Abstract
Bone morphogenetic protein 2 (BMP2) promotes the differentiation of undifferentiated mesenchymal cells into adipocytes. To investigate the molecular mechanisms that regulate this differentiation process, we studied the relationship between BMP2 signaling and peroxisome proliferator-activating receptor γ (PPARγ) during adipogenesis of mesenchymal cells by using pluripotent mesenchymal cell line C3H10T1/2. In C3H10T1/2 cells, BMP2 induced expression of PPARγ along with adipogenesis. Overexpression of Smad6, a natural antagonist for Smad1, blocked PPARγ expression and adipocytic differentiation induced by BMP2. Overexpression of dominant-negative PPARγ also diminished adipocytic differentiation of C3H10T1/2 cells, suggesting the central role of PPARγ in BMP2-induced adipocytic differentiation. Specific inhibitors for p38 kinase inhibited BMP2-induced adipocytic differentiation and transcriptional activation of PPARγ, whereas overexpression of Smad6 had no effect on transcriptional activity of PPARγ. Furthermore, activation of p38 kinase by overexpression of TAK1 and TAB1, without affecting PPARγ expression, led the up-regulation of transcriptional activity of PPARγ. These results suggest that both Smad and p38 kinase signaling are concomitantly activated and responsible for BMP2-induced adipocytic differentiation by inducing and up-regulating PPARγ, respectively. Thus, BMP2 controls adipocytic differentiation by using two distinct signaling pathways that play differential roles in this process in C3H10T1/2 cells.
INTRODUCTION
Adipocytes play a major role in controlling lipid homeostasis and energy balance. Adipocytes derive from undifferentiated mesenchymal cells that have the capacity to differentiate into myoblasts, osteoblasts, and chondrocytes as well as adipocytes (Prockop, 1997; Pittenger et al., 1999). A variety of hormones, growth factors, and cytokines are involved in this differentiation program of adipocytes (Spiegelman, 1998; Rosen and Spiegelman, 2000). Among these factors, bone morphogenetic protein 2 (BMP2), a member of the transforming growth factor-β (TGF-β) superfamily that is implicated in embryogenesis, organogenesis, and morphogenesis by controlling differentiation of variety types of cells (Kingsley, 1994; Hogan, 1996), promotes adipocytic differentiation of undifferentiated mesenchymal cells (Ahrens et al., 1993; Ji et al., 2000; Sottile and Seuwen, 2000). However, it is unknown how BMP2 promotes differentiation of mesenchymal cells into adipocytes.
BMP2 exhibits its biological effects through the sequential activation of two types of transmembrane receptors, namely, type I (BMPIR) and type II (BMPIIR), which posses intrinsic serine/threonine kinase activity (Heldin et al., 1997; Massague and Wotton, 2000). After forming heterocomplex between BMPIR and BMPIIR, activated BMPIR phosphorylates and activates Smad1 and Smad5, which subsequently associate with Smad4, a common partner for receptor-regulated Smads. These complexes then relocate to the nucleus and regulate the expression of target genes, leading to an elicitation of biological effects of BMP2 (Heldin et al., 1997; Massague and Wotton, 2000).
In addition to Smad signaling pathway, a mitogen-activated protein (MAP) kinase, p38 kinase, which is involved in growth and differentiation of many types of cells, is also activated by BMP2 (Iwasaki et al., 1999; Kimura et al., 2000). In some types of cells, BMP2 activates MAP kinase kinases kinase, including TAK1, and consequently the MAP kinase kinases kinase elicits MKK3 or MKK6 that directly phosphorylates and activates p38 kinase (Raingeaud et al., 1996; Shibuya et al., 1996, 1998; Davis, 2000; Kimura et al., 2000). The phosphorylated p38 kinase, in turn, controls gene expression in the nucleus. Although p38 kinase has been described to be implicated in regulation of adipogenesis (Engelman et al., 1998, 1999), the precise role of p38 kinase in adipogenesis remains elusive.
A nuclear hormone receptor, peroxisome proliferator-activating receptor γ (PPARγ), plays a central role in differentiation program of adipocytes by controlling the expression of specific genes for adipocytes such as lipoprotein lipase and aP2 through a specific binding element for PPARγ in their promoter regions (Wu et al., 1999; Rosen and Spiegelman, 2000). In fact, overexpression of PPARγ in fibroblasts causes adipocytic differentiation of the cells (Tontonoz et al., 1994). Furthermore, the mice deficient for PPARγ gene showed markedly impaired adipogenesis (Kubota et al., 1999; Rosen et al., 1999). Because BMP2 promotes the adipocytic differentiation of mesenchymal cells, it is possible that the linkage between BMP2 and PPARγ signaling may account for adipogenesis of mesenchymal cells.
To determine the molecular mechanisms by which BMP2 promotes adipocytic differentiation, we investigated the role of Smad signaling and p38 kinase in adipogenesis of undifferentiated mesenchymal cells by using murine pluripotent mesenchymal cell line C3H10T1/2 (Ahrens et al., 1993; Asahina et al., 1996). We found that Smad1 is essential for induction of PPARγ, which is necessary for BMP2-induced adipocytic differentiation. We also shown herein that p38 kinase acts as an activator for PPARγ in C3H10T1/2 cells. Our results indicate that two distinct signaling cascades that are concomitantly elicited by BMP2 control adipocytes differentiation of mesenchymal cells in the different manners.
MATERIALS AND METHODS
Cell, Antibodies, and Reagents
C3H10T1/2 cells were purchased from RIKEN (Saitama, Japan) and were cultured in DMEM containing 10% fetal bovine serum. Anti-PPARγ monoclonal (E-8) and polyclonal antibody (H-100), anti-hemagglutinin polyclonal antibody, anti-Smad1 antibody, and anti-β-actin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p38 kinase and anti-phospho-p38 kinase antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-Myc monoclonal, anti-Flag monoclonal, and anti-phosphoserine polyclonal antibodies were purchased from BD Biosciences PharMingen (San Diego, CA), Kodak Scientific Imaging Systems (New Haven, CT), and Zymed Laboratories (South San Francisco, CA), respectively, and used as described previously (Nishimura et al., 1998). SB203580 and PD169316 were purchased from Calbiochem (San Diego, CA). FR167653 was kindly provided by Fujisawa Pharmaceutical (Osaka, Japan).
Constructs and Transfection
6xMyc-Smad 1 (Imamura et al., 1997), Flag-Smad6 (Imamura et al., 1997), dominant-negative form MKK3 (Enslen et al., 1998), and TAK1 and TAB1 (Shibuya et al., 1996) cDNA were kindly gifted by Drs. Kohei Miyazono, Roger Davis, and Hiroshi Shibuya. Flag-Smad1, Flag-Smad 4, PPARγ expression vector, and PPRE-Luc reporter gene have been described previously (Hu et al., 1996; Nishimura et al., 1998). To generate dominant-negative mutant form of PPARγ (Barroso et al., 1999), proline 467 was point mutated into leucine by performing in vitro mutagenesis, and the sequence of the mutant PPARγ was confirmed by DNA sequence analysis. Transfection of C3H10T1/2 cells was carried out using FuGENE6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol.
Generation of Adenovirus
The recombinant adenovirus carrying wild-type or dominant-negative form of PPARγ, dominant-negative MKK3, or Flag-Smad6 was constructed by homologous recombination between the expression cosmid cassette and the parental virus genome in 293 cells as described previously (Miyake et al., 1996) by using adenovirus construction kit (Takara, Kyoto, Japan). The viruses were confirmed to retain no proliferative activity in the cells other than 293 cells, because of lacking E1A-E1B (Miyake et al., 1996). Titers of the viruses were determined by modified point assay (Miyake et al., 1996).
Oil Red O Staining
C3H10T1/2 cells were washed with phosphate-buffered saline (PBS) and fixed with 10% formalin for 20 min. After washing the cells twice with PBS and once with 60% isopropyl-alcohol, the cells were stained with Oil Red O solution (Sigma-Aldrich, St. Louis, MO). The area of the cells stained with Oil Red O was measured by ImagePro Plus analyzer (Palmerton).
Immunoprecipitation and Immunoblotting
Cells were washed three times with ice-cold buffer PBS and solubilized in lysis buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM sodium orthovanadate). The lysates were centrifuged for 20 min at 4°C, 16,000 × g. The supernatants of the centrifuged cell lysates were recovered, boiled in SDS sample buffer containing 0.5 M β-mercaptoethanol, and used as total cell lysates. For immunoprecipitation, the lysates were incubated with antibodies for 4 h at 4°C, followed by immunoprecipitation with protein A-Sepharose (Zymed Laboratories) or protein G-agarose (Roche Diagnostics). Immunoprecipitates were washed five times with lysis buffer and boiled in SDS sample buffer containing 0.5 M β-mercaptoethanol, and supernatants were recovered as immunoprecipitates sample. These samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with appropriate antibodies. The samples were visualized with horseradish peroxidase coupled to protein A (KPL) or horseradish peroxidase-coupled anti-mouse IgG antibodies (Cappel Laboratories, Durham, NC), and enhanced by enhanced chemiluminescence detection kits (Pharmacia AB, Uppsala, Sweden).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from C3H10T1/2 cells by RNeasy kit (QIAGEN, Valencia, CA). After denaturation of total RNA at 70°C for 10 min, cDNA was synthesized with oligo-dT primer and reverse transcriptase (QIAGEN). PCR amplification was performed by using specific primers for PPARγ (forward primer, 5′-TATGGAGTTCATGCTTGTGA-3′; reverse primer, 5′-CGGGAAGGACTTTATGTATG-3′). PCR products were loaded to agarose gel, and stained with ethidium bromide. After PCR, products were subcloned into TA-cloning vector, and DNA sequences of PCR products were determined.
Transcriptional Activity of PPARγ
Luciferase reporter constructs containing three repeats of PPARγ binding elements (PPRE-Luc) were cotransfected with TK-renilla luciferase construct (Promega, Madison, WI) into C3H10T1/2 cells. Two days after transfection, cells were lysed and luciferase activity was determined using specific substrates in a luminometer (Promega) according to manufacturer's protocol. Transfection efficiency was normalized by determining the activity of renilla luciferase.
Statistical Analysis
All data were analyzed by analysis of variance followed by a paired t test. Values shown are mean ± SD.
RESULTS
BMP2 Promotes Adipocytic Differentiation of C3H10T1/2 Cells by Inducing PPARγ
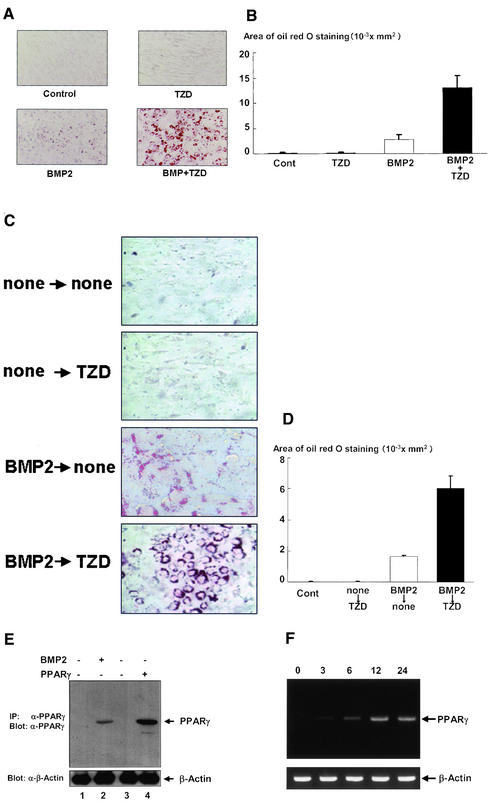
To investigate the relationship between BMP2 signaling and PPARγ during adipocytic differentiation, we first evaluated the effects of BMP2 and a specific ligand for PPARγ, troglitazone (TZD) (Sarraf et al., 1999), on adipocytic differentiation of C3H10T1/2 cells (Ahrens et al., 1993; Asahina et al., 1996). As shown in Figure 1, A and B, BMP2 induced differentiation of C3H10T1/2 cells into adipocytes, whereas TZD had little effect on adipocytic differentiation of C3H10T1/2 cells. Treatment with both BMP2 and TZD more efficiently induced adipocytes differentiation of C3H10T1/2 cells than that with BMP2 alone (Figure 1, A and B). Interestingly, we found that pretreatment with BMP2 is able to trigger the adipogenic effects of TZD on C3H10T1/2 cells (Figure 1, C and D). Collectively, these data suggest that BMP2 induced PPARγ expression, resulting in the induction of adipocytic differentiation in C3H10T1/2 cells. We therefore assessed the role of BMP2 in the regulation of PPARγ expression in C3H10T1/2 cells as determined by Western blotting analysis and RT-PCR experiment. Although expression of PPARγ was not detected in untreated C3H10T1/2 cells, C3H10T1/2 cells treated with BMP2 expressed PPARγ (Figure 1, E and F). The expression of PPARγ was observed 3 h after BMP2 treatment and increased in a time-dependent manner (Figure 1F). The data indicate that BMP2 induced expression of PPARγ in C3H10T1/2 cells.
Figure 1.
BMP2 induced adipogenesis in C3H10T1/2 cells. C3H10T1/2 cells were cultured with BMP2 (300 ng/ml), troglitazone (10−6 M), or both for 10 d. After 10 d, the cells were stained with Oil Red O, photographed under a microscope (A), and the area stained with Oil Red O in the cells was assessed as described in the text (B). (C and D) Treatment of BMP2 initiated the adipogenic effects of troglitazone in C3H10T1/2 cells. C3H10T1/2 cells precultured with or without BMP2 for 3 d (300 ng/ml) were incubated with or without troglitazone (10−6 M) for 4 d. The cells were stained with Oil Red O, photographed under a microscope (C), and the areas stained with Oil Red O in the cells were assessed (D). (E) BMP2 induced expression of PPARγ in C3H10T1/2 cells. C3H10T1/2 cells were untransfected (lanes 1 and 2) or transfected with pcDNA3 (lane 3) or PPARγ expression vector (lane 4). Twelve hours after transfection, the cells were incubated with (lane 2) or without (lanes 1, 3, and 4) BMP2 for 3 d and lysed. The cell lysates were immunoprecipitated with anti-PPARγ polyclonal antibody and immunoblotted with anti-PPARγ mAb (top). The lysates were also determined by immunoblotting with anti-β-actin antibody (bottom). (F) BMP2 induced expression of PPARγ mRNA in a time-dependent manner in C3H10T1/2 cells. Total RNA was isolated from C3H10T1/2 cells that were incubated with or without BMP2 for 3, 6, 12, or 24 h and subjected to RT-PCR experiments by using specific primers for PPARγ (top) or β-actin (bottom).
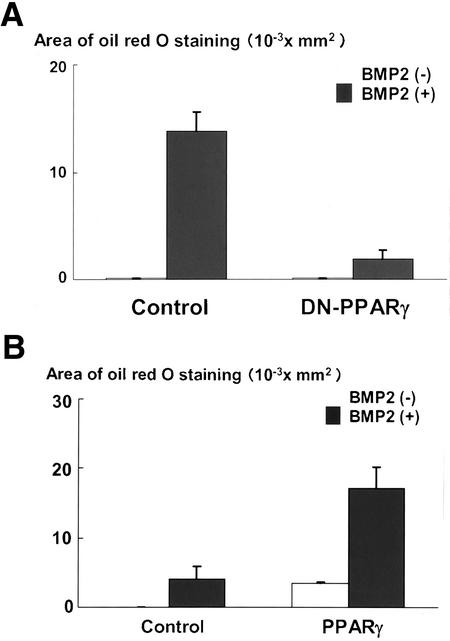
We next asked the importance of PPARγ in BMP2-induced adipocytic differentiation. To address this, we overexpressed a dominant-negative mutant of PPARγ (Barroso et al., 1999) in C3H10T1/2 cells by using adenovirus system and determined its effects on adipocytic differentiation of C3H10T1/2 cells. As shown in Figure 2A, overexpression of dominant-negative PPARγ markedly inhibited adipocytic differentiation induced by BMP2. The data suggest that PPARγ is critical for BMP2 to exhibit the adipogenic effect in C3H10T1/2 cells. To further verify this notion, we examined whether expression of PPARγ is sufficient to induce adipocytic differentiation of C3H10T1/2 cells. As shown in Figure 2B, exogenous introduction of PPARγ was able to promote the adipocytic differentiation of C3H10T1/2 cells. Interestingly, this effect of PPARγ was profoundly enhanced by BMP2. Together, the data suggested that expression of PPARγ is sufficient for adipocytic differentiation of C3H10T1/2 cells and that BMP2 exhibits adipogenic actions by regulating expression and function of PPARγ.
Figure 2.
(A) Dominant-negative PPARγ blocked BMP2-induced adipogenesis of C3H10T1/2 cells. C3H10T1/2 cells infected with control or dominant-negative PPARγ adenovirus at 100 multiplicity of infection were incubated with or without BMP2 (300 ng/ml) for 10 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed. (B) Overexpression of PPARγ-induced adipogenesis. C3H10T1/2 cells infected with control or PPARγ adenovirus at 100 multiplicity of infection were incubated with or without BMP2 (300 ng/ml) for 7 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.
Smad1 Is Mediator for BMP2-induced Adipocytic Differentiation in C3H10T1/2 Cells by Inducing PPARγ Expression
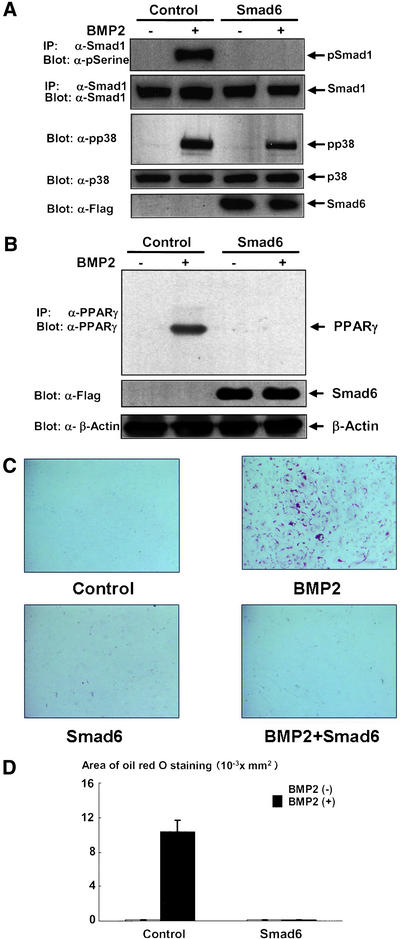
Because Smad1 has been shown to play important roles in BMP2 signaling (Heldin et al., 1997), we next examined whether activation of Smad1 is involved in the induction of PPARγ and adipocytic differentiation of C3H1 0T1/2 cells. To address this, we overexpressed Smad6, a natural antagonist for Smad1 in BMP2 signaling (Imamura et al., 1997), and determined the effects of Smad6 on PPARγ expression and adipocytic differentiation. We confirmed that Smad6 was effectively overexpressed and completely blocked activation of Smad1 elicited by BMP2 in C3H10T1/2 cells (Figure 3A). Western blotting experiment indicated that overexpression of Smad6 abolished BMP2-induced expression of PPARγ (Figure 3B). Moreover, overexpression of Smad 6 blocked adipocytic differentiation promoted by BMP2 treatment (Figure 3, C and D). These results indicated that activation of Smad1 is necessary for induction of PPARγ and subsequent adipocytic differentiation of C3H10T1/2 cells.
Figure 3.
Overexpression of Smad6 inhibited activation of Smad1 (A), BMP2-regulated induction of PPARγ (B), and adipogenesis (C and D). (A) C3H10T1/2 cells infected with control or Flag-Smad6 adenovirus at 100 multiplicity of infection were starved with 0.2% fetal calf serum-DMEM for 16 h and then stimulated with or without BMP2 (300 ng/ml) for 10 min and lysed. The cell lysates were immunprecipitated with anti-Smad1 antibody and immunoblotted with anti-phsophoserine antibody (top). The amount of Smad1 in the immunoprecipitates was determined by immunoblotting with anti-Smad1 (second panel). The lysates were also determined by immunoblotting with antiphsopho-p38 (third panel) or antip38 (forth panel) antibodies. The expression of Flag-Smad6 was determined by immunoblotting with anti-Flag antibody (bottom). (B) C3H10T1/2 cells infected with control or Flag-Smad6 adenovirus at 100 multiplicity of infection were incubated with or without BMP2 (300 ng/ml) for 4 d and lysed. The lysates were determined by immunoblotting with anti-PPARγ mAb followed by immunoprecipitation with anti-PPARγ polyclonal antibody (top). The lysates were also determined by immunoblotting with anti-Flag (middle) or anti-β-actin antibodies (bottom). (C and D) 10T1/2 cells infected with control or Flag-Smad6 adenovirus at 100 multiplicity of infection were incubated with or without BMP2 (300 ng/ml) for 10 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.
BMP2 Up-Regulates Transcriptional Activity of PPARγ Not through Smad Signaling
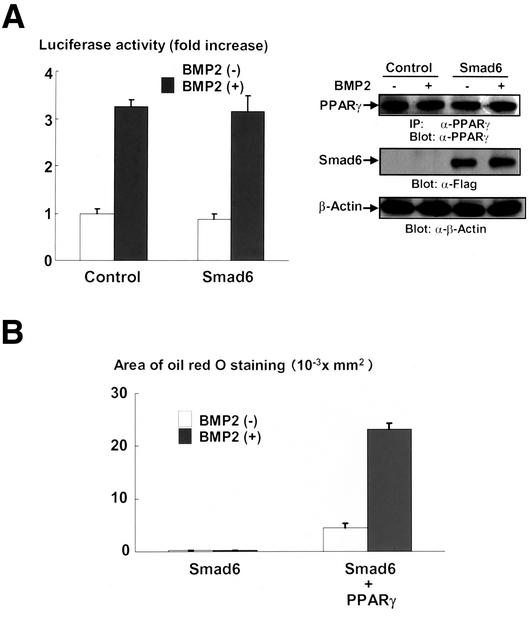
We observed that BMP2 enhanced the adipogenic effects of PPARγ as shown in Figure 2B; therefore, we determined the effects of Smad signaling on the transcriptional activity of PPARγ by using a firefly luciferase reporter construct containing PPARγ-responsible elements, PPRE-Luc (Hu et al., 1996). As shown in Figure 4A (left), BMP2 up-regulated the transcriptional activity of PPARγ. However, overexpression of Smad6 failed to block the effects of BMP2 on transcriptional activity of PPARγ (Figure 4A, left). Western blotting analyses indicate that treatment of BMP2 or overexpression of Smad6 had little influence on the expression of PPARγ introduced by transfection in this experimental condition (Figure 4A, right). Thus, the data suggest that BMP2 up-regulated the function of PPARγ not through Smad1 signaling. To confirm this, we determined whether BMP2 is able to enhance adipogenic function of PPARγ in the condition where Smad signaling is inactivated. As shown in Figure 4B, in spite of blockade of Smad signaling by overexpression of Smad6, overexpression of PPARγ could differentiate C3H10T1/2 cells into adipocytes. More importantly, BMP2 further promoted the adipogenic effects of PPARγ in C3H10T1/2 cells even in the presence of Samd6. Together, these results suggested that BMP2 enhanced the adipogenic function of PPARγ by up-regulating its transcriptional in Smad-independent mechanisms.
Figure 4.
(A) BMP2 up-regulated the transcriptional activity of PPARγ in C3H10T1/2 cells. Left, pcDNA3 (control) (1.6 μg) or Smad6 expression vector (1.6 μg) was transfected into C3H10T1/2 cells together with PPARγ (0.1 μg) and RXR (0.1 μg) expression vectors and PPRE-Luc (0.1 μg) and TK-renilla (0.1 μg) reporter constructs. Twelve hours after transfection, cells were incubated with or without BMP2 (300 ng/ml) for 2 d. At the end of the culture, cells were lysed and the luciferase activity was measured and normalized by determining renilla luciferase activity as described in the text. Data were shown as mean ± SD. Right, expression of PPARγ and Smad6 were monitored by immunoblotting with anti-PPARγ (top) and anti-Flag (middle) antibodies in the same set of transfection experiments. The lysates were also determined by immunoblotting with anti-β-actin antibody (bottom). (B) Introduction of PPARγ rescued the adipogenesis blocked by Smad6. C3H10T1/2 cells were infected with Flag-Smad6 (100 multiplicity of infection) and/or PPARγ (100 multiplicity of infection) adenoviruses and incubated with or without BMP2 (300 ng/ml) for 7 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.
p38 Kinase Up-Regulates Transcriptional Activity of PPARγ during Adipocytic Differentiation
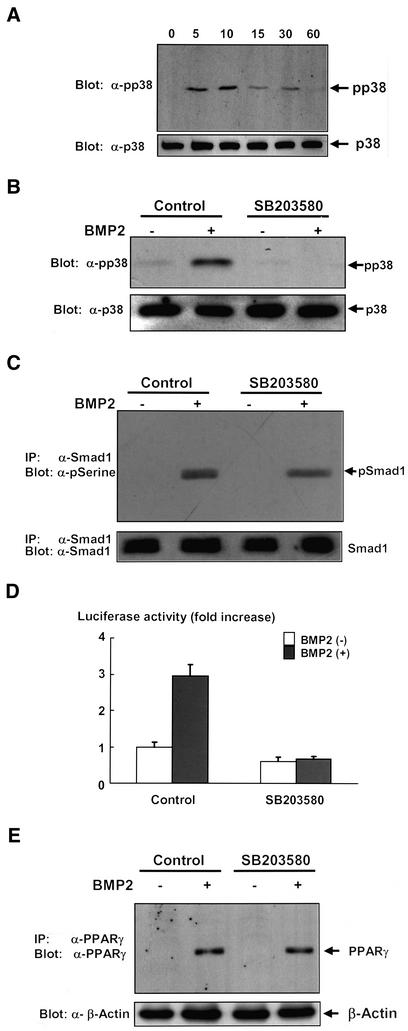
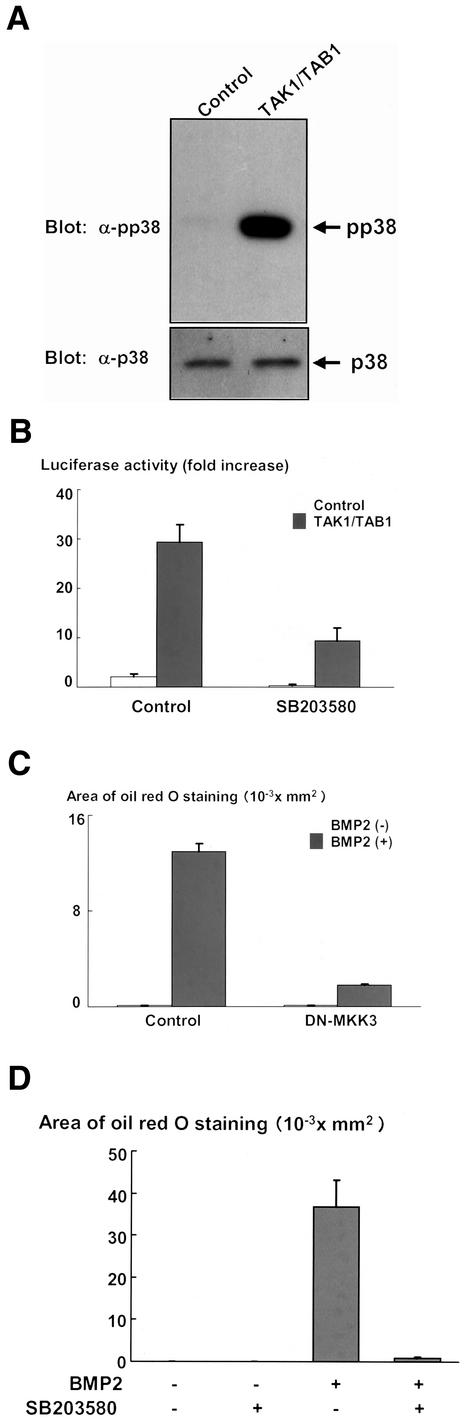
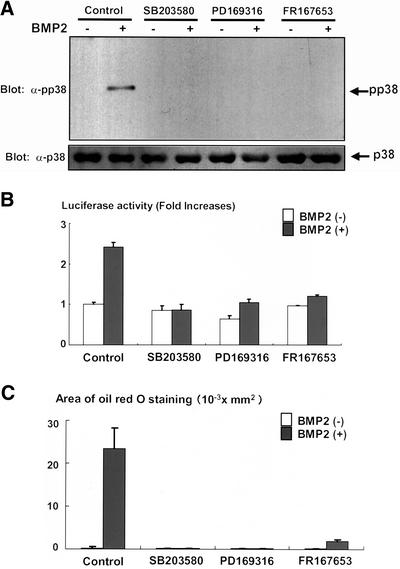
BMP2 has been shown to activate not only Smad signaling but also elicit p38 kinase pathway in the variety types of cells (Iwasaki et al., 1999; Kimura et al., 2000; Hay et al., 2001). In C3H10T1/2 cells, p38 kinase was activated upon treatment with BMP2, and the activation reached maximum at 10 min and thereafter declined (Figure 5A). Consistent with our results shown in Figure 4, A and B, we observed that activation of p38 kinase was only modestly inhibited by overexpression of Smad6 (Figure 3A), which abolished phosphorylation of Smad1 induced by BMP2 (Figure 3A). We, therefore, assessed whether p38 kinase is responsible for activation of PPARγ function by using a specific inhibitor for p38 kinase, SB203580 (Enslen et al., 1998; Iwasaki et al., 1999). SB203580 efficiently inhibited activation of p38 kinase elicited by BMP2 without affecting the activation of Smad1 in C3H10T1/2 cells (Figure 5, B and C). As shown in Figure 5D, treatment with SB203580 inhibited the BMP2-dependent transcriptional activation of PPARγ. In contrast, SB203580 did not affect PPARγ expression (Figure 5E). To further evaluate the importance of p38 kinase in the transactivation of PPARγ, we attempted to examine the transcriptional activity of PPARγ when p38 kinase is specifically activated in the absence of BMP2. Because p38 kinase is regulated by TAK1 (Davis, 2000), and overexpression of TAK1 together with TAB1 is able to activate the down stream signaling (Shibuya et al., 1996), we examined the effects of overexpression of TAK1 and TAB1 on the transcriptional activity of PPARγ. We observed that overexpression of TAK1 together with TAB1 efficiently leads activation of p38 kinase in C3H10T1/2 cells (Figure 6A). Overexpression of TAK1 and TAB1 in C3H10T1/2 cells dramatically enhanced the transcriptional activity of PPARγ (Figure 6B). In addition, treatment with SB203580 inhibited this effect of TAK1 and TAB1 (Figure 6B). These results suggest that p38 kinase accounts for up-regulation of transcriptional activity of PPARγ, and thereby promotes its adipogenic function. To assess the role of p38 kinase in BMP2-induced adipocytic differentiation, we next tested the effects of dominant-negative form of MKK3, which is a direct regulator for p38 kinase (Davis, 2000). Overexpression of dominant-negative MKK 3 inhibited BMP2-induced adipocytic differentiation (Figure 6C). Consistently, we also found that treatment with SB203580 inhibited adipocyte differentiation of C3H10T1/2 cells in the presence of BMP2 (Figure 6D). To further confirm the roles of p38 kinase in the regulation of PPARγ function, we also examined the effects of other p38 kinase inhibitors, PD169316 (Kummer et al., 1997; Assefa et al., 1999) and FR167653 (Yoshinari et al., 2001; Kobayashi et al., 2002). Both PD169316 and FR167653 abolished BMP2-induced p38 phosphorylation (Figure 7A) and suppressed the transactivation of PPARγ by BMP2 (Figure 7B) and BMP2-induced adipogenesis of C3H10T1/2 cells (Figure 7C). Together, these results suggested that p38 kinase promotes the BMP2-induced adipocytic differentiation by activating function of PPARγ.
Figure 5.
(A) Time-dependent activation of p38 kinase by BMP2 in C3H10T1/2. C3H10T1/2 cells were serum starved in 0.2% fetal calf serum-DMEM for 16 h. The cells were then stimulated with BMP2 (300 ng/ml) for 5, 10, 15, 30, or 60 min and lysed, and the cell lysates were determined by immunoblotting with anti-phospho-p38 kinase (top) and anti-p38 kinase antibodies (bottom). (B) Activation of p38 kinase in C3H10T1/2 and its inhibition by SB203580. C3H10T1/2 cells were serum starved in 0.2% fetal calf serum-DMEM for 12 h. The cells were further incubated with or without p38 kinase inhibitor SB203580 (10 μM), for 4 h. The cells were then stimulated with BMP2 (300 ng/ml) for 10 min and lysed, and the cell lysates were determined by immunoblotting with anti-phospho-p38 kinase (top) and anti-p38 kinase antibodies (bottom). (C) SB203580 did not affect the phosphorylation of Smad1. C3H10T1/2 cells were serum starved in 0.2% fetal calf serum-DMEM for 12 h. The cells were further incubated with or without SB203580 (10 μM) for 4 h. The cells were then stimulated with BMP2 (300 ng/ml) for 10 min and lysed. The cell lysates were immunoprecipitated with anti-Smad1 and immunoblotting with anti-phosphoserine (top) or anti-Smad1 antibodies (bottom). (D) SB203580 inhibited transcriptional activity of PPARγ induced by BMP2. C3H10T1/2 cells were transfected into together with PPARγ (0.1 μg) and RXR (0.1 μg) expression vectors, and PPRE-Luc (0.1 μg) and TK-renilla (0.1 μg) reporter constructs, and pcDNA3 (1.6 μg). Twelve hours after transfection, cells were incubated with or without SB203580 (10 μM) for 2 d. At the end of the culture, cells were lysed and the luciferase activity was measured and normalized by determining renilla luciferase activity as described in the text. Data are shown as mean ± SD. (E) SB203580 did not affect the expression of PPARγ. C3H10T1/2 cells were incubated with BMP2 (300 ng/ml), SB203580 (10 μM), or both for 3 d. The cells were lysed, and the expression of PPARγ was determined by immunoblotting with anti-PPARγ monoclonal antibody, followed by immunoprecipitation with anti-PPARγ polyclonal antibody (top). The lysates were also determined by immunoblotting with anti-β-actin antibody (bottom).
Figure 6.
(A) Overexpression of TAK1- and TAB1-induced activation of p38 kinase in C3H10T1/2 cells. C3H10T1/2 cells were transfected with pcDNA3 vector or both TAK1 and TAB1 expression constructs. Twenty hours after transfection, the cells were serum starved for 16 h and lysed. The cell lysates were determined by immunoblotting with anti-phospho-p38 kinase (top) or anti-p38 kinase (bottom) antibodies. (B) Overexpression of TAK1 and TAB1 up-regulated transcriptional activity of PPARγ. pcDNA3 (control) (1.6 μg) or both TAK1 (0.8 μg) and TAB1 (0.8 μg) expression vectors (1.6 μg) were transfected into C3H10T1/2 cells together with PPARγ (0.1 μg) and RXR (0.1 μg) expression vectors and PPRE-Luc (0.1 μg) and TK-renilla (0.1 μg) reporter constructs. Twelve hours after transfection, cells were incubated with or without SB203580 (10 μM) for 2 d. At the end of the culture, cells were lysed and the luciferase activity was measured and normalized by determining renilla luciferase activity as described in the text. Data were shown as mean ± SD. (C) Dominant-negative MKK3 inhibited BMP2-induced adipogenesis in C3H10T1/2 cells. C3H10T1/2 cells infected with control or dominant-negative MKK3 adenovirus at 100 multiplicity of infection were incubated with or without BMP2 (300 ng/ml). The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed. (D) SB203580 inhibited BMP2-induced adipogenesis. C3H10T1/2 cells were incubated with BMP2 (300 ng/ml), SB203580 (10 μM), or both for 10 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.
Figure 7.
(A) Inhibition of p38 kinase activation by PD169316 and FR167653. C3H10T1/2 cells were serum starved in 0.2% fetal calf serum-DMEM for 12 h. The cells were further incubated with or without p38 kinase inhibitor SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 4 h. The cells were then stimulated with BMP2 (300 ng/ml) for 10 min and lysed, and the cell lysates were determined by immunoblotting with anti-phospho-p38 kinase (top) and anti-p38 kinase antibodies (bottom). (B) PD169316 and FR167653 inhibited transcriptional activity of PPARγ induced by BMP2. C3H10T1/2 cells were transfected together with PPARγ (0.1 μg) and RXR (0.1 μg) expression vectors and PPRE-Luc (0.1 μg) and TK-renilla (0.1 μg) reporter constructs, and pcDNA3 (1.6 μg). Twelve hours after transfection, cells were incubated with or without SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 2 d. At the end of the culture, cells were lysed and the luciferase activity was measured and normalized by determining renilla luciferase activity as described in the text. Data were shown as mean ± SD. (C) PD169316 and FR167653 inhibited BMP2-induced adipogenesis. C3H10T1/2 cells were incubated with or without BMP2 (300 ng/ml), in the presence or absence of SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 10 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.
DISCUSSION
Although BMP2 is well known to induce adipogenesis of mesenchymal cells (Ahrens et al., 1993; Ji et al., 2000; Sottile and Seuwen, 2000), to date, the molecular mechanisms by which BMP2 promotes adipocytic differentiation are elusive. In this study, we have shown that BMP2 induces the expression of PPARγ in association with adipocytic differentiation of C3H10T1/2 cells. We also found that dominant-negative PPARγ markedly blocked BMP2-induced adipocytic differentiation of C3H10T1/2 cells. Furthermore, introduction of PPARγ is enough to induce adipogenesis of C3H10T1/2 cells in the absence of BMP2. Collectively, it is most likely that BMP2 exhibits its adipogenic effects by inducing PPARγ. This conclusion is supported by the results that pretreatment of C3H10T1/2 cells with BMP2 was able to bring out the adipogenic effects of TZD. Moreover, overexpression of Smad6, which blocked activation of Smad1 in response to BMP2 stimulation, abolished BMP2-induced adipogenesis of C3H10T1/2 cells as well as the induction of PPARγ expression. Thus, activation of Smad family signaling is a prerequisite for the induction of PPARγ expression, thereby inducing differentiation of C3H10T1/2 cell into adipocytes.
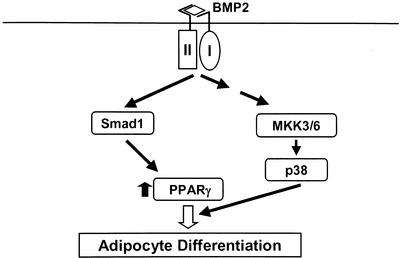
Recently, the data that Smad regulates the function of transcriptional factors, nuclear receptors, or coactivator through its physical association are accumulated (Massague and Wotton, 2000). We have shown herein that BMP2 increased the transcriptional activity of PPARγ. BMP2 also enhanced adipogenesis caused by overexpression of PPARγ. It is, therefore, possible that Smad signaling promoted the adipogenesis in cooperation with PPARγ. However, Smad6 failed to inhibit the transcriptional activity of PPARγ enhanced by BMP2. In addition, overexpression of Smad1 or Smad5 together with Smad4 had no effects on transcriptional activity of PPARγ (our unpublished data). Furthermore, BMP2 enhanced PPARγ-promoted adipogenesis of C3H10T1/2 cells in which Smad signaling is blockaded by overexpression of Smad6. These data suggest that Smad signaling is implicated in the regulation of the PPARγ expression but not in activation of its function. We found that specific inhibitors for p38 kinase, SB203580, PD169316, and FR167653, decreased the transcriptional activity of PPARγ enhanced by BMP2 treatment to the basal level and markedly inhibited BMP2-induced adipocyte differentiation of C3H10T1/2 cells. Consistently, treatment with SB203580 or overexpression of dominant-negative MKK3 inhibited BMP2-induced adipogenesis of C3H10T1/2 cells. Furthermore, overexpression of TAK1 and TAB1, which markedly induced activation of p38 kinase, was sufficient to up-regulate the transcriptional activity of PPARγ. This effect of TAK1 and TAB1 was also suppressed by treatment with SB203580. These results indicate that p38 kinase but not Smad signaling accounts for up-regulation of PPARγ activity, which leads to further promotion of adipogenesis. The potential schematic model in which BMP2 regulates adipogenesis through activation of Smad1 and p38 kinase is delineated in Figure 8.
Figure 8.
Schematic model of BMP2-induced adipogenesis. Activation of Smad1 is essential for induction of PPARγ expression, which is critical for adipogenic effects of BMP2. In contrast, activation of p38 kinase, presumably through MKK3 and MKK6, plays roles in the up-regulation of transcriptional activity of PPARγ.
The mechanism for the up-regulation of PPARγ by p38 kinase remains unknown. The direct up-regulation of PPARγ by p38 kinase through phosphorylation is unlikely because PPARγ possesses only one consensus phosphorylation site by MAP kinases at serine112, which is shown to be phosphorylated by extracellular signal-regulated kinase kinase, thereby inhibiting the transcriptional activity of PPARγ (Hu et al., 1996). Consistent with this report, we confirm that a specific inhibitor for mitogen-activated protein kinase kinase, PD98059, enhanced the function of PPARγ- and BMP2-induced adipogenesis in C3H10T1/2 cells (our unpublished data). Another possibility is that PPARγ might be up-regulated by forming the complex with a certain transcription factor. ATF-2 is known to be regulated by p38 kinase and to control transcription through cross-talk with other transcriptional factors or coactivators (Davis, 2000). We observed that BMP2 phosphorylates ATF-2 in C3H10T1/2 cells (our unpublished data). It is, therefore, possible that interaction of ATF-2 with PPARγ might account for the up-regulation of PPARγ by p38 kinase, although the interaction of ATF-2 and PPARγ, and the biological relevance of this interaction are needed to be addressed.
Recently, Sano et al. (1999) have reported the direct cooperation between Smad3 and p38 kinase signaling. Similarly, it has been shown that p38 pathway is involved in transforming growth factor-β–induced gene expression by interacting with Smad signaling (Hanafusa et al., 1999, Watanabe et al., 2001). In contrast, our results with SB203580 indicated that p38 kinase pathway is not involved in the regulation of PPARγ expression, which is controlled by Smad signaling. Kimura et al. (2000) and Yanagisawa et al. (2001) reported that activation of p38 signaling was blocked by Smad6 or Smad7 in mouse hybridoma or PC12 cells. On the other hand, we showed herein that Smad6 failed to inhibit the transactivation of PPARγ by BMP2 and that BMP2 increased adipogenic function of PPARγ even in the presence of Smad 6, suggesting that Smad6 has little effect on the up-regulation of PPARγ by p38 kinase. Because we observed that SB203580 had no effect on phosphorylation of Smad1, and that the inhibitory effect of Smad6 on p38 activation was modest in C3H10T1/2 cells, the extent of interaction of both Smad pathway and p38 signaling might depend on types of cells or tissues. Alternatively, it is also possible that experimental models and/or levels of Smad6 expression may account for these differences. Further dissection of relationship of both pathways would solve this issue.
Because we have shown that BMP2 induced expression of PPARγ in C3H10T1/2 cells and that Smad6 blocked the effects of BMP2, we were concerned that these effects of BMP2 or Smad6 might affect our transcriptional assay for PPARγ. To eliminate this possibility, we exogenously introduced the appropriate amount of PPARγ by transfection when we examined the effects of BMP2 on the transcriptional activity of PPARγ. As we expected, the amount of PPARγ introduced by transfection was much larger than that induced by BMP2 (Figure 1E). Moreover, when we performed the reporter assay, the amounts of PPARγ were not affected by treatment with BMP2 or Smad6 (Figure 4A). It is, therefore, likely that our transcriptional assay for PPARγ was independent of levels of PPARγ induced by BMP2 treatment or effect of Smad6.
It is known that adipocytes share their origin in bone marrow with osteoblasts (Prockop, 1997; Pittenger et al., 1999). We and others have demonstrated that BMP2 also differentiates pluripotent mesenchymal cells toward osteoblasts by activating Smad signaling (Yamamoto et al., 1997; Nishimura et al., 1998). In this study, we showed that BMP2 regulated PPARγ expression via Smad signaling. Interestingly, we also observed that overexpression of PPARγ in bone marrow stromal cells or primary osteoblasts inhibited the osteoblastic differentiation process (Hata and Nishimura, unpublished data). Abnormally accelerated adipogenesis in bone marrow, also known as fatty marrow, is often observed in the patients with osteoporosis, which is a common metabolic bone disease characterized by the impaired function and differentiation of osteoblasts (Ducy et al., 2000). Therefore, the identification of molecular mechanisms that regulate the direction between adipogenesis and osteoblastogenesis may contribute to the further understanding of pathogenesis of metabolic bone disease such as osteoporosis.
In conclusion, our data suggest that BMP2 induces the differentiation of undifferentiated mesenchymal cells into adipocytes by induction of PPARγ expression through activation of Smad1 and up-regulation of its transcriptional activity via activation of p38 kinase. We believe that these results further our understanding of the molecular mechanism underlying the BMP2-induced adipogenesis.
ACKNOWLEDGMENTS
We thank Dr. Bruce M Spiegelman (Harvard Medical School, Cambridge, MA) for PPARγ expression vector and PPRE-Luc, Dr. Kohei Miyazono (Cancer Institute) for 6xMyc-Smad1 and Flag-Smad6 cDNA, Dr. Roger Davis (University of Massachusetts Medical Center) for MKK3 cDNA, Dr. Hiroshi Shibuya (National Institute for Basic Biology) for TAK1 and TAB1 cDNA, Dr. Akira Miyamoto for troglitazone (Sankyo Pharmacia), Yamanouchi Pharmacia for recombinant BMP2, and Fujisawa Pharmaceutical for FR167653. We also thank Dr. Izumu Saito (Tokyo University) for providing useful information about generation of recombinant adenovirus. Part of this work was supported by the Ministry of Education, Science, Sports and Culture Grant-in-Aid for Scientific Research A 11307041 and C 10671739, Senri Life Science Foundation, and by National Institutes of Health grants P01-CA40035, R01-AR28149, and R01-DK45229.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–06–0356. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–06–0356.
REFERENCES
- Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- Assefa Z, Vantieghem A, Declercq W, Vandenabeele P, Vandenheede JR, Merlevede W, de Witte P, Agostinis P. The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J Biol Chem. 1999;274:8788–8796. doi: 10.1074/jbc.274.13.8788. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Saibo. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Berg AH, Lewis RY, Lin A, Lisanti MP, Scherer PE. Constitutively active mitogen-activated protein kinase kinase 6 (MKK6) or salicylate induces spontaneous 3T 3-L1 adipogenesis. J Biol Chem. 1999;274:35630–35638. doi: 10.1074/jbc.274.50.35630. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3–L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hay E, Lemonnier J, Fromigue O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028–29036. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Iguchi M, Watanabe K, Hoshino R, Tsujimoto M, Kohno M. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J Biol Chem. 1999;274:26503–26510. doi: 10.1074/jbc.274.37.26503. [DOI] [PubMed] [Google Scholar]
- Ji X, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3–F442A. J Bone Miner Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1–p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–1765. doi: 10.1074/jbc.M908622199. 2. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. What do BMPs do in mammals? Clues from the mouse short-ear mutation. Trends Genet. 1994;10:16–21. doi: 10.1016/0168-9525(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131:344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- Kubota N, et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C. Loss-of-function mutations in PPARγ associated with human colon cancer. Mol Cell. 1999;3:799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475:201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM. PPARγ in monocytes: less pain, any gain? Saibo. 1998;93:153–155. doi: 10.1016/s0092-8674(00)81567-6. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ 2, a lipid-activated transcription factor. Saibo. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Spiegelman BM. Transcriptional activation of adipogenesis. Curr Opin Cell Biol. 1999;11:689–694. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276:14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takeda K, Ochiai W, Takizawa T, Ueno M, Takizawa M, Shibuya H, Taga T. Inhibition of BMP2-induced, TAK1 kinase-mediated neurite outgrowth by Smad6 and Smad7. Genes Cells. 2001;6:1091–1099. doi: 10.1046/j.1365-2443.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Yoshinari D, Takeyoshi I, Kobayashi M, Koyama T, Iijima K, Ohwada S, Matsumoto K, Morishita Y. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to University of Wisconsin solution on reperfusion injury in liver transplantation. Transplantation. 2001;72:22–27. doi: 10.1097/00007890-200107150-00007. [DOI] [PubMed] [Google Scholar]