Abstract
We previously demonstrated, using fluorescence recovery after photobleaching, that clathrin in clathrin-coated pits at the plasma membrane exchanges with free clathrin in the cytosol, suggesting that clathrin-coated pits are dynamic structures. We now investigated whether clathrin at the trans-Golgi network as well as the clathrin adaptors AP2 and AP1 in clathrin-coated pits at the plasma membrane and trans-Golgi network, respectively, also exchange with free proteins in the cytosol. We found that when the budding of clathrin-coated vesicle is blocked without significantly affecting the structure of clathrin-coated pits, both clathrin and AP2 at the plasma membrane and clathrin and AP1 at the trans-Golgi network exchange rapidly with free proteins in the cytosol. In contrast, when budding of clathrin-coated vesicles was blocked at the plasma membrane or trans-Golgi network by hypertonic sucrose or K+ depletion, conditions that markedly affect the structure of clathrin-coated pits, clathrin exchange was blocked but AP2 at the plasma membrane and both AP1 and the GGA1 adaptor at the trans-Golgi network continue to rapidly exchange. We conclude that clathrin-coated pits are dynamic structures with rapid exchange of both clathrin and adaptors and that adaptors are able to exchange independently of clathrin when clathrin exchange is blocked.
INTRODUCTION
Clathrin-mediated endocytosis at the plasma membrane is thought to be initiated by binding of the clathrin adaptor AP2 to various plasma membrane receptors and possibly synaptotagmin as well. AP2 is thought to then recruit clathrin to the nascent clathrin-coated pits followed by invagination of the clathrin-coated pits to form clathrin-coated vesicles. The budding of clathrin-coated vesicles also involves other proteins such as dynamin, amphiphysin, epsin, eps15, endophilin, and synaptojanin but, in most cases, the exact functions of these proteins remain speculative (Brodin et al., 2000; Marsh and McMahon, 1999). In addition, dephosphorylation of AP2 (Wilde and Brodsky, 1996) and several of these other proteins apparently plays an important role in formation of the clathrin-coated pit as does interaction of AP2 and several of these other proteins with the phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) on the membrane (Beck and Keen, 1991; Voglmaier et al., 1992). In addition to budding of clathrin-coated vesicles at the plasma membrane, clathrin-coated vesicles also bud from the trans-Golgi network (TGN), transporting enzymes complexed with the mannose-6-phosphate receptor to late endosomes and thence to lysosomes. Clathrin coat formation at the TGN is initiated by the binding of ARF1-GTP, which in turn recruits the clathrin adaptors AP1 and GGA (Robinson and Bonifacino, 2001). Although dynamin isozymes are involved in scission and perhaps signaling at both the plasma membrane and the TGN, relatively little is known about the other proteins involved in budding of clathrin-coated vesicles from the TGN (McNiven et al., 2000; Danino and Hinshaw, 2001).
Despite differences in the proteins involved in the budding of clathrin-coated vesicles from the plasma membrane and the TGN, it is clear that in both cases as the curvature of the membrane increases the polymerized clathrin must be transformed from a planar hexagonal array into a curved mixture of hexagons and pentagons. One way this process could occur is through addition of clathrin at the edges of a growing clathrin-coated pit so that pentagons are formed de novo from newly attached clathrin triskelions (Kirchhausen, 2000). Alternatively, hexagons may be transformed into pentagons through the exchange of bound and free clathrin triskelions as the curvature of the planar clathrin-coated pit increases (Wu et al., 2001).
We recently demonstrated, using fluorescence recovery after photobleaching (FRAP), that clathrin exchange occurs during clathrin-mediated endocytosis at the plasma membrane (Wu et al., 2001). Furthermore, there was little effect on the rate of replacement of the bleached clathrin when endocytosis was blocked by cholesterol depletion, expression of the dynamin mutant K44A, or a decrease in temperature. On the other hand, we found that treatment of the cells by hypertonic sucrose or K+ depletion, conditions that have been shown to affect profoundly the structure of the pits, not only blocked clathrin-mediated endocytosis but also completely blocked clathrin exchange. In addition, ATP depletion also blocked clathrin exchange. We concluded that ATP-dependent clathrin exchange is a fundamental property of intact clathrin-coated pits at the plasma membrane, a property that may be involved in the transformation of clathrin structure that occurs as clathrin-coated pits invaginate. In this regard, it is interesting that, in addition to clathrin exchange at the plasma membrane, both ARF1 and COPI bound to the membranes of the Golgi apparatus have been shown to exchange with cytosolic ARF1 and COPI, respectively (Presley et al., 2002).
In the present study, we extended our investigation into the exchange of clathrin coats, addressing several questions raised by the results we obtained in our initial study. First, we asked whether exchange of clathrin occurs at the TGN where clathrin budding is initiated by a different mechanism than occurs at the plasma membrane and, if so, whether this exchange is affected by the same agents that affect clathrin exchange and clathrin-mediated endocytosis at the plasma membrane. Second, we asked whether the major clathrin adaptors, AP2 at the plasma membrane and AP1 at the TGN, also exchange and, if so, whether their exchange is linked to clathrin exchange. Our results show that, when clathrin-mediated endocytosis is blocked under conditions where clathrin-coated pits remain intact, AP2 at the plasma membrane exchanges at about the same rate as clathrin. Likewise, both clathrin and AP1 bound to the TGN exchange at the same rate when vesicle budding is blocked by decreasing the temperature to 20°C. However, although clathrin exchange is blocked at the TGN as well as at the plasma membrane by K+ depletion or treatment of the cells by hypertonic sucrose, AP2 and AP1 exchange continue to occur under these conditions, although at slightly decreased rates. Likewise, GGA1 localized at the TGN continues to exchange when clathrin exchange is blocked. We conclude that not only can exchange of clathrin adaptors occur at the same rate as clathrin exchange but it can also occur independently of clathrin exchange in vivo.
MATERIALS AND METHODS
2-(N-Morpholino)ethanesulfonic acid, HEPES, deoxyglucose, imidazole, tetracycline, brefeldin A, methyl-β-cyclodextrin, oubain, and anti-AP1 monoclonal antibody (mAb) (100/3) were from Sigma-Aldrich (St. Louis, MO). Anti-clathrin mAb (X22) and anti-AP2 mAb (AP.6) were from Affinity Bioreagents (Golden, CO). Secondary antibodies and cy5-transferrin were from Jackson Immunoresearch Laboratories (West Grove, PA). All media and supplements were obtained from Biofluids (Rockville, MD). Fugene6 was from Roche Diagnostics (Indianapolis, IN).
DNA Constructs
Green fluorescent protein (GFP)-clathrin light chain and GFP-GGA1 were cloned as described previously (Puertollano et al., 2001; Wu et al., 2001). GFP-AP2 was imaged using the rat AP2 α chain (X53773) with a GFP attached at its NH2 terminus. The rat AP2 α chain (X53773) in pACT2 was subcloned into pEGFP-N1 (BD Biosciences Clontech, Palo Alto, CA) by using the polymerase chain reaction (PCR) primers CTCGAGATGCCGGCGTATCCAAGGGGGAG and GGATCCCGGAACTGTTCCGACAGCAATTC. The PCR fragment was first cloned into XhoI and BamHI sites of pEGFP-N1 and then replaced with cDNA fragment via SacI and Acl I sites. DNA sequencing confirmed the correct sequences cloned in the pEGFP-N1. The GFP-AP1 was derived from the mouse cDNA AP1 γ-adaptin (NM 009677) in pACT2. The PCR primers used were CGAATTCTGATGCCAGCCCCCATCAGATTG and GGATCCCGTTGCCAGGACTGAGGGGGGAAATTG. The PCR fragment was cloned into a TA vector first and then replaced with cDNA fragment via Age I and StuI sites. After DNA sequencing confirmed the correct sequences was cloned, the EcoRI/BamHI fragment was then cloned into pEGFP-C3 vector.
Cell Culture and Transfection
HeLa cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin (100 unit/ml), and streptomycin (100 unit/ml) in a humidified incubator with 5% CO2 at 37°C. HeLa cells stably expressing mutant (K44A) dynamin were grown under control of a tetracycline promoter and induced by removal of tetracycline (Damke et al., 1995). Cells were transfected with the GFP-constructs with Fugene6. Hypertonic treatment was performed by incubating the cells in 0.2 M sucrose for 45 min at 37°C. K+ depletion was performed by hypotonic swelling of the cells in 50% diluted DMEM containing 1 mM oubain and then incubating the cells in ice-cold K+-free medium (100 mM NaCl, 50 mM HEPES, 1 mM MgCl2, 0.1 mM CaCl2, 10 mM glucose at pH 7.3) for 20 min (Lukacs et al., 1997). Cholesterol was depleted by incubating the cells in 10 mM methyl-β-cyclodextrin for 30 min at 37°C (Rodal et al., 1999; Subtil et al., 1999).
Confocal Microscopy
Cells were grown in chamber slides and imaged using a confocal microscope (Carl Zeiss, Thornwood, NY) as described previously. All FRAP experiments were done using a 40×, 1.4 numerical aperture objective, whereas a 63×, 1.4 numerical aperture objective was used to examine the plasma membrane at higher magnification. GFP- and CFP-constructs were imaged and photobleached using 488- and 413-nm laser, respectively. When GFP- and CFP-constructs were simultaneously imaged, the configuration was designed and tested to ensure there was no spill over of the CFP-fluorescence into the GFP-channel. A defined region was photobleached at a high laser power to reduce the fluorescence intensity by 50 to 80%. The recovery of fluorescence was monitored by scanning the whole cell at low laser power. Temperature was regulated at 37°C using a heating fan and a precision thermometer (YSI, Yellow Springs, OH) to check the temperature and at 15 or 20°C by using a Micro Devices microincubator (20/20 Technology, Wilmington, NC). Data were analyzed as described previously (Wu et al., 2001).
RESULTS
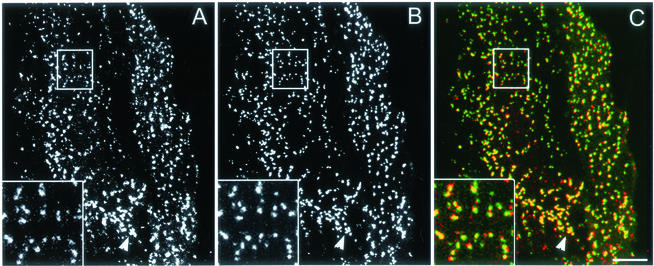
We began our study by investigating whether fluorescently tagged AP2 colocalizes with clathrin in clathrin-coated pits on the plasma membrane of HeLa cells. We constructed and transfected HeLa cells with a plasmid encoding the rat AP2 α chain with GFP attached at its amino terminus. Immunofluorescence studies using both anti-clathrin and anti-AP2 antibodies showed that most of the GFP-AP2 colocalized with the clathrin-coated pits. We also found that expression of GFP-AP2 had no effect on transferrin uptake, suggesting that labeling the α chain of AP2 with GFP did not affect the function of the AP2 (our unpublished data). Figure 1, A–C, shows that, as expected, essentially all of the GFP-AP2 colocalizes with cyan fluorescent protein (CFP)-clathrin. Many of the GFP-AP2 pits at the cell periphery were ∼0.3 μm or smaller in diameter, about the same size we obtained for the pits with CFP-clathrin (Wu et al., 2001). However, whether we used CFP-clathrin or GFP-AP2 to visualize the pits, the cells always had areas where pits were clustered (Figure 1, arrow); these areas may represent hot spots of pit formation (Gaidarov et al., 1999).
Figure 1.
Colocalization of clathrin and AP2 on the plasma membrane. Cells were imaged using CFP-clathrin and GFP-AP2 by using the 63× objective. (A) CFP-clathrin. (B) GFP-AP2. (C) Overlap between clathrin (red) and AP2 (green). The arrow points to an area of clustered pits. The insets show the distribution of clathrin, AP2, and overlap between clathrin and AP2. Bar, 9 μm.
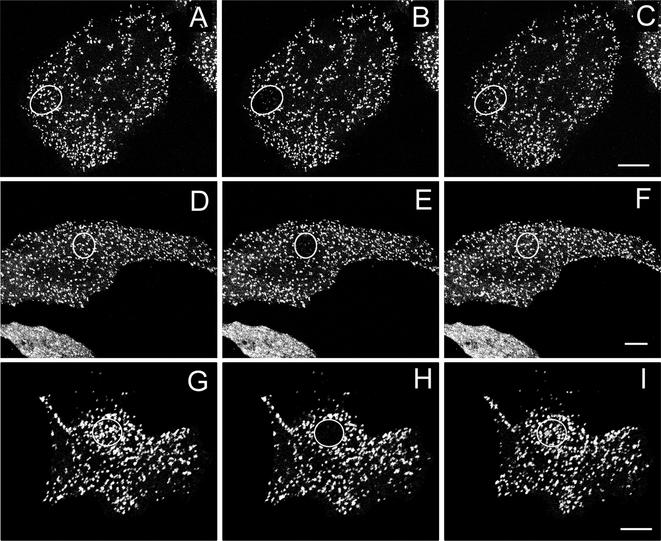
We next determined whether the fluorescence of GFP-AP2 in clathrin-coated pits recovered after photobleaching. Figure 2, A–C, shows that after photobleaching a small region of the plasma membrane of control cells at 37°C there was an immediate decrease in the fluorescence of GFP-AP2 followed by nearly full fluorescence after 2 min. Of course, because clathrin-mediated endocytosis was occurring in these cells, the fluorescence recovery was at least partly due to old pits invaginating and new pits forming. Although it seems that the new pits reformed at the same position as the old pits (Figure 2C), this is because to avoid damage in the FRAP studies we used a 40× objective rather than a 63× objective so that clusters of pits tended to be brighter than individual pits. This was much less of problem when we imaged GFP-clathrin because the individual pits were brighter than with GFP-AP2. When we used a 63× objective to follow the fluorescence of GFP-AP2 it was clear that old pits disappeared and new pits formed because there was little overlap of fluorescent pits over a period of 1 min (our unpublished data).
Figure 2.
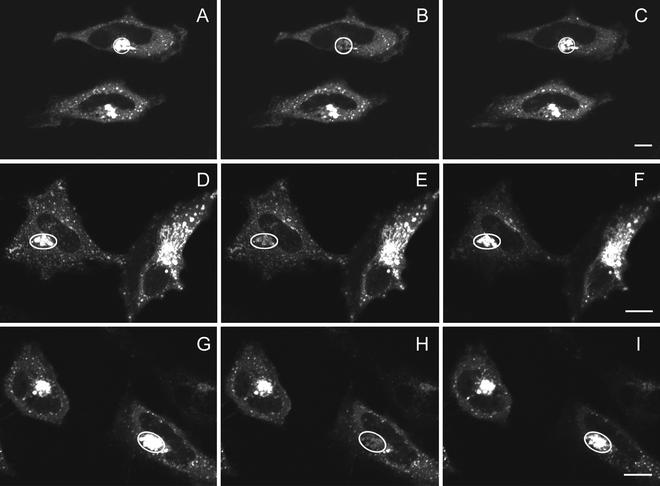
Fluorescence recovery after photobleaching GFP-AP2 in HeLa cells at 37°C under different conditions. (A–C) Control HeLa cells. (D–F) HeLa depleted of cholesterol. (G–I) HeLa cells expressing K44A-dynamin. (A, D, and G) Images obtained directly before being photobleached. (B, E, and H) Images immediately after photobleaching. (C, F, and I) Images 2 min after photobleaching. The photobleached area is indicated in each figure. Bars, A–C, 6.5 μm; D–F; 9.5 μm; and G–I, 9 μm.
To determine whether replacement of AP2 occurred in clathrin-coated pits even when endocytosis was blocked we carried out FRAP experiments on GFP-AP2 in cells either depleted of cholesterol or expressing the dynamin mutant K44A, treatments that block endocytosis but do not significantly affect the structure of clathrin-coated pits (Damke et al., 1994; Rodal et al., 1999; Subtil et al., 1999). We found that with both treatments, GFP-AP2 fluorescence recovered after photobleaching (Figure 2, D–I). Thus, as occurred with clathrin, even though endocytosis is blocked the bleached GFP-AP2 in the pits is replaced by unbleached GFP-AP2.
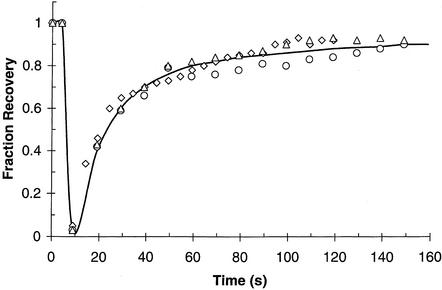
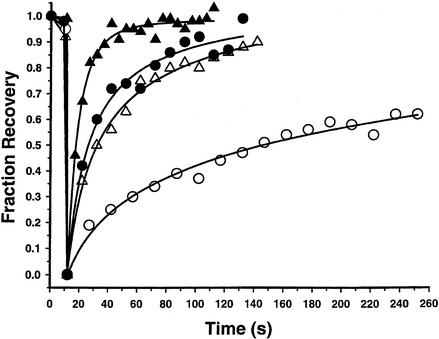
Figure 3 shows the time course of recovery of GFP-AP2 fluorescence after photobleaching both in control cells and in cells expressing K44A dynamin or depleted of cholesterol to block endocytosis. As summarized in Table 1, our data show that blocking clathrin-mediated endocytosis had little effect on the rate or extent of fluorescence recovery, demonstrating that GFP-AP2 exchange occurs at a rapid rate even when clathrin-mediated endocytosis is blocked. Another method of blocking clathrin-mediated endocytosis is to decrease the temperature to 15°C, which we previously found greatly reduced the rate of transferrin uptake so that over a period of 5 min almost no uptake occurred (Wu et al., 2001). Over the same period of time almost complete recovery of AP2 fluorescence occurred (Table 1), and the time course of this recovery in all cases was essentially the same as that previously found for clathrin at the plasma membrane (Wu et al., 2001). Therefore, consistent with our previous observations (Wu et al., 2001), rapid replacement of bleached GFP-AP2 occurs in clathrin-coated pits even when clathrin-mediated endocytosis is blocked.
Figure 3.
Kinetics of GFP-AP2 recovery after photobleaching at 37°C. HeLa control cells (triangles). HeLa cells depleted of cholesterol (circles). HeLa cells expressing K44A-dynamin (diamonds). Cells were photobleached at 10 s and then scanned at low laser power.
Table 1.
Fluorescence recovery after photobleaching
| GFP construct | Condition | Temp, °C | % Recovery | t½ (s) |
|---|---|---|---|---|
| GFP-AP2 | Control | 37 | 81 ± 8 | 14 ± 4 (n = 15) |
| K44A dynamin | 37 | 85 ± 10 | 15 ± 5 (n = 10) | |
| Cholesterol depleted | 37 | 86 ± 6 | 16 ± 5 (n = 7) | |
| Sucrose | 37 | 93 ± 6 | 19 ± 7 (n = 7) | |
| K+ depleted | 37 | 90 ± 9 | 15 ± 5 (n = 11) | |
| Control | 15 | 76 ± 11 | 39 ± 10 (n = 9) | |
| GFP-clathrin (TGN) | Control | 37 | 83 ± 10 | 14 ± 4 (n = 10) |
| Control | 20 | 56 ± 8 | 43 ± 9 (n = 8) | |
| GFP-AP1 | Control | 37 | 90 ± 12 | 10 ± 3 (n = 8) |
| Control | 20 | 75 ± 10 | 20 ± 6 (n = 8) | |
| Sucrose | 37 | 60 ± 3 | 14 ± 4 (n = 6) | |
| K+ depleted | 37 | 70 ± 15 | 14 ± 3 (n = 10) | |
| GFP-GGA1 | Control | 37 | 77 ± 11 | 12 ± 4 (n = 10) |
| Sucrose | 37 | 69 ± 11 | 27 ± 7 (n = 9) | |
| K+ depleted | 37 | 77 ± 14 | 16 ± 5 (n = 7) |
The values represent average ± SEM.
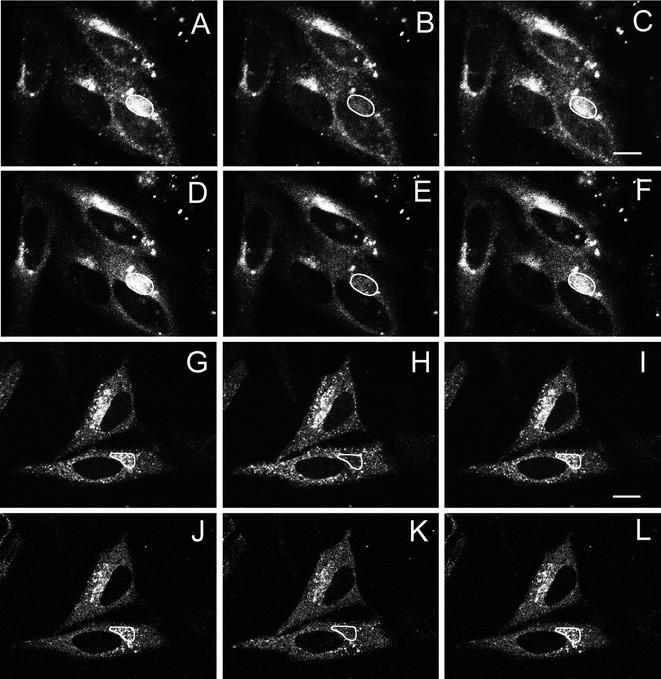
Our data with AP2 at the plasma membrane show that clathrin-coated pits are dynamic structures in which both clathrin and AP2 rapidly exchange during clathrin-mediated endocytosis. However, clathrin and adaptors are present not only in clathrin-coated pits at the plasma membrane but also at the TGN where AP1 is a major clathrin adaptor. Because we were interested in examining the fluorescence recovery during clathrin budding at the TGN of AP1 we constructed a GFP-AP1. Immunofluorescence studies using antibodies showed that the GFP-AP1 colocalized with AP1 and clathrin at the TGN. We also found that, after treatment with brefeldin A, GFP-AP1 dissociated from the TGN with the same time course as native AP1 (our unpublished data), suggesting that labeling the γ chain of AP1 with GFP does not affect AP1 function. AP1 has also been labeled with YFP on its μ1A chain to study vesicle movement form the TGN (Huang et al., 2001). Figure 4, A–F, shows the colocalization of GFP-AP1 and CFP-clathrin at the TGN as well as their recovery after photobleaching at 37°C. It should be noted that although much of the perinuclear clathrin and AP1 is undoubtedly present in the TGN, some may also be present in endosome, which we cannot distinguish from the TGN in the 2-min period of these studies. As expected, in control cells, after bleaching of both CFP-clathrin and GFP-AP1, essentially complete replacement of the bleached clathrin and AP1 occurred at 37°C. Because CFP-constructs cause more UV damage to proteins upon photobleaching than GFP, this result was confirmed using GFP-clathrin, and we found that, as with CFP-clathrin, after photobleaching the fluorescence of the GFP-clathrin at the TGN recovered over a short period of time (Table 1).
Figure 4.
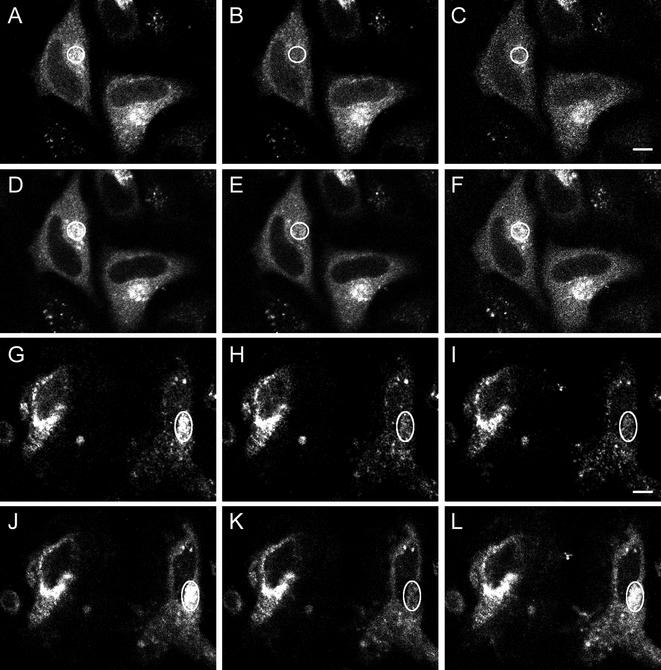
Fluorescence recover after photobleaching of CFP-clathrin and GFP-AP1 at the trans-Golgi network at 37 and 20°C. HeLa cells at 37°C (A–F) and 20°C (G–L) were imaged for CFP-clathrin (A–C and G–I) and GFP-AP1 (D–F and J–L) before photobleaching (A, D, G, and J), immediately after photobleaching (B, E, H, and K), and either 2 min (C and F) or 5 min (I and L) after photobleaching. The photobleached area is indicated in each figure. Bars, A–F, 11 μm; G–L, 13 μm.
We next determined whether this same recovery of fluorescence occurs at 20°C, a temperature that blocks transport from the TGN (Griffiths et al., 1985). As shown in Figure 4, G–L, the fluorescence of both the bleached CFP-clathrin and the bleached GFP-AP1 recovered over a period of 5 min, demonstrating that replacement of both AP1 and clathrin occur at the TGN under conditions where clathrin budding is prevented. Figure 5 and Table 1 show the time course of the fluorescence recovery of GFP-clathrin and GFP-AP1 at the TGN both at 37 and 20°C. At both temperatures, particularly at 20°C, the time course of recovery of GFP-AP1 is faster than that of GFP-clathrin. Perhaps because the GFP-clathrin on the TGN was unusually sensitive to damage by photobleaching at 20°C, we observed only ∼55% recovery of the GFP-clathrin fluorescence after photobleaching at 20°C but fluorescence loss in photobleaching experiments confirmed that all of the bound clathrin was freely exchangeable at this temperature (our unpublished data). We conclude that replacement of clathrin and adaptors during budding of clathrin-coated pits is a general phenomenon that occurs at both the plasma membrane and the TGN. Of course, although we could observe individual clathrin-coated pits at the plasma membrane and, therefore, conclude that replacement was due to clathrin exchange (Wu et al., 2001), at the TGN we could not observe individual pits and therefore this replacement could have been caused by either exchange or dissolution and replacement of whole clathrin-coated pits.
Figure 5.
Kinetics of recovery after photobleaching of CFP-clathrin and GFP-AP1 at the TGN at 37°C (closed symbols) and 20°C (open symbols). Time course of recovery are shown for GFP-clathrin (circles) and GFP-AP1 (triangles).
The observation that at 20°C AP1 exchange is somewhat faster than clathrin exchange at the TGN suggests that adaptor exchange may not always be linked with clathrin exchange. Another way of approaching this question is to treat the cells with hypertonic sucrose or by K+ depletion. Although cholesterol depletion and expression of K44A block endocytosis without significantly affecting the structure of the clathrin-coated pits, hypertonic sucrose and K+ depletion also block endocytosis but they do so by altering the structure of the clathrin-coated pits. It has been reported that both hypertonic sucrose and K+ depletion reduce the number of clathrin-coated pits at the plasma membrane and at the same time induce the formation of clathrin microcages near the remaining clathrin latices on the plasma membrane (Heuser and Anderson, 1989). Hansen et al. (1993), however, using ultrastructural immunogold microscopy, did not detect either clathrin on the plasma membrane or clathrin microcages in HEp-2 cells in hypertonic sucrose and K+ depletion. In our previous study on clathrin exchange, we found that both hypertonic sucrose and K+ depletion not only blocked endocytosis but also almost completely blocked clathrin exchange at the plasma membrane. Therefore, we were interested in determining whether AP2 exchange was also blocked by these treatments.
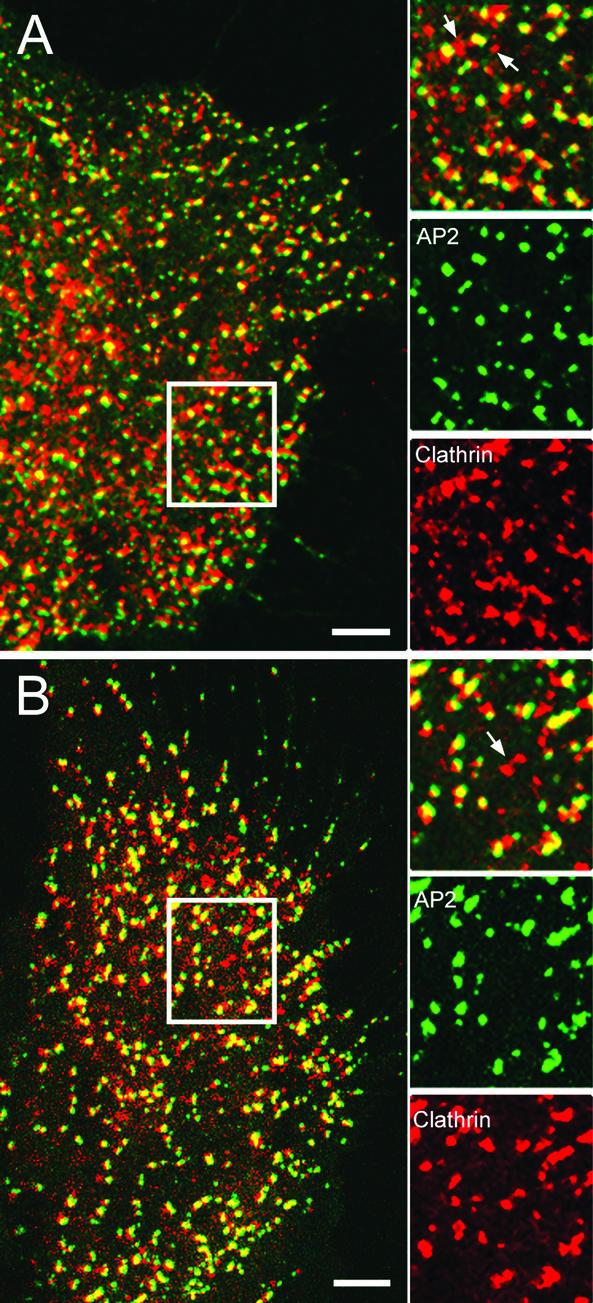
As we showed previously, these treatments caused a marked alteration in clathrin distribution (Figure 6, A and B). Both treatments tended to cause formation of clusters of clathrin structures some of which were not colocalized with AP2 (arrows) and therefore may represent the the clathrin microcages observed by freeze-fracture electron microscopy. In addition, based on MetaMorph (Universal Imaging, Downingtown, PA) analysis we found that ∼50% of the AP2 colocalized with both clusters of clathrin structures and with smaller clathrin fluorescent spots. Therefore, these structures may represent clusters of clathrin-coated pits and individual clathrin-coated pits, respectively. In support of this view, electron microscopy of the treated samples indeed showed that there were clathrin-coated pits present after treatment with either hypertonic sucrose or K+ depletion (our unpublished data). Finally we observed AP2 structures devoid of clathrin similar to the structures that were observed by Brown et al., 1999. Therefore, although clathrin microbaskets without AP2 and AP2 pits without clathrin are present, considerable clathrin-coated pits also occur in the treated samples.
Figure 6.
Colocalization of CFP-clathrin and GFP-AP2 in cells treated with hypertonic sucrose or depleted of K+. HeLa cells were treated as described in MATERIALS OF METHODS to block clathrin exchange at the coated pits. HeLa cells were then imaged at 63× to examine clathrin and AP2 in K+-depleted cells (A) or cells treated with hypertonic sucrose (B). The inset shows the distribution of clathrin, AP2, and overlap between clathrin and AP2. The arrows point to the clathrin clusters. Bar, 5 μm.
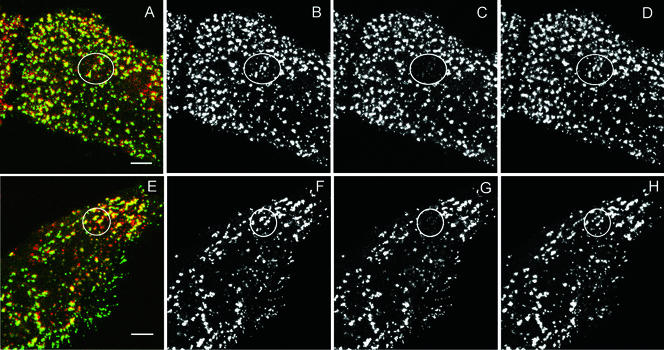
This allowed us to test whether, after treatment of the cells to hypertonic sucrose or K+ depletion, clathrin and AP2 exchange occurred in the fluorescent structures on the plasma membrane that contained both clathrin and AP2. Table 1 shows that, in contrast to the previous results we obtained in which clathrin exchange was completely blocked (Wu et al., 2001), more than 90% of the GFP-AP2 fluorescence recovered after photobleaching, and this AP2 exchange occurred at essentially the same rate as occurs in untreated cells. Furthermore, we found that this AP2 exchange specifically occurred in structures where AP2 and clathrin were colocalized (Figure 7). Because our fluorescence studies do not reveal whether AP2 is present in any of the clathrin microcages, we obviously cannot determine whether AP2 exchange occurs in the microcages. However, because all of the clathrin we observe on the plasma membrane shows no exchange, whereas 90% of the AP2 we observe does show exchange, including the AP2 colocalized with clathrin, it seems that AP2 is exchanging in clathrin-coated pits on the plasma membrane where clathrin exchange is blocked.
Figure 7.
Fluorescence recovery after photobleaching GFP-AP2 in HeLa cells in cells depleted of K+ (B–D) or treated with hypertonic sucrose (F–H) at 37°C. (A and E) Image of CFP-clathrin and GFP-AP2 in treated HeLa cells. (B and F) AP2 image obtained directly before being photobleached. (C and G) Image immediately after photobleaching. (D and H) Image 2 min after photobleaching. The photobleached area is indicated in each figure. Bars, 5 μm.
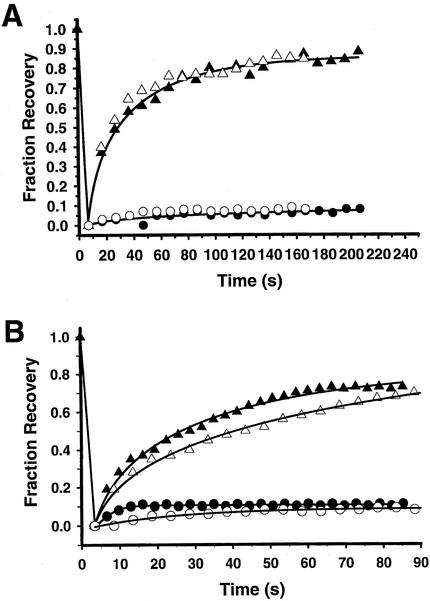
We next investigated how hypertonic sucrose and K+ depletion affect clathrin and AP1 dynamics at the TGN. Hypertonic sucrose and K+ depletion were previously found to markedly decrease the number of clathrin buds at the TGN as well as at the plasma membrane in HEp-2 cells (Hansen et al., 1993). However, we observed no significant decrease in fluorescence of the GFP clathrin on the plasma membrane and TGN in HeLa cells after treatment with hypertonic sucrose or K+ depletion. Furthermore, we found that recovery of clathrin fluorescence at the TGN was completely blocked by both K+ depletion and hypertonic sucrose just as we observed at the plasma membrane (Figures 8B and 9, A–C and G–I). In addition, like AP2 at the plasma membrane, AP1 at the TGN showed significant fluorescence recovery (Figures 8B and 9, D–F and J–L), although the rate of AP1 fluorescence recovery was slightly slower than in control cells (Table 1). Therefore, AP1 bound to the TGN is in equilibrium with free AP1 in the cytosol even when clathrin is immobilized on the TGN by treatment with hypertonic sucrose or K+ depletion.
Figure 8.
Time course of fluorescence recovery after photobleaching of adaptors and clathrin in cells depleted of K+ (open symbols) or treated with hypertonic sucrose at 37°C filled symbols) (A) Time course of recovery of GFP-AP2 (triangles) and GFP-clathrin (circles) at the plasma membrane. (B) Time course of recovery of GFP-AP1 (triangles) and GFP-clathrin (circles) at the TGN.
Figure 9.
Fluorescence recovery after photobleaching of CFP-clathrin and GFP-AP1 at the trans-Golgi network of cells depleted of K+ or treated with hypertonic sucrose at 37°C. HeLa cells were imaged for CFP-clathrin (A–C and G–I) and GFP-AP1 (D–F and J–L) before photobleaching (A, D, G, and J), immediately after photobleaching (B, E, H, K), and 2 min (C, F, I, L) after photobleaching in cells K+ depleted (A–F) or treated with hypertonic sucrose (G–L). The photobleached area is indicated in each figure. Bar, A–F, 7.5 μm; G–L, 5.5 μm.
Fluorescence recovery after photobleaching of GFP-GGA1, a recently discovered AP (Dell'Angelica et al., 2000; Hirst et al., 2000; Costaguta et al., 2001) at the TGN, also occurred at about the same rate as clathrin (Figure 10; Table 1). Furthermore in HeLa cells depleted of K+ or treated with hypertonic sucrose, after photobleaching GFP-GGA1 showed ∼70% fluorescence recovery at a rate slightly slower than the rate of GFP-AP1 fluorescence recovery under the same conditions (Figure 10; Table 1). Therefore, when clathrin exchange at the TGN is blocked by treatment with hypertonic sucrose or K+ depletion, like AP1, GGA1 is still able to detach from and reattach to the TGN. It should be noted that treatment of the TGN with hypertonic sucrose or K+ depletion caused fragmentation (our unpublished data). Nevertheless, even after fragmentation occurred, clathrin exchange was still blocked while both AP1 and GGA1 continued to exchange.
Figure 10.
Fluorescence recovery after photobleaching of GFP-GGA1 at the TGN of cells under different conditions at 37°C. HeLa cells were imaged for GFP-GGA1 before photobleaching (A, D, and G), immediately after photobleaching (B, E, and H), and 2 min (C, F, and I) after photobleaching in control cells (A–C), in cells K+ depleted (D–F) or treated with hypertonic sucrose (G–I). The photobleached area is indicated in each figure. Bar, 12 μm.
DISCUSSION
In a previous study, we showed that clathrin in clathrin-coated pits on the plasma membrane exchanges with free clathrin in the cytosol both during clathrin-mediated endocytosis and when clathrin-mediated endocytosis is blocked by cholesterol depletion or expression of a dynamin mutant (Wu et al., 2001). On the basis of these data, we suggested that clathrin exchange may be a fundamental characteristic of the budding of clathrin-coated vesicles, perhaps involved in the structural change that occurs in the clathrin lattice as clathrin-coated pits invaginate. Clathrin-coated vesicles not only bud from the plasma membrane but also from the TGN where the initiation of budding occurs by a very different mechanism requiring ARF1. In the present study, we found that both at 37°C where clathrin budding takes place and at 20°C where it is blocked, clathrin exchange also occurs at the TGN and, furthermore, at nearly the same rate as at the plasma membrane, supporting our view that clathrin exchange is a fundamental property of the budding of clathrin-coated vesicles.
Previous experiments (Greener et al., 2001) suggested that auxilin is required in Caenorhabditis elegans, for both clathrin-mediated endocytosis and exchange of clathrin on existing clathrin-coated pits. We, therefore, proposed that Hsc70 and auxilin may be involved in both the irreversible dissociation of clathrin that occurs after clathrin-coated vesicles bud off from the plasma membrane and in the clathrin exchange that occurs during clathrin-mediated endocytosis. At the same time, PIP2 binds directly to AP2 (Beck and Keen, 1991; Voglmaier et al., 1992), and there is evidence that the level of PIP2 on the plasma membrane, affected in part by the PIP2 phosphatase synaptojanin, controls whether clathrin-coated vesicles are uncoated after they pinch off from the plasma membrane (Cremona et al., 1999; Gad et al., 2000; Stenmark, 2000). Therefore, it was not clear whether clathrin, which binds to the β chain of AP2, and AP2 would show similar exchange properties or exchange at different rates.
Our results clearly show that photobleached AP2 was replaced at about the same rate as photobleached clathrin when clathrin budding from the plasma membrane was blocked by agents that did not affect the structure of the clathrin-coated pits. This is an intriguing finding given the recent determination of the core structure of AP2 that indicated AP2 undergoes a large-scale conformational change upon binding cargo (Collins et al., 2002). We also found that when clathrin-coated vesicle budding was blocked at the TGN by reducing the temperature to 20°C, AP1 exchanged at a two- to threefold faster rate than clathrin exchanged. Therefore, we conclude that clathrin-coated pits are not only dynamic structures in regard to clathrin but also in regard to the two major clathrin adaptors AP2 and AP1. Furthermore, at the TGN, the rates of AP1 and clathrin exchange seem to be at least partially uncoupled, which we also observed using fluorescence loss in photobleaching (our unpublished data).
Treatment of cells with hypertonic sucrose or K+ depletion supported this observation. We (Wu et al., 2001) previously found that both hypertonic sucrose and K+ depletion blocked clathrin exchange. Both of these treatments are thought to act in part by removing clathrin from clathrin-coated pits to form clathrin microcages (Heuser and Anderson, 1989). We, therefore, suggested that because clathrin-coated vesicles and clathrin baskets show no clathrin exchange in vitro, clathrin-microcages in vivo would likewise show no clathrin exchange. In the present study, although hypertonic sucrose and K+ depletion blocked clathrin exchange at both the plasma membrane and the TGN, AP2, AP1 and GGA1 continued to exchange at almost the same rate as in untreated cells. Furthermore, closer examination of the effect of hypertonic sucrose and K+ depletion suggested that, although some clathrin no longer colocalizes with AP2, there was still a considerable amount of colocalization of clathrin and AP2 at the plasma membrane in what seemed to be clathrin-coated pits. In confirmation of this view, electron microscopy studies showed that clathrin-coated pits still occurred on the plasma membrane of HeLa cells after treatment with hypertonic sucrose and K+ depletion. Heuser and Anderson (1989) likewise saw clathrin-coated pits in fibroblasts under these conditions, but they were not observed in HEp-2 cells (Hansen et al., 1993). Therefore, our data suggest that even though clathrin that remains in clathrin-coated pits after hypertonic sucrose and K+ depletion no longer exchanges, AP2 in these pits does exchange. This is rather surprising because global treatments such as hypertonic sucrose and K+ depletion might be expected to have global effects in which case there may be an alteration of the PIP2 level on the membrane and the phosphorylation level of the AP2 both of which are thought to affect the binding strength of AP2 to the clathrin-coated pits (Wilde and Brodsky, 1996; Jost et al., 1998). Yet neither of these treatments significantly affected the rate of AP2 exchange. Furthermore, AP2 is such a large molecule that immobilization of the clathrin lattice by hypertonic sucrose and K+ depletion might be expected to inhibit AP2 exchange sterically. Yet, it is clear from out data that adaptor exchange is not directly linked to clathrin exchange.
On the other hand, it seems unlikely that adaptor exchange and clathrin exchange are completely independent because in control cells the rates of AP2 and clathrin exchange at the plasma membrane are almost identical and there is only a twofold difference in the rates of AP1 and clathrin exchange at the TGN. Also, PIP2 has been shown to affect the rate at which clathrin dissociates from clathrin-coated vesicles at the plasma membrane and, because AP2 directly interacts with PIP2 and clathrin does not, it seems likely that PIP2 indirectly affects the rate of clathrin dissociation through its effect on AP2 dissociation. Therefore, we favor a model in which clathrin exchange is linked to adaptor exchange, which, in turn, is linked to the PIP2 level. Furthermore, we think it likely that, rather than clathrin passively dissociating when adaptors dissociate, Hsc70 and auxilin are required to unravel individual clathrin molecules from the intricate clathrin lattice present in clathrin-coated pits (Wu et al., 2001).
Further evidence that Hsc70 may be involved in this process comes from the data of Schmid and colleagues (Hannan et al., 1998) who showed that Hsc70 is not only required for dissociation of clathrin from clathrin-coated vesicles in vitro but also, in an ATP-dependent process, is involved along with another cofactor in AP2 dissociation. Hsc70 may also be involved in dissociation of clathrin from clathrin-coated pits at the TGN. ARF1-GFP recruits AP1 to the TGN but Kornfeld and coworkers (Zhu et al., 1998), using a reconstituted assay, showed that after the membrane is primed by ARF1 to bind AP1, the ARF1 dissociates, leaving AP1 and clathrin bound to the membrane. Therefore, factors other than ARF1 could be involved in dissociation of clathrin and AP1 from clathrin-coated vesicles produced at the TGN, raising the possibility that Hsc70 may be involved in uncoating at the TGN as well as at the plasma membrane. Clearly, much more work will be required to understand the coordinated control of adaptor and clathrin dissociation at both the TGN and the plasma membrane.
ACKNOWLEDGMENTS
We thank Myoung-Soon Cho for preparing the electron micrographs of the HeLa cells under different conditions, Dr. James Keen for informing us before publication how to prepare GFP-AP2, and Samir Bhasin for assistance in analysis of the FRAP data.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–06–0353. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–06–0353.
REFERENCES
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- Brodin L, Low P, Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol. 2000;10:312–320. doi: 10.1016/s0959-4388(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Brown CM, Roth MG, Henis YI, Petersen NO. An internalization-competent influenza hemagglutinin mutant causes the redistribution of AP-2 to existing coated pits and is colocalized with AP-2 in clathrin free clusters. Biochemistry. 1999;38:15166–15173. doi: 10.1021/bi991170v. [DOI] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture, and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell. 2001;6:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling: sequential steps in clathrin-mediated synaptic vesicle endocytosis. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Gossen M, Freundlieb S, Bujard H, Schmid SL. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–220. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- Danino D, Hinshaw JE. Dynamin family of mechanoenzymes. Curr Opin Cell Biol. 2001;13:454–460. doi: 10.1016/s0955-0674(00)00236-2. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, Brodin L. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells: sequential steps in clathrin-mediated synaptic vesicle endocytosis. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- Greener T, Grant B, Zhang Y, Wu X, Greene LE, Hirsh D, Eisenberg E. Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo. Nat Cell Biol. 2001;3:215–219. doi: 10.1038/35055137. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Pfeiffer S, Simons K, Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan LA, Newmyer SL, Schmid SL. ATP- and cytosol-dependent release of adaptor proteins from clathrin-coated vesicles: a dual role for Hsc70. Mol Biol Cell. 1998;9:2217–2229. doi: 10.1091/mbc.9.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation: sequential steps in clathrin-mediated synaptic vesicle endocytosis. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2002;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Nesterov A, Carter RE, Sorkin A. Trafficking of yellow-fluorescent-protein-tagged mu1 subunit of clathrin adaptor AP-1 complex in living cells. Traffic. 2001;2:345–357. doi: 10.1034/j.1600-0854.2001.25020506.x. [DOI] [PubMed] [Google Scholar]
- Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol. 1998;8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem J. 1997;328:353–361. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis: sequential steps in clathrin-mediated synaptic vesicle endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- Presley JF, Ward TH, Pfeifer AC, Siggia ED, Phair RD, Lippincott-Schwartz J. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417:187–193. doi: 10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Cycling lipids. Curr Biol. 2000;10:R57–R59. doi: 10.1016/s0960-9822(00)00279-7. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Keen JH, Murphy JE, Ferris CD, Prestwich GD, Snyder SH, Theibert AB. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem Biophys Res Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhao X, Baylor L, Kaushal S, Eisenberg E, Greene LE. Clathrin exchange during clathrin-mediated endocytosis. J Cell Biol. 2001;155:291–300. doi: 10.1083/jcb.200104085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]