-target and other genes (Kao et al., 1998; Hsieh et al., 2000). Notch-IC disrupts the interaction between the repressor complexes and RBPjκ, leading to activation of gene transcription (reviewed in Kadesch, 2000).
We previously described that expression of a cytoplasmic p65 mutant lacking the transcriptional activation domain (TAD) upregulates different promoters by translocating NCoRs to the cytoplasm (Espinosa et al., 2002). We have now investigated the physiological relevance of this mechanism by using different stimuli that modify IκB stability and p65 subcellular localization.
MATERIALS AND METHODS
Plasmids
The N1-IC and N2-IC expression vectors have been previously described (Bigas et al., 1998). Expression vectors for pCMV-p65, pCMV-IκBα32–36, pCMV-flag-N-CoR, pCMV-flag-SMRT, eGFP-p65, Ha-CBP, Gal4-SMRT-D, activated PKA, and reporter plasmids for Hes1-luc, 2xκB-luc have been previously described (Orellana and McKnight, 1992; Jarriault et al., 1995; DiDonato et al., 1996; Miralles et al., 1998; Harhaj and Sun, 1999; Lee et al., 2000; Chen et al., 2001). myc-SMRT was obtained by digesting full-length SMRT with SacII and EcoRV, blunt-ended and cloned into the pCS2 vector in frame with the myc-tag. flp65(1–551) was cloned in frame into the VP16-TAD expression vector. To obtain p65(96–551) we digested p65 with BglI, blunt-ended, and cloned it in frame into the same vector. The rest of the p65 constructs were obtained by PCR using the following primers: forward (5′-gaggatccATGGACGAACTGTTCCC-3′) and reversed for p65(96–443) (5′-catgcggccgc CAAGCAAGGCCCCCA-3′), p65(96–455) (5′-catgcggccgcAGCTGCAGCAGGGCCT-3′), p65(96–470) (5′-catgcggccgcCTCTGACAGCGTTC-CTTC-3′). PCR products were digested with BglI, blunt-ended, and cloned in frame into the VP16-TAD vector. All constructs contain an N-terminal Ha-tag and were confirmed by automated sequencing.
Antibodies
Antiflag (clone M2) was purchased from Sigma Chemical CO. (St. Louis, MO) and used 1:1000 for Western blot and 1:750 for immunofluorescence; anti–p65-NFκB (sc-109, Santa Cruz Biotechnology, Santa Cruz, CA) was used 1:400 for Western blot and immunofluorescence; anti-IκBα was purchased from Santa Cruz (sc-1643) and used 1:1000 for Western blot; anti-Ha was purchased from Babco (Berkley, CA) and used 1:1000 for Western blot; anti–N-CoR (sc-109, Santa Cruz Biotechnology) was used 1:200 for immunofluorescence; secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from DAKO (Carpinteria, CA) and used 1:2000 for Western blot. Fluorescein-conjugated goat anti-mouse or Cy3-conjugated goat anti-rabbit (Amersham Pharmacia Biotechnology, Piscataway, NJ) secondary antibodies were used 1:200 and 1:1000 respectively. Fluorescein-conjugated rabbit anti-goat antibody (Amersham) was used 1:50.
Cell Culture and Transfections
NIH-3T3 and 293T cells were cultured in DMEM and 10% FBS (fetal bovine serum). p65+/+ and p65−/− murine embryonic fibroblasts (MEF) were obtained from D. Baltimore (CalTech) and have been previously described (Beg et al., 1995). Cells were plated at subconfluence and transfected by calcium phosphate. Medium was changed after 12 h, and cells were processed 24 h later for luciferase assays, immunofluorescence, or Western blot. Murine TNF-α and human TNF-α were purchased from Peprotech EC Ltd and Upstate Biotechnology Incorporated (Lake Placid, NY), respectively, and used at 25–40 ng/ml. LPS from Sigma was used at 1 μg/ml. PDTC (pyrrolidinedithiocarbamic acid; Sigma) was used at the indicated concentrations, depending on the cell line and incubation hours required. Trichostatine A (TSA) was purchased from Calbiochem (La Jolla, CA) and used at 0.6 μM for 4 h in coprecipitation experiments or overnight incubation (0.6–1.2 μM) in the Gal4 experiments. Zinc-inducible promoter of the PKA-activated construct and controls were incubated with 100 μM ZnCl2. 32D cells (Greenberger et al., 1983) were maintained in Iscove's modified Dulbecco's medium with 10% fetal calf serum (FCS) and 10% WEHI-conditioned medium (WCM) as a source of interleukin-3 (IL-3). Cells were maintained undifferentiated and mycoplasma free and were checked regularly for the capacity to differentiate in G-CSF as previously described (Ingles-Esteve et al., 2001). Transfection of 32D cells has been previously described (Ingles-Esteve et al., 2001), and individual clones were evaluated for construct expression by Western blot or by flow cytometry for enhanced green fluorescence protein (eGFP) expression.
Flow Cytometry Analysis
Cells were analyzed for eGFP expression at different times of incubation in G-CSF. Dead cell exclusion was performed by incubation with 7-amino-actinomycin D (7-AA) and gating out the positive cells. The geometrical mean value of the eGFP-positive cells measures the intensity of green fluorescence and correlates with the quantity of eGFP protein in each sample. Cells were analyzed in a FACS Scalibur, Becton Dickinson, and the WinMDI 2.8 software.
Coimmunoprecipitation Assays
Forty-eight hours after transfection, cells were lysed for 30 min at 4°C in 500 μl of immunoprecipitation (IP) buffer (phosphate-buffered saline [PBS] containing 0.5% Triton X-100, 1 mM EDTA, 100 μM Na-orthovanadate, 0.25 mM PMSF, and complete protease inhibitor cocktail; Roche, Germany). After centrifugation, supernatants were incubated for 3 h at 4°C with 7.5 μg of anti-myc antibody (9E10) coupled to A-Sepharose beads. The immunocomplexes were extensively washed with IP buffer, and samples were boiled in Laemmli buffer and assayed by Western blot.
Western Blot
Forty-eight hours after transfection, cells were lysed for 30 min at 4°C in a buffer containing 1% Nonidet P-40, 10 mM Tris-HCl, pH 7.5, 140 mM NaCl, 5 mM EDTA, 50 mM sodium fluoride, 0.4 mM sodium orthovanadate, 1 mM PMSF, and 10 μg/ml leupeptin and aprotinin. Protein extracts were electrophoresed in polyacrylamide gels and transferred to PVDF membranes. Membranes were blocked with 5% nonfat dried milk in TBS and incubated with the appropriate antibody in TBS and 0.5% Tween 20 (TBS-T) with 5% nonfat dried milk for 90 min. Membranes were washed and incubated with a secondary HRP-conjugated antibody for 1 h. After extensive washing, immunoreactive proteins were detected by using the Enhanced Chemiluminescent Detection System (ECL; Amersham Pharmacia Biotechnology) as specified by the manufacturer.
Luciferase Assays
NIH-3T3 were plated on 12-well plates and transfected with the indicated expression vectors or the empty vector as a control. In the different experiments we used 0.5 μg of Hes1-luc, 2xκB-luc or 5xGal4-luc as reporter plasmids and 0.25 μg RSV-β-gal as internal control. pCS2 vector was added when necessary to keep DNA amount constant. Luciferase assay (Luciferase Assay System; Promega, Madison, WI) was performed 48 h after transfection, following the manufacturer's instructions. Luciferase values were normalized for β-galactosidase activity. At least three independent experiments were performed in duplicate.
Immunofluorescence
NIH-3T3 or MEF cells were seeded on slides at 20% confluence and transfected with the indicated plasmids. After 48 h, cells were fixed in 3% paraformaldehyde in PBS for 25 min at 4°C, washed in PBS, permeabilized in 0.1% Triton X-100 in PBS, and 5% nonfat dry milk for 25 min at 4°C. After washing, cells were incubated with the indicated primary antibody for 90 min at 4°C and extensively washed in PBS 1% nonfat dry milk. After 90 min incubation with the appropriate secondary antibody slides were extensively washed and mounted with Vectashield plus DAPI (Vector Laboratories, Burlingame, CA). Cells were visualized in an Olympus BX-60 microscope with the appropriate filters. Representative images were taken with a Spot 4.3 digital camera and software and edited in Adobe Photoshop (San Jose, CA).
Northern Blot Analysis
Total RNA was extracted from cells using Chomczynski and Sacchi method (Chomczynski and Sacchi, 1987). RNAs were size-fractionated by electrophoresis, transferred onto Hybond-N+ nylon membranes (Amersham), and then hybridized with a radiolabeled Hes1, Herp2, or IκBα probes.
RESULTS
Incubation with PDTC or Expression of IκBα32–36 Induces Cytoplasmic Translocation of NCoRs
We previously described that ectopic expression of a p65-NFκB mutant that lacks the transactivation domain (p65ΔTA) and primarily localizes in the cell cytoplasm induces the cytoplasmic translocation of N-CoR (Espinosa et al., 2002), thus resulting in the upregulation of different N-CoR repressed promoters such as Hes1. Moreover, we demonstrated that incubation with leptomycin B or deletion of the nuclear export signal of transfected p65 abolishes cytoplasmic translocation of both p65 and N-CoR proteins (Espinosa et al., 2002). We have now investigated whether changes in subcellular localization of endogenous p65 would modify the distribution of NCoRs.
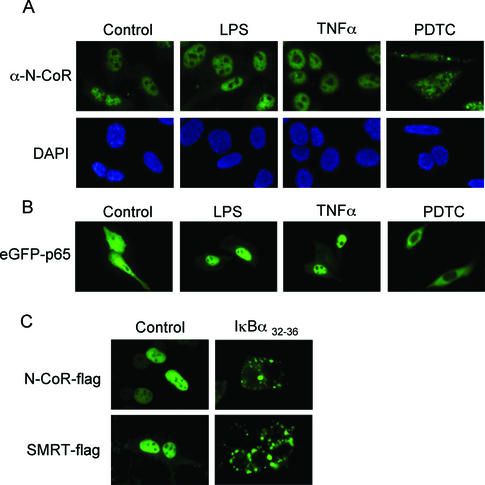
As shown in Figure 1A, stimuli that lead to IκB degradation and p65 nuclear translocation such as TNF-α and LPS did not change the nuclear localization of the endogenous N-CoR protein. Conversely, incubation of cells with PDTC, which stabilizes IκB and leads to p65 cytoplasmic retention, resulted in a partial and dose-dependent (unpublished data) cytoplasmic translocation of N-CoR in the totality of cells displaying a homogenous punctuate staining pattern (Figure 1A). The effect of the different stimuli on the subcellular localization of p65 was confirmed by transfection of an eGFP-p65 construct into NIH-3T3 cells and further exposure to the same experimental conditions (Figure 1B).
Figure 1.
Stabilization of IκBα induces cytoplasmic translocation of NCoRs. (A and B) Subcellular localization of endogenous N-CoR protein in NIH-3T3 cells (A, upper panel) and nuclear staining with DAPI (A, lower panel) or transfected eGFP-p65 fusion protein (B) in cells incubated for 1 h in control media, LPS (1 μg/ml), mTNF-α (25 ng/ml), or PDTC (100 μM). α-N-CoR antibody was detected with FITC-conjugated secondary antibody. (C) NIH-3T3 cells were cotransfected with N-CoR (upper panels) or SMRT (lower panels) along with empty vector (left panels) or IκBα32–36 (right panels). Subcellular localization of the ectopic N-CoR and SMRT proteins was detected with α-flag antibody and FITC-conjugated secondary antibody. These are representative images of three independent experiments.
Because PDTC treatment can exert different effects other than IκBα stabilization, we coexpressed N-CoR and SMRT with the IκBα32–36 mutant, a nondegradable IκBα molecule that constitutively sequesters p65 in the cytoplasm. In 293T cells (Figure 1C) or NIH-3T3 cells (unpublished data) we observed an apparent cytoplasmic translocation of both N-CoR and SMRT proteins in the presence of IκBα32–36. Together, these results strongly suggest that IκBα protein levels can modulate not only p65 subcellular localization but also NCoRs distribution.
Incubation with PDTC or Expression of IκBα32–36 Exert a Positive Effect on the Transcriptional Activation of the Notch-dependent Hes1 Promoter
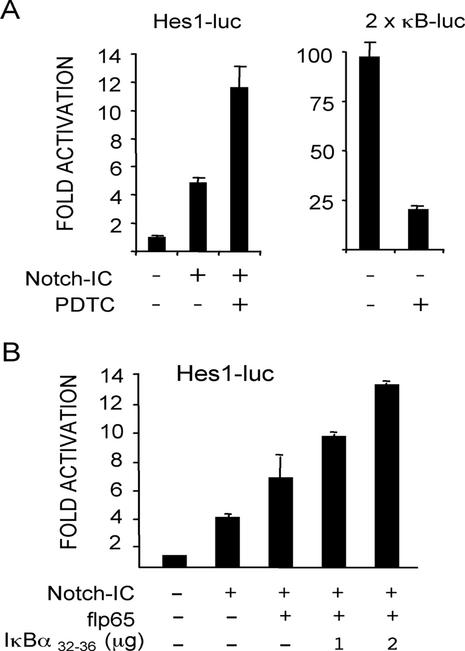
We previously described that cytoplasmic translocation of N-CoR mediated by a p65ΔTA mutant resulted in the transcriptional upregulation of different N-CoR/SMRT repressed promoters such as SRE-, AP-1-, or Hes1. Because our interest is focused on Notch pathway regulation, we have now investigated whether cytoplasmic translocation of NCoRs mediated by IκB stabilization had any effect on the transcriptional activation of the Hes1 promoter. To examine this, we cotransfected the Hes1-luc promoter with the Notch1 intracellular (N1-IC) construct in NIH-3T3 cells and measured its transcriptional activity in the absence or presence of PDTC. We observed a threefold upregulation of the promoter in the cells incubated with PDTC relative to the control, whereas most of the NFκB transcriptional activity was eliminated as expected (Figure 2A). We previously reported that upregulation of Hes1 by p65 is maintained or even increased in the presence of IκBα32–36 (Espinosa et al., 2002). Moreover, cotransfection of higher doses of IκBα32–36 with flp65 and N1-IC resulted in a dose-dependent upregulation of the Hes1 promoter (Figure 2B), thus suggesting that PDTC modulates Hes1 transcription by stabilizing IκBα.
Figure 2.
Stabilization of IκBα exerts a positive effect on the transcriptional activation of the Notch-dependent Hes1 promoter. (A) N1-IC (0.75 μg) was cotransfected along with either Hes1-luc or 2xκB-luc reporter in NIH-3T3 cells. Luciferase activity was measured in the cells after 12 h incubation in the presence or absence of 20 μM PDTC. (B) Hes1-luciferase activity of NIH-3T3 cells cotransfected with N1-IC (0.75 μg) and pCMV-flp65 (0.25 μg) plasmids and increasing amounts of the IκBα32–36 mutant. Luciferase activity is represented as the fold induction relative to cells transfected with empty vector. CMV-β-galactosidase was cotransfected as internal control in all experiments and equivalent amounts of N1-IC protein and increasing amounts of IκBα32–36 were confirmed by immunoblotting (unpublished data). The average and SD of duplicates from one of three independent experiments are presented.
p65-NFκB and IκBα32–36 Bind to SMRT Corepressor
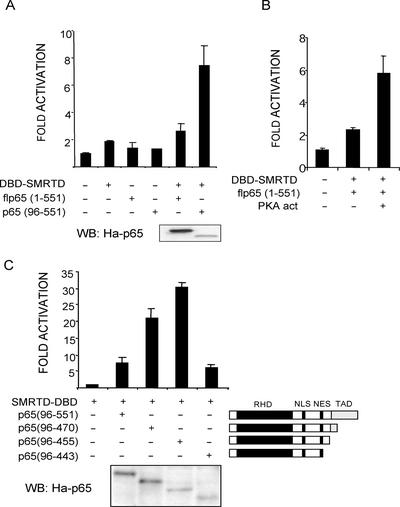
Inhibition of NFκB-dependent promoters by SMRT and interaction between p65 and SMRT (C-terminal fragment, SMRT-D) has been reported (Lee et al., 2000). We confirmed the interaction of p65 with SMRT-D by a mammalian two-hybrid system in NIH-3T3 cells using different p65 constructs fused to the VP16-TAD. Surprisingly, coexpression of SMRT-D with a full-length p65 construct failed to activate the 5xGal4-luciferase reporter. However, the use of a truncated N-terminal form of p65(96–551) resulted in a six- to eightfold increase of luciferase activity (Figure 3A). Because conformational changes of flp65 after PKA phosphorylation have been proposed to modulate its binding to coactivators (Zhong et al., 1998), we repeated the two-hybrid assay coexpressing flp65 and SMRT-D along with the active catalytic subunit of PKA. In these conditions, we observed that interaction of flp65 with SMRT-D was comparable to the one obtained with p65(96–551) (Figure 3B). To determine the binding domain of p65 to SMRT we assayed different p65 constructs containing serial deletions of the C-terminal end (Figure 3C). As seen in Figure 3C, p65(96–455) most efficiently binds to SMRT-D (30- to 40-fold activation of the reporter), whereas deletion of aa 444–455 completely abrogated this interaction (Figure 3B). Altogether these results indicate that p65 can interact with SMRT-D through a region containing aa 444–455 and that the interaction of p65 with both coactivators and corepressors may be regulated by phosphorylation.
Figure 3.
p65-NFκB physically interacts with SMRT corepressor. (A and B) NIH-3T3 cells were cotransfected with the Gal4DBD-SMRT-D (5 ng) and (A) two different p65 constructs fused to the VP16 TA domain (0.5 μg) or (B) VP16-TAD-p65(1–551) in the presence or absence of 0.5 μg of the activated PKA catalytic subunit mutant (His87, Trp196) along with the 5xGal4-luc reporter. (C) Amino acids 444–455 of p65 are required for the p65/SMRT interaction. Cells were cotransfected with the indicated p65 constructs fused to VP16-TA (0.5 μg). Numbers in brackets correspond to the amino acids present in the p65 construct. p65 constructs are represented (RHD, rel homology domain; NLS, nuclear localization signal; NES, nuclear export signal; TAD, transactivation domain). Luciferase activity is represented as the fold induction relative to cells transfected with empty vector. The average and SD of duplicates from one representative of three independent experiments are presented. CMV-β-galactosidase was cotransfected as internal control in all experiments. Expression levels of the different p65 constructs were determined by Western blot using an α-Ha antibody (lower panels).
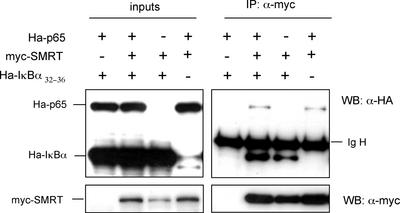
To corroborate this interaction in vivo, we performed a coimmunoprecipitation assay from 293T-transfected cell extracts using the myc-tag antibody. As shown in Figure 4, Ha-p65(96–551) was detected in the immunoprecipitates of myc-SMRT by using an anti-Ha antibody. Because IκBα is crucial for p65 cytoplasmic translocation (Huang and Miyamoto, 2001), we also investigated whether IκBα was able to bind SMRT in the presence or in the absence of transfected p65. In both situations we consistently detected the presence of IκBα32–36 in the myc-SMRT immunoprecipitates (Figure 4). These results demonstrate that both p65 and IκBα are able to bind to the SMRT protein and suggest that the p65/IκBα dimers may participate in the cytoplasmic shuttling of NCoRs-containing complexes.
Figure 4.
p65-NFκB and IκBα32–36 coimmunoprecipitates with SMRT. Whole-cell lysates from 293T cells cotransfected with the indicated plasmids were immunoprecipitated with the anti-myc antibody followed by immunoblotting with anti-Ha. 1/50 of the input is showing in the left panel. Lower panels show the same membrane stripped and reprobed with anti-myc to detect SMRT. (Ig H, immunoglobulin heavy chain)
Both p65-NFκB and IκBα Are Implicated in the Cytoplasmic Translocation of N-CoR
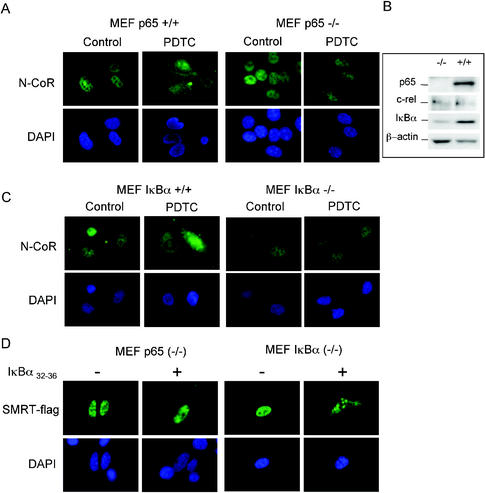
To examine whether p65 is required for the cytoplasmic translocation of NCoRs observed after IκBα stabilization, we studied the effect of PDTC on the subcellular localization of endogenous N-CoR protein in murine embryo fibroblasts (MEFs) isolated from wild-type or p65−/− mice produced by targeted gene disruption. As shown in Figure 5A, when p65+/+ MEF were exposed to PDTC, they showed a consistent cytoplasmic shuttling of the endogenous N-CoR as observed in NIH-3T3 cells (Figure 1A). By contrast, in p65-deficient cells an extremely reduced cytoplasmic N-CoR staining was observed in the presence of PDTC (Figure 5A).
Figure 5.
Both p65-NFκB and IκBα are implicated in the cytoplasmic translocation of NCoRs. (A) Endogenous N-CoR subcellular localization was determined in p65−/− and p65+/+ MEFs after 2 h incubation in the presence or absence of PDTC (100 μM). α-N-CoR was detected with FITC-labeled secondary antibody (upper panels), and nuclei were visualized by DAPI staining (lower panels). (B) Expression of NFκB family members in p65−/− and p65+/+ MEFs. Cell lysates were electrophoresed and immunoblotted with anti-p65, anti-c-rel, and anti-IκBα antibodies. β-Actin was detected to ensure equal protein loading. (C) Endogenous N-CoR subcellular localization was determined in IκBα−/− and IκBα+/+ MEFs after 1 h incubation in the presence or absence of PDTC (100 μM). Cells were stained as above. (D) p65−/− and IκBα−/− MEFs were cotransfected with SMRT-flag and an empty vector or IκBα32–36 as indicated. SMRT protein was detected with α-flag antibody and FITC-conjugated secondary antibody. These are representative images of three independent experiments.
Because stabilization of IκB proteins is responsible for p65 cytoplasmic retention in response to PDTC treatment and we detected the presence of IκBα in the SMRT immunoprecipitates (Figure 4), we decided to investigate the importance of this protein in NCoRs translocation. For this purpose we reproduced the above-mentioned experiments using wild-type and IκBα-deficient MEFs. Treatment of IκBα−/− cells with PDTC did not result in N-CoR cytoplasmic translocation, similar to that observed in p65−/− MEF. Conversely, in IκBα+/+ MEFs cytoplasmic translocation of N-CoR was very apparent in PDTC-treated cells (Figure 5C).
Because we detected very reduced levels of IκBα in the p65−/− MEFs (Figure 5B), we considered the possibility that only IκBα was necessary to induce NCoRs translocation. To test this hypothesis, we transfected p65−/− MEFs with SMRT alone or with IκBα32–36. Cells transfected with both molecules showed very low levels of cytoplasmic SMRT staining, suggesting that p65 is required for the IκB-mediated cytoplasmic translocation of NCoRs (Figure 5D). Nevertheless, because some cytoplasmic SMRT was present in the p65−/− cells, it is formally possible that in the absence of p65 other NFκB members (such as c-rel; see Figure 5B) may compensate for its deficiency. As a control, reconstitution of IκBα-deficient MEFs with IκBα32–36 resulted in SMRT cytoplasmic translocation as expected (Figure 5D). These results indicate that both IκBα and p65 cooperate in the cytoplasmic translocation of NCoRs.
Binding of SMRT Corepressor to p65 and IκBα Is Negatively Regulated by CBP Coactivator or TNF-α Treatment
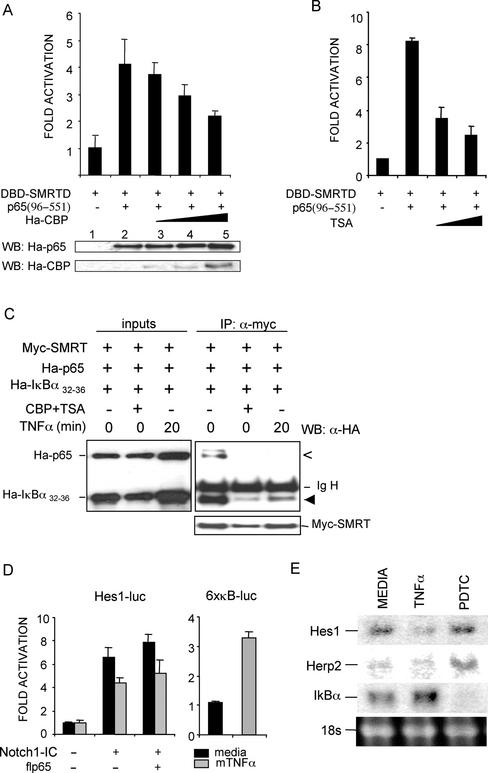
It has been described that corepressors and coactivators compete for binding to nuclear hormone receptors (Perissi et al., 1999). In this system, ligand binding is thought to induce conformational changes in the receptor that lead to release of the corepressors and recruitment of the coactivators. To test whether there is competition between coactivators and corepressors for binding to p65, we measured the p65/SMRT interaction in the Gal4-luciferase system when cotransfecting increasing amounts of the coactivator CBP. We observed that interaction between SMRT and p65(96–551) is disrupted in a dose-dependent manner by the addition of CBP (Figure 6A), thus suggesting that CBP can modulate SMRT and p65 binding.
Figure 6.
Binding of SMRT corepressor to p65 and IκBα is negatively regulated by CBP coactivator or TNF-α treatment. (A) CBP coactivator competes with SMRT for interacting with p65. NIH-3T3 cells were cotransfected with DBD-SMRT-D and VP16-TAD-p65(96–551) with increasing amounts (125, 250, and 500 ng) of the CBP coactivator. Lower panels show the immunoblot to confirm the expression of equivalent amounts of p65 and the increasing amounts of CBP. (B) DBD-SMRT-D and VP16-TAD-p65(96–551) interaction was assayed in the presence of 0.6 and 1.2 μM of the HDAC inhibitor TSA. (C) 293T cells were transfected with the indicated constructs and incubated with media alone, 0.6 μM TSA (4 h), or 40 ng/ml hTNF-α. Whole-cell lysates were immunoprecipitated with the anti-myc antibody followed by immunoblotting with anti-Ha. One-fiftieth of the input is showing in the left panel. Arrowheads indicate coimmunoprecipitated p65 (open) and IκBα (filled); IgH, immunoglobulin heavy chain. (D) Hes1-luc (left) and 6xκB-luc (right) activity measured in NIH-3T3 cells cotransfected with N1-IC (0.75 μg) and pCMV-flp65 (0.25 μg) when indicated, after 12 h incubation in medium alone (black bar) or 25 ng/ml mTNF-α (gray bar). Luciferase activity is represented as the fold induction, relative to cells transfected with empty vector. CMV-β-galactosidase was cotransfected as internal control. The average and SD of duplicates from one of three independent experiments are presented. (E) Activation by TNF-α or inhibition by PDTC of the NFκB pathway modifies the mRNA levels of different Notch-target genes. Northern blot of total RNA from 293T cells exposed to media, hTNF-α (40 ng/ml) or PDTC (100 μM) for 3 h. Membranes were hybridized with 32P-labeled DNA probes for the Notch-target genes Hes1 and Herp2. IκBα probe was used as a control for NFκB activity. Ethidium bromide–stained 18s rRNA is shown as loading control.
Chen et al. (2001) recently demonstrated that TNF-α induces acetylation of p65 by p300/CBP, thus abrogating the binding of p65 to IκBα. Conversely, deacetylation of p65 by HDAC3 stabilizes its binding to IκBα and promotes the nuclear export of the p65/IκBα complexes. To test whether acetylation was responsible for inhibiting the binding of p65 and/or IκBα to SMRT, we reproduced the interaction experiments in the presence of the HDAC inhibitor trichostatin A (TSA). In the Gal4 system, incubation with TSA results in a dose-dependent inhibition of the p65 and SMRT interaction (Figure 6B) similar to that observed by cotransfecting CBP. Additionally, in coprecipitation experiments, overexpression of CBP plus TSA treatment resulted in an important decrease in the amount of both p65 and IκBα detected in the SMRT immunoprecipitates (Figure 6C). Consistent with this, a 20-min TNF-α treatment had a similar effect on the IκBα/p65/SMRT interaction (Figure 6C). These results suggest that binding of p65/IκBα complexes to SMRT is modulated by TNF-α, probably through CBP-mediated acetylation of p65 (Chen et al., 2001).
We have shown that stimuli that stabilize IκBα and induce SMRT/N-CoR cytoplasmic translocation exert a positive regulation of the Hes1 transcription (Figures 1 and 2). On the basis of our results, we speculated that activators of the NFκB pathway such as TNF-α (which induce IκBα degradation and p65 acetylation) might release NCoRs from the p65/IκBα complexes, thus increasing nuclear levels of active NCoRs and playing a repressive effect on Hes1 activity. In NIH-3T3 cells transfected with N1-IC, incubation with TNF-α resulted in a consistent inhibition of the Hes1 promoter activity (Figure 6D). Conversely, TNF-α activated the NFκB-dependent promoter, as expected.
To examine whether this regulation of the Notch-target promoters by TNF-α and PDTC may be operating in vivo, we performed Northern blot experiments to determine the expression levels of four different Notch-target genes (Hes1, Hes5, Herp1, and Herp2) after 3 h treatment with TNF-α or PDTC in 293T cells. Although transcription of Notch/RBP-Jκ–regulated genes is dependent on Notch activation in most systems, we detected basal expression of the Hes1 and Herp2 in 293T cells. In contrast, no expression of Hes5 or Herp1 genes was observed (unpublished data). Incubation with TNF-α resulted in an important decrease in the levels of Hes1 mRNA, whereas not much effect was observed on the expression of Herp2 (Figure 6E). Conversely, PDTC treatment had almost no effect on the basal transcription of the Hes1 gene in 293T cells, whereas we did observe upregulation of the Herp2 gene in these conditions (Figure 6E). To confirm that in these experiments TNF-α and PDTC were activating or inhibiting the NFκB pathway, we evaluated the expression levels of the NFκB-target gene IκBα. As expected, IκBα mRNA levels increased after incubation with TNF-α but decreased after incubation with PDTC (Figure 6E). These results indicate that different endogenous Notch-target genes can be modulated by stimuli that induce IκB degradation or stabilization, in an opposite manner to that described for NFκB-dependent genes.
Stabilization of IκBα by PDTC Results in the Cytoplasmic Translocation of N-CoR, the Upregulation of the Hes1 Promoter, and the Inhibition of the G-CSF–induced Differentiation in N2-IC–expressing 32D Cells
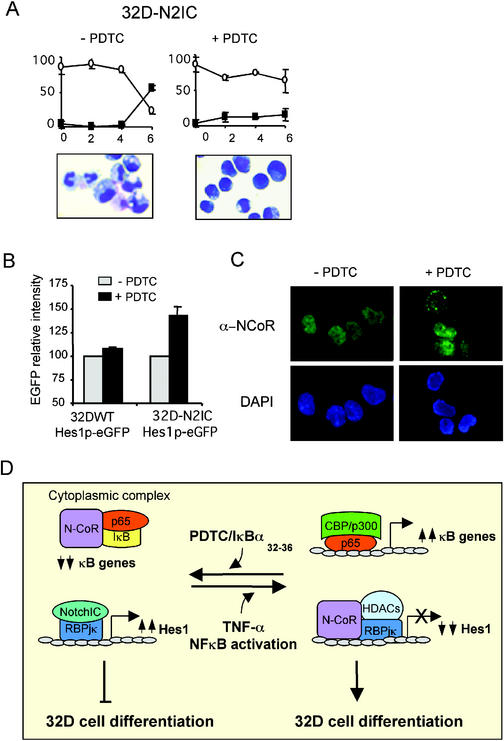
The role of Notch and NFκB pathways in the progress of hematopoietic differentiation still remains unclear. We have previously shown that constitutively active forms of Notch1, but not of Notch2, inhibit G-CSF–induced differentiation in the 32D myeloid progenitor cell line (Bigas et al., 1998). To investigate whether activation of Notch and cytoplasmic retention of NCoRs by p65/IκB may cooperate to modulate cell differentiation, we used two different 32D clones stably expressing the Notch2 intracellular (N2-IC) construct and containing a Hes1 promoter driving the expression of eGFP. Using these cells, we can simultaneously assay the capacity of differentiation and the Notch-dependent activity by measuring the fluorescence intensity. First, we examined the phenotype of N2-IC–expressing cells after 6 d in G-CSF in the presence or absence of PDTC. Figure 7A shows that PDTC-treated cells cannot differentiate when incubated in G-CSF compared with control cells. At day 6 of culture, 60% of cells in the absence of PDTC showed a neutrophil-like differentiated phenotype, whereas only 15% of these cells appeared in the PDTC-treated cultures. Then, we measured eGFP intensity of N2-IC–expressing cells in the presence or absence of PDTC during G-CSF–induced differentiation. In four independent experiments, we observed a similar increase in the fluorescence intensity of cells incubated with PDTC (Figure 7B), which was maintained all along the differentiation culture period (unpublished data). The expression of N2-IC was required for PDTC-mediated activation of the Hes1 promoter because PDTC had no effect in wild-type 32D cells containing the eGFP construct (Figure 7B). Next, we asked whether incubation with PDTC was also able to modify N-CoR subcellular localization in these cells. Figure 7C shows that 32D control cells have an almost exclusively nuclear staining of α-N-CoR antibody (90% of cells with exclusive nuclear staining) and this staining pattern changes to predominantly cytoplasmic after 2-h incubation in PDTC (60% of cells showing cytoplasmic staining). Together, our results indicate that inhibition of myeloid differentiation by PDTC in the N2-IC–expressing 32D cells involves both, relocalization of NCoRs by p65/IκBα and activation of Notch-target genes, including Hes1.
Figure 7.
Stabilization of IκB by PDTC inhibits G-CSF–induced differentiation in N2-IC–expressing 32D cells. (A) N2-IC–expressing cells were induced to differentiate in the presence or absence of PDTC (10 μM). Graphs represent the percentage of blast-like undifferentiated cells (○) and band neutrophil-like differentiated cells (▪) during 6 d incubation in G-CSF. Cells with intermediate phenotype are not represented. Graphs show the average and SD of two different clones. Photographs of representative cells at day 6 of differentiation in the absence (left) or presence (right) of PDTC are shown in the lower panels. (B) Hes1p-eGFP activity was assayed in control and N2-IC-32D cells (used in A), after 24 h in G-CSF with or without PDTC. eGFP intensity was measured by flow cytometry and is represented relative to the control in the absence of PDTC for each cell type. One stably transfected Hes1p-eGFP clone for 32D wt and two clones for N2-IC-32D cells were evaluated. Values are the average and SD of three independent experiments. (C) N-CoR subcellular localization in 32D control cells or cells incubated 1 h with PDTC (100 μM). α-N-CoR was detected with a FITC-labeled secondary antibody (upper panels), and nuclei were visualized by DAPI staining (lower panels). (D) Model for Notch and NFκB cross-talk to regulate myeloid 32D cell differentiation. See DISCUSSSION for details.
DISCUSSION
Notch and NFκB pathways are key regulators in the control of numerous cellular events. Putative cross-talk mechanism involving both pathways may contribute to generate additional complexity to the regulation of cell behavior. Many cellular processes have now been reported where the activation of Notch signaling correlates with the inhibition of the NFκB pathway (Kopan et al., 1994; Kaliman et al., 1999; Kuroda et al., 1999). In this study we address the question on how NFκB regulates the nuclear levels of NCoRs and how this mechanism influences Notch-dependent gene transcription and myeloid cell differentiation. We have previously shown that overexpression of a truncated form of p65 that primarily localizes in the cytoplasm induced cytoplasmic translocation of N-CoR nuclear corepressor and enhances the transcriptional activity of Notch-dependent promoters. Now, we demonstrate that stabilization of IκBα by PDTC or ectopic expression of IκBα32–36 mimics the previously described phenomenon. Moreover, we demonstrate that SMRT directly interacts with p65 and IκBα, competing with the CBP coactivator for this binding. In agreement with this, stimuli that promote IκB degradation, p65 acetylation, and NFκB activation, such as TNF-α, inhibit Notch-dependent transcriptional activity.
We propose that p65/IκBα complexes that are stabilized by inhibitory NFκB stimuli are able to translocate specific N-CoR/SMRT complexes to the cytoplasm, thus converting NCoRs-repressed genes into potentially active ones. On the other hand activating NFκB stimuli that promote IκB degradation and enhance the binding of p65 to nuclear coactivators (for example, CBP/p300) may simultaneously release NCoRs to inhibit specific genes such as Hes1. The composition of the NCoRs complexes that translocate to the cytoplasm associated with p65/IκBα is currently under investigation. We hypothesize that distinct repressor complexes may translocate to the cytoplasm in response to different stimuli, thus permitting the activation of specific sets of genes. In this sense, it has recently been shown that IL1β can induce nuclear export of a N-CoR/TAB2/HDAC3 complex, leading to the activation of a NFκB-dependent subset of genes (Baek et al., 2002). To illustrate how this mechanism may be relevant in specific cellular processes such as cell differentiation, we have shown that stabilization of IκB by PDTC inhibits G-CSF–induced differentiation in N2-IC–expressing 32D cells. Altogether, these results provide evidence that integration of the Notch and NFκB pathways by competing for common corepressors regulate gene expression and affect myeloid cell differentiation.
Competitive Binding of Corepressors and Coactivators to the NFκB Member p65
NCoRs were first described as transcriptional inhibitors of the nuclear hormone receptors. After ligand binding, coactivators displace corepressors from the hormone receptor, thus leading to the activation of its target genes. Both, NCoRs and coactivators bind to nuclear receptors through similar LXXLL motifs. Moreover, nuclear hormone receptors themselves contain leucine-rich motifs that participate in this binding. We have identified a p65 region comprising aa 444–455 containing a leucine-rich motif, which is required to bind SMRT in the mammalian two-hybrid assay. A similar adjacent motif located in aa 440–443 has been shown to be required for p65-induced cytoplasmic translocation of NCoR (Espinosa et al., 2002). Together, these results suggest that the two contiguous hydrophobic cores may be involved in the binding of p65 to NCoRs and show the parallelism existing between this new mechanism and that described for activation/repression of nuclear hormone receptors.
Moreover, it has been shown that CBP can bind to two different domains of p65, one of them dependent on Ser 276 phosphorylation by PKA and the other, located at the C-terminal TAD, phosphorylation independent (Zhong et al., 1998). This, together with the fact that coactivators and corepressors compete for binding to transcription factors, may explain the higher affinity of SMRT for p65 constructs lacking the N-terminal (1–95 aa) and C-terminal TAD. Our results suggest that conformational changes induced by phosphorylation may be necessary for the accessibility of both coactivators and corepressors to p65 binding domains. On the other hand, because acetylation events inhibit the binding of SMRT to p65 and IκBα, it is tempting to speculate that HDAC molecules will play an important role in the regulation of this interaction.
Functional Significance of p65/IκBα-mediated Cytoplasmic Shuttling of NCoRs
NCoRs play an important role in remodeling chromatin structure and regulating gene transcription. The subcellular localization of these molecules is primarily nuclear; however, cytoplasmic translocation of SMRT in response to phosphorylation events has been reported (Hong and Privalsky, 2000). Besides, SMRT has been shown to regulate the nuclear translocation of RBPjκ (Zhou and Hayward, 2001). Once in the nucleus RBPjκ and SMRT cooperate in the silencing of promoters containing the GTGGGAA sequence through the recruitment of a corepressor complex that includes CIR, Sin3A, and different HDACs (Kao et al., 1998; Hsieh et al., 1999). After Notch-pathway activation, Notch-IC translocates to the nucleus and disrupts the interaction of RBPjκ with corepressors, leading to the activation of the same target genes (Kao et al., 1998; Hsieh et al., 2000). We have demonstrated that after NFκB inhibition (incubation with PDTC or expression of constitutively active IκBα) NCoRs are induced to translocate to the cytoplasm. We previously demonstrated that nuclear-cytoplasmic shuttling of p65 is necessary for inducing cytoplasmic translocation of NCoRs (Espinosa et al., 2002). The NFκB inhibitor IκBα contains a functional nuclear export signal (Johnson et al., 1999), and it has recently been demonstrated that it is involved in the nuclear cytoplasmic shuttling of p65 (Huang and Miyamoto, 2001), being crucial for the control of the NFκB signaling termination. Because IκBα has a central role in the p65 nuclear export, it is tempting to speculate that corepressors exit the nucleus accompanied not only by p65 but also in a complex containing IκBα. In agreement with this our results indicate that both IκBα and p65 can physically bind to SMRT, preferentially in the absence of NFκB stimulation. Moreover we have demonstrated that expression of IκBα in p65−/− MEF has no significant effect on SMRT localization, suggesting that cytoplasmic shuttling of SMRT requires both proteins. These observations reveal a different and unexpected role for p65 and IκBα in the shuttling of corepressor complexes from the nucleus to the cytoplasm. Thus, the interaction between corepressor molecules and IκBα/p65 may not only result in the silencing of NFκB-dependent genes, but also would be important for the activation of other sets of genes. In agreement with this, we have previously described that different promoters are upregulated by this mechanism (Espinosa et al., 2002). Nevertheless, the fact that only part of the endogenous N-CoR protein is translocated to the cytoplasm in response to PDTC suggests that specific repressor elements may dictate the sensibility of the complexes for exiting the nucleus with p65/IκB. Because different corepressor complexes interact with NFκB (Lee et al., 2000; Ashburner et al., 2001; Baek et al., 2002) and other transcription factors (Bailey et al., 1999; Wu et al., 2001; Melnick et al., 2000), we are currently examining the composition of the putative complex that may translocate with p65 and IκBα.
Notch and NFκB Pathways Cooperate in Regulating Myeloid Cell Differentiation
Cellular differentiation is controlled by the coordinated activation and silencing of specific subsets of genes. Both Notch and NFκB pathways are involved in the regulation of gene expression and differentiation in many different systems (Egan et al., 1998; Feng and Porter, 1999; Kaliman et al., 1999; Guttridge et al., 2000; Kaisho et al., 2001), including hematopoietic cells (Boothby et al., 1997; Milner and Bigas, 1999). The effect of each pathway in inhibiting or promoting differentiation is controversial, and it could depend on the cellular context. In the myeloid 32D cell line, other groups and ourselves have observed that activation of Notch1 correlates with inhibition of G-CSF–induced differentiation (Milner et al., 1996; Kumano et al., 2001), while activated Notch2 has no effect (Bigas et al., 1998). Now, we have demonstrated that inhibition of NFκB activity by incubation with PDTC relocates endogenous N-CoR, enhances Notch2-dependent transcription and cooperates with activated Notch2 in the maintenance of the undifferentiated 32D phenotype. In our model (Figure 7D) we propose that after NFκB activation, p65 binds to nuclear coactivators (such as p300/CBP), resulting in the transcriptional activation of the κB-regulated genes and permits corepressors to inhibit Notch-dependent promoters. In this situation 32D cells can differentiate. When NFκB is inhibited, p65 and NCoRs are retained in the cytoplasm bound to IκBα and reduced nuclear levels of NCoRs contribute to decrease the threshold for Notch-dependent gene activation. In this situation, κB-regulated genes are silenced, expression of Hes-related genes is increased, and cell differentiation is inhibited.
We are further investigating the importance of this new mechanism in the control of cell differentiation and cancer.
ACKNOWLEDGMENTS
We thank M. Karin, J. Caamaño, R. Evans, S. Sun, A. Israel, J. W. Lee, W. Greene, Y. Hamamori, GS Mcknight for kindly providing plasmids and A. Hoffman/D. Baltimore for p65−/−, IκBα−/− and control MEFs. We are thankful to P. Muñoz-Canoves for helpful suggestions to improve the manuscript, A. Nonell for helping with Gal4 constructs, and H. Evans for helping in language correction. We thank FAPS for financial support for L.E. A.R.M. is a recipient of a CIRIT predoctoral fellowship (2002-SI00791). This work was supported by a grant (SAF2001–1191) from the Comisión Interministerial de Ciencia y Tecnología, Plan Nacional de Salud.
Abbreviations used:
- CBP
CREB binding protein
- eGFP
enhanced green fluorescence protein
- HDAC
histone deacetylase
- MEF
murine embryonic fibroblast
- N1-IC
Notch1-intracellular
- N2-IC
Notch2-intracellular
- N-CoR
nuclear corepressor
- PDTC
pyrrolidinedithiocarbamic acid
- SMRT
silence mediator of retinoic acid and thyroid hormone
- TAD
transactivation domain
- TNF-α
tumor necrosis factor alpha
- TSA
trichostatin A
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0404. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0404.
REFERENCES
- Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-B and -amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- Bailey P, Downes M, Lau P, Harris J, Chen SL, Hamamori Y, Sartorelli V, Muscat GE. The nuclear receptor corepressor N-CoR regulates differentiation: N-CoR directly interacts with MyoD. Mol Endocrinol. 1999;13:1155–1168. doi: 10.1210/mend.13.7.0305. [DOI] [PubMed] [Google Scholar]
- Baonza A, Garcia-Bellido A. Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc Natl Acad Sci USA. 2000;97:2609–2614. doi: 10.1073/pnas.040576497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y, Gelinas C. Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J. 1999;18:2803–2811. doi: 10.1093/emboj/18.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 1998;18:2324–2333. doi: 10.1128/mcb.18.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93:838–848. [PubMed] [Google Scholar]
- Carlotti F, Dower SK, Qwarnstrom EE. Dynamic shuttling of nuclear factor kappa B between the nucleus and cytoplasm as a consequence of inhibitor dissociation. J Biol Chem. 2000;275:41028–41034. doi: 10.1074/jbc.M006179200. [DOI] [PubMed] [Google Scholar]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity. 2000;13:73–84. doi: 10.1016/s1074-7613(00)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan SE, St Pierre B, Leow CC. Notch receptors, partners and regulators: from conserved domains to powerful functions. Curr Top Microbiol Immunol. 1998;228:273–324. doi: 10.1007/978-3-642-80481-6_11. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Santos S, Ingles-Esteve J, Munoz-Canoves P, Bigas A. p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J Cell Sci. 2002;115:1295–1303. doi: 10.1242/jcs.115.6.1295. [DOI] [PubMed] [Google Scholar]
- Feng Z, Porter AG. NF-kappaB/Rel proteins are required for neuronal differentiation of SH-SY5Y neuroblastoma cells. J Biol Chem. 1999;274:30341–30344. doi: 10.1074/jbc.274.43.30341. [DOI] [PubMed] [Google Scholar]
- Greenberger JS, Sakakeeny MA, Humphries RK, Eaves CJ, Eckner RJ. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan E, Wang J, Laborda J, Norcross M, Baeuerle PA, Hoffman T. T cell leukemia-associated human Notch/translocation-associated Notch homologue has I kappa B-like activity and physically interacts with nuclear factor-kappa B proteins in T cells. J Exp Med. 1996;183:2025–2032. doi: 10.1084/jem.183.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun SC. Regulation of RelA subcellular localization by a putative nuclear export signal and p50. Mol Cell Biol. 1999;19:7088–7095. doi: 10.1128/mcb.19.10.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T, Leiden JM. NF-kappa B is required for the positive selection of CD8+ thymocytes. J Immunol. 2000;165:5004–5010. doi: 10.4049/jimmunol.165.9.5004. [DOI] [PubMed] [Google Scholar]
- Hong SH, Privalsky ML. The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol Cell Biol. 2000;20:6612–6625. doi: 10.1128/mcb.20.17.6612-6625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JJ, Zhou S, Chen L, Young DB, Hayward SD. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. J Virol. 2000;74:1939–1947. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JJ, Zhou S, Chen L, Young DB, Hayward SD. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc Natl Acad Sci USA. 1999;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Miyamoto S. Postrepression activation of NF-kappaB requires the amino-terminal nuclear export signal specific to IkappaBalpha. Mol Cell Biol. 2001;21:4737–4747. doi: 10.1128/MCB.21.14.4737-4747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles-Esteve J, Espinosa L, Milner LA, Caelles C, Bigas A. Phosphorylation of Ser2078 modulates the Notch2 function in 32D cell differentiation. J Biol Chem. 2001;276:44873–44880. doi: 10.1074/jbc.M104703200. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signaling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Isral A. Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol. 1998;18:7423–7431. doi: 10.1128/mcb.18.12.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn BM, Bielke W, Pear WS, Osborne BA. Protective effects of notch-1 on TCR-induced apoptosis. J Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- Johnson C, Van Antwerp D, Hope TJ. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IkappaBalpha. EMBO J. 1999;18:6682–6693. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T. Notch signaling: A dance of proteins changing partners. Exp Cell Res. 2000;260:1–8. doi: 10.1006/excr.2000.4921. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. IkappaB kinase alpha is essential for mature B cell development and function. J Exp Med. 2001;193:417–426. doi: 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix- loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- Kumano K, Chiba S, Shimizu K, Yamagata T, Hosoya N, Saito T, Takahashi T, Hamada Y, Hirai H. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood. 2001;98:3283–3289. doi: 10.1182/blood.v98.12.3283. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T. Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J Biol Chem. 1999;274:7238–44. doi: 10.1074/jbc.274.11.7238. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim JH, Lee YC, Cheong J, Lee JW. Silencing mediator of retinoic acid and thyroid hormone receptors, as a novel transcriptional corepressor molecule of activating protein-1, nuclear factor-kappaB, and serum response factor. J Biol Chem. 2000;275:12470–12474. doi: 10.1074/jbc.275.17.12470. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Melnick A, Carlile GW, McConnell MJ, Polinger A, Hiebert SW, Licht JD. Cell signaling switches HOX-PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Blood. 2000;96:3939–3947. [Google Scholar]
- Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- Milner LA, Bigas A, Kopan R, Brashem-Stein C, Bernstein ID, Martin DI. Inhibition of granulocytic differentiation by mNotch1. Proc Natl Acad Sci USA. 1996;93:13014–13019. doi: 10.1073/pnas.93.23.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Parra M, Caelles C, Nagamine Y, Felez J, Munoz-Canoves P. UV irradiation induces the murine urokinase-type plasminogen activator gene via the c-Jun N-terminal kinase signaling pathway: requirement of an AP1 enhancer element. Mol Cell Biol. 1998;18:4537–4547. doi: 10.1128/mcb.18.8.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Flowers D, Anasetti C, Myerson D, Bernstein ID. Monocytes express high amounts of Notch and undergo cytokine specific apoptosis following interaction with the Notch ligand, Delta-1. Blood. 2000;95:2847–2854. [PubMed] [Google Scholar]
- Orellana SA, McKnight GS. Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci USA. 1992;89:4726–4730. doi: 10.1073/pnas.89.10.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin MJ, Kadonaga JT. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- Perissi V, et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- Shelly LL, Fuchs C, Miele L. Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem. 1999;73:164–175. doi: 10.1002/(sici)1097-4644(19990501)73:2<164::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha, and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B, and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- Wang J, Shelly L, Miele L, Boykins R, Norcross MA, Guan E. Human notch-1 inhibits nf-kappab activity in the nucleus through a direct interaction involving a novel domain. J Immunol. 2001;167:289–95. doi: 10.4049/jimmunol.167.1.289. [DOI] [PubMed] [Google Scholar]
- Wu XY, Li H, Park EJ, Chen JD. SMRTe inhibits MEF2C transcriptional activation by targeting HDAC4 and 5 to nuclear domains. J Biol Chem. 2001;276:24177–24185. doi: 10.1074/jbc.M100412200. [DOI] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- Zhou S, Hayward SD. Nuclear localization of CBF1 is regulated by interactions with the SMRT corepressor complex. Mol Cell Biol. 2001;21:6222–6232. doi: 10.1128/MCB.21.18.6222-6232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]