Abstract
Glycerophosphoinositol 4-phosphate (GroPIns-4P) is a biologically active, water-soluble phospholipase A metabolite derived from phosphatidylinositol 4-phosphate, whose cellular concentrations have been reported to increase in Ras-transformed cells. It is therefore important to understand its biological activities. Herein, we have examined whether GroPIns-4P can regulate the organization of the actin cytoskeleton, because this could be a Ras-related function involved in cell motility and metastatic invasion. We find that in serum-starved Swiss 3T3 cells, exogenously added GroPIns-4P rapidly and potently induces the formation of membrane ruffles, and, later, the formation of stress fibers. These actin structures can be regulated by the small GTPases Cdc42, Rac, and Rho. To analyze the mechanism of action of GroPIns-4P, we selectively inactivated each of these GTPases. GroPIns-4P requires active Rac and Rho, but not Cdc42, for ruffle and stress fiber formation, respectively. Moreover, GroPIns-4P induces a rapid translocation of the green fluorescent protein-tagged Rac into ruffles, and increases the fraction of GTP-bound Rac, in intact cells. The activation of Rac by GroPIns-4P was near maximal and long-lasting. Interestingly, this feature seems to be critical in the induction of actin ruffles by GroPIns-4P.
INTRODUCTION
The phosphoinositides are multifunctional lipids that play major roles in the modulation of many cellular events, such as membrane traffic, intracellular signaling, cytoskeleton organization, and apoptosis (Berridge, 1993; Czech, 2000; De Matteis et al., 2002). Phosphoinositides may act either by directly affecting the function of a variety of proteins involved in specific cellular activities (Toker, 1998; Lemmon and Ferguson, 2000) or as precursors of second messenger molecules via the activity of specific phospholipases (Berridge, 1993; Rhee and Bae, 1997). An example of the former possibility is the formation of molecular complexes where the phosphoinositides behave as specific docking sites at the membrane for proteins containing appropriate recognition domains, such as pleckstrin homology, FYVE (Stenmark and Aasland, 1999; Lemmon and Ferguson, 2000), and Phox homology (Xu et al., 2001) domains. The second case (phosphoinositides acting as precursors of second messengers) is exemplified by the formation of diacylglycerol and of the water-soluble inositol 1,4,5-trisphosphate via hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C (Berridge, 1993). Of note, soluble phosphoinositide derivatives may also be relevant in the regulation of the interaction of proteins with cellular membranes because they share the polar head group with the phosphoinositides. They have, therefore, the potential to compete with their parent lipids and to interfere with protein–lipid interactions in different cell compartments (De Matteis et al., 2002).
Thus, despite their relatively low abundance (10% of the total cellular phospholipids; Downes et al., 1989), the phosphoinositides and their derivatives are functionally crucial. Accordingly, the interest of cell biologists in determining the localization and the metabolism of all of the inositol-containing molecules is constantly increasing, as is the knowledge of the processes in which the phosphoinositides and their products play major roles (Czech, 2000; Sechi and Wehland, 2000).
Over the last few years, we have characterized a category of water-soluble phosphoinositide metabolites produced by the sequential action of phosphoinositide-specific phospholipase A2 (PLA2) and lysolipase activities (reviewed in Corda et al., 2002), i.e., lysophosphatidylinositol and the glycerophosphoinositols. These molecules have been associated with the expression of oncogenic Ras (Alonso et al., 1988; Alonso and Santos, 1990; Valitutti et al., 1991; Falasca et al., 1995) and are biologically active. Lysophosphatidylinositol acts as a mitogen in Ras-transformed cells (Falasca and Corda, 1994; Falasca et al., 1995, 1998), and among the glycerophosphoinositols, glycerophosphoinositol 4-phosphate (GroPIns-4P) has been shown to inhibit the heterotrimeric G protein Gs in thyroid cells, thereby decreasing the cellular levels of cAMP and inhibiting cAMP-dependent functions, such as cell proliferation and iodide uptake (Iacovelli et al., 1993). However, in nonthyroid cells, the glycerophosphoinositols do not have significant effects on cell growth (Corda and Falasca, 1996). In view of this observation, it is reasonable to hypothesize that the glycerophosphoinositols may play a role in other cell functions affected by Ras. One such function is cell motility and invasiveness.
Herein, we test this hypothesis and examine the effects of GroPIns-4P on the dynamics and organization of the actin cytoskeleton. This approach is made feasible by our previous observation that GroPIns-4P readily crosses the plasma membrane and can easily reach its potential intracellular targets (Berrie et al., 1999). We report that GroPIns-4P added in the extracellular medium exerts a powerful control on the organization of cellular actin, consisting of the induction of membrane ruffles and of stress fibers. The mechanism of action of GroPIns-4P requires the independent activation of the small GTPases Rac and Rho, but not of Cdc42. The ruffling effect, in particular, is accompanied by the recruitment of Rac to the cell membrane, and by the near maximal and sustained activation of Rac. We believe that these observations are important on two accounts. First, they define a new biological activity of an endogenous, freely diffusable inositol-containing molecule. Moreover, because GroPIns-4P is a natural metabolite of the phosphoinositides generated by the activation of the Ras cascade (Falasca et al., 1995, 1997), the actin remodeling it induces is likely to play a role in motility and metastatic invasion. Second, because GroPIns-4P is a small, water-soluble molecule that is active when added extracellularly, our observations suggest that it has the potential to serve as a prototype of a new class of pharmacologically active agents.
MATERIALS AND METHODS
Reagents
Anti-Myc antibody (clone 9E10) was from Roche Diagnostics (Indianapolis, IN). Fluorescein dextran and Alexa 488 secondary antibody were from Molecular Probes (Eugene, OR). The glycerophosphoinositols and tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin were from Sigma-Aldrich (St. Louis, MO). Epidermal growth factor (EGF) and Mowiol were from Calbiochem (La Jolla, CA). The Rac activation kit was from Upstate Biotechnology (Lake Placid, NY). pRK5myc encoding Rac or Cdc42 mutants and the cDNA encoding for the human isoform of Rac1 were kindly provided by Dr. A. Hall (University College London, London, United Kingdom). All other reagents were obtained from standard commercial sources and were of the highest purities available.
Cell Culture and Microinjection
Swiss 3T3 cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM containing 10% calf serum. Cells were plated at a density of 3.5 × 104 onto 13-mm glass coverslips; the day after, the calf serum concentration was reduced to 0.5% for 18 h before injections and/or the addition of stimuli. pRK5myc vector encoding either the Rac or Cdc42 mutants was microinjected at 0.1–0.5 μg/μl in phosphate-buffered saline (PBS) into the nucleus of ∼50 cells over a period of 1 h by using a manual injection system (Eppendorf, Hamburg, Germany). Cells were returned to the incubator for a further 4 h before treatment with stimuli. Clostridium botulinum C3 transferase was injected at 160 μg/ml; fluorescein dextran (1 mg/ml) was coinjected with the transferase to localize injected cells. Cells were returned to the incubator for 15 min and then treated with stimuli. During microinjection, cells were maintained at ∼35°C in DMEM containing 10 mM HEPES (pH 7.4).
Immunofluorescence Analysis
After treatment, cells were fixed in 4% (wt/vol) paraformaldehyde for 10 min, permeabilized in 0.2% Triton X-100 for 5 min, and for filamentous actin visualization, incubated with 0.1 μg/ml TRITC-labeled phalloidin for 40 min. All steps were carried out at room temperature, and coverslips were rinsed in PBS after each step. To localize cells expressing the proteins encoded by the injected plasmids, cells were incubated in the presence of the mouse monoclonal anti-Myc antibody (clone 9E10), diluted in PBS, for 60 min. Coverslips were treated with Alexa 488 goat anti-mouse secondary antibody together with 0.1 μg/ml TRITC-labeled phalloidin for 30 min. Coverslips were mounted by inverting them onto 8 μl of Mowiol mountant. After 2 h at room temperature, the cells were examined with an Axiophot microscope using a 40× 1.3 objective (Carl Zeiss, Jena, Germany). Fluorescence samples were recorded on T-MAX 400 ASA film (Eastman Kodak, Rochester, NY). All micrographs shown are representative of 3 to 10 independent experiments.
Morphological Scoring
Samples of cells from five independent experiments were subjected to double-blind scoring for ruffle and stress fiber formation. The assessment was on the basis of a null score (zero) for the absence of the feature, followed by a score of one or two according to the level of response of each individual cell (one, partial response; two, full response). Cell phenotypes were quantified by counting more than 200 cells in each experiment. Percentage of score (±SE) represents the score for each morphological feature expressed as a percentage of the potential maximum score (2 × cell number). Scoring of the control cells was performed in a similar manner, evaluating the presence of the features of interest in untreated cells.
Affinity Precipitation of Cellular GTP-Rac, Rho, and Cdc42
Swiss 3T3 cells were lysed and incubated with Pak-1 binding domain (PBD) according to the manufacturer instructions (Upstate Biotechnology). For positive and negative controls, guanosine 5′-O-(3-thio)triphosphate (GTPγS) (100 μM) and GDP (1 mM) were used, respectively. Samples, subjected to SDS-PAGE and transferred to nitrocellulose, were revealed with a mouse monoclonal antibody (mAb) against Rac (Upstate Biotechnology) by using the ECL detection method (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). Densitometry analyses were performed on a Macintosh computer with NIH Image 1.61/fat. The amount of PBD-bound Rac was normalized to the total amount of protein content in cell lysates.
Preparation and Purification of Recombinant Rac1
Glutathione S-transferase (GST)-Rac1 expression was induced in the Escherichia coli strain BL21, carrying pGEX-4T vector encoding for GST-Rac1 with isopropyl 1-thio-β-d-galactopyranoside (1 mM) (Self and Hall, 1995a). Cells were lysed by sonication in 20 mM Tris-HCl pH 7.6, 100 mM NaCl, 5 mM MgCl2, 0.5% (vol/vol) Nonidet P-40, 1 mM dithiothreitol (DTT), 10 μM GDP, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin and then centrifuged at 10,000 × g for 10 min at 4°C. The protein was purified using glutathione-Sepharose 4B beads (Amersham Biosciences AB, Uppsala, Sweden). The GST-fusion protein was eluted with 5 mM reduced glutathione (Merck, Darmstadt, Germany) and dialyzed against dialysis buffer (10 mM Tris-HCl pH 7.6, 2 mM MgCl2, 0.1 mM DTT). Samples were stored at −80°C.
In Vitro GDP/GTP Exchange and GTPase Assays
The guanine nucleotide off-rate assays were performed as described previously (Self and Hall, 1995b). The GDP/GTP exchange assays were carried out by the filter binding method (Zheng et al., 1995). GST-Rac1 was preloaded with 10 μCi of [3H]GDP (11.7 Ci/mmol) (PerkinElmer Life Sciences, Boston, MA) in 25 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM EDTA, 0.1 mM DTT, and 1 mg/ml bovine serum albumin for 20 min at 30°C. MgCl2 (25 mM final concentration) was added for the further 10 min of incubation at 30°C. Nucleotides exchange reactions were performed at 30°C by sampling the [3H]GDP-loaded GST-Rac1 (0.5 μg) in 25 mM Tris-HCl pH 7.5, 100 mM NaCl, 11 mM MgCl2, 1 mM DTT, 1 mg/ml bovine serum albumin, 2 mM GTP (final concentrations), in the presence of 300 μg of cellular lysates (400 μl of final volume). Forty microliters of each reaction mixture was sampled at each time point, filtered over nitrocellulose filters, and followed by scintillation counting. The results are expressed as percentages of the [3H]GDP bound at each time point for the guanine nucleotide exchange factor (GEF)-catalyzed reactions relative to the same time points of the unanalyzed reactions, after subtracting the background counts released in control reactions (with recombinant Rac1 denatured at 100°C for 10 min). The Rac GTPase activity was assayed as described previously (Self and Hall, 1995b), with minor modifications.
Rac1 Expression in Swiss 3T3 Cells
cDNA encoding for the human isoform of Rac1 was inserted into the BglII-EcoRI restriction sites of the pEGFP-C2 vector (BD Biosciences Clontech, Palo Alto, CA). Swiss 3T3 cells were transfected using the gene pulser electroprotocol (according to the manufacturer's instructions) and plated in multiwell plates containing uncoated glass coverslips. After 6 h, the growth medium was replaced, cells were cultured for an additional 24 h, and serum starved for 12 h before being used for immunofluorescence analysis. Cell imaging was performed on a laser scanner microscope 510 (LSM510; Carl Zeiss), by using a 40× objective. Control experiments were performed in Swiss 3T3 cells transfected with the pEGFP-C2 empty vector, stimulated, and analyzed as described previously.
Statistical Analysis
All experiments are presented as the average of duplicate or triplicate determinations repeated at least three times. Statistical analysis was carried out using paired Student's t test.
RESULTS
GroPIns-4P Induces the Formation of Actin Ruffles and Stress Fibers
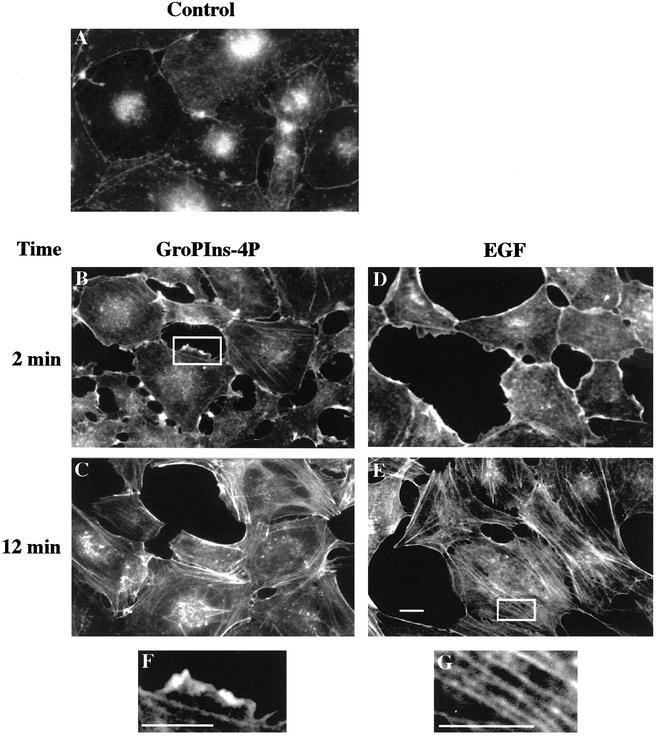
The possible role of GroPIns-4P in the organization of the actin cytoskeleton was studied in Swiss 3T3 fibroblasts. The addition of GroPIns-4P (50 μM) to serum-starved Swiss 3T3 fibroblasts (Figure 1A) caused the rapid formation of membrane ruffles (detectable within 1 min and maximal at 2 min; Figure 1B), followed by the formation of stress fibers (at 10–15 min; Figure 1C). These GroPIns-4P effects were dose dependent between 1 μM (a near-threshold concentration that induced ruffle formation in ∼10–20% of the cells observed) and 100 μM (when the effect was maximal, and virtually all cells presented ruffle formation). Most of the experiments in the rest of this study were therefore performed at 50 μM GroPIns-4P, a concentration that induced a nearly maximal effect (∼80–90% of cells presented ruffles). These concentrations are also compatible with the evaluated cellular levels of GroPIns-4P, which we have estimated to be in the low micromolar range under basal conditions and to increase from 1.5- to 8-fold after stimulation (the basal levels are <3% of total glycerophosphoinositols contents, and these range between ∼40 and 930 μM in the cell lines tested so far; Iacovelli et al., 1993; Berrie et al., 2002; Corda et al., 2002).
Figure 1.
GroPIns-4P– and EGF-induced modifications of the actin cytoskeleton in Swiss 3T3 cells. Serum-starved Swiss 3T3 cells (A) were treated with either 50 μM GroPIns-4P (B and C) or 20 ng/ml EGF (D and E) for 2 and 12 min, respectively. Cells were fixed and stained with TRITC-labeled phalloidin as described under MATERIALS AND METHODS. The results shown are representative of at least 30 independent experiments performed in triplicate. (F and G) Higher magnification of the boxed areas in B and E, respectively. Bar, 20 μm.
The specificity of the action of GroPIns-4P was then examined. Under conditions identical to those reported above, other glycerophosphoinositols, i.e., glycerophosphoinositol (GroPIns) and glycerophosphoinositol 4,5-bisphosphate (GroPIns-4,5P2), both at 50 and 100 μM, had no measurable effect on actin organization (our unpublished data). The possible products of the hydrolysis of GroPIns-4P, inositol 1-phosphate, inositol 4-phosphate, and inositol 1,4-bisphosphate were also tested; none of these compounds added at concentrations up to 50 μM caused any change in the actin cytoskeleton. In addition, although our previous in vitro work did not show any reacylation of GroPIns-4P to form lysophosphatidylinositol, or further metabolism to lysophosphatidic acid (LPA) (Berrie et al., 1999), we also examined the effects of these lysolipids (at 10 μM) on actin polymerization. This might be of interest because LPA has been shown to be a modulator of the actin cytoskeleton. Lysophosphatidylinositol was without effect, and LPA, as already reported (Ridley and Hall, 1992), induced the formation of stress fibers (upon 12 min of incubation; our unpublished data), but had no effect on ruffle formation.
Thus, in Swiss 3T3 fibroblasts, the GroPIns-4P-dependent induction of ruffle and stress fiber formation seems to be GroPIns-4P specific and independent of the possible metabolism of this compound.
Mechanism of Action of GroPIns-4P Involves Activation of Rac and Rho
To investigate the mechanism of action of GroPIns-4P, we examined whether its effect on the actin cytoskeleton was exerted upstream or downstream of the small G proteins of the Rho family; this is a reasonable hypothesis because the effect of several cytoskeletal modulators has been shown to be mediated by this family of small GTPases, which coordinately control distinct actin structures (Zohn et al., 1998): Cdc42 induces the formation of filopodia and can activate Rac (Nobes and Hall, 1995); Rac regulates the formation of lamellipodia and membrane ruffles, and can activate Rho (Ridley et al., 1992); and Rho itself is involved in the formation of stress fibers and focal adhesion sites (Ridley and Hall, 1992). Of note, the cell system used in this study is one in which the role of the small G proteins Cdc42, Rac, and Rho, as well as the actions of growth factors, have been extensively documented (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995).
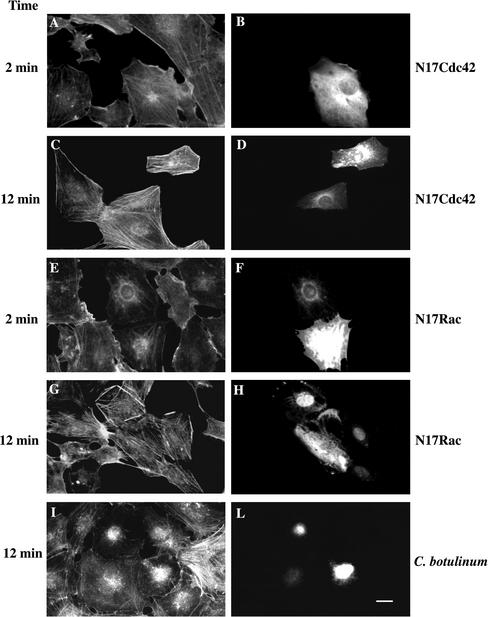
The Myc-tagged, dominant-negative mutants of Rac (N17Rac) and Cdc42 (N17Cdc42), as well as C. botulinum C3 transferase (that acts on Rho), were microinjected in serum-starved Swiss 3T3 cells to prevent the action of the endogenous Rac, Cdc42, or Rho (see MATERIALS AND METHODS; Ridley et al., 1992). Cells expressing the Myc-tagged proteins were revealed using an anti-Myc antibody (Figure 2, B, D, F, and H). The expression of N17Cdc42 had no influence on the actin cytoskeleton modifications induced by GroPIns-4P (Figure 2, A and C). Instead, N17Rac completely inhibited the membrane ruffling induced by GroPIns-4P (Figure 2E). The same construct (N17Rac) had no effect, however, on the stress fiber formation induced by this compound (Figure 2G). Thus, the membrane ruffling induced by GroPIns-4P, is dependent on a functional Rac protein, but stress fiber formation is not. To examine whether the latter effect required Rho, serum-starved Swiss 3T3 cells were microinjected with C. botulinum C3 transferase (160 μg/ml), which ADP ribosylates and inactivates Rho, leaving the functions of Rac and Cdc42 unchanged (Ridley and Hall, 1992; Ridley et al., 1992) (Figure 2L). GroPIns-4P-dependent stress fiber formation was completely inhibited by C. botulinum C3 (Figure 2I), indicating that this GroPIns-4P effect needs a functional Rho protein. However, the formation of membrane ruffling induced by GroPIns-4P was not influenced by C. botulinum C3 (our unpublished data). A summary of a series of experiments, analyzed in a double-blind manner, is presented in Table 1, whereas, Figure 1, F and G, shows the high magnification of the actin structures scored as ruffles and stress fibers, respectively. Together, the results lead to the conclusion that GroPIns-4P acts upstream of Rac and Rho and requires active Rac (but not Cdc42 and Rho) to form ruffles and active Rho (but not Rac and Cdc42) to form stress fibers (see above; Table 1).
Figure 2.
Effects of the inactivation of Cdc42, Rac, and Rho on the GroPIns-4P–induced actin cytoskeleton reorganization. Serum-starved Swiss 3T3 cells microinjected with expression constructs encoding a Myc-tagged version of N17Cdc42 (A–D), N17Rac1 (E–H), or with C. botulinum C3 transferase and fluorescein isothiocyanate-dextran (I and L) were treated with 50 μM GroPIns-4P for 2 min (A and E) or 12 min (C, G, and I). Cells were fixed and costained either for the Myc-tag by using an anti-Myc specific mAb (9E10) (B, D, F, and H) or for filamentous actin by using TRITC-labeled phalloidin (A, C, E, G, and I) or revealed for the presence of fluorescein-dextran staining (L). The N17Rac1 and N17Cdc42 constructs were microinjected at 0.5 μg/μl 4 h before the addition of GroPIns-4P. C. botulinum C3 transferase was microinjected at 160 μg/ml 15 min before the addition of GroPIns-4P. The results shown are representative of three to five independent experiments performed in triplicate. Bar, 20 μm.
Table 1.
Effects of N17Rac, N17Cdc42, and C. botulinum C3 transferase microinjection on the GroPIns-4P–induced actin cytoskeleton reorganization
| Ruffles
|
Stress Fibers
|
|||
|---|---|---|---|---|
| Injected | Noninjected | Injected | Noninjected | |
| N17 Cdc42 | ||||
| GroPIns-4P (2 min) | 21.9 ± 4.0 | 32.7 ± 6.0 | 29.7 ± 5.2 | 23.8 ± 10.1 |
| GroPIns-4P (12 min) | 0.7 ± 0.5 | 1.1 ± 0.8 | 73 ± 2.6 | 73.1 ± 4.9 |
| N17 Rac | ||||
| GroPIns-4P (2 min) | 0.7 ± 0.6* | 36.7 ± 6.0 | 24.7 ± 6.5 | 22.0 ± 6.1 |
| GroPIns-4P (12 min) | 0 | 0.4 ± 0.3 | 56.9 ± 5.2 | 66.7 ± 7.7 |
| C. botulinum | ||||
| GroPIns-4P (12 min) | 10.6 ± 1.5* | 40.8 ± 2.5 | ||
Serum-starved Swiss 3T3 cells microinjected with N17Rac, N17Cdc42, or C. botulinum C3 transferase were treated with GroPIns-4P (50 μM). Cells were fixed and stained with TRITC-labeled phalloidin as described under MATERIALS AND METHODS. Samples of control (noninjected) and injected cells from three to five independent experiments were subjected to double-blind morphological scoring as described under MATERIALS AND METHODS. An example of the actin structures identified as ruffles and stress fibers is shown in Figure 1 (F and G, respectively).
Statistically significant difference p < 0.05 (injected vs. noninjected). A parallel scoring was also performed in control cells, resulting in a level of ruffling and stress fibers of 0.7 ± 0.2 and 4.1 ± 0.4, respectively. The total number of injected cells assessed under each condition ranged from 20 to 50, and equivalent numbers of noninjected cells were selected at random and also scored.
GroPIns-4P Pathway for Rac Activation Differs from That of Growth Factors
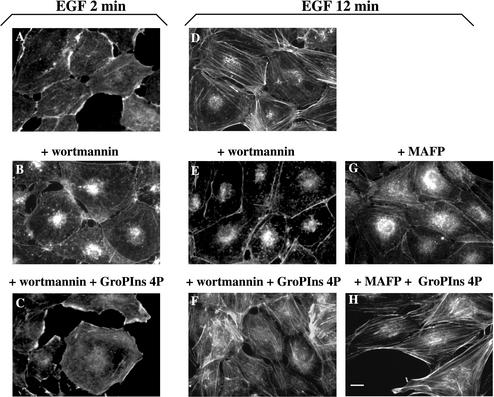
Because several growth factors, typically EGF, cause, like GroPIns-4P, the formation of actin ruffles and stress fibers, we compared the effects of GroPIns-4P (50 μM) with those of EGF (20 ng/ml) under identical conditions. EGF added to serum-starved Swiss 3T3 cells caused membrane ruffles (Figure 1D, maximal effect at 2 min) and stress fiber formation (Figure 1E, maximal effect at 10 min). The effects of GroPIns-4P and EGF were similar in type, extent, and time course. In the signaling cascade mediating the growth factor-dependent organization of the actin cytoskeleton, enzymes such as phosphoinositide 3-kinase (PI3K) and PLA2 are intimately connected to the activation of Rac and Rho (Nobes et al., 1995; Kjoller and Hall, 1999). We thus examined whether these enzymes might be involved also in the reorganization of the cytoskeleton by GroPIns-4P. In the presence of either the PI3K inhibitor wortmannin or methyl arachidonyl fluorophosphonate (MAFP), a cell-membrane-permeant, irreversible inhibitor of PLA2 (Lio et al., 1996), GroPIns-4P was still able to induce the formation of ruffles and stress fibers. In contrast, in experiments in which the cytoskeletal changes were induced by EGF, both these inhibitors blocked the effects of the growth factor. Figure 3 shows that a 10-min preincubation with the inhibitor wortmannin (100 nM) completely prevented the EGF-induced formation of ruffles and stress fibers (Figure 3, A, B, D, and E), whereas it did not affect the modifications induced by GroPIns-4P by itself (our unpublished data) or in combination with EGF (Figure 3, C and F). Similarly, GroPIns-4P can induce stress fiber formation in serum-starved Swiss 3T3 cells where the EGF stimulation of stress fibers was markedly (albeit not completely) prevented by MAFP (15 μM) (Figure 3, G and H). Notably, this inhibitor did not affect the EGF-induced membrane ruffling. Similar results were obtained with another inhibitor of PLA2, AACOCF3 (10 μM; 15-min preincubation).
Figure 3.
GroPIns-4P rescues the EGF-induced effects on the actin cytoskeleton in Swiss 3T3 cells pretreated with MAFP or wortmannin. Serum-starved Swiss 3T3 cells without pretreatment (A and D) or pretreated with MAFP (15 μM for 15 min) (G and H) or wortmannin (100 nM for 10 min) (B, C, E, and F) were stimulated with EGF (20 ng/ml) alone or in the presence of GroPIns-4P (50 μM) for 2 (C) and 12 min (F and H). Cells were fixed and stained with TRITC-labeled phalloidin as described under MATERIALS AND METHODS. The results are representative of at least three independent experiments performed in triplicate. Bar, 20 μm.
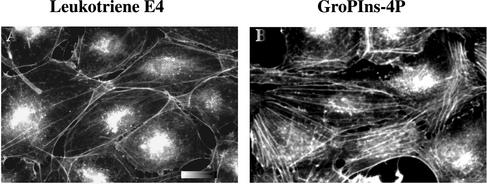
Also of note, the effect of EGF has been shown to be mediated by another PLA2 metabolite, the 5-lipoxygenase product leukotriene E4, which induces stress fiber formation in fibroblasts via the activation of the small G protein Rho (Peppelenbosch et al., 1995). Because these studies were based in part on the use of chemical enzymatic inhibitors, it is conceivable that other metabolites deriving from the PLA2 pathway, such as the glycerophosphoinositols, might act per se or cooperate with the leukotrienes at the level of the cytoskeleton. We compared the effects of leukotriene E4 and GroPIns-4P in Swiss 3T3 cells under the experimental conditions described above (Figure 4). High concentrations of leukotriene E4 (up to 5 μM) were indeed able to induce stress fiber formation (Figure 4A). However, the effect of GroPIns-4P was more pronounced (Figure 4B) and was not modified by the presence of the lipoxygenase inhibitor nordihydroguaretic acid (5–10 μM; 10-min preincubation; our unpublished data), indicating that GroPIns-4P does not act by inducing the formation of leukotrienes.
Figure 4.
Comparison between the stress fiber formation induced by leukotriene E4 and by GroPIns-4P. Serum-starved Swiss 3T3 cells were treated with either leukotriene E4 (5 μM) (A) or GroPIns-4P (50 μM) (B) for 12 min. Cells were fixed and stained with TRITC-labeled phalloidin as described under MATERIALS AND METHODS. The results are representative of three independent experiments performed in triplicate. Bar, 20 μm.
Collectively, the above-mentioned results indicate that GroPIns-4P modifies the actin cytoskeleton via a mechanism of action different from that of EGF and suggest that it may act on a molecular target close to the activation of Rac and Rho themselves.
GroPIns-4P Promotes Localization of Green Fluorescent Protein (GFP)-tagged Rac in Actin Ruffles
We next examined the effect of GroPIns-4P on the Rac GTPase in living cells. To this end, we expressed the GFP-tagged protein in Swiss 3T3 fibroblasts (see MATERIALS AND METHODS). The same construct has been previously shown to behave like the endogenous Rac (Michaelson et al., 2001). As indicated in Figure 5, in serum-starved cells, Rac-GFP showed predominantly a cytosolic localization, both diffuse and in scattered spots (Figure 5B). During the first 2 min of stimulation of living cells with GroPIns-4P (50 μM), a clear accumulation of Rac-GFP became detectable at the plasma membrane within specific structures exhibiting the morphology and dynamics typical of actin ruffles. When cells were fixed and stained for actin by phalloidin, it seemed that actin and Rac-GFP indeed colocalized in ruffles at the plasma membrane (Figure 5, D and F). A similar analysis was performed after 12 min of stimulation with GroPIns-4P (50 μM). At this time, the images revealed the formation of stress fibers (Figure 5G) and a diffuse localization of Rac-GFP, with no clear ruffling (Figure 5H), in line with the data reported above. Control cells transfected with a pEGFP-C2 empty vector showed a predominant nuclear localization of GFP; moreover, no colocalization with F-actin, or concentration in ruffles, of this protein was observed upon GroPIns-4P addition (our unpublished data).
Figure 5.
Localization of Rac and actin in GroPIns-4P-induced membrane ruffles. Subconfluent Swiss 3T3 cells were transfected with the pEGFP-Rac construct as described under MATERIALS AND METHODS. Twenty-four hours after transfection, cells were treated with GroPIns-4P (50 μM) for 2 min (D–F) or 12 min (G–I), fixed, and stained with TRITC-labeled phalloidin. Unstimulated control cells are shown in A–C. Arrowheads show areas of extensive membrane ruffles where Rac colocalizes with F-actin. The results are representative of three independent experiments performed in triplicate. Bar, 20 μm.
These data are clearly in line with the conclusion that a major effect of GroPIns-4P on Swiss 3T3 cells is to activate Rac and thereby induce its interaction with, and reorganization of, the actin cytoskeleton.
GroPIns-4P Induces a Long-lasting GTP-bound Fraction of Cellular Rac
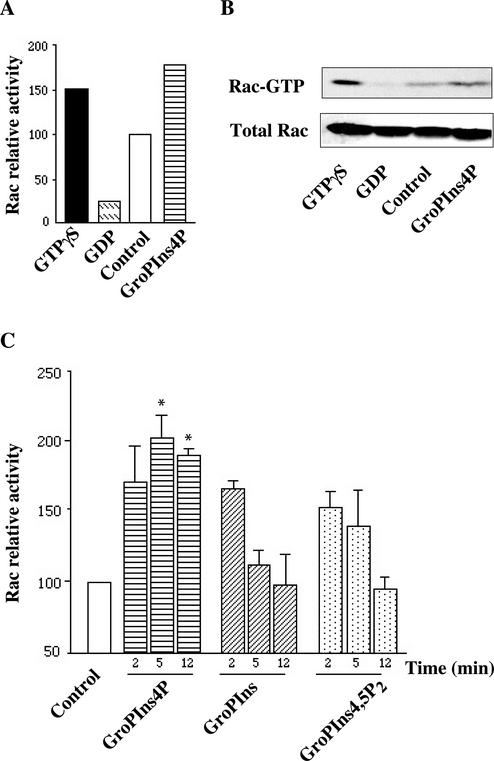
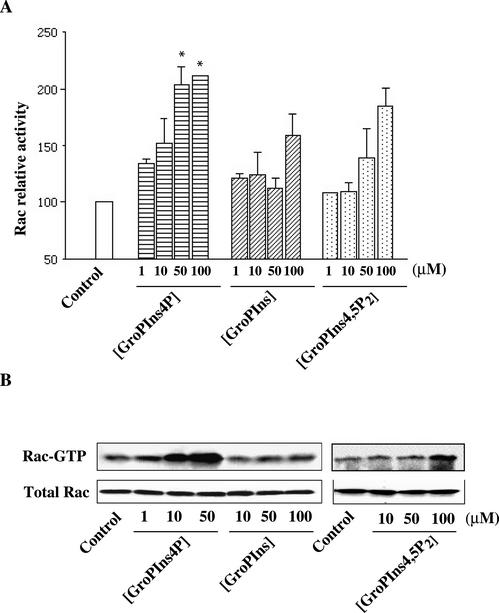
To directly evaluate the effects of GroPIns-4P on Rac, we assessed the ability of this agent to activate Rac in living cells by assaying the fraction of the active (GTP-bound) protein in cells exposed to this agent. Figure 6, A and B, shows that the binding of GTP to Rac in serum-starved Swiss 3T3 cells was stimulated by GroPIns-4P (50 μM), which induced a two-fold increase in the cellular fraction of GTP-bound Rac (GTP-Rac). Remarkably, this effect was comparable to the in vitro activation induced by 100 μM GTPγS (Figure 6, A and B), suggesting that GroPIns-4P activates Rac nearly maximally. Similar experiments were performed by treating Swiss 3T3 cells with GroPIns and GroPIns-4,5P2, which as mentioned above do not detectably affect the actin cytoskeleton organization at this concentration (50 μM). These compounds induced a degree of activation of Rac, but their effects differed markedly from that of GroPIns-4P in two ways. The first concerned the time course of the effects of the glycerophosphoinositols. As shown in Figure 6C, the activation induced by GroPIns and GroPIns-4,5P2 was transient, whereas GroPIns-4P induced a sustained activation of Rac. The second difference can be seen in Figure 7, which shows the dose responses of the various glycerophosphoinositols (1–100 μM). Although the GroPIns-4P-induced Rac activation was already noticeable at 1 μM and reached the maximal twofold increase at 50 μM, in good agreement with the effect of this compound on ruffle formation, the effects of GroPIns and GroPIns-4,5P2 were evident only at higher concentrations (50–100 μM) and were significantly lower in maximal extent.
Figure 6.
Regulation of Rac activity by GroPIns-4P. (A) Serum-starved Swiss 3T3 cells were treated with 50 μM GroPIns-4P for 2 min. Lysates from unstimulated and stimulated cells were processed as indicated under MATERIALS AND METHODS and analyzed by Western blotting with a mAb against Rac. Lysates from control cells were incubated with GTPγS (100 μM) or GDP (1 mM) for positive and negative controls, respectively. Rac-relative activation was calculated as the amount of PBD-bound Rac in stimulated cells, and normalized vs. the amount of Rac in unstimulated cells. (B) Western blots from one of the representative experiments showing activation of Rac. (C) Time course of Rac activation by GroPIns, GroPIns-4P, and GroPIns-4,5P2. Serum-starved Swiss 3T3 cells were treated with these stimuli (50 μM) for the indicated times. Rac-relative activation was calculated as the amount of PBD-bound Rac in stimulated cells, and normalized vs. the amount of Rac in unstimulated cells. Data are means ± SE of three independent experiments. *p < 0.05, with respect to unstimulated cells, as analyzed by paired Student's t test.
Figure 7.
Dose response of GroPIns, GroPIns-4P, and GroPIns-4,5P2 on Rac activation. (A) Serum-starved Swiss 3T3 cells were treated with the indicated amounts of GroPIns, GroPIns-4P, or GroPIns-4,5P2 for 5 min; lysates from unstimulated and stimulated cells were processed as indicated under MATERIALS AND METHODS and analyzed by Western blotting with a mAb against Rac. Rac-relative activation was calculated as the amount of PBD-bound Rac in stimulated cells, and normalized vs. the amount of Rac in unstimulated cells. Data are means ± SE of three experiments. *p < 0,05, with respect to unstimulated cells, as analyzed by paired Student's t test. (B) Western blots from one of the representative experiments showing the amount of activated Rac present in the affinity precipitations and the constant level of endogenously expressed Rac in the different cell lysates.
These data suggest that the duration and the extent of the Rac activation induced by these compounds might play important roles in initiating the signal cascade leading to actin rearrangements and might in fact reflect a different mechanism of activation (see DISCUSSION). In line with this, we observed that LPA, which induces stress fiber formation (see above; Ridley and Hall, 1992) but, like GroPIns and GroPIns-4,5P2, does not cause ruffling, increased by only ∼50% the cellular fraction of GTP-Rac (our unpublished data). A similar (i.e., limited) LPA-dependent increase in GTP-Rac has been recently reported and related to the Gi-dependent ability of LPA to induce NIH-3T3 cell spreading over fibronectin layers (Ueda et al., 2001). Another possibility is that the effect of GroPIns-4P on ruffling might involve not only Rac activation but also a concomitant action on another effector, which would be unaffected by the other two glycerophosphoinositols. A full elucidation of these questions requires further investigation and is beyond the scope of the present report.
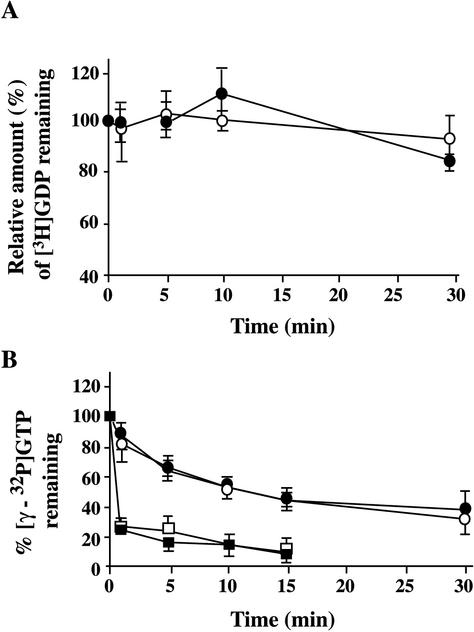
Finally, we attempted to assess the effects of GroPIns-4P on the GDP/GTP exchange and GTPase activities of recombinant Rac (Figure 8). Nucleotide exchange was evaluated by preloading Rac with [3H]GDP and then measuring the time course of [3H]GDP release under various conditions (in the presence of low or high magnesium concentrations or cell lysate; see MATERIALS AND METHODS). No change in the rate of GDP release was induced by GroPIns-4P (50 μM) (Figure 8A). The Rac GTPase activity was evaluated by preloading recombinant Rac with γ[32P]GTP and measuring the rate of GTP hydrolysis. Again, GroPIns-4P (50–100 μM) had no effect, either in the presence or in the absence of cell lysates (Figure 8B). Thus, GroPIns-4P does not seem to directly activate Rac under the conditions so far used to assay the enzymatic activity of this GTPase in cell-free extracts.
Figure 8.
Nucleotide exchange and GTPase activity of Rac1. (A) Time course of the [3H]GDP/GTP exchange reaction on bacterially expressed GST-Rac1. The exchange reactions were performed in a GEF buffer as described under MATERIALS AND METHODS, in the absence (empty circles) or presence (filled circles) of 50 μM GroPIns-4P. The reactions were terminated by nitrocellulose filtration at the indicated times. The results are expressed as percentage of the [3H]GDP remaining bound at each time point vs. the amount of radioactivity at time 0 (100%). (B) Intrinsic GTPase activity of Rac1 was determined by a filter binding assay (see MATERIALS AND METHODS) in the absence (empty circles) or presence (filled circles) of 50 μM GroPIns-4P. The same reactions were performed in a GAP buffer containing 300 μg of lysates from Chinese hamster ovary cells in the absence (empty squares) or presence (filled squares) of 50 μM GroPIns-4P. Data in A are the averages of two independent experiments, expressed as means ± SE (n = 5) and in B they are from representative experiments expressed as means ± SE (n = 4).
DISCUSSION
The main finding in the present study is that GroPIns-4P, a small, water-soluble phosphoinositide metabolite that can be generated intracellularly by PLA enzymes, modulates the organization of the actin cytoskeleton toward the formation of ruffles and stress fibers, through the activation of the small GTPases Rac and Rho.
Mechanism of Action of GroPIns-4P on the Actin Cytoskeleton Differs from That of Growth Factors
The effects of GroPIns-4P on the actin cytoskeleton are phenomenologically similar to those induced by an important class of physiological agents, the growth factors. However, the mechanisms of action of these two types of agents seem to be different. The well-characterized mitogen EGF, for instance, affects the cytoskeleton via a receptor-induced signaling cascade that includes as key elements the transduction enzymes PI3K and PLA2 and the formation of arachidonic acid metabolites, the leukotrienes (Kjoller and Hall, 1999). Although the role of the leukotrienes has been demonstrated only for EGF-induced stress fiber formation, PI3K is considered a rather general mediator of the growth factor effects on the actin cytoskeleton (Kjoller and Hall, 1999). Yet, the cytoskeletal reorganization induced by GroPIns-4P involves neither of these two enzymes, nor does it require the leukotrienes. These findings are consistent with our previous proposal that GroPIns-4P acts, rather than by stimulating a membrane receptor, by crossing the plasma membrane into the cytosol, where it would reach its potential targets (Berrie et al., 1999; Corda et al., 2002). This hypothesis is attractive because 1) it fits with our observation that GroPIns-4P can be generated in the cell cytoplasm and is therefore likely to exert its action in the cell interior; 2) in previous studies, we found no evidence of specific GroPIns-4P binding to the plasma membrane; and 3) this compound is actively internalized into cultured cells (Swiss 3T3 fibroblasts; Berrie et al., 1999). If this is the case, the passage through the plasma membrane of the exogenously added GroPIns-4P, which is highly hydrophilic (i.e., nonmembrane permeant), should occur via a specific transporter protein. The identity of such a transporter is unknown, but a reasonable hypothesis is that it might be the mammalian ortholog of the GroPIns transmembrane carrier already identified and studied in yeast (Patton-Vogt and Henry, 1998). In yeast, the glycerophosphoinositols are released (Hawkins et al., 1993) and can be taken back up as a source of the essential nutrient inositol when this component is lacking in the growth medium (Patton et al., 1995). In higher eukaryotes, the glycerophosphoinositols are unlikely to have nutritional roles, but the molecules presiding over their transport and metabolism might be conserved. Clearly, more work is needed to clarify this point.
Effect of GroPIns-4P on Rac: Requirement for a Long-lasting Activation
Because GroPIns-4P acts by activating Rac, we have characterized the manner in which this activation is induced. The GDP/GTP cycle of the Rho GTPases is regulated by three distinct classes of proteins (Takai et al., 2001): GEFs that stimulate the exchange of GDP for GTP; the intrinsic GTPase-activating proteins (GAPs); and the GDP-dissociation inhibitory factors. These proteins also play an important role in the regulation of the association of Rho proteins with the membrane, via the presence of lipid-binding domains in their sequence (see INTRODUCTION; Takai at al., 2001). The field of potential candidates is therefore large. If one of the Rac accessory proteins is the target of GroPIns-4P, it could be one (or more) of the >40 members of these Rho family protein modulators (Bishop and Hall, 2000). A possible way to reduce the number of candidates is to consider that GroPIns-4P could act by binding to a specific lipid-binding domain (e.g., a pleckstrin homology domain) of one of the GEFs or GAPs, thus affecting the interaction of this domain with the membrane phosphoinositides. Interestingly, in preliminary experiments, we indeed find that GroPIns-4P facilitates the translocation of Tiam, a Rac GEF characterized by two pleckstrin domains (Habets et al., 1994), to the plasma membrane. Because Tiam has been implicated in metastatic invasion (Michiels et al., 1995), this observation is also broadly in line with a role of GroPIns-4P in Ras transformation.
Alternatively, we cannot yet exclude that to exert its effects on Rac, GroPIns-4P might affect one of the proteins in the cascade upstream of the Rac interactors, along a cascade different, however, from that initiated by EGF (see above). A signaling complex that might be involved in the effect of GroPIns-4P is the Gs/adenylyl cyclase system, because this compound directly inhibits Gs and thus affects cAMP-dependent functions (Iacovelli et al., 1993). Evidence of an interplay between cAMP and Rho GTPases has recently been reported: for example, in platelets cAMP inhibits both Rac and Rho (Gratacap et al., 2001). Moreover, increases in cAMP levels result in the inhibition of RhoA, with respect to its ability to induce either morphological changes in neuroblastoma cells or cell migration in colon carcinoma and hepatoma cells (Dong et al., 1998; Mukai et al., 2000; O'Connor et al., 2000). Thus, a decrease in cAMP induced in Swiss 3T3 cells by GroPIns-4P (Falasca et al., 1997) might play at least an accessory role in Rac and Rho activation also in fibroblasts. Irrespective of the precise manner in which Rac is activated, an intriguing and potentially important mechanistic aspect emerges from the comparison between the stimulatory effects of GroPIns-4P and those of the other glycerophosphoinositols. Whereas also GroPIns and GroPIns-4,5P2 can induce Rac charging, their effect is transient, whereas the effect of GroPIns-4P is long-lasting. This difference might explain the inability of GroPIns and GroPIns-4,5P2 to induce ruffle formation, in contrast to the marked ruffling effect of GroPIns-4P. Possibly, to lead to ruffling, the Rac cellular pool must remain in the active state for a time sufficient for the interaction with the effectors controlling actin polymerization or translocation to the membrane. If this is the case, the duration of the Rac activation would be more important than the extent of activation, a notion in line with our observations that GroPIns-4P, at low concentrations that induce levels of cellular GTP-Rac similar to those supported by GroPIns and GroPIns-4,5P2, is able to produce ruffles, whereas the other two glycerophosphoinositols are not.
CONCLUSION
In summary, GroPIns-4P activates intracellular molecular steps leading to a reorganization of the actin cytoskeleton via the activation of the small GTPase Rac. This Rac activation is mediated by a mechanism different from that initiated by growth factors, and is long-lasting. Because agents that produce a transient activation of Rac (such as LPA; see RESULTS) do not induce ruffling, the latter feature might prove relevant toward clarifying both the mode of Rac activation by GroPIns-4P and the manner in which activated Rac leads to ruffling.
Because the glycerophosphoinositols have been implicated in the Ras cascade both in mitogen-activated and in Ras-transformed cells, these observations might be relevant to our understanding of Ras signaling. In addition, there are several possible physiological and pathological conditions where GroPIns-4P formation might be induced and play a role. The synthesis of GroPIns-4P involves the complete deacylation of phosphatidylinositol 4-phospate, or of phosphatidylinositol 4,5-bisphosphate followed by dephosphorylation. Such a deacylation could be brought about by several PLA2 isoforms, because these enzymes may act sequentially at positions sn-2 and sn-1 of the glycerol backbone (de Carvalho et al., 1995; Ma and Turk, 2001). It will be of great interest to define the conditions where this metabolism can take place. Finally, because the glycerophosphoinositols are soluble, small-molecular-weight compounds amenable to chemical modification, are able to easily cross the plasma membrane and are active when added extracellularly, our observations suggest that they should serve as valuable lead compounds for the development of molecules active in the control of motility and invasiveness of cancer cells, for both research and therapeutic purposes.
ACKNOWLEDGMENTS
We thank Drs. C.D. Nobes and A. Hall (University College London, United Kingdom) for help in setting up the microinjection experiments, for kindly providing cDNAs, and for helpful discussions; and Dr. A. Luini and M. Baldassarre (Consorzio Mario Negri Sud, Chieti, Italy) for helpful discussions and help in setting confocal microscopy experiments, respectively. This study was supported in part by the Italian Association for Cancer Research (Milano, Italy), Telethon (Italy) grant E.841, and the Italian National Research Council (Consiglio Nazionale delle Ricerche, Rome, Italy) Progetto Finalizzato “Biotecnologie” 01.00027.PF49.
Abbreviations used:
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GroPIns
glycerophosphoinositol
- GroPIns-4P
glycerophosphoinositol 4-phosphate
- GroPIns-4,5P2
glycerophosphoinositol 4,5-bisphosphate
- GST
glutathione S-transferase
- LPA
lysophosphatidic acid
- PLC
phospholipase C
- PLA2
phospholipase A2
- PI3K
phosphoinositide 3-kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0179. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0179.
REFERENCES
- Alonso T, Morgan RO, Marvizon JC, Zarbl H, Santos E. Malignant transformation by ras and other oncogenes produces common alterations in inositol phospholipid signaling pathways. Proc Natl Acad Sci USA. 1988;85:4271–4275. doi: 10.1073/pnas.85.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso T, Santos E. Increased intracellular glycerophosphoinositol is a biochemical marker for transformation by membrane-associated and cytoplasmic oncogenes. Biochem Biophys Res Commun. 1990;171:14–19. doi: 10.1016/0006-291x(90)91349-w. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berrie CP, Iurisci C, Corda D. Membrane transport and in vitro metabolism of the Ras cascade messenger, glycerophosphoinositol 4-phosphate. Eur J Biochem. 1999;266:413–419. doi: 10.1046/j.1432-1327.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- Berrie CP, Dragani LK, van der Kaay J, Iurisci C, Brancaccio A, Rotilio D, Corda D. Maintenance of PtdIns45P2 pools under limiting inositol conditions, as assessed by liquid-chromatography-tandem mass spectrometry and PtdIns45P2 mass evaluation in Ras-transformed cells. Eur J Cancer. 2002;38:2463–2475. doi: 10.1016/s0959-8049(02)00485-9. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases, and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Corda D, Falasca M. Glycerophosphoinositols as potential markers of ras-induced transformation and novel second messengers. Anticancer Res. 1996;16:1341–1350. [PubMed] [Google Scholar]
- Corda D, Iurisci C, Berrie CP. Biological activities and metabolism of the lysophosphatidylinositol and glycerophosphoinositols. Biochim Biophys Acta. 2002;1582:52–69. doi: 10.1016/s1388-1981(02)00137-3. [DOI] [PubMed] [Google Scholar]
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- de Carvalho MG, Garritano J, Leslie CC. Regulation of lysophospholipase activity of the 85-kDa phospholipase A2 and activation in mouse peritoneal macrophages. J Biol Chem. 1995;270:20439–20446. doi: 10.1074/jbc.270.35.20439. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Godi A, Corda D. Phosphoinositides and the Golgi complex. Curr Opin Cell Biol. 2002;14:434–447. doi: 10.1016/s0955-0674(02)00357-5. [DOI] [PubMed] [Google Scholar]
- Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKα. J Biol Chem. 1998;273:22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]
- Downes CP, Hawkins P, Stephens L. Identification of the stimulated reaction in intact cells, its substrate supply and the metabolism of inositol phosphates. In: Michell RH, Drummond AH, Downes CP, editors. Inositol Lipids in Cell Signaling. London, United Kingdom: Academic Press; 1989. pp. 3–38. [Google Scholar]
- Falasca M, Carvelli A, Iurisci C, Qiu RG, Symons MH, Corda D. Fast receptor-induced formation of glycerophosphoinositol-4-phosphate, a putative novel intracellular messenger in the Ras pathway. Mol Biol Cell. 1997;8:443–453. doi: 10.1091/mbc.8.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M, Corda D. Elevated levels and mitogenic activity of lysophosphatidylinositol in k-ras-transformed epithelial cells. Eur J Biochem. 1994;221:383–389. doi: 10.1111/j.1432-1033.1994.tb18750.x. [DOI] [PubMed] [Google Scholar]
- Falasca M, Iurisci C, Carvelli A, Sacchetti A, Corda D. Release of the mitogen lysophosphatidylinositol from H-Ras-transformed fibroblasts; a possible mechanism of autocrine control of cell proliferation. Oncogene. 1998;16:2357–2365. doi: 10.1038/sj.onc.1201758. [DOI] [PubMed] [Google Scholar]
- Falasca M, Silletta MG, Carvelli A, Di Francesco AL, Fusco A, Ramakrishna V, Corda D. Signaling pathways involved in the mitogenic action of lysophosphatidylinositol. Oncogene. 1995;10:2113–2124. [PubMed] [Google Scholar]
- Gratacap MP, Payrastre B, Nieswandt B, Offermanns S. Differential regulation of Rho and Rac through heterotrimeric G-proteins and cyclic nucleotides. J Biol Chem. 2001;276:47906–47913. doi: 10.1074/jbc.M104442200. [DOI] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Stephens LR, Piggott JR. Analysis of inositol metabolites produced by Saccharomyces cerevisiae in response to glucose stimulation. J Biol Chem. 1993;268:3374–3383. [PubMed] [Google Scholar]
- Iacovelli L, Falasca M, Valitutti S, D'Arcangelo D, Corda D. Glycerophosphoinositol 4-phosphate, a putative endogenous inhibitor of adenylylcyclase. J Biol Chem. 1993;268:20402–20407. [PubMed] [Google Scholar]
- Kjoller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253:166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J. 2000;350:1–18. [PMC free article] [PubMed] [Google Scholar]
- Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Ma Z, Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog Nucleic Acid Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells. Regulation by hypervariable regions and Rho GDI binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Mukai M, Nakamura H, Tatsuta M, Iwasaki T, Togawa A, Imamura F, Akedo H. Hepatoma cell migration through a mesothelial cell monolayer is inhibited by cyclic AMP-elevating agents via a rho-dependent pathway. FEBS Lett. 2000;484:69–73. doi: 10.1016/s0014-5793(00)02129-3. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the α6β4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–258. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JL, Pessoa Brandao L, Henry SA. Production and reutilization of an extracellular phosphatidylinositol catabolite, glycerophosphoinositol, by Saccharomyces cerevisiae. J Bacteriol. 1995;177:3379–3385. doi: 10.1128/jb.177.12.3379-3385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton-Vogt JL, Henry SA. GIT1, a gene encoding a novel transporter for glycerophosphoinositol in Saccharomyces cerevisiae. Genetics. 1998;149:1707–1715. doi: 10.1093/genetics/149.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch MP, Qiu RG, de Vries Smits AM, Tertoolen LG, de Laat SW, McCormick F, Hall A, Symons MH, Bos JL. Rac mediates growth factor-induced arachidonic acid release. Cell. 1995;81:849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J Cell Sci. 2000;113:3685–3695. doi: 10.1242/jcs.113.21.3685. [DOI] [PubMed] [Google Scholar]
- Self AJ, Hall A. Measurement of intrinsic nucleotide exchange and GTP hydrolysis rates. Methods Enzymol. 1995a;256:67–76. doi: 10.1016/0076-6879(95)56010-6. [DOI] [PubMed] [Google Scholar]
- Self AJ, Hall A. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 1995b;256:3–10. doi: 10.1016/0076-6879(95)56003-3. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Aasland R. FYVE-finger proteins–effectors of an inositol lipid. J Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Ueda H, Morishita R, Yamauchi J, Itoh H, Kato K, Asano T. Regulation of Rac and Cdc42 pathways by G(i) during lysophosphatidic acid-induced cell spreading. J Biol Chem. 2001;276:6846–6852. doi: 10.1074/jbc.M007541200. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Cucchi P, Colletta G, Di Filippo C, Corda D. Transformation by the k-ras oncogene correlates with increases in phospholipase A2 activity, glycerophosphoinositol production and phosphoinositide synthesis in thyroid cells. Cell Signal. 1991;3:321–332. doi: 10.1016/0898-6568(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Seet LF, Hanson B, Hong W. The Phox homology (PX) domain, a new player in phosphoinositide signaling. Biochem J. 2001;360:513–530. doi: 10.1042/0264-6021:3600513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Hart MJ, Cerione RA. Guanine nucleotide exchange catalyzed by dbl oncogene product. Methods Enzymol. 1995;256:77–84. doi: 10.1016/0076-6879(95)56011-4. [DOI] [PubMed] [Google Scholar]
- Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]