Abstract
The inhibitory Smad7, a direct target gene for transforming growth factor-β (TGF-β), mediates TGF-β1–induced apoptosis in several cell types. Herein, we report that apoptosis of human prostate cancer PC-3U cells induced by TGF-β1 or Smad7 overexpression is caused by a specific activation of the p38 mitogen-activated protein kinase pathway in a TGF-β–activated kinase 1 (TAK1)- and mitogen-activated protein kinase kinase 3 (MKK3)-dependent manner. Expression of dominant negative p38, dominant negative MKK3, or incubation with the p38 selective inhibitor [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole], prevented TGF-β1–induced apoptosis. The expression of Smad7 was required for TGF-β–induced activation of MKK3 and p38 kinases, and endogenous Smad7 was found to interact with phosphorylated p38 in a ligand-dependent manner. Ectopic expression of wild-type TAK1 promoted TGF-β1–induced phosphorylation of p38 and apoptosis, whereas dominant negative TAK1 reduced TGF-β1–induced phosphorylation of p38 and apoptosis. Endogenous Smad7 was found to interact with TAK1, and TAK1, MKK3, and p38 were coimmunoprecipitated with Smad7 in transiently transfected COS1 cells. Moreover, ectopically expressed Smad7 enhanced the coimmunoprecipitation of HA-MKK3 and Flag-p38, supporting the notion that Smad7 may act as a scaffolding protein and facilitate TAK1- and MKK3-mediated activation of p38.
INTRODUCTION
Members of the transforming growth factor (TGF)-β family, e.g., TGF-βs, activins, and bone morphogenetic proteins (BMPs), are important regulators of proliferation, differentiation, apoptosis, and migration of cells during embryogenesis, and are also involved in the maintenance of tissue homeostasis in the adult (Attisano and Wrana, 2000; Massagué, 2000; Miyazono et al., 2000). The cellular responses evoked by TGF-β are mediated by type II and type I transmembrane serine/threonine kinase receptors. On ligand binding to the constitutively active type II receptor, the type I receptor is recruited into the receptor complex and becomes phosphorylated by the type II receptor kinase. The activated type I receptor kinase subsequently phosphorylates members of the intracellular Smad signal transduction pathway. In the TGF-β pathway, the receptor-regulated Smad2 and Smad3 (R-Smads) form complexes with coSmad4 that accumulate in the nucleus, where they regulate transcriptional responses by direct or indirect interactions with promoter regions of specific genes.
Smad6 and Smad7 are classified as inhibitory Smads (I-Smads), because their interactions with the activated type I receptors prevent phosphorylation of the R-Smads (Attisano and Wrana, 2000; Massagué, 2000; Miyazono et al., 2000). Moreover, Smad7 binds ubiquitin ligases of the Smurf family, which cause ubiquitination and proteasomal degradation of the TGF-β receptors (Kavsak et al., 2000; Ebisawa et al., 2001). Initial studies demonstrated that the expressions of Smad6 and Smad7 are increased by BMP and TGF-β (Hayashi et al., 1997; Imamura et al., 1997; Nakao et al., 1997). Subsequently, interferon γ, tumor necrosis factor-α, as well as other growth factors have been reported to regulate the expression of Smad6 and Smad7 (Afrakhte et al., 1998; Ulloa et al., 1999; Bitzer et al., 2000).
The R- and CoSmad proteins consist of two highly conserved amino- and carboxy-terminal parts, the Mad homology 1 (MH1) and MH2 domains, which binds DNA and has transactivation effect, respectively. The MH1 and MH2 domains interact reciprocally and thereby inhibit each others' function, but this autoinhibition is overcome when the R-Smads become phosphorylated in their extreme C-terminal part by the activated type I receptor kinase (Heldin et al., 1997). The I-Smads have conserved MH2 domains, like R-Smads and Smad4, whereas the MH1 domain is not conserved in the I-Smads; however, the N-terminal parts of Smad6 and Smad7 show 37% sequence similarity to each other. The I-Smads, as well as the other Smads, shuttle between the nucleus and the cytoplasm (Itoh et al., 1998; Kavsak et al., 2000; Hanyu et al., 2001). Smad6 has been shown to act as a transcriptional corepressor in the BMP signaling pathway (Bai et al., 2000). The same group recently reported that the N-terminal part of Smad6 can bind to DNA, whereas the MH2 domain recruits both Hoxc-8 and histone deacetylases, resulting in repression of the osteopontin promoter (Bai et al., 2002). Smad7 was also shown to interact with histone deacetylase 1 (Bai and Cao, 2002). In resting cells, Smad7 is localized in the nucleus; in response to TGF-β stimulation Smad7 is exported to the cytoplasm (Itoh et al., 1998). Smad7 has recently been reported to regulate gene transcription, an ability that is modulated by its phosphorylation status (Pulaski et al., 2001). Thus, all Smads contribute to regulation of transcriptional activities in cells.
We have previously reported that Smad7 is necessary for TGF-β–induced apoptosis of epithelial cells (Landström et al., 2000). Moreover, it has been shown that Smad7 overexpression in Madine-Darby canine kidney (MDCK) cells enhances the apoptosis of MDCK cells caused by treatment with TGF-β or tumor necrosis factor-α, growth factor withdrawal, or loss of cell adhesion (Lallemand et al., 2001). This effect was found to be mediated by a decreased expression of nuclear factor-κB, a molecule known to promote survival of cells. Moreover, podocytes, a highly specialized cell type in the kidney, have been shown to undergo apoptosis after TGF-β1 stimulation or overexpression of Smad7 through adenoviral infection (Schiffer et al., 2001).
The mitogen-activated protein (MAP) kinase pathways regulate cell growth, differentiation, and stress responses (Cowley et al., 1994; Davis, 2000; Nebreda and Porras, 2000; Chang and Karin, 2001). Both positive and negative cross-talk between MAP kinase pathways and TGF-β/Smad pathways has been reported (Kretzschmar et al., 1997; Zhang et al., 1998; Sano et al., 1999). TGF-β has been found to activate one or more of three different MAP kinase pathways in different cell types, i.e., the extracellular signal-regulated kinases (ERKs) (Hu et al., 1999; Yue and Mulder, 2000), c-Jun N-terminal kinases (JNK)/stress-activated protein kinases (SAPKs) (Engel et al., 1999; Hocevar et al., 1999), and the p38 kinases (Hanafusa et al., 1999). Previous studies have shown that the SAPK/JNK pathway is activated by TGF-β1 in MDCK cells (Atfi et al., 1997a,b; Wang et al., 1997; Hanafusa et al., 1999). In contrast, TGF-β treatment of C2C12 cells resulted in specific activation of the MKK6 and p38 MAP kinase pathway, without any effect on the SAPK/JNK pathway (Hanafusa et al., 1999). In Drosophila melanogaster, the TGF-β–like secreted ligand decapentaplegic has been shown to activate the Drosophila homolog of p38 (Adachi-Yamada et al., 1999).
The p38 MAP kinase pathway has been implicated in regulation of apoptosis in adipocytes and in neurons induced by withdrawal of growth factors (Ichijo et al., 1997; Kummer et al., 1997; Xia et al., 1995; Yamagishi et al., 2001). Like the other MAP kinases, the p38 group of kinases is activated by the MAP kinase kinases at conserved Thr-Xaa-Tyr dual phosphorylation sites. MKK6 can activate all of the four different isoforms of p38; MKK3 preferentially activates p38α, p38δ, and p38γ; and MKK4 has been reported to activate p38α and p38δ (Davis, 2000). TGF-β–activated kinase 1 (TAK1) was originally identified as an MAP kinase kinase kinase, activated downstream of TGF-β/BMP receptors, positively regulating the SAPK/JNK and p38 kinase pathways (Yamaguchi et al., 1995). BMP-2 treatment of a mouse hybridoma cell line (MH60) results in apoptosis, mediated by activation of TAK1 and p38, whereas ectopic expression of Smad6 inhibited the BMP-induced activation of TAK1 and p38, resulting in inhibition of apoptosis (Kimura et al., 2000). In contrast, overexpression of Smad7 has recently been demonstrated to cause activation of the JNK pathway and apoptosis in MvLu1, MDCK and COS7 cells, suggesting different roles of the I-Smads in regulation of the MAP kinase pathways (Mazars et al., 2001). In a recent report, TGF-β was found to induce apoptosis of the BL41 Burkitt's lymphoma cells via p38-dependent activation of caspase 8 (Schrantz et al., 2001). However, TGF-β seems also to be important for survival of some epithelial cell lines such as HaCaT and NMuMG cells, where TGF-β increased cell survival through its positive effects on the Akt kinase, whereas it inhibited the effects of the forkhead factor FKHRL1, a transcription factor known to positively regulate apoptosis-related genes (Shin et al., 2001). These reports underline that TGF-β can affect survival of cells, both positively and negatively, depending on the cellular context.
In the present study, we have examined further the molecular mechanisms of TGF-β1 and Smad7-induced apoptosis of PC-3U prostate cancer cells. Our data implicate an involvement of TAK1, as well as MKK3 and the p38 MAP kinase in TGF-β1– and Smad7-induced apoptosis. Smad7 was found to interact with TAK-1, MKK3, and p38. Importantly, Smad7 enhanced the interaction between MKK3 and p38, indicating that Smad7 may act as a scaffolding protein facilitating TGF-β–induced p38 activation and apoptosis.
MATERIALS AND METHODS
Cell Culture
The human prostate cancer cell line PC-3U, originating from PC-3 cells (Franzén et al., 1993), the stably transfected pMEP4-FlagSmad7 (Clone I and Clone K), and antisense Smad7 PC-3U cells (AS-S7) were routinely grown in RPMI 1640 with 10% fetal bovine serum (FBS), in the presence of their specific antibiotics (to maintain selection pressure), as described previously (Landström et al., 2000). Clone I and Clone K cells were stimulated with 1.0 μM CdCl2 for indicated time periods to induce expression of F-Smad7. PC-3U cells treated with 1.0 μM CdCl2 for similar time periods were used as control. COS1 cells were maintained in DMEM with 10% FBS. In all assays, TGF-β1 treatment was given at 10 ng/ml in medium containing 1% FBS. For inhibition studies, [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole], (Calbiochem, San Diego, CA) was used at a concentration of 10 μM. The inhibitor was added to media one hour before stimulation of cells with TGF-β1 or CdCl2.
Analyses by Western Blotting, Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL), DNA Fragmentation, and Mitosensor
Different time periods after treatment with TGF-β1, cells were lysed in sample buffer and subjected to SDS-gel electrophoresis in a 10% polyacrylamide gel, and then transferred to Immobilon (Millipore, Bedford, MA) by using a semidry transfer apparatus (Bio-Rad, Hercules, CA), as described previously (Itoh et al., 1998; Landström et al., 2000). For detection of phosphorylated and total ATF-2, MKK3/MKK6, p38, and SAPK/JNK, affinity-purified polyclonal antibodies from Cell Signaling Technology (Beverly, MA) were used at recommended concentrations. PC-3U cells and AS-S7 cells treated with osmotic shock (OS, 0.7 M NaCl for 30 min) were used as positive control for phospho-ATF-2, phospho-MKK3/MKK6, phospho-p38, and phospho-SAPK/JNK. Affinity-purified polyclonal antibodies against phospho-ERK1/2 and total ERK2 were kind gifts from Dr. L. Rönnstrand at our institute. DNA-fragmentation assays and 4′-6 diamidino-2-phenylindoledihydrochloride (DAPI) stainings, to identify and quantify apoptotic nuclei, were performed as described previously (Landström et al., 2000). Apoptotic cells were identified by TUNEL (Roche Diagnostics, Mannheim, Germany) and Mitosensor (BD Biosciences Clontech, Palo Alto, CA) stainings, and the assays were carried out following the manufacturers' instructions. Monoclonal M30 antibodies (Roche Diagnostics), which recognize caspase-cleaved cytokeratin 18, were used in immunofluorescence analyses of apoptosis. Cells were photographed by Hamamatsu ORCA charge-coupled device digital camera by using the QED Imaging System software with an Axioplan2 microscope (Carl Zeiss, Jena, Germany). The number of cells positive for M30, hemagglutinin (HA) (transfected with wild-type or dominant negative [DN] MKK3), or TAK1 was counted at 40× magnification; in total, 500-1000 cells for each group were examined.
Immunofluorescence, Transfections, and Immunoprecipitations
Immunofluorescence, transient transfections of PC-3U and COS1 cells, and immunoprecipitations were performed as described previously (Itoh et al., 1998; Landström et al., 2000; Edlund et al., 2002).
Protein Kinase Assays
His-MKK6 was expressed in Escherichia coli and affinity purified. TAK1 was immunoprecipitated from total cell lysates with anti-TAK1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The immuncomplexes were subjected to kinase assays in a final volume of 50 μl in kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol, 5 mM MgCl2, 0.1 mM ATP, and 3 μCi of [γ-32P]ATP, and 3 μg of His-MKK6. Samples were incubated at 37°C for 20 min. Reactions were terminated by the addition of sample buffer and boiling. Substrate phosphorylations were detected and quantified by autoradiography and image analysis (BAS-reader; Fuji, Tokyo, Japan).
RESULTS
TGF-β1 Specifically Activates the p38 Pathway in PC-3U Cells, Resulting in Apoptosis
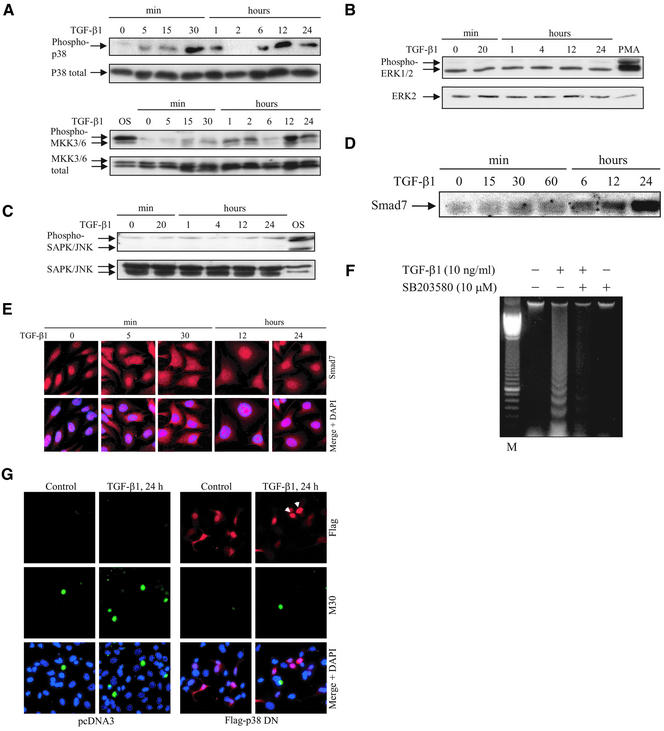
To investigate whether TGF-β1 treatment of PC-3U cells specifically activated any of the MAP kinase signaling pathways, phospho-specific antibodies for the activated forms of p38, ERK1/2, MKK3/6, and SAPK/JNK were used in Western blot analyses of cell lysates. Phospho-p38 antibodies, which recognize specifically the activated form of p38 phosphorylated on Thr180 and Tyr182, revealed a TGF-β1–induced p38 phosphorylation 5 min after stimulation of the PC-3U cells; a pronounced response was observed at 30 min and a second wave of activation occurred after 12 h (Figure 1A, phospho-p38). The total amount of p38 remained unchanged during the treatment period (Figure 1A, p38 total). We also analyzed whether MKK3/6 is activated by TGF-β in PC-3U cells. The phospho-MKK3/MKK6–specific antibodies (recognizing MKK3 and MKK6 phosphorylated on Ser189 and Ser207) used in immunoblotting of total cell extracts showed a slight activation of MKK3/6, detected after 5–15 min and peaking at 12 h of TGF-β treatment (Figure 1A, phospho-MKK3/6). The total amount of MKK3/6 remained essentially constant during the treatment period (Figure 1A, total MKK3/6). In contrast, TGF-β1 did not affect the constitutive phosphorylation of ERK1/2 in PC-3U cells (Figure 1B) and did not lead to phosphorylation of SAPK/JNK, whereas the phosphorylation of these kinases was increased after treatment with phorbol 12-myristate 13-acetate (PMA) or osmotic shock, respectively (Figure 1C).
Figure 1.
Specific activation of p38 by TGF-β1 causes apoptosis. Time courses of phosphorylation of endogenous p38 and MKK3/6 (A), ERK1/2 (B), and SAPK/JNK (C), and expression levels of endogenous Smad7 (D) after TGF-β stimulation. Total cell lysates were prepared from TGF-β1–treated or untreated PC-3U cells and used for immunoblotting. In the top and bottom panels, the phosphorylated and the nonphosphorylated forms, respectively, of p38, MKK3/6, ERK1/2, and SAPK/JNK are indicated. Cell lysates from PC-3U cells exposed to 0.7 M NaCl for 30 min (OS) or 10 nM PMA for 20 min (PMA) were used as positive controls. (E) Subcellular localization of endogenous Smad7 in untreated PC-3U cells or PC-3U cells treated for 5 min, 30 min, 12 h, or 24 h with TGF-β, as investigated by immunostainings with antibodies against Smad7 and additional staining of the nuclei by DAPI. An overlay of both pictures (merge + DAPI) shows that Smad7 is predominantly localized in nuclei of untreated cells, whereas 5 min after TGF-β treatment an export to the cytoplasm is observed, followed by accumulation in the nucleus after 12 and 24 h. (F) Analysis of fragmentation of DNA isolated from PC-3U cells, TGF-β1–treated or not for 24 h in the presence or absence of SB203580. (G) Apoptosis of PC-3U cells, transiently transfected with pcDNA3 (control) and dominant negative Flag p38 (Flag-p38 DN), treated with TGF-β1 for 24 h, as analyzed by immunostainings with antibodies against Flag and the apoptotic marker M30. An overlay of both pictures with additional staining of nuclei with DAPI (merge + DAPI) showed a reduced number of M30-positive cells per field in cells expressing Flag-p38 DN after treatment with TGF-β1, compared with control cells. Note that cells expressing Flag-p38 DN, indicated by arrowheads, do not undergo apoptosis.
TGF-β treatment of PC-3U cells led to increased levels of Smad7 protein with the highest levels detected after 24 h (Figure 1D). The subcellular localization of endogenous Smad7 was determined by immunofluorescence stainings by using an affinity-purified rabbit antibody. As previously reported by us (Itoh et al., 1998), TGF-β stimulation caused an export of Smad7 that was observed already after 5 min; upon prolonged incubation in the presence of TGF-β for 6–24 h, Smad7 was increased in amount and found to accumulate predominantly in the nucleus (Figure 1E).
Because TGF-β1 was previously shown to induce apoptosis in PC-3U cells, we investigated the role of p38 MAP kinase in apoptosis, by treatment of cells with the pyridinyl imidazole derivative SB203580, which inhibits p38α and p38β (Young et al., 1997), but not ERKs and JNKs (Lee et al., 1994; Cuenda et al., 1995; Jiang et al., 1996; Sano et al., 1999). Interestingly, SB203580 treatment reduced the TGF-β1–induced apoptotic response, as analyzed by DNA fragmentation (Figure 1F) and TUNEL assay (our unpublished data). It should be noted that, at high doses, SB203580 can also inhibit TGF-β1 receptor-mediated phosphorylation of Smad2 (Eyers et al., 1998); however, at the concentration used in our experiments (10 μM), no effect on TGF-β–induced Smad2 phosphorylation was observed (our unpublished data). Moreover, in a chondrogenic cell line (ATDC5), concentrations of SB203580 up to 20 μM did not affect TGF-β–induced Smad2 phosphorylation and nuclear translocation (Watanabe et al., 2001).
To explore the possibility that TGF-β initiates apoptosis by activating the p38 MAP kinase pathway, we transiently transfected PC-3U cells with a mutant Flag-p38α construct containing T180A and Y182F amino acid substitutions; this p38 mutant cannot be activated and therefore act in a dominant negative manner (Han et al., 1994). Dominant negative Flag-p38 was found to protect cells against TGF-β1–induced apoptosis in PC-3U cells, resulting in a reduction of apoptosis by 75% compared with control cells (Figure 1G), as shown by coimmunofluorescence analysis by using the M30 antibody, which specifically recognizes caspase-cleaved cytokeratin 18, and the Flag-antibody, to identify cells expressing dominant negative Flag-p38. Therefore, we conclude that treatment of PC-3U cells with TGF-β1 results in activation of the p38 MAP kinase pathway and subsequent apoptosis of the cells.
Smad7 Is Required for Activation of p38
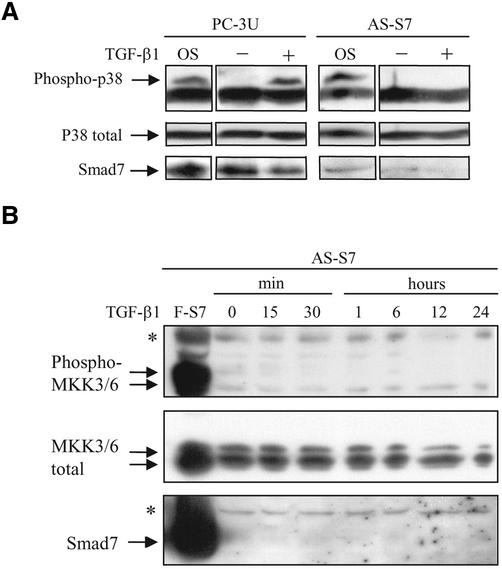
We have previously demonstrated that Smad7 is necessary for TGF-β1–induced apoptosis in PC-3U cells (Landström et al., 2000). Therefore, we investigated whether Smad7 expression is also needed for TGF-β1–induced activation of the p38 pathway, by using PC-3U cells stably transfected with an antisense Smad7 construct (AS-S7; Landström et al., 2000). Treatment of AS-S7 cells with TGF-β1 up to 48 h did not lead to phosphorylation of p38, whereas osmotic shock resulted in phosphorylation of p38 (Figure 2A; our unpublished data). To confirm that expression of Smad7 is reduced in AS-S7, the filter used in Figure 2A was stripped, blocked, and reproved with Smad7-specific antisera, demonstrating the reduced expression of Smad7 protein in AS-S7 cells. In PC-3U cells treated with TGF-β1 for 2–24 h, the phosphorylated p38 accumulated in the nucleus, as investigated by immunofluorescence stainings. In contrast, the nuclei of the AS-S7 cells treated with TGF-β for similar time periods did not show staining for phosphorylated p38 (our unpublished data). Moreover, treatment of AS-S7 cells with TGF-β1 for 15 min to 24 h did not lead to phosphorylation of MKK3/6, whereas a strong signal for phosphorylated MKK3/6 was observed in lysates from PC-3U cells infected with adenoviral Flag-Smad7 (Figure 2B). We therefore conclude that Smad7 is required for TGF-β1–induced phosphorylation of MKK3/6 and p38 in PC-3U cells.
Figure 2.
Smad7 is necessary for TGF-β–induced phosphorylation of p38 and MKK3/6. (A) Phosphorylation of endogenous p38 in total cell lysates prepared from PC-3U and antisense Smad7 PC-3U cells (AS-S7), untreated or treated with TGF-β1 for 1 h, was analyzed by immunoblotting. The phosphorylated and nonphosphorylated forms of p38 are shown in the top and bottom panels, respectively. Cell lysates from PC-3U cells exposed to 0.7 M NaCl for 30 min (OS) were used as positive control. The same filter was stripped, blocked, and reprobed with antibodies against Smad7. Note the lowered Smad7 expression and the lack of phosphorylated p38 in AS-S7 cells. (B) Phosphorylation of endogenous MKK3/6 in total cell lysates prepared from AS-S7 cells, untreated or treated with TGF-β1 for 15 min to 24 h, was analyzed by immunoblotting. The phosphorylated and nonphosphorylated forms of MKK3/6 are shown in the top and bottom panels, respectively. Cell lysates from PC-3U cells infected with adenoviral Flag-Smad7 (F-S7) was used as positive control. The same filter was stripped, blocked, and reprobed with antibodies against Smad7. Note the lowered Smad7 expression and the lack of phosphorylated MKK3/6 in AS-S7 cells. A background band is indicated by an asterisk (*).
Smad7 Overexpression Causes Activation of the p38 MAP Kinase Pathway and Apoptosis in PC-3U Cells
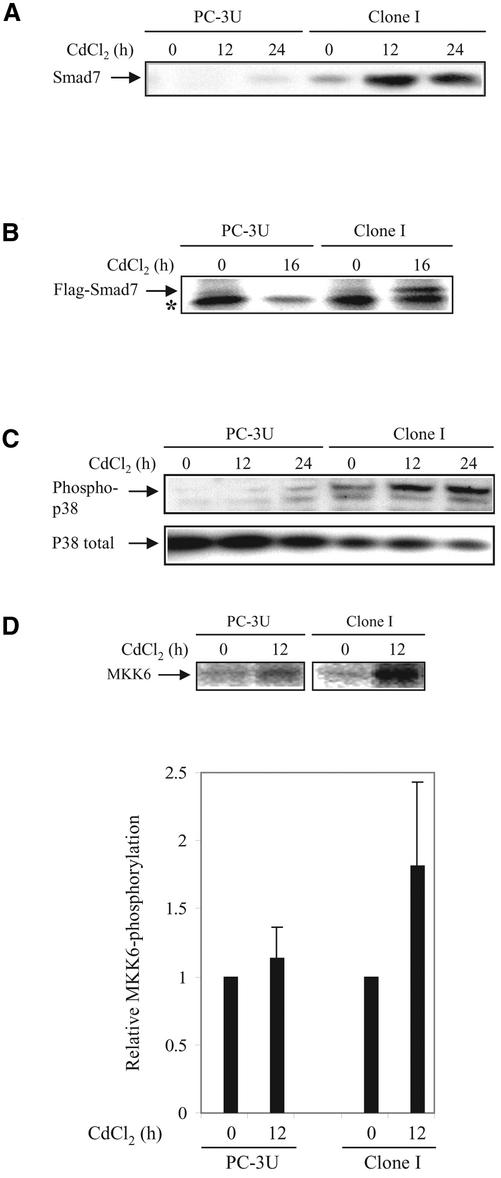
Given that Smad7 is essential for TGF-β1–induced phosphorylation of p38, we determined the effect of Smad7 overexpression on the activation of the p38 MAP kinase pathway. In PC-3U cells stably transfected with pMEP4-Smad7 (Clone I), Smad7 expression is under control of the CdCl2-inducible metallothionein promoter (Landström et al., 2000). As reported previously by us (Itoh et al., 1998) and others (Kavsak et al., 2000; Ebisawa et al., 2001), ectopically expressed Smad7 is predominantly localized in the nucleus, whereas after TGF-β treatment a portion of Smad7 is exported to the cytoplasm, where it interacts with the activated TGF-β receptor complex. Treatment of Clone I cells with 1.0 μM CdCl2 for 12 or 24 h, significantly increased the levels of Smad7, as shown by immunoblotting with a Smad7 antibody (Figure 3A), or immunoprecipitation from metabolically labeled cells by using a Flag-antibody (Figure 3B). Overexpression of Smad7 significantly increased the level of phosphorylated p38 after 12 and 24 h, compared with control cells, as shown by immunoblotting (Figure 3C). CdCl2 treatment of control PC-3U cells only caused a minor induction of Smad7, with a concomitant activation of p38.
Figure 3.
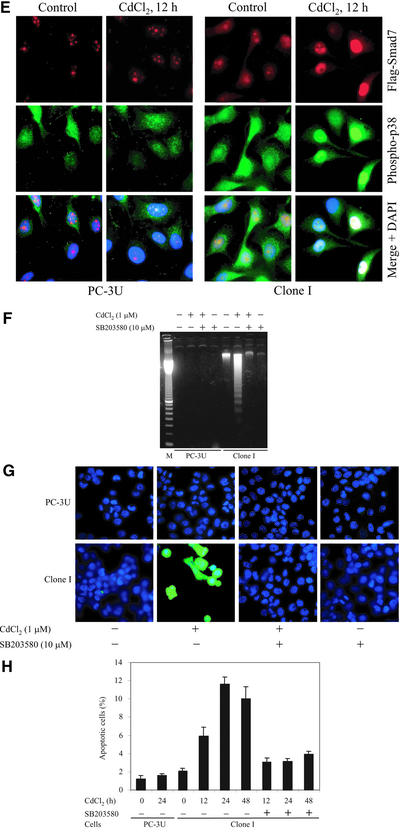
Ectopic expression of Smad7 induces increased levels of p38 phosphorylation and apoptosis. (A) Time course of Smad7 levels in PC-3U and PC-3U cells stably transfected with pMEP-4 F-Smad7 (Clone I cells). Total cell lysates were prepared from wild-type PC-3U cells and Clone I cells, treated or not with CdCl2 and subjected to immunoblotting. (B) Ectopic expression of Flag-Smad7 is induced by CdCl2 stimulation (16 h) of Clone I cells as shown by metabolic labeling and immunoprecipitation with Flag antibody. A background band is indicated by an asterisk (*). (C) Time course of phosphorylation of endogenous p38. Total cell lysates were prepared from wild-type PC-3U cells and Clone I cells, treated or not with CdCl2 and subjected to immunoblotting. The phosphorylated p38 (phospho-p38) and the total p38 are shown in the top and bottom panels, respectively. (D) Activation of MKK6 by Smad7 overexpression is shown. PC-3U and Clone I cells were treated or not with CdCl2 for 12 h. Cell lysates were immunoprecipitated with anti-TAK1 antibodies and immunoprecipitates were subjected to in vitro kinase assay by using His-MKK6 as substrate. The phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography (top). The mean relative values from three independently performed experiments including scanning electron microscopy are presented (bottom). (E) Immunofluorescence analyses of phosphorylated p38 in PC-3U cells and Clone I cells treated or not with CdCl2 for 12 h. Cells were also analyzed for Flag-Smad7 expression by immunofluorescence staining by using Flag antibodies. An overlay of both pictures with additional staining of nuclei by DAPI (merge + DAPI) reveals several nuclei with a colocalization of Smad7 and phospho-p38 in Clone I cells. (F) Analysis of fragmentation of DNA isolated from control PC-3U cells and Clone I cells treated or not for 24 h with CdCl2, in the presence or absence of SB203580. (G) Stainings with DAPI and TUNEL (fluorescein isothiocyanate) of control PC-3U cells and Clone I cells treated or not for 24 h with CdCl2 in the presence or absence of SB203580. Note the enhanced TUNEL staining of nuclei showing morphological criteria for apoptosis in cells overexpressing Flag-Smad7, which is inhibited by SB203580. (H) Apoptosis of PC-3U cells and Clone I cells treated or not with CdCl2, alone or together with SB203580, as revealed by immunostaining with the apoptotic marker M30; staining was quantified as described in MATERIALS AND METHODS. Note that the increase of M30 staining in Clone I cells treated with CdCl2 is reduced by SB203580, whereas only a minor apoptotic effect was detected in PC-3U cells after 24 h treatment with CdCl2.
To further investigate the importance of Smad7 for the activation of the TAK1-MKK3/6-p38 MAP kinase pathway, we performed in vitro kinase assays. Endogenous TAK1 was immunoprecipitated from PC-3U and Clone I cells, untreated or treated with 1.0 μM CdCl2 for 12 h, and His-MKK6 was used as a substrate. Ectopical expression of Smad7 resulted in an almost twofold increase of phosphorylation of MKK6 in TAK1 immunoprecipitates, as shown in Figure 3D.
Immunofluorescence analysis of phosphorylated p38 in cells overexpressing Smad7 demonstrated a strong nuclear staining of phosphorylated p38, as well as of Flag-Smad7 (Figure 3E).
When Smad7 expression was induced by incubation of Clone I cells with CdCl2, the apoptotic rate was increased as shown by a DNA-fragmentation assay. Interestingly, simultaneous treatment of cells with SB203580 inhibited Smad7-induced apoptosis (Figure 3F). This inhibitory effect of SB203580 on Smad7-induced apoptosis was confirmed using a Mitosensor assay (our unpublished data), by counting the number of TUNEL-positive apoptotic cells, and by counting the percentage of fragmented nuclei, as visualized by DAPI staining (Figure 3G and Table 1). In addition, we used the M30 antibody as a marker for apoptotic cells, and again confirmed an increase of the number of apoptotic cells in Smad7-overexpressing cells, an effect that was prevented by SB203580. Similar results were obtained using another Smad7-overexpressing clone (Clone K; our unpublished data). Treatment of control PC-3U cells with CdCl2 for 24 h caused a minor increase in the number of apoptotic cells, which probably is due to a response to stress (Table 1 and Figure 3H). Thus, our data suggest that Smad7-induced apoptosis is mediated by activation of p38 in PC-3U cells.
Table 1.
Effect of the p38 inhibitor SB203580 on Smad7-induced apoptosis
| Cells | Treatmenta | TUNEL-positive cells/fieldb | DAPI (%)b |
|---|---|---|---|
| PC-3U | None | 0.1 | 0.3 |
| CdCl2 | 2.0 | 0.9 | |
| CdCl2 + SB | 0.2 | 0.6 | |
| SB | 0.4 | 0.5 | |
| Clone I | None | 1.8 | 3.7 |
| CdCl2 | 7.5 | 25.0 | |
| CdCl2 + SB | 1.0 | 0.8 | |
| SB | 0.8 | 0.8 |
CdCl2, 1.0 μM CdCl2, 24 h; SB, 10 μM SB203580, 24 h.
The number of TUNEL-positive cells were counted in 10 different and randomly chosen fields and the mean number is presented. Nuclei were stained with DAPI 1000 cells were counted in each group. Fragmented and isolated condensed nuclei were counted as apoptotic cells. Investigations were performed using a Zeiss microscope (40× magnification).
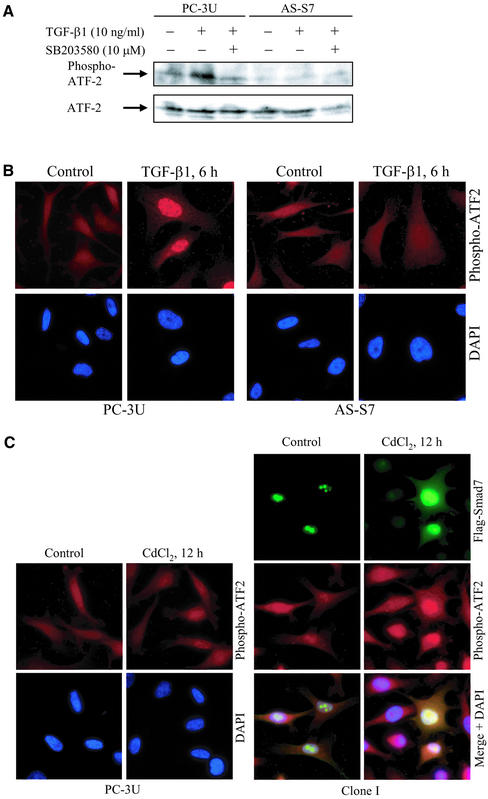
Smad7 Is Important for TGF-β1–induced Phosphorylation of ATF-2
We next studied whether the p38 substrate ATF-2 (Gupta et al., 1995; Livingstone et al., 1995; van Dam et al., 1995; Sano et al., 1999) was affected by TGF-β1 treatment or expression of Smad7. TGF-β1 treatment of PC-3U cells for 5 min up to 24 h resulted in a considerable enhancement of the level of ATF-2 phosphorylated at Thr69 and Thr71 (phospho-ATF-2), as detected by phospho-ATF-2–specific antibody in immunoblots. The increase was detected after 30 min of TGF-β stimulation and remained increased up to 24 h (Figure 4A; our unpublished data). When the p38 inhibitor SB203580 was added, the phosphorylation of ATF-2 decreased, consistent with the report from Sano et al. (1999), which showed that 1.0 μM–10 μM SB203580 blocked TGF-β–induced phosphorylation of ATF-2. No phosphorylation of ATF-2 was detected in AS-S7 cells 6 h after TGF-β treatment (Figure 4A). TGF-β treatment for 6 h of PC-3U cells resulted in accumulation of phospho-ATF-2 in the nucleus, whereas no such response was observed in the AS-S7 cells as investigated by immunofluorescence (Figure 4B). When the Smad7-overexpressing cells (Clone I) were treated with CdCl2 for 12 h to induce Flag-Smad7 expression, immunofluorescence stainings with the phospho-ATF-2–specific antibody revealed an increased intensity of phospho-ATF-2, which was found to colocalize with Flag-Smad7 in the nucleus. Treatment of PC-3U cells for 12 h with CdCl2 did not affect the immunofluorescence staining for phospho-ATF-2 (Figure 4C). These data suggest that phosphorylation of ATF-2 by p38 in TGF-β–treated cells is dependent on the expression of Smad7.
Figure 4.
Smad7 is necessary for TGF-β–induced phosphorylation of ATF-2. (A) PC-3U and AS-S7 cells were treated or not with TGF-β for 6 h in the absence or presence of SB203580. Total cell lysates were then prepared and subjected to immunoblotting, by using antibodies against ATF-2 phosphorylated at Thr69 and Thr71 (phospho-ATF-2) (top) or total ATF-2 (bottom). (B) Immunofluorescence investigations of phospho-ATF-2 in PC-3U and AS-S7 cells treated with TGF-β1 for 6 h. Note the lack of staining for phospho-ATF-2 in AS-S7 cells compared with control PC-3U cells. (C) Coimmunofluorescence stainings of Flag-Smad7 and phospho-ATF-2 in Clone I cells treated for 12 h with CdCl2. An overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI) shows colocalization of Smad7 and phospho-ATF-2 in the nucleus. Immunofluorescence stainings of phospho-ATF-2 in PC-3U cells untreated or treated for 12 h with CdCl2 are shown as control. Immunofluorescence staining with Flag antibodies of PC-3U cells treated for 12 h with CdCl2 as control for Flag, is shown in Figure 3E.
TAK1 Enhances TGF-β1–induced Phosphorylation of p38 and Apoptosis
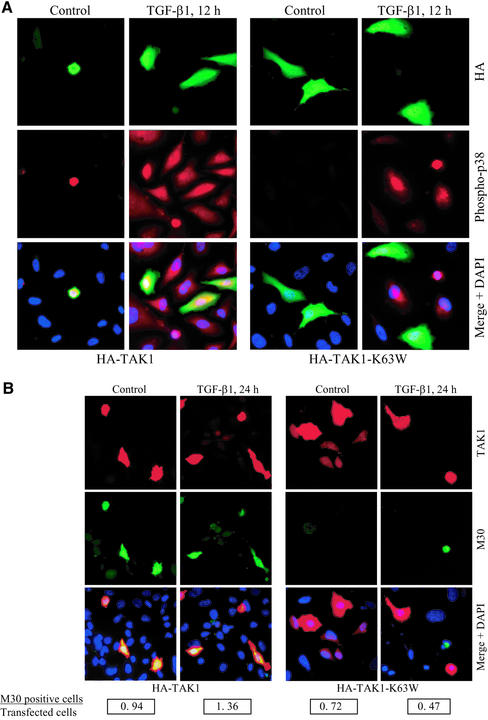
TAK1 is a member of the MAP kinase kinase kinase family previously implicated in TGF-β signaling (Yamaguchi et al., 1995). Recently, TAK1 was shown to be a mediator of TGF-β1–induced phosphorylation of ATF-2 via the p38 MAPK pathway (Hanafusa et al., 1999; Sano et al., 1999). Therefore, we examined whether TAK1 contributes to the TGF-β–induced phosphorylation of p38 and apoptosis in PC-3U cells. PC-3U cells were transiently transfected with wild-type HA-TAK1 and then treated with TGF-β1 for 24 h. By immunofluorescence stainings, we observed a colocalization of phosphorylated p38 and HA-TAK1. In contrast, when PC-3U cells were transiently transfected with the kinase inactive form of TAK1, in which Lys63 of the ATP-binding site was replaced by tryptophan (HA-TAK1-K63W), a reduction of TGF-β–induced phosphorylation of p38 was seen (Figure 5A). Moreover, in PC-3U cells transfected with wild-type TAK1 we observed an increased number of apoptotic cells 24 h after TGF-β treatment compared with nontransfected cells as determined by M30 staining, whereas in HA-TAK1-K63W–transfected cells a decrease in the number of apoptotic cells was observed (Figure 5B). The number of cells expressing high levels of TAK1 (wild-type or K63W) was detected by coimmunofluorescence stainings, by using a polyclonal antibody against TAK1. The rate of apoptosis was determined by screening at least 1000 cells from randomly chosen fields at 40× magnification. The ratio between M30-positive cells and transfected cells expressing high levels of TAK1, is shown in Figure 5B. These results show that expression of wild-type TAK1 caused cells to undergo apoptosis already in the absence of TGF-β and enhanced the TGF-β–induced apoptotic response, whereas the cells expressing the kinase inactive TAK1, were protected from TGF-β–induced apoptosis. From these results, we conclude that ectopic expression of wild-type TAK1 in PC-3U cells, enhances TGF-β–induced phosphorylation of p38 and apoptosis, whereas ectopic expression of kinase-inactive TAK1, decreases the TGF-β–activated phosphorylation of p38 and subsequent apoptosis.
Figure 5.
TAK1 enhances TGF-β1–induced phosphorylation of p38 and apoptosis in PC-3U cells. PC-3U cells were transfected with either wild-type HA-TAK1 or kinase inactive HA-TAK1-K63W and treated with TGF-β1 for 12 or 24 h. (A) Coimmunofluorescence stainings for HA, detected by fluorescein isothiocyanate and phosphorylated p38 (phospho-p38) by tetramethylrhodamine B isothiocyanate. An overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI) reveals a high degree of colocalization of HA-TAK1 and phospho-p38 in PC-3U cells expressing wild-type HA-TAK1, both before and after TGF-β treatment for 12 h. In contrast, PC-3U cells expressing HA-TAK1-K63W, do not express phospho-p38 after TGF-β1 treatment, as shown in the overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI). (B) Coimmunofluorescence stainings for endogenous TAK1 (by using polyclonal antibodies against TAK1) as shown by tetramethylrhodamine B isothiocyanate and the apoptotic marker M30, as shown by fluorescein isothiocyanate, was used to identify apoptotic cells after TGF-β treatment for 24 h. An overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI) reveals a high degree of colocalization of TAK1 and M30, in PC-3U cells expressing wild-type HA-TAK1. In contrast, in PC-3U cells expressing HA-TAK1-K63W, no colocalization of mutant TAK1 and M30 was observed, as shown in the overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI). The number of transfected cells, expressing high levels of TAK1, and the number of M30-positive cells, were counted in several, randomly chosen fields. At total of at least 1000 cells was counted. The ratio between M30-positive cells and transfected cells is shown below the picture.
MKK3 Enhances TGF-β1–induced Phosphorylation of p38 and Apoptosis
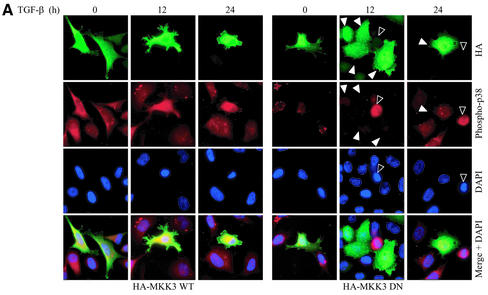
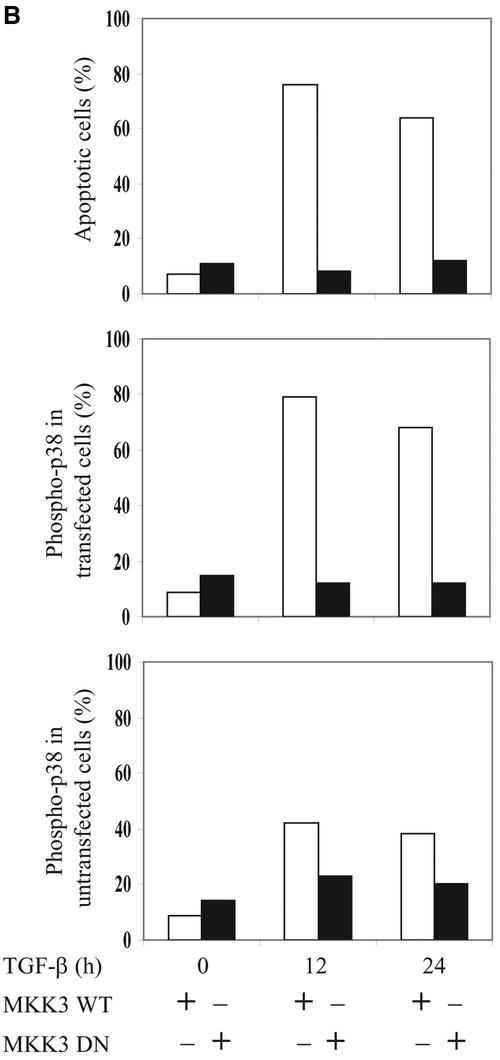
MKK3 and MKK6 have been shown to specifically phosphorylate and activate p38 but not JNK. Therefore, we examined whether MKK3 also contributes to the TGF-β–induced phosphorylation of p38 and apoptosis in PC-3U cells. PC-3U cells were transiently transfected with wild-type HA-MKK3 and then treated with TGF-β1 for 12 or 24 h. By immunofluorescence stainings, we observed an increase of cells positive for phosphorylated p38 and with fragmented nuclei as sign of apoptosis, already without TGF-β treatment (Figure 6A). In contrast, when PC-3U cells were transiently transfected with the dominant negative MKK3, a reduction of TGF-β–induced phosphorylation of p38 and apoptosis was seen (Figure 6A). Phospho-p38–positive cells and apoptotic cells, identified as pycnotic or fragmented nuclei, were counted in PC-3U cells transfected with either wild-type or dominant negative MKK3, and the mean values are presented in Figure 6B. In conclusion, in PC-3U cells transfected with wild-type MKK3, we observed an increased number of phospho-p38–positive and apoptotic cells 12 and 24 h after treatment, whereas PC-3U cells transiently transfected with DN MKK3 were protected against TGF-β–induced phosphorylation of p38 and apoptosis.
Figure 6.
MKK3 enhances TGF-β1–induced phosphorylation of p38 and apoptosis in PC-3U cells. PC-3U cells were transfected with either wild-type HA-MKK3 or DN HA-MKK3 and treated with TGF-β1 for 12 or 24 h. (A) Coimmunofluorescence stainings for HA, detected by fluorescein isothiocyanate and phospho-p38 by tetramethylrhodamine B isothiocyanate. An overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI) reveals a high degree of colocalization of wild-type HA-MKK3 and phospho-p38 in PC-3U cells expressing wild-type HA-MKK3 both before and after TGF-β treatment for 12 h and 24 h. In contrast, in PC-3U cells expressing DN HA-MKK3 indicated by arrowheads, no colocalization of mutant MKK3 and phospho-p38, indicated by an open arrowhead, is observed, as shown in the overlay of both pictures with additional stainings of nuclei with DAPI (merge + DAPI). The number of transfected cells, expressing high levels of MKK3, the number of phospho-p38-positive cells and apoptotic nuclei indicated by an open arrowhead (identified by DAPI), were counted in several, randomly chosen fields. A total of at least 500 cells was counted. The percentage of MKK3-expressing cells, positively stained for HA and phospho-p38 and apoptotic cells are shown in Figure 6B.
Smad7 Interacts with p38, MKK3 and TAK1
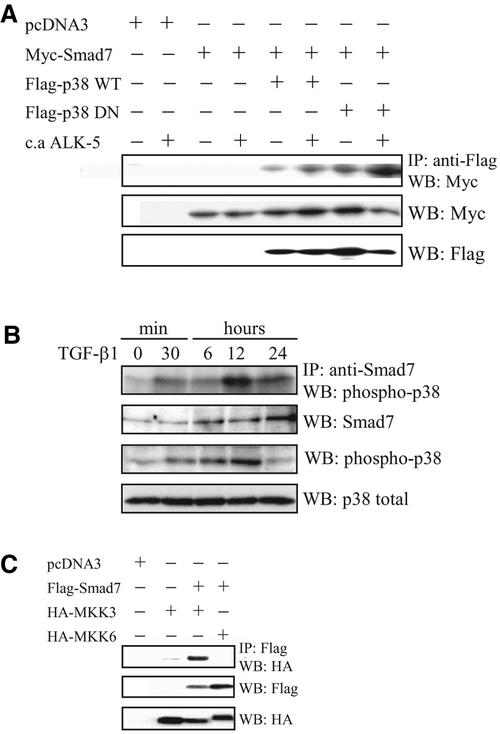
The observations that Flag-Smad7 and phosphorylated p38 colocalized in the nucleus of Clone I cells and that Smad7 seems to be required for TGF-β1–induced activation of the p38 MAP kinase pathway, tempted us to investigate whether Smad7 physically interacts with p38. In transiently transfected COS1 cells, coimmunoprecipitations of Myc-Smad7 with both wild-type and dominant negative Flag-p38 were detected (Figure 7A). The other inhibitory Smad, Smad6, was also found to coimmunoprecipitate with p38 upon transient transfections in COS1 cells (our unpublished data). We thus conclude that p38 can interact with Smad7 in a manner that is not dependent on its phosphorylation status.
Figure 7.
Interaction between Smad7, p38, and MKK3/6 in vivo. (A) Smad7 binds to p38 in vivo. COS1 cells were transfected with Myc-tagged Smad7 alone or together with wild-type (WT) or DN Flag-tagged p38, with or without constitutively active TGF-β type I receptor (c.a. ALK-5). Lysates were immunoprecipitated with anti-Flag antibodies and the immunoblots were incubated with antibodies specific for Myc or Flag. (B) Endogenous Smad7 binds to phosphorylated p38 in vivo. PC-3U cells were treated or not with TGF-β1 for the indicated time periods. Cell lysates were immunoprecipitated with anti-Smad7 antibodies and the immunoblots were incubated with antibodies specific for phospho-p38. Immunoblots from total cell lysates were incubated with antibodies specific for Smad7, phosphorylated p38, and p38. Smad7 binds to p38 in vivo. (C) COS1 cells were transfected with Flag-tagged Smad7 alone or together with wild-type HA MKK3 or HA-MKK6. Lysates were immunoprecipitated with anti-Flag antibodies and the immunoblots were probed with antibodies specific for HA. Immunoblots from total cell lysates were incubated with antibodies specific for HA and Flag. A clear MKK3 band is seen; upon prolonged exposure, a faint, specific MKK6 band is also seen.
We next investigated whether endogenous p38 coimmunoprecipitates with endogenous Smad7 in cell lysates from TGF-β1–stimulated PC-3U cells. When lysates from cells stimulated with TGF-β for different time periods were subjected to immunoprecipitation with a Smad7 antiserum followed by immunoblotting with phospho-p38 antibodies (Figure 7B), a band corresponding to p38 was detected. The same result was obtained by using another Smad7 antibody (our unpublished data). Maximal interaction was seen after 30 min and after 12 h of stimulation with TGF-β, which is consistent with the observation that TGF-β stimulation gives two waves of p38 phosphorylation (Figure 1). Immunoblotting of whole cell lysates with Smad7 antibodies shows that TGF-β treatment increases the Smad7 protein in PC-3U cells (Figure 6B), in agreement with our previous findings (Landström et al., 2000).
Because the p38 MAP kinase pathway is known to be activated by the MAP kinases MKK3 and MKK6, we also examined whether Smad7 interacts with MKK3 and/or MKK6. In transiently transfected COS1 cells, coimmunoprecipitations of Flag-Smad7 with MKK3, and less efficiently with MKK6, were detected (Figure 7C).
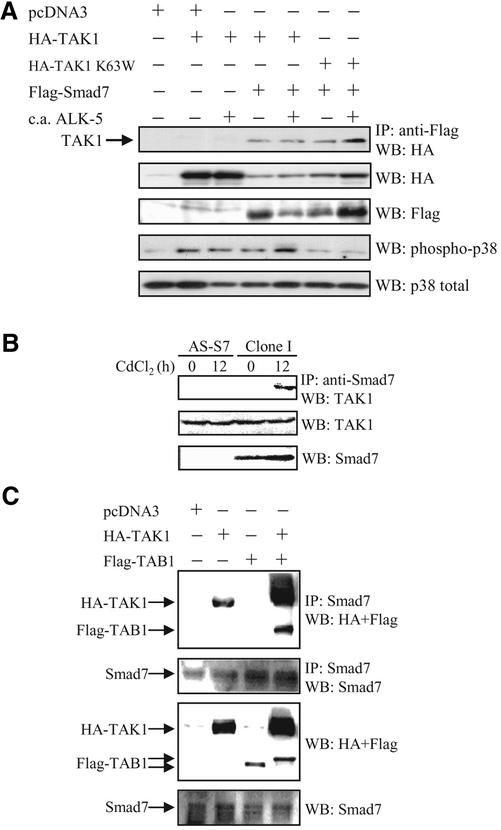
To explore a possible mechanism whereby Smad7 activates p38, we investigated whether Smad7 physically interacts with TAK1. In transiently transfected COS1 cells, Flag-Smad7 was coimmunoprecipitated with wild-type as well as kinase inactive HA-TAK1 (K63W; Figure 8). The interaction between Smad7 and TAK1 did not show any clear dependence on the presence of an activated TGF-β type I receptor (constitutively active [c.a.] ALK-5). When both Flag-Smad7 and Myc-Smad6 were cotransfected with HA-TAK1, the interaction between Flag-Smad7 and HA-TAK1 decreased, indicating that Smad6 and Smad7 may compete for binding to TAK1 (our unpublished data). In COS1 cells transfected with wild-type TAK1, alone or together with Smad7 and in the absence or presence of c.a. ALK-5, a band corresponding to phosphorylated p38 was detected. There was a slight increase of the level of phosphorylated p38, when wild-type TAK1, Smad7 and the c.a. ALK-5 were cotransfected, suggesting a synergistic effect of TAK1 and Smad7, in the presence of the ligand. However, when the kinase inactive TAK1-K63W was cotransfected with Smad7, the level of phosphorylated p38 was clearly reduced (Figure 8A). When lysates from Clone I cells stimulated with CdCl2 for 12 h to induce Smad7 were subjected to immunoprecipitation with a Smad7 antiserum followed by immunoblotting with TAK1 antibodies, a band corresponding to TAK1 was observed, whereas no TAK1 band was seen in corresponding immunoprecipitates from AS-S7 cells (Figure 8B). Immunoblotting of whole cell lysates with Smad7 antibodies shows that CdCl2 treatment increases the Smad7 protein in Clone I cells (Figure 8B), whereas the endogenous levels of TAK1 were similar in AS-S7 and Clone I cells independent of treatment with CdCl2.
Figure 8.
Smad7 interacts with TAK1 in vivo. (A) COS1 cells were transfected with Flag-tagged Smad7 alone or together with wild-type TAK1 or kinase inactive (K63W) HA-tagged TAK1, with or without constitutively active TGF-β type I receptor (c.a. ALK-5). Lysates were immunoprecipitated with anti-Flag antibodies and the immunoblots were probed with antibodies specific for HA. Immunoblots from total cell lysates were incubated with antibodies specific for HA, Flag, phosphorylated p38, and p38. (B) Antisense Smad7 PC-3U cells (AS-S7) cells and PC-3U cells stably transfected with pMEP-4 F-Smad7 (Clone I cells), treated or not with CdCl2 for 12 h to induce Smad7 expression in Clone I cells. Lysates were immunoprecipitated with anti-Smad7 antibodies and the immunoblots were probed with antibodies specific for TAK1. Immunoblots from total cell lysates were incubated with antibodies specific for Smad7 and TAK1. (C) PC-3U cells were transiently transfected with HA-TAK1, Flag-TAB1, either alone or together. Lysates were immunoprecipitated with anti-Smad7 antibodies and the immunoblots were probed with antibodies specific for HA and Flag. Immunoblots from total cell lysates were incubated with antibodies specific for Smad7, Flag, and HA.
TAK1-binding protein 1 (TAB1) binds and activates TAK1 (Shibuya et al., 1996). Therefore, we investigated whether TAB1 also interacts with Smad7. PC-3U cells were transiently transfected with a control vector, or vectors for HA-TAK1, Flag-TAB1, and HA-TAK1 together with Flag-TAB1; a polyclonal Smad7 antibody was used for immunoprecipitation. HA-TAK1, but not Flag-TAB1, was coimmunoprecipitated with endogenous Smad7 in PC-3U cells; however, an increased interaction between Smad7 and TAK1 was observed in the presence of ectopically expressed TAB1 (Figure 8C). Immunoblotting of whole cell lysates with antibodies against Smad7, Flag, and HA showed the expression of endogenous Smad7 protein and ectopically expressed HA-TAK1 and Flag-TAB1 (Figure 8C).
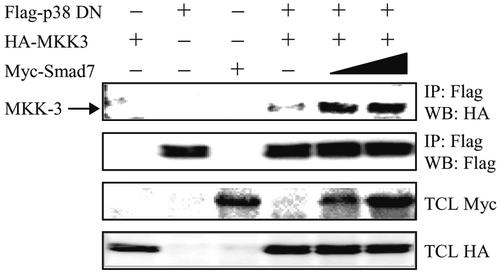
To investigate whether Smad7 can act as a scaffolding protein for MKK3 and p38, we ectopically overexpressed in COS1 cells wild-type HA-MKK3 and dominant negative (DN) Flag-p38 together with increasing amounts of Myc-Smad7. DN Flag-p38 was used because we, in initial experiments, observed massive cell death when wild-type Flag-p38 was cotransfected with Myc-Smad7 in COS1 cells. Expression of Myc-Smad7 led to enhanced coimmunoprecipitation of HA-MKK3 with DN Flag-p38 (Figure 9). We therefore conclude that Smad7 enhances the interaction between HA-MKK3 and Flag-p38 DN, which supports the notion that Smad7 can act as a scaffolding protein, facilitating the activation of the p38 MAP kinase pathway.
Figure 9.
Expression of Smad7 facilitates the interaction between MKK3 and p38 MAP kinase. COS1 cells were transfected with wild-type HA-MKK3, DN Flag-tagged p38, and Myc-tagged Smad7 either alone or together. Increasing amount of Myc-tagged Smad7 (0.5 or 1.5 μg) was used for cotransfections. Lysates were immunoprecipitated with anti-Flag M2 antibodies and the immunoblots were incubated with antibodies specific for HA and Flag. Immunoblots from total cell lysates were incubated with antibodies specific for HA and Myc.
DISCUSSION
TGF-β and Smads are important regulators of cell fate and apoptosis in different cell types, although the molecular mechanisms involved are not well understood. In addition to activation of Smad pathways, TGF-β stimulation leads to activation of ERK, p38, and SAPK/JNK MAP kinase pathways in certain cell types (Attisano and Wrana, 2000; Massagué, 2000; Miyazono et al., 2000). In the present study, we demonstrate that the specific activation of the p38 MAP kinase pathway is essential for TGF-β1– and Smad7-induced apoptosis in human prostate cancer cells.
TGF-β1 treatment of PC-3U cells specifically activated the TAK1-MKK3-p38 pathway, whereas no effect on the constitutively phosphorylated ERK, or any activation of SAPK/JNK phosphorylation, was observed. The TGF-β–induced phosphorylation of p38 proceeded the onset of apoptosis, and the p38-kinase inhibitor SB203580 significantly reduced the TGF-β–induced apoptotic response in PC-3U cells. Moreover, transient transfection of dominant negative p38 or dominant negative MKK3 protected the PC-3U cells from TGF-β–induced apoptosis. We thus conclude that TGF-β–induced p38 activation mediates an apoptotic response in PC-3U cells. In PC-3U cells stably expressing antisense Smad7, TGF-β-treatment did not cause p38 activation or phosphorylation of MKK3 at any time point investigated, whereas Smad7 overexpression caused an activation of the p38 MAP kinase pathway, indicating that Smad7 plays an important role in TGF-β–induced activation of p38 MAP kinase and apoptosis.
In our study, we found that Smad7 colocalizes with phosphorylated p38 and ATF-2 in the nucleus of Smad7-overexpressing cells. ATF-2 is a known nuclear substrate for p38; it is a basic region-leucine zipper transcription factor that can mediate a diverse range of transcriptional responses, including those generated by various forms of cellular stress (Davis, 2000). Activation of ATF-2 in response to stress stimuli occurs through posttranslational modifications, in particular phosphorylation of Thr69 and Thr71 (Gupta et al., 1995). Recently, it was reported that ATF-2 is phosphorylated after TGF-β1 stimulation, in a TAK1- and p38-dependent manner (Sano et al., 1999). Moreover, Smad3 and Smad4 were reported to interact with ATF-2 in transiently transfected 293 and COS cells, and to act in synergy with TAK1, MKK6, and ATF-2 in transcriptional assays (Hanafusa et al., 1999; Sano et al., 1999). In neuronal cells, phosphorylation of both c-Jun and ATF-2 has been shown to correlate with apoptosis induced by stress-inducing factors such as ischemia or treatment with cyclosporine A (Walton et al., 1998; Pyrzynska et al., 2000). It is an interesting possibility, which remains to be elucidated, that Smad7 together with p38 and ATF-2 initiate apoptosis by regulating the expression of proapoptotic or antiapoptotic proteins. Of interest in this context is the observation that the expression of the Fas ligand has been shown to be induced by overexpressed p38 in activated T cells (Hsu et al., 1999).
Smad7 has recently been shown to induce apoptosis by activation of the JNK pathway in other epithelial cells such as mink lung epithelial (Mv1Lu) and MDCK cells (Mazars et al., 2001). Together with our observation that Smad7 activates p38 in a prostate cancer cell line, this suggests that TGF-β1 through Smad7 can activate the p38 and/or JNK MAP kinase pathways in several epithelial cells and mediate an apoptotic response. Also in the human embryonal kidney cell line 293, TGF-β1 stimulation or overexpression of Smad7 leads to phosphorylation of both p38 and JNK, and apoptosis (our unpublished data), supporting the notion that Smad7 and p38 can have a general proapoptotic effect in epithelial cells. This finding suggests different functions of the I-Smads, because Smad6 has recently been reported to inhibit BMP/TAK1-induced phosphorylation of p38 and apoptosis in a mouse hybridoma cell line, MH60 cells (Kimura et al., 2000). Recently, both Smad6 and Smad7 were found to be able to interact with TAB1 (Yanagisawa et al., 2001), an adaptor protein that is known to activate TAK1 (Shibuya et al., 1996). We observed that Smad6 competed with Smad7 for binding to TAK1 (our unpublished data). We did not detect any interaction between Smad7 and TAB1 in PC-3U cells; however, TAB1 was found to increase the interaction between TAK1 and Smad7. Further studies are required to elucidate the interactions of Smad6 and Smad7 with TAK1 and TAB1, and to determine whether Smad6 and Smad7 have different effects on the TAK1-p38 MAP kinase pathway.
Our observations indicate a role for TAK1 in Smad7-mediated activation of p38 in TGF-β–stimulated cells. The mechanism whereby TGF-β stimulation leads to activation of TAK1 kinase and the role of Smad7 in this process remains to be elucidated. We report herein that Smad7 can interact with TAK1, MKK3 as well as p38, which thereby could bring the upstream kinase TAK1 together with MKK3 and p38. In pull down experiments, we have observed that the interaction of both TAK1 and p38 occurs with the N-terminal part of Smad7 (our unpublished data). Our finding that ectopic Smad7 expression facilitates the interaction between HA-MKK3 and dominant negative Flag-p38, observed by coimmunopreciptations in transiently transfected COS1 cells, favor the idea that Smad7 can act as a scaffolding protein in the activation of the p38 MAP kinase pathway. However, further studies are needed in order to investigate the kinetics of interaction within this complex, and also where the complex is localized. Smad7 is predominantly localized in the nucleus, but shuttle between the nucleus and the cytoplasm, e.g., in response to TGF-β treatment. Moreover, it is not known how the TGF-β signal causes activation of the TAK1 kinase. Because Smad7 can interact with the activated TGF-β receptor complex, it is possible that Smad7 acts as an adaptor protein, bridging between the activated receptor and TAK1.
It will be interesting to investigate whether Smad7 also interacts with JNK, because TAK1 is known to activate both JNK and p38 MAP kinase pathways. TAK1 has been shown to significantly contribute to an apoptotic signal in vivo through activation of JNK in the retina of D. melanogaster (Takatsu et al., 2000). In Xenopus, ectopic expression of TAK1 has been demonstrated to induce apoptosis in early embryos (Shibuya et al., 1998). In transgenic mice expressing an activating mutation of TAK1 in the heart, apoptosis was induced by activation of p38, in response to mechanistic overload (Zhang and Derynck, 2000). These reports suggest that activation of the TAK1 pathway plays an important role also for apoptosis in vivo.
In conclusion, we have shown that TGF-β1 stimulation causes phosphorylation and activation of p38, in a Smad7-, MKK3-, and TAK1-dependent manner, resulting in apoptosis. The activation of p38 occurs in two waves: the first, within 5 min after TGF-β stimulation, probably involves a low basal level of Smad7 present in resting cells, whereas the second, occurring 12 h after TGF-β stimulation, coincides with the increase in Smad7 levels seen in response to TGF-β stimulation. The observed interactions between Smad7 and the TAK1, MKK3, and p38 kinases, in addition to the finding that ectopic expression of Smad7 enhances the interaction between MKK-3 and the p38 MAP kinase, suggests that Smad7 can act as a scaffolding protein. An important goal for our future studies is to further investigate how Smad7 activates the TGF-β type I receptor-TAK1-MKK3-p38 pathway and to identify genes transcriptionally regulated by this pathway.
ACKNOWLEDGMENTS
We thank J. Han (The Scripps Research Institute, La Jolla, CA) for the Flag-p38, HA-MKK3, and HA-MKK6 (wild-type and dominant negative) cDNA expression constructs; K. Matsumoto and J. Ninomiya-Tsuji (Department of Molecular Biology, Graduate School of Science, Nagoya University, Chikusa-ku, Nagoya, Japan) for different cDNA-constructs for TAK1 and His-MKK6; N. Ferrara (Genentech for TGF-β1; and L. Rönnstrand at our institute for phospho-ERK1/2 and ERK2 antibodies. We also thank Aris Moustakas for providing constructive comments on this manuscript, Marie Andersson for technical assistance, and Ingegärd Schiller for help in the preparation of the manuscript. This work was supported in part by the Swedish Cancer Society (to P.A., M.L., N-E.H.), the Swedish Medical Research Council, Swedish Society of Medicine (to M.L), and the Dutch Cancer Society (NKI 2001-2481; to P.t.D.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–03–0037. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–03–0037.
REFERENCES
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor-β superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrakhte M, Morén A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin C-H, Heldin N-E, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem Biophys Res Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- Atfi A, Buisine M, Mazars A, Gespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-β through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997a;272:24731–24734. doi: 10.1074/jbc.272.40.24731. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997b;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Bai S, Cao X. A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-β signaling. J Biol Chem. 2002;277:4176–4182. doi: 10.1074/jbc.M105105200. [DOI] [PubMed] [Google Scholar]
- Bai S, Shi X, Yang X, Cao X. Smad6 as a transcriptional corepressor. J Biol Chem. 2000;275:8267–8270. doi: 10.1074/jbc.275.12.8267. [DOI] [PubMed] [Google Scholar]
- Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP. A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- Edlund S, Landström M, Heldin C-H, Aspenström P. Transforming growth factor-β-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Eyers PA, Craxton M, Morrice N, Cohen P, Goedert M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem Biol. 1998;5:321–328. doi: 10.1016/s1074-5521(98)90170-3. [DOI] [PubMed] [Google Scholar]
- Franzén P, Ichijo H, Miyazono K. Different signals mediate transforming growth factor-β1-induced growth inhibition and extracellular matrix production in prostatic carcinoma cells. Exp Cell Res. 1993;207:1–7. doi: 10.1006/excr.1993.1156. [DOI] [PubMed] [Google Scholar]
- Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-β signaling. J Cell Biol. 2001;155:1017–1027. doi: 10.1083/jcb.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, et al. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Gavrilin MA, Tsai MH, Han J, Lai MZ. p38 mitogen-activated protein kinase is involved in Fas ligand expression. J Biol Chem. 1999;274:25769–25776. doi: 10.1074/jbc.274.36.25769. [DOI] [PubMed] [Google Scholar]
- Hu PP, Shen X, Huang D, Liu Y, Counter C, Wang XF. The MEK pathway is required for stimulation of p21(WAF1/CIP1) by transforming growth factor-β. J Biol Chem. 1999;274:35381–35387. doi: 10.1074/jbc.274.50.35381. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by A.S.K1, a mammalian M.A.P.K.K.K. that activates S.A.P.K/J.N.K. and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Itoh S, Landström M, Hermansson A, Itoh F, Heldin C-H, Heldin N-E, ten Dijke P. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273:29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1–p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signaling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Mazars A, Prunier C, Bertrand F, Kornprost M, Gallea S, Roman-Roman S, Cherqui G, Atfi A. Smad7 inhibits the survival nuclear factor κB and potentiates apoptosis in epithelial cells. Oncogene. 2001;20:879–884. doi: 10.1038/sj.onc.1204167. [DOI] [PubMed] [Google Scholar]
- Landström M, Heldin N-E, Bu S, Hermansson A, Itoh S, ten Dijke P, Heldin C-H. Smad7 mediates apoptosis induced by transforming growth factor β in prostatic carcinoma cells. Curr Biol. 2000;10:535–538. doi: 10.1016/s0960-9822(00)00470-x. [DOI] [PubMed] [Google Scholar]
- Lee JC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Mazars A, Lallemand F, Prunier C, Marais J, Ferrand N, Pessah M, Cherqui G, Atfi A. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem. 2001;276:36797–36803. doi: 10.1074/jbc.M101672200. [DOI] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Heldin C-H. TGF-β signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- Nakao A, et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Porras A. p38 MAP kinases. beyond the stress response. Trends Biochem Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- Pulaski L, Landström M, Heldin C-H, Souchelnytskyi S. Phosphorylation of Smad7 at Ser-249 does not interfere with its inhibitory role in transforming growth factor-β-dependent signaling but affects Smad7-dependent transcriptional activation. J Biol Chem. 2001;276:14344–14349. doi: 10.1074/jbc.M011019200. [DOI] [PubMed] [Google Scholar]
- Pyrzynska B, Mosieniak G, Kaminska B. Changes of the trans-activating potential of AP-1 transcription factor during cyclosporin A-induced apoptosis of glioma cells are mediated by phosphorylation and alterations of AP-1 composition. J Neurochem. 2000;74:42–51. doi: 10.1046/j.1471-4159.2000.0740042.x. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Shin I, Bakin AV, Rodeck U, Brunet A, Arteaga CL. Transforming growth factor β enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol Biol Cell. 2001;12:3328–3339. doi: 10.1091/mbc.12.11.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantz N, Bourgeade MF, Mouhamad S, Leca G, Sharma S, Vazquez A. p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFβ-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol Biol Cell. 2001;12:3139–3151. doi: 10.1091/mbc.12.10.3139. . 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu Y, Nakamura M, Stapleton M, Danos MC, Matsumoto K, O'Connor MB, Shibuya H, Ueno N. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol Cell Biol. 2000;20:3015–3026. doi: 10.1128/mcb.20.9.3015-3026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massagu J. Inhibition of transforming growth factor-β/SMAD signaling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M, Woodgate AM, Sirimanne E, Gluckman P, Dragunow M. ATF-2 phosphorylation in apoptotic neuronal death. Brain Res Mol Brain Res. 1998;63:198–204. doi: 10.1016/s0169-328x(98)00275-7. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou G, Hu MC-T, Yao Z, Tan T-H. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor β (TGF-β)-activated kinase (TAK1), a kinase mediator of TGF β signal transduction. J Biol Chem. 1997;272:22771–22775. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276:14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Yamada M, Ishikawa Y, Matsumoto T, Ikeuchi T, Hatanaka H. p38 mitogen-activated protein kinase regulates low potassium-induced c-Jun phosphorylation, and apoptosis in cultured cerebellar granule neurons. J Biol Chem. 2001;276:5129–5133. doi: 10.1074/jbc.M007258200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takeda K, Ochiai W, Takizawa T, Ueno M, Takizawa M, Shibuya H, Taga T. Inhibition of BMP2-induced, TAK1 kinase-mediated neurite outgrowth by Smad6 and Smad7. Genes Cells. 2001;6:1091–1099. doi: 10.1046/j.1365-2443.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Young PR, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor β 1 production in a Smad-dependent pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Derynck R. Transcriptional regulation of the transforming growth factor-β-inducible mouse germ line Ig α constant region gene by functional cooperation of Smad, CREB and AML family members. J Biol Chem. 2000;275:16979–16985. doi: 10.1074/jbc.M001526200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]