Abstract
Several findings have revealed a likely role for DNA ligase IV, and interacting protein XRCC4, in the final steps of mammalian DNA double-strand break repair. Recent evidence suggests that the human DNA ligase IV protein plays a critical role in the maintenance of genomic stability. To identify protein–protein interactions that may shed further light on the molecular mechanisms of DSB repair and the biological roles of human DNA ligase IV, we have used the yeast two-hybrid system in conjunction with traditional biochemical methods. These efforts have resulted in the identification of a physical association between the DNA ligase IV polypeptide and the human condensin subunit known as hCAP-E. The hCAP-E polypeptide, a member of the Structural Maintenance of Chromosomes (SMC) super-family of proteins, coimmunoprecipitates from cell extracts with DNA ligase IV. Immunofluorescence studies reveal colocalization of DNA ligase IV and hCAP-E in the interphase nucleus, whereas mitotic cells display colocalization of both polypeptides on mitotic chromosomes. Strikingly, the XRCC4 protein is excluded from the area of mitotic chromosomes, suggesting the formation of specialized DNA ligase IV complexes subject to cell cycle regulation. We discuss our findings in light of known and hypothesized roles for ligase IV and the condensin complex.
INTRODUCTION
Cellular resistance to ionizing radiation as well as normal development of the mammalian immune system require the ability to repair DNA double-strand breaks (DSBs). Although capable of homologous recombination, higher eukaryotic species appear to repair DSBs predominantly via a pathway of nonhomologous end-joining (NHEJ) or illegitimate recombination (Robins et al., 1981; Smith and Berg, 1984; Lin et al., 1985; Roth and Wilson, 1985; Smithies et al., 1985; Thomas and Capecchi, 1986). Although the molecular details of NHEJ remain to be elucidated, a number of studies have established the involvement of the DNA-dependent protein kinase, XRCC4 and DNA ligase IV (for review, see Khanna and Jackson, 2001; Smith and Jackson, 1999). The formation of intricately orchestrated DNA-protein and protein–protein complexes is a recurring theme in many aspects of nuclear metabolism including NHEJ. One such complex that has been extensively characterized and that appears to be central to the detection, signaling, and repair of DSBs is the tripartite DNA-dependent protein kinase (Dvir et al., 1992, 1993; Gottlieb and Jackson, 1993; Cary et al., 1997, 1998; Yaneva et al., 1997; Hammarsten and Chu, 1998). The requirement for DNA-PK in the NHEJ pathway together with the biochemical properties of the complex have led to the formulation of a model for DSB repair in which an early step in the repair process is the binding of a DSB by the Ku heterodimer followed by the recruitment of DNA-PKcs and activation of the protein kinase which, in turn, activates the NHEJ pathway (Smith and Jackson, 1999).
Although evidence suggests a role for DNA-PK in the detection and signaling steps of DSB repair, other multiprotein complexes are likely to be involved in subsequent NHEJ steps. Exonucleolytic end processing before DSB repair may occur through the activities of MRE11/RAD50/NBS1 or the WRN protein in association with DNA-PK or its subunits (Maser et al., 1997; Goedecke et al., 1999; Paull and Gellert, 1998, 1999; Yannone et al., 2001), whereas the DNA ligase IV/XRCC4 complex is implicated in the final, covalent-rejoining steps of repair (Critchlow et al., 1997; Grawunder et al., 1997, 1998a, 1998b). Recently, it has been reported that the DNA ligase IV/XRCC4 complex associates at DNA ends with the DNA-PK holoenzyme (Chen et al., 2000) and may be recruited to DNA via a physical interaction with the Ku heterodimer (Nick McElhinny et al., 2000), an observation supported by prior reports that the XRCC4 protein interacts with DNA and is a substrate for the DNA-PK (Leber et al., 1998; Modesti et al., 1999).
Deletion of DNA ligase IV or XRCC4 genes in mouse results in late embryonic lethality and apoptotic loss of neuronal cells (Barnes et al., 1998; Frank et al., 1998; Gao et al., 1998). Embryonic lethality observed in XRCC4−/− mice is rescued by loss of p53 function but with an associated increase in proB-cell lymphoma, and chromosomal abnormalities such as translocations and amplifications (Gao et al., 2000). These data point to involvement of p53-dependent pathways in the XRCC4 phenotype, implying that it is the failure to repair double-strand breaks that gives rise to neuronal apoptosis and ultimately embryonic lethality. The observation that DNA ligase IV or XRCC4 deficiency results in an increase in genomic instability suggests that the DNA ligase IV protein and its complexes play critical roles in the maintenance of genomic integrity in addition to established roles in V(D)J recombination and cellular resistance to ionizing radiation (Ferguson et al., 2000; Frank et al., 1998, 2000; Gao et al., 1998).
Given the importance of protein–protein interactions in NHEJ and the evidence that DNA ligase IV plays critical roles in the maintenance of genomic stability, we sought to identify proteins that associate with DNA ligase IV. We used the yeast two-hybrid screen to identify novel binding partners for human DNA ligase IV. These studies resulted in the identification of a physical interaction between a condensin subunit, known as hCAP-E, and DNA ligase IV. First identified in Xenopus laevis, condensins are thought to play an active role in the packaging of mitotic chromosomes. The hCAP-E subunit, along with its heterodimeric partner hCAP-C, are members of the Structural Maintenance of Chromosomes (SMC) super-family of proteins. SMC proteins are associated with many aspects of DNA metabolism, including DNA repair, chromosome condensation, and chromosome cohesion (for review, see Ball and Yokomori, 2001). The hCAP-E/C heterodimer is thought to form a core complex that serves, in part, to nucleate the association of other proteins involved in mitotic chromosome condensation (Kimura et al., 2001). The interaction of DNA ligase IV with hCAP-E suggests that the core SMC component of the condensin complex may play a novel role in DNA repair and genomic stability.
MATERIALS AND METHODS
Yeast Two-Hybrid Constructs
Yeast two-hybrid vectors pACT2 and pAS2-1 (Clontech, Inc., Palo Alto, CA) were used in conjunction with Saccharomyces cerevisiae strain Y190 (MATa, ura3-52, his3--200, lys2--801, ade2--101, trp1--901, leu2--3, 112, gal4D, gal80D, cyhr2, LYS2::GAL1UAS-HIS3TATA-HIS3, URA3::GAL1UAS-GAL1TATA-lacZ) for all two-hybrid assays (Durfee et al., 1993; Harper et al., 1993). The full-length coding sequence of human DNA ligase IV was subcloned into the pAS2-1 vector using a combination of PCR and trimolecular ligation. Specifically, the 5′ portion of the DNA ligase IV cDNA was amplified using the upstream primer: 5′ CTC TGC GAA TTC ATG GCT GCC TCA CAA ACT 3′ and downstream primer: 5′ TTT TTC CGG ATC CGT AGT GAC ATT 3′, which adds an EcoRI site to the 5′ end (underlined) and changes the endogenous BglII site to a BamHI site (underlined) by silent mutation. This fragment was cloned into the EcoRI and BamHI sites of pAS2-1 to form pAS-lig4EB. The 3′ portion of DNA ligase IV was amplified using the upstream primer: 5′ GCC TGC ATG GCT TTT AGA 3′ and downstream primer: 5′ CTC TGC AGG TCG ACT TAA ATC AAA TAC TGG TTT TC 3′. The resulting fragment contained a naturally occurring NcoI site near the 5′ end and a SalI site added to the 3′ end via PCR (site underlined). This fragment and an internal fragment of the human DNA ligase IV cDNA resulting from digestion with BglII and NcoI were ligated to BamHI/SalI digested pAS-lig4EB in a trimolecular ligation. The resulting clone was confirmed by sequence analysis to be the full-length human DNA ligase IV cDNA fused in frame to the GAL4 DNA-binding domain of pAS2-1 .
To isolate the plasmids responsible for the positive yeast two-hybrid interaction with DNA ligase IV, we selected for cyclohexamide-resistant yeast. Because the pAS2-1 plasmid carries a cyclohexamide sensitivity marker, Y190 carrying pAS-LigaseIV and the interaction suspects in pACT2 were plated onto minimal media plates lacking leucine but containing 100 μg/ml cyclohexamide. Yeast capable of growth under these conditions have lost the pAS-LigaseIV bait plasmid but retain the pACT2-based library plasmid. Bacterial transformants were generated by electroporation with crude DNA isolated from cultures of cyclohexamide segregants. Automated DNA sequencing was used to determine the nucleotide sequence of isolated plasmids.
Cloning of the hCAP-E cDNA
Yeast two-hybrid library screens resulted in the identification of a fragment of the human condensin subunit, hCAP-E, that associates with ligase IV. The fragment of hCAP-E identified in yeast two-hybrid library screens was highly homologous to nucleotides 2561 through 3140 of the published hCAP-E sequence (Schmiesing et al., 1998). To clone the full-length hCAP-E cDNA, we used this fragment to probe a human cDNA library. Clones isolated from these library screens represented either 5′ or 3′ ends of the coding sequence; no full-length clones were isolated. The failure to isolate full-length clones may have been the result of an adenine-rich region in the 5′ half of the coding sequence (see for example, Hirano and Mitchison, 1994). Nonetheless, overlapping clones were identified and used to generate a full-length clone. Full-length hCAP-E was assembled using a trimolecular ligation strategy and the addition of convenient restriction sites to the 5′ and 3′ ends using PCR. Our partial hCAP-E clone containing the 5′ portion of the coding sequence, designated RBC8–5, was amplified by low-cycle PCR to generate a fragment containing a BglII site at the 5′ end and the naturally occurring NdeI site at the 3′ end. This fragment was generated using the upstream primer: 5′ CTA TAG ATC TTA ATG CAT ATT AAG TCA ATT ATT C 3′ and the downstream primer: 5′ TAG AGT CGT TCT CCA GCC 3′. After digestion with BglII and NdeI the 1.492-kb fragment was ligated to the 3′ half of the coding sequence digested with NdeI, which cuts in the region of overlap between 5′ and 3′ clones, and XhoI, which restricts downstream of the stop codon in the vector sequence.
Polyclonal Antibody Preparation
Recombinant hCAP-E C-terminal fragment was produced in Escherichia coli strain BL21(DE3) pLysS (Novagen, Inc., Madison, WI) transformed with pET28c carrying a cDNA encoding amino acids 783–976 of our hCAP-E clone. This hCAP-E C-terminal peptide was purified as a His-Tag fusion protein using TALON resin (Clontech, Inc.) and denaturing guanidinium-based buffers as recommended by the resin manufacturer. After peptide binding, TALON columns were washed first with five bed volumes of guanidinium based buffer followed by five bed volumes of 5 M urea-based wash buffer and then eluted by low-pH, 5 M urea buffer. Dialysis of protein preparations in 5 M urea against phosphate-buffered saline (PBS) resulted in the production of soluble hCAP-E C-terminal polypeptide that was suitable for injection into New Zealand white rabbits following established protocols (Harlow and Lane, 1988).
For the production of DNA Ligase IV antibodies, PCR was used to amplify a region of the human DNA ligase IV gene that corresponds to the C-terminal 200 amino acids of the protein. The resulting PCR product was cloned in the pET28c expression vector (Novagen, Inc.) and confirmed by sequencing. Protein was expressed in and purified from E. coli strain BL21(DE3) using denaturing immobilized metal affinity chromatography and Talon resin (Clontech, Inc.) according to the manufacturer's instructions. Purified protein was used as antigen as described above.
Baculovirus carrying the human XRCC4 cDNA was used to infect Sf9 insect cells growing in suspension at a multiplicity of infection of ∼5. The cells were cultured for 72 h after infection and then harvested by centrifugation, washed once with PBS, and stored at −80°C. Cells were later thawed in 5 packed cell volumes 50 mM HEPES, pH 7.5, 400 mM NaCl, 10% glycerol, 1 mM EDTA, 0.01% Igepal and sonicated to complete lysis, and the debris removed by centrifugation at for 30 min at 10,000 × g at 4°C. The resulting extract was loaded directly onto a DEAE column, and the flow through and 1 M NaCl peaks were collected. The flow through was then dialyzed to 100 mM NaCl HCB and sequentially purified over Q-sepharose, SP-sepharose, S-200 gel filtration, and finally Mono-Q; the active fractions were detected by Coomassie-stained SDS-PAGE gels.
Human Cell Protein Extracts
HeLa cell extracts were prepared from HeLa cells grown in suspension in RPMI (Life Technologies BRL, Inc., Rockville, MD) supplemented with 10% fetal calf serum (Hyclone, Inc., Logan, UT). Harvested cells were washed three times in PBS (Life Technologies, Inc.) and resuspended in hypotonic lysis buffer (10 mM Tris, pH 7.9, 10 mM KCl, 1 mM DTT) containing 20 μg/ml phenylmethylsulfonyl fluoride and a cocktail of additional protease inhibitors each present at a final concentration of 1 μg/ml (aprotinin, leupeptin, and pepstatin A). Phosphatase inhibitors 0.1 mM sodium orthovanadate, 20 mM β-glycerophosphate, and 20 mM sodium fluoride were included. Hypotonic lysis was allowed to proceed by incubation on ice for 10 min. Cell lysate was then centrifuged to yield cytoplasmic extract and nuclei. To prepare nuclear extract, nuclei were subjected to salt extraction using 50 mM Tris, pH 7.9, 420 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 20% glycerol and 10% sucrose, and protease inhibitors. Cytoplasmic and nuclear extracts were dialyzed extensively against 50 mM Tris, pH 7.9, 100 mM KCl, 12.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 20% glycerol with protease inhibitors and phosphatase inhibitors. Mitotic chromosomes were isolated from HT1080 cells, arrested in metaphase by colcemid treatment, as described by van den Engh et al. (1984). Chromosomes were harvested by centrifugation and boiled in SDS-PAGE sample buffer before Western blot analysis.
Immunoprecipitation
For each immunoprecipitation nuclear extracts from 5 × 107 HeLa cells were brought to 50 μg/ml ethidium bromide, centrifuged, and transferred to a prechilled tube to remove any precipitated material before the addition of antibody. Incubations with antibody were allowed to tumble at 4°C for at least 8 h. Protein A beads (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) were added, and the incubations continued for an additional 2 h. Beads were washed five times with 1 ml of IP wash buffer (50 mM Tris, pH 8, 150 mM NaCl, 1 mM EDTA, 0.25% NP-40, 5 mM MgCl2, 5% glycerol), resuspended in SDS-PAGE sample buffer, and boiled before electrophoresis.
In Vitro Binding Assay
Full-length human DNA ligase IV cDNA was subcloned into the pFastBac vector using EcoRI and SalI. Full-length human XRCC4 was subcloned into the pFastBac vector using BamHI and SalI. These constructs were used in the Bac-To-Bac system (Life Technologies BRL, Inc.) to obtain Sf9 cells expressing human DNA ligase IV or human XRCC4. Cells were grown in suspension and infected with virus 48 h before extract preparation. Harvested cells were washed twice in cold PBS followed by lysis in 5 ml lysis buffer (50 mM Tris, pH 8.0, 100 mM KCl, 10% glycerol, 1% Nonidet P-40, 5 mM 2-mercaptoethanol) per gram of cells. After centrifugation extract was used in GST pull-down experiments or stored at −70°C.
GST and a GST-hCAP-E fusion protein consisting of amino acids 783–976 of hCAP-E (GST-hCAP-E783–976) were expressed in E. coli DH5a using the pGEX-5X-3 vector (Amersham Pharmacia Biotech, Inc.). GST and GST-hCAP-E783–976 were purified from cleared lysates prepared by sonication of induced E. coli resuspended in PBS. Cellular debris was removed by centrifugation before incubation of lysates with glutathione Sepharose 4B (Amersham Pharmacia Biotech, Inc.) beads at room temperature for 30 min. After incubation, beads were washed extensively with PBS.
For GST pull-down experiments, beads bound to GST or GST-hCAP-E783–976 were incubated for 30 min in PBS containing 750 μg/ml IgG-free BSA (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to block nonspecific binding. Beads were then introduced to Sf9 cell extract diluted 1:10 in PBS and incubated at room temperature for 1 h. Beads were washed once with 1 ml of PBS and five times with 1 ml wash buffer (50 mM Tris, pH 8.0, 300 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.25% Nonidet P-40, 1 mM DTT, 10% glycerol). Washed beads were boiled in SDS sample buffer and analyzed by Western blot.
Indirect Immunofluorescence
Human fibrosarcoma cell line HT1080 were plated onto coverslips and grown in DMEM supplemented with 10% fetal calf serum. Cells were washed three times in PBS before fixation with cold methanol. Methanol fixation was compatible with the visualization of all antigens without preceding detergent extraction. Fixed cells were rehydrated in Tris-buffered saline (TBS) and blocked in 3% IgG-free BSA in TBS for 15 min before the addition of primary antibody. All antibodies were diluted in 4% normal goat serum (Jackson ImmunoResearch Laboratories, Inc.), 1% IgG-free BSA (Jackson ImmunoResearch Laboratories, Inc.) in TBS. Coverslips carrying primary antibody were incubated at room temperature in a humid chamber for 1 h followed by extensive washing in TBS. Incubation in secondary antibody, FITC-conjugated goat anti-rabbit, was allowed to proceed for 1 h at room temperature followed by washing in TBS and mounting in ProLong antifade reagent (Molecular Probes, Inc., Eugene, OR). Cells were visualized on an inverted epifluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) and images recorded using an Orca cooled CCD camera (Hamamatusu, Inc., Malvern, PA). For double-labeling experiments, Rhodamine Red-X–conjugated goat anti-rabbit Fab fragment was used as the first secondary antibody. In some cases, staining with conjugated Fab fragment was followed by incubation with unconjugated goat anti-rabbit Fab fragment to assure complete blocking of rabbit Ig epitopes before initiating the second staining reaction. Fab fragments were used to avoid subsequent reactivity with the second primary antibody through the bivalence of whole IgG. In all cases, parallel negative controls employing rabbit preimmune serum as a mock second primary antibody confirmed the specificity of double-labeling.
RESULTS
Yeast Two-Hybrid Screens Identify hCAP-E as a DNA Ligase IV Interacting Protein
To identify candidate proteins that may form physical associations with the human DNA ligase IV protein, we used yeast two-hybrid screens of a human lymphocyte cDNA library directionally cloned into pACT2 (Clontech, Inc.). Fusions to the GAL4 DNA-binding domain were constructed for the full-length human DNA ligase IV sequence. No β-galactosidase reporter gene activation was detected in yeast strains carrying pAS-LigaseIV and pACT2 vector with no insert, confirming that expression of the GAL4 DNA-binding domain-DNA ligase IV fusion protein does not generate false-positive results under the two-hybrid assay conditions. Yeast strain Y190 transformed with the pAS-LigaseIV plasmid was used in large-scale lithium acetate–mediated transformations with a human lymphocyte cDNA library cloned into the pACT2 vector. Approximately 400,000 independent transformants were selected for prototrophy on drop-out plates lacking tryptophan, leucine, and histidine. From this selection, colonies were screened further using a β-galactosidase/X-gal assay. Only those suspects that were both capable of growth in the absence of histidine and that were positive in subsequent β-galactosidase assays were scored as positive for interaction with DNA ligase IV in the two-hybrid system.
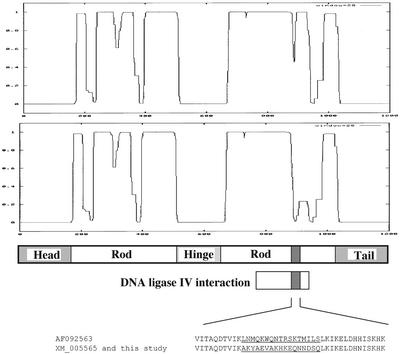
Of the identified positive clones, one isolated plasmid, dubbed pACT3–4, carried an insert highly homologous to the recently published sequence for the hCAP-E subunit of the human condensin complex. Negative controls established that the pACT3–4 plasmid was not capable of reporter gene activation in presence of the pAS2-1 vector lacking the DNA ligase IV cDNA insert. The pACT3–4 insert was used as a probe to screen a human cDNA library. The identified clones were used to assemble a full-length hCAP-E cDNA clone. Automated sequencing and subsequent analysis revealed the clone to be identical to GenBank accession number XM005565 with the exception of two amino acids: 998 Y → C and 1080 E → K. Sequence comparison between the hCAP-E clone reported by Schmiesing et al. (1998; GenBank accession number AF092563) and our clone revealed three single amino acid changes and a short frame shift resulting in a stretch of 17 amino acids (891 through 907), which differ from the AF092563 sequence—after this short stretch the sequence shifts back to the same frame as in AF092563 (Schmiesing et al., 1998). The single amino acid differences are 294V→G, 916H →N, and 1080E→K, the 17 amino acid changes resulting from the temporary change in frame are illustrated in Figure 1. Using the COILS program (Lupas et al., 1991) to examine the effects of the 17 amino acid frame shift on the predicted ability of the protein to form coiled-coils revealed our clone to have a higher probability of coiled-coil formation in this region than the sequence previously reported by Schmiesing et al. (1998). In addition, BLAST searches against the human genome reveal that our clone and XM005565 agree perfectly with the genomic sequence in this region, further suggesting that our sequence is the correct coding sequence for hCAP-E.
Figure 1.
Predicted secondary structure of hCAP-E. Output from the COILS program (Lupas et al., 1991) indicating predicted coiled-coil formation for our hCAP-E clone described in this study (top) and the previously reported sequence (GenBank accession number AF092563; Schmiesing et al., 1998; (bottom) that contains a frame shift changing amino acids 891 through 907 (shaded box in schematic). The amino acid sequence of the region differing between the two clones is shown (underlined). Our clone contains a predicted DNA-PK phosphorylation site (DSQ) not present in the previously described sequence.
Production of Antibodies Specifically Recognizing hCAP-E, XRCC4, and DNA Ligase IV
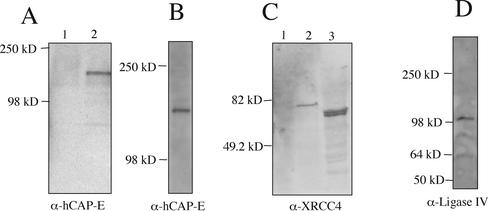
We raised rabbit polyclonal antibodies against hCAP-E, DNA ligase IV, and XRCC4. To establish the specificity of these reagents, we examined Western transfers of cell extracts from HeLa cells or Saccharomyces cerevisiae cells expressing recombinant forms of XRCC4 or hCAP-E. Immunoblots of cell extracts from yeast strains expressing a GAL4 DNA binding domain-hCAP-E fusion protein showed a single band of expected mobility when probed with anti–hCAP-E antiserum at a dilution of 1:3000 (Figure 2A lane 2). A control lane containing extract from yeast transformed with the GAL4 DNA binding domain expression vector without the hCAP-E insert displayed no anti–hCAP-E reactive bands (Figure 2A, lane 1). Western transfer of HeLa cell nuclear extracts revealed that the hCAP-E antibody recognized a single polypeptide exhibiting mobility consistent with a protein of ∼135 kDa (Figure 2B).
Figure 2.
Characterization of polyclonal antiserum. (A) Western blots probed with anti–hCAP-E antibody at a 1:3000 dilution. Protein extracts from yeast strain Y190 transformed with pAS2-1 (lane 1) or pAS-hCAP-E (lane 2). (B) When probed with anti–hCAP-E, Western blots of HeLa cell extract reveal a single band with mobility consistent with the predicted molecular weight of hCAP-E. (C) Western blot probed with anti-XRCC4 antibody at a 1:5000 dilution. Protein extract from yeast strain Y190 transformed with pAS2-1 (lane 1) or pAS-XRCC4 (lane 2). Extract from HeLa cells reveals a doublet that migrates at ∼55 kDa consistent with prior reports of XRCC4 behavior on SDS-PAGE gels (lane 3). (D) Western blot of HeLa cell extract probed with anti-DNA ligase IV at a 1:500 dilution.
Polyclonal anti-XRCC4 antibodies were generated using recombinant full-length human XRCC4 purified from insect Sf9 cells infected with a recombinant baculovirus carrying the human XRCC4 cDNA. The resulting antibodies recognized a single band in a yeast strain transformed with an expression vector encoding a GAL4 DNA-binding domain-XRCC4 fusion protein (Figure 2C, lane 2) but exhibited no reactivity with extract from yeast cells transformed with the expression vector lacking the XRCC4 insert (Figure 2C, lane 1). Human cell extracts displayed a doublet of the expected size based on prior reports of XRCC4 migrating as a ∼55-kDa species despite a predicted molecular mass of ∼38 kDa (Critchlow et al., 1997; Grawunder et al., 1997; Figure 2C, lane 3). Polyclonal DNA ligase IV antiserum predominantly recognizes a single band migrating at ∼100 kDa in HeLa cell extracts, consistent with the molecular weight of DNA ligase IV (Figure 2D).
DNA Ligase IV Colocalizes with hCAP-E throughout the Cell Cycle
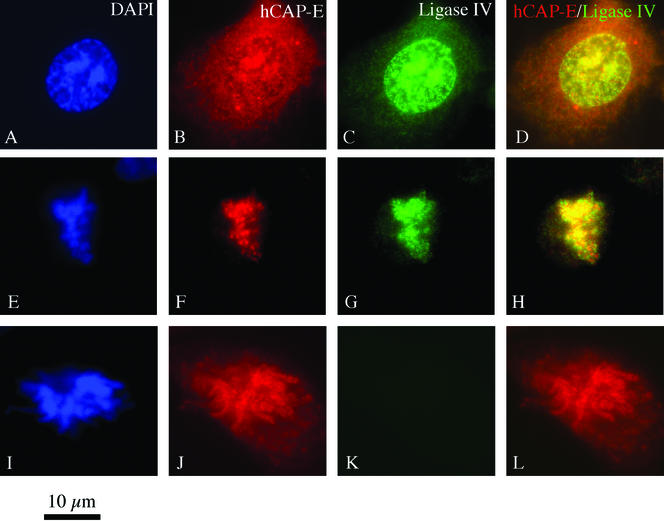
To determine the extent of DNA ligase IV colocalization with hCAP-E within cells, interphase and mitotic HT1080 human cultured cells were processed for double-label immunofluorescence to detect these two antigens. In the interphase cell, others have observed hCAP-E staining in both the cytoplasmic and nuclear compartments (Schmiesing et al., 2000). During mitosis, the condensin complex undergoes relocalization to condensing chromatin, giving rise to the hypothesis that the condensin complex is sequestered in the cytoplasm until mitosis (Schmiesing et al., 2000). We observed staining patterns in HT1080 cells consistent with prior reports; both cytoplasmic and nuclear hCAP-E staining was easily visualized in the majority of cells. Nuclear hCAP-E staining has been described as associated with both nucleoli and with nuclear foci localized to regions of increased DAPI staining (Schmiesing et al., 2000). Nucleolar association has been reported for other SMC proteins and condensin subunit hCAP-H as well (Cabello et al., 2001; Andersen et al., 2002). We noted hCAP-E foci associated with regions of increased DAPI staining intensity in some cells (Figure 3, A and B), consistent with the association of hCAP-E with sites of early chromatin condensation. DNA ligase IV displayed a similar nuclear distribution and localized throughout the nucleus with hCAP-E, including nuclear foci at sites of increased DAPI staining (Figure 3C). Cells in mitosis displayed hCAP-E and DNA ligase IV colocalization associated with condensed mitotic chromosomes (Figure 3, F and G). Negative controls confirmed colocalization was not a staining artifact (Figure 3, I–L). These in vivo observations support our yeast two-hybrid finding that DNA ligase IV associates with hCAP-E.
Figure 3.
Double-label immunofluorescence staining of HT1080 cells for hCAP-E and DNA ligase IV. (A–D) Interphase nuclei display significant colocalization of hCAP-E and DNA ligase IV. Although cytoplasmic staining by the hCAP-E antibody is prominent, DNA ligase IV is largely localized to the nucleus, although some sparse cytoplasmic staining is occasionally visible. (E–H) As cells enter mitosis, colocalization of hCAP-E and DNA ligase IV on condensing chromatin accompanies morphological changes in DNA visualized by DAPI staining. Negative controls in which the second primary antibody is preimmune serum reveal that the observed colocalization of hCAP-E and DNA ligase IV is not an artifact of the staining method (I–L).
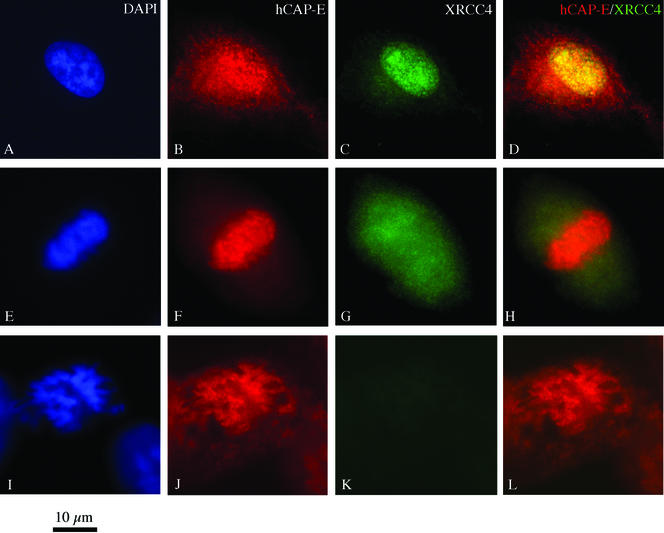
Interphase cells stained for hCAP-E and XRCC4 revealed significant colocalization of these antigens (Figure 4, A–D). Mitotic cells, however, displayed distinct staining patterns for hCAP-E and XRCC4. Although hCAP-E localized to mitotic chromosomes, XRCC4 assumed a diffuse finely granular distribution and appeared excluded from regions of condensed chromosomes such as the metaphase plate (Figure 4G).
Figure 4.
Double-label immunofluorescence staining of HT1080 cells for hCAP-E and XRCC4. (A–D) Significant colocalization of XRCC4 and hCAP-E could be detected in the interphase nucleus. (E–H) Mitotic cells displayed a markedly different distribution of the antigens. hCAP-E staining was localized to mitotic chromosomes (F); however, XRCC4 staining was not detected on mitotic chromosomes and often appeared excluded from the metaphase plate (G). These data are consistent with the distribution of XRCC4 observed in XRCC4/ligase IV double-label experiments. (I–L) Negative controls using preimmune serum as the second primary antibody confirmed the specificity of the dual-staining method.
Immunofluorescence Analysis Reveals DNA ligase IV and XRCC4 Colocalize During Interphase but Not Mitosis
Double-label immunofluorescence was used to examine the colocalization of DNA ligase IV and XRCC4 in interphase and mitotic cells. As expected, interphase nuclei displayed significant colocalization of XRCC4 and DNA ligase IV (Figure 5, A–D). Although the distribution of both antigens was similar in the interphase nucleus, DNA ligase IV staining appeared as slightly larger foci relative to the finer granular staining seen with anti-XRCC4. In addition, DNA ligase IV staining often displayed an apparent enrichment in the nuclear periphery while XRCC4 did not. Colocalization of DNA ligase IV and XRCC4 was limited to the interphase nucleus. As cells entered mitosis, as evidenced by chromatin condensation and the formation of mitotic chromosomes, DNA ligase IV staining was localized to the condensing DNA (Figure 5, G and K). XRCC4 staining was excluded from areas of chromatin condensation (Figure 5, F and J), yet; nonetheless, strong XRCC4 staining persisted in the cell as diffuse punctate staining.
Figure 5.
Double-label immunofluorescence staining of HT1080 cells for XRCC4 and DNA ligase IV. (A–D) Interphase cells displayed partial but significant colocalization of XRCC4 and DNA ligase IV in the nucleus. (E–L) Cells in mitosis, determined by DNA organization as visualized by DAPI staining, revealed little or no colocalization of DNA ligase IV and XRCC4. Although DNA ligase IV localized to punctate structures coincident with DNA staining, XRCC4 staining was present as a diffuse halo throughout the cell. Exclusion of XRCC4 staining could often be seen in regions containing condensed chromosomes, especially in metaphase cells where mitotic chromosomes packed densely along the metaphase plate (e.g., F). Negative controls (M–P) using preimmune serum as the second primary antibody were performed in parallel to confirm independent labeling of the two antigens.
Preferential Association of DNA ligase IV but not XRCC4 with Mitotic Chromosomes
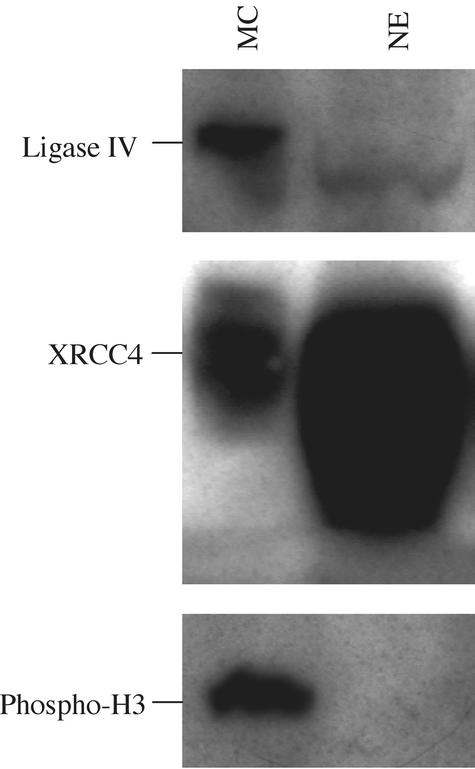
Given the unexpected distribution of DNA ligase IV and XRCC4 during mitosis, we sought to determine the relative abundance of these two proteins in nuclear extract versus isolated mitotic chromosomes. Chromosomes were isolated from HT1080 cells arrested in metaphase with colcemid and compared with extract from untreated cell nuclei. Western blot analysis revealed chromosome preparations were significantly enriched for DNA ligase IV relative to XRCC4 when compared with protein extracts from untreated cell nuclei (Figure 6). XRCC4 reactivity was significantly stronger relative to DNA ligase IV in untreated cell extracts. Although differences in antibody affinities preclude absolute comparisons of the protein levels in these studies, mitotic chromosome preparations revealed a pronounced relative loss of XRCC4-reactive material while displaying strong DNA ligase IV reactivity. As a control for the presence of mitotic chromosomes, blots were also analyzed for the presence of phosphorylated H3 (Wei et al., 1999).
Figure 6.
The relative abundance of DNA ligase IV and XRCC4 on mitotic chromosomes from colcemid-arrested cells and in untreated cell nuclear extracts. Chromosomes harvested from colcemid-treated cells and nuclear extract from untreated cells were analyzed for the presence of DNA ligase IV, XRCC4 and phosphorylated H3 by Western blot as indicated. Identical sample loadings were used for each blot. Untreated cell nuclear extract, NE, exhibits weak DNA ligase IV reactivity, whereas XRCC4 is readily detected as a strongly reactive band. Mitotic chromosome preparations, MC, on the other hand, displayed prominent DNA ligase IV reactivity, whereas the relative intensity of anti-XRCC4 staining was markedly reduced when compared with extracts from untreated cells. DNA ligase IV from mitotic chromosome isolations displayed reduced mobility relative to that of nuclear extract, suggesting a possible posttranslational modification.
A Region in the C-terminal Coiled-Coil Domain of hCAP-E Mediates Interaction with DNA Ligase IV In Vitro
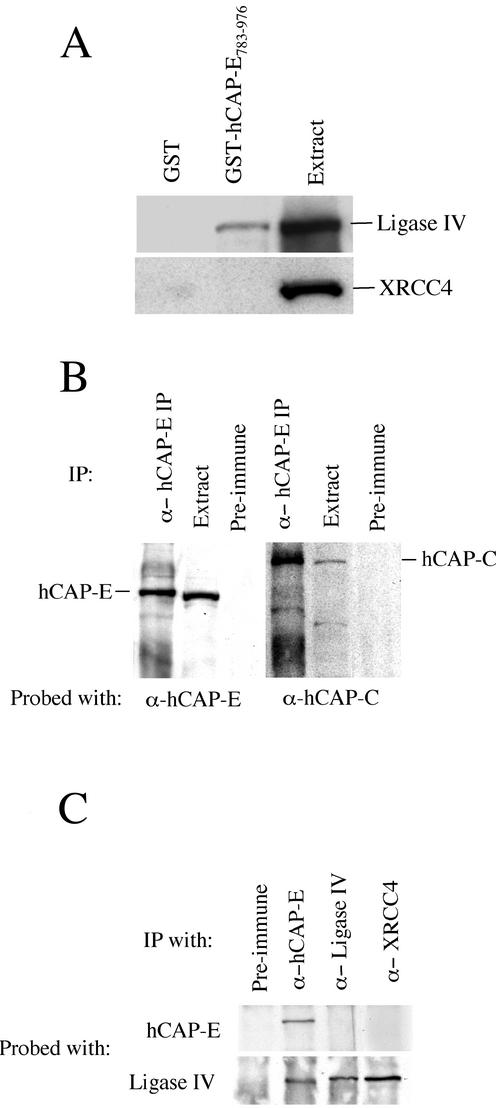
To confirm two-hybrid findings and determine if DNA ligase IV was capable of binding hCAP-E in the absence of endogenous yeast proteins, we examined the capacity of a region in the C-terminal coiled-coil domain of hCAP-E, found in two-hybrid studies to mediate the association with DNA ligase IV, to bind to recombinant DNA ligase IV in vitro. A bacterially expressed GST fusion protein carrying amino acids 783–976 of hCAP-E was used in GST pull-down experiments with recombinant human DNA ligase IV. Glutathione Sepharose 4B beads bound to GST or to GST-hCAP-E783–976 were incubated with diluted Sf9 cell extract containing overexpressed human DNA ligase IV or XRCC4 as a negative control. After a 1-h incubation at room temperature, beads were washed extensively with 300 mM NaCl wash buffer and analyzed by Western blot with anti-DNA ligase IV or anti-XRCC4 antibodies. Under these conditions we observed no binding of DNA ligase IV or XRCC4 to GST bound glutathione Sepharose 4B beads. Beads carrying the GST-hCAP-E783–976 fusion protein, however, bound recombinant DNA ligase IV but not to XRCC4 (Figure 7A). These data confirm that the interaction identified in the yeast two-hybrid system is not the result of artifactual transcriptional activation.
Figure 7.
hCAP-E interacts with DNA ligase IV. (A) Glutathione Sepharose 4B beads bound to GST or to a GST-hCAP-E fusion protein were incubated with diluted extract from Sf9 cells expressing either recombinant human DNA ligase IV or recombinant human XRCC4. After extensive washing, beads were boiled in sample buffer, and proteins were analyzed by Western blot. The GST fusion to the region of hCAP-E identified in yeast two-hybrid studies (amino acids 783–976) binds specifically to recombinant DNA ligase IV (top). As a control for nonspecific interactions, we examined the binding of GST and GST-hCAP-E783–976 to XRCC4 under identical reaction conditions and found no detectable binding to XRCC4 (bottom). (B) To determine if our anti–hCAP-E antibody immunoprecipitated the core condensin SMC heterodimer, we probed anti–hCAP-E immunoprecipitates from HeLa cell extract with anti–hCAP-C. These data show that the hCAP-C partner of hCAP-E is coimmunoprecipitated by the anti–hCAP-E antibody used in this study. (C) Immunoprecipitations from HeLa cell extracts reveal that anti–hCAP-E coimmunoprecipitates DNA ligase IV. Neither anti-XRCC nor anti-DNA ligase IV antibody was able to coprecipitate hCAP-E.
Anti–hCAP-E Coimmunoprecipitates DNA Ligase IV from HeLa Cell Extract
Our anti–hCAP-E antibody was capable of immunoprecipitating itself and its heterodimeric partner hCAP-C from HeLa cell nuclear extract (Figure 7B). This observation supports the conclusion that our antibody recognizes hCAP-E and immunoprecipitates the core SMC heterodimer of the condensin complex. Using anti–hCAP-E for immunoprecipitation from HeLa extract we found that DNA ligase IV was coprecipitated (Figure 7C). To reduce the likelihood that coimmunoprecipitations were mediated by contaminating DNA, 50 μg/ml ethidium bromide was included in all immunoprecipitation reactions.
Given prior reports of a stable physical association between DNA ligase IV and XRCC4, we sought to determine the extent to which anti-XRCC4 antibodies were capable of immunoprecipitating the hCAP-E polypeptide. Although DNA ligase IV was coprecipitated by anti-XRCC4 from HeLa cell nuclear extracts, these experiments failed to demonstrate coprecipitation of hCAP-E (Figure 7C). Similarly, when we generated immunoprecipitates using our anti-DNA ligase IV antibody, we were able to detect the immunoprecipitation of DNA ligase IV but not hCAP-E. Unfortunately, XRCC4 protein migrates closely with IgG heavy chain and is thus difficult to detect on Western blots of immunoprecipitated material. We were, therefore, unable to determine unambiguously if XRCC4 was coimmunoprecipitated by anti-DNA ligase IV antibody. However, the DNA ligase IV antibody was produced using the C-terminal portion of DNA ligase IV that contains two BRCT domains and the XRCC4 interacting region, which has been found to reside between the BRCT domains (Grawunder et al., 1998). Given that this C-terminal portion of the DNA ligase IV molecule is unique among the characterized mammalian DNA ligases, that the region contains two BRCT domains and that this portion of the molecule mediates interactions with XRCC4, it is likely that this region of the molecule is hidden within protein–protein interfaces and inaccessible to precipitating antibody when participating in protein–protein interactions. This explanation is also consistent with the sensitivity of our DNA ligase IV antibody to fixation and extraction conditions used for immunofluorescence. Although methanol fixation was compatible with immunofluorescence visualization of the distribution of DNA ligase IV, extraction with 0.1% Triton X-100 before fixation was required to visualize DNA ligase IV staining of paraformaldehyde fixed cells (unpublished observations).
DISCUSSION
A Physical Interaction between DNA Ligase IV and the Condensin Subunit hCAP-E Suggests a Link between the DSB Repair Pathway and a Key Modulator of Chromatin Structure
Using the yeast two-hybrid system and coimmunoprecipitation, we have demonstrated a physical interaction between DNA ligase IV and one of the SMC subunits of the condensin complex known as hCAP-E (Schmiesing et al., 2000). The colocalization of DNA ligase IV and hCAP-E in both interphase and mitotic cells, as visualized by indirect immunofluorescence, further supports our biochemical evidence for the existence of a complex containing DNA ligase IV and hCAP-E. The hCAP-E protein forms a heterodimer with hCAP-C, together these two SMC proteins form a stable heterodimeric SMC core of a larger protein complex required for mitotic chromosome condensation known as the condensin complex (Schmiesing et al., 1998, 2000; Kimura et al., 2001). Although the hCAP-E/C heterodimer has been shown to exist in the cell in a form not associated with the other condensin subunits, other biological roles and physical partners for the SMC core have not been described (Kimura et al., 2001).
The SMC super-family is a growing family of proteins involved in diverse aspects of DNA metabolism, including DNA repair, chromosome condensation, and cohesion (for recent reviews, see Hirano, 2000; Ball and Yokomori, 2001). The condensin complex is particularly fascinating in that the mechanism hypothesized to mediate chromosome condensation is by an active molecular motor like process (Kimura et al., 1999). In this model, the elongated SMC core binds DNA at the ends and, in an ATP-dependent process, packs chromatin into a condensed state through bending at the central “hinge” domain. The condensin complex was first identified in Xenopus laevis early embryonic cell extracts as a13S complex required for mitotic chromosome condensation (Hirano and Mitchison, 1994; Hirano et al., 1997). Recently a similar complex from human cells has been isolated using an antibody against a non-SMC subunit known as hCAP-G (Kimura et al., 2001). We have shown here that our hCAP-E–directed antibody coimmunoprecipitates the hCAP-C partner as well as DNA ligase IV, indicating that our antibody recognizes hCAP-E in the context of the heterodimeric SMC complex.
SMC Proteins Have Been Identified in a Number of Multiprotein Complexes Involved in DNA Repair and Recombination
The formation of multiprotein complexes containing SMC and non-SMC proteins appears to be a recurring theme. The hRad50/hMre11/NBS1 complex, of which hRad50 is a SMC protein, has been implicated in DSB repair, most likely playing a role in homologous recombination (Yamaguchi-Iwai et al., 1999; Petrini, 2000). Rad18, a SMC protein implicated in DNA repair in Schizosaccharomyces pombe, is required for excision repair of UV damage and the repair of ionizing radiation–induced DSBs (Lehmann et al., 1995; Verkade et al., 1999). S. pombe Rad18 was found to be a component of a multiprotein complex containing at least six other proteins (Fousteri and Lehmann, 2000). Similarly, the condensin complex has been hypothesized to consist of a SMC core regulated, with respect to chromosome condensation functions, by association with additional subunits (Kimura and Hirano, 2000). In bovine, two SMC proteins have been identified as part of the high-molecular-weight DNA recombination complex, known as RC-1, that also contains DNA ligase III and DNA polymerase ε (Jessberger et al., 1996). Thus, the formation of protein complexes about a SMC core may represent a common strategy for the nucleation and regulation of molecular machines involved in diverse aspects of DNA metabolism. In this light, it is of particular interest to note that structural studies of the XRCC4 protein have revealed a three-dimensional structure strikingly similar to the predicted secondary structure of the SMC super-family (Junop et al., 2000). It remains to be determined if DNA ligase IV/hCAP-E complexes contain XRCC4 or represent an alternative complex that may form as a result of cell cycle signals, DNA damage signaling cascades, or chromatin or histone modifications.
During Mitosis XRCC4 and DNA Ligase IV Do Not Colocalize by Immunofluorescence
In the course of characterizing the subcellular distribution of DNA ligase IV, XRCC4, and hCAP-E, we have found that, although DNA ligase IV and hCAP-E colocalize throughout the cell cycle, XRCC4 and DNA ligase IV do not colocalize during mitosis. Prior biochemical characterization has shown that XRCC4 and DNA ligase IV form a stable complex and that the XRCC4 protein stimulates DNA ligase IV activity in vitro (Critchlow et al., 1997; Grawunder et al., 1997, 1998a, 1998b). Our immunofluorescence studies indicate that interactions between DNA ligase IV and XRCC4 and other cellular factors are subject to cell cycle regulation. Although DNA ligase IV and hCAP-E colocalize on mitotic chromosomes, XRCC4 appears to be excluded from these structures. These data indicate a profound change in the conformation, and perhaps the composition, of XRCC4 complexes during mitosis. The lack of XRCC4 staining on mitotic chromosomes, where DNA ligase IV is enriched, may represent changes in the accessibility of XRCC4 epitopes to the XRCC4 antibody. If XRCC4 remains associated with DNA ligase IV on mitotic chromosomes, but unavailable for antibody binding, we would predict a significant reduction in XRCC4 staining intensity during mitosis. However, XRCC4 staining remains robust during mitosis, suggesting the majority of the protein is available for antibody binding. Additionally, Western analysis of mitotic chromosomes, isolated from colcemid-treated cells, revealed a marked relative enrichment of DNA ligase IV in mitotic chromosomes when compared with nuclear extracts from untreated cells. Taken together these data indicate suggest profound changes in the physical partners for DNA ligase IV during mitosis.
Localized Changes in Chromatin Structure May Play a Critical Role in the Cellular Response to DNA Damage
Although evidence from yeast suggests that H2A phosphorylation may relax chromatin, it is not clear if this observation extends to higher eukaryotic systems (Downs et al., 2000). In fact, indirect lines of evidence suggest condensation or compaction of chromatin may also be a response to DNA damage. Exposure to ionizing radiation can lead to both increases and decreases in the viscosity of cell lysates depending on the dosage of radiation. Assuming a relationship between lysate viscosity and chromatin conformation, these data suggest that a complex choreography of chromatin structural changes may accompany exposure to DNA damaging agents and furthermore that both condensation and relaxation may be differentially induced depending on the nature or extent of damage (Belyaev and Harms-Ringdahl, 1996; Belyaev et al., 1996).
Recently uncovered behaviors of the Ku heterodimer and its subunits also suggest a link between DNA repair and chromatin structure. Specifically, the role of Ku in telomere maintenance and silencing in yeast and mammals suggest this NHEJ protein is associated with compacted chromatin structures (Boulton and Jackson, 1998; Laroche et al., 1998; Bailey et al., 1999; Hsu et al., 1999; Martin et al., 1999; Mishra and Shore, 1999). This possible link has recently been strengthened by the report from Song et al. (2001) that Ku70 interacts with HP1α, a heterochromatin protein. Others and we have also shown that the Ku protein displays characteristics consistent with end linking or end alignment roles in DSB repair (Cary et al., 1997; Pang et al., 1997; Ramsden and Gellert, 1998). Compaction of chromatin at or near a break site could further limit the movement of damaged DNA, tethering it to nuclear matrix structures and facilitating a physical conformation conducive to repair and the assembly of a repair complex. Localized changes in chromatin at or near DSB sites could be modulated by a number of reinforcing factors. It is tempting to speculate that, once a DSB is introduced, a DNA damage sensing protein kinase, such as ATM (Smith et al., 1999), initiates a localized alteration of chromatin structure through the phosphorylation of histone H2AX (Rogakou et al., 1998, 1999, 2000; Paull et al., 2000; Burma et al., 2001), the binding of Ku (Cary et al., 1997; Pang et al., 1997; Ramsden and Gellert 1998; Song et al., 2001) and the recruitment of DNA ligase IV/XRCC4 associated with core condensin subunits.
ACKNOWLEDGMENTS
We are grateful to Kyoko Yokomori (UCI) for providing hCAP-C antibody, and to Carolyn S. Bell and the National Flow Cytometry Resource for assistance with chromosome preparations. We thank Scott R. Peterson and Bruce E. Lehnert for helpful comments and discussions. This work was funded by National Institutes of Health grants CA82198 to R.B.C. and CA50519 to D.J.C., by the United States Department of Energy and by Los Alamos National Laboratory Directed Research and Development (LA-UR-02–6645).
Abbreviations used:
- DSB
double-strand break
- NHEJ
nonhomologous end-joining
- SMC
structural maintenance of chromosomes
- DNA-PK
DNA-dependent protein kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E01–11–0117. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E01–11–0117.
REFERENCES
- Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE, Goodwin EH. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball AR, Jr, Yokomori K. The structural maintenance of chromosomes (SMC) family of proteins in mammals. Chromosome Res. 2001;9:85–96. doi: 10.1023/a:1009287518015. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8:1395–8. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Belyaev I, Harms-Ringdahl M. Effects of gamma rays in the 0.5–50-cGy range on the conformation of chromatin in mammalian cells. Radiat Res. 1996;145:687–693. [PubMed] [Google Scholar]
- Belyaev I, Spivak IM, Kolman A, Harms-Ringdahl M. Relationship between radiation induced adaptive response in human fibroblasts and changes in chromatin conformation. Mutat Res. 1996;358:223–230. doi: 10.1016/s0027-5107(96)00124-8. [DOI] [PubMed] [Google Scholar]
- Boulton SJ, Jackson SP. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 1998;17:1819–1828. doi: 10.1093/emboj/17.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI. Directed proteomic analysis of the human nucleolus. Curr Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- Cabello OA, Eliseeva E, He WG, Youssoufian H, Plon SE, Brinkley BR, Belmont JW. Cell cycle-dependent expression, and nucleolar localization of hCAP-H. Mol Biol Cell. 2001;12:3527–3537. doi: 10.1091/mbc.12.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary RB, Chen F, Shen Z, Chen DJ. A central region of Ku80 mediates interaction with Ku70 in vivo. Nucleic Acids Res. 1998;26:974–979. doi: 10.1093/nar/26.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary RB, Peterson SR, Wang J, Bear DG, Bradbury EM, Chen DJ. DNA looping by Ku and the DNA-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94:4267–4272. doi: 10.1073/pnas.94.9.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Trujillo K, Sung P, Tomkinson AE. Interactions of the DNA ligase IV-XRCC4 complex with DNA ends and the DNA-dependent protein kinase. J Biol Chem. 2000;275:26196–26205. doi: 10.1074/jbc.M000491200. [DOI] [PubMed] [Google Scholar]
- Critchlow SE, Bowater RP, Jackson SP. Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Curr Biol. 1997;7:588–598. doi: 10.1016/s0960-9822(06)00258-2. [DOI] [PubMed] [Google Scholar]
- Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature. 2000;408:1001–1004. doi: 10.1038/35050000. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Dvir A, Peterson SR, Knuth MW, Lu H, Dynan WS. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci USA. 1992;89:11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir A, Stein LY, Calore BL, Dynan WS. Purification and characterization of a template-associated protein kinase that phosphorylates RNA polymerase II. J Biol Chem. 1993;268:10440–10447. [PubMed] [Google Scholar]
- Ferguson DO, Sekiguchi JM, Chang S, Frank KM, Gao Y, DePinho RA, Alt FW. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci USA. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri MI, Lehmann AR. A novel SMC protein complex in Schizosaccharomyces pombe contains the Rad18 DNA repair protein. EMBO J. 2000;19:1691–1702. doi: 10.1093/emboj/19.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL, Davidson L, Kangaloo L, Alt FW. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, Chaudhuri J, Horner J, DePinho RA, Alt FW. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- Goedecke W, Eijpe M, Offenberg HH, van Aalderen M, Heyting C. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23:194–198. doi: 10.1038/13821. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–42. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Grawunder U, Wilm M, Wu X, Kulesza P, Wilson TE, Mann M, Lieber MR. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 1997;388:492–425. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- Grawunder U, Zimmer D, Kulesza P, Lieber MR. Requirement for an interaction of XRCC4 with DNA ligase IV for wild-type V(D)J recombination and DNA double-strand break repair in vivo. J Biol Chem. 1998a;273:24708–24714. doi: 10.1074/jbc.273.38.24708. [DOI] [PubMed] [Google Scholar]
- Grawunder U, Zimmer D, Leiber MR. DNA ligase IV binds to XRCC4 via a motif located between rather than within its BRCT domains. Curr Biol. 1998b;8:873–876. doi: 10.1016/s0960-9822(07)00349-1. [DOI] [PubMed] [Google Scholar]
- Hammarsten O, Chu G. DNA-dependent protein kinase: DNA binding and activation in the absence of Ku. Proc Natl Acad Sci USA. 1998;95:525–530. doi: 10.1073/pnas.95.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hirano T. Chromosome cohesion, condensation, and separation. Annu Rev Biochem. 2000;69:115–144. doi: 10.1146/annurev.biochem.69.1.115. [DOI] [PubMed] [Google Scholar]
- Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP- C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Hsu HL, Gilley D, Blackburn EH, Chen DJ. Ku is associated with the telomere in mammals. Proc Natl Acad Sci USA. 1999;96:12454–8. doi: 10.1073/pnas.96.22.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R, Riwar B, Baechtold H, Akhmedov AT. SMC proteins constitute two subunits of the mammalian recombination complex RC-1. EMBO J. 1996;15:4061–4068. [PMC free article] [PubMed] [Google Scholar]
- Junop MS, Modesti M, Guarne A, Ghirlando R, Gellert M, Yang W. Crystal structure of the Xrcc4 DNA repair protein and implications for end joining. EMBO J. 2000;19:5962–5970. doi: 10.1093/emboj/19.22.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- Kimura K, Cuvier O, Hirano T. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J Biol Chem. 2001;276:5417–5420. doi: 10.1074/jbc.C000873200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hirano T. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc Natl Acad Sci USA. 2000;97:11972–11977. doi: 10.1073/pnas.220326097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Laroche T, Martin SG, Gotta M, Gorham HC, Pryde FE, Louis EJ, Gasser SM. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol. 1998;8:653–656. doi: 10.1016/s0960-9822(98)70252-0. [DOI] [PubMed] [Google Scholar]
- Leber R, Wise TW, Mizuta R, Meek K. The XRCC4 gene product is a target for and interacts with the DNA-dependent protein kinase. J Biol Chem. 1998;273:1794–1801. doi: 10.1074/jbc.273.3.1794. [DOI] [PubMed] [Google Scholar]
- Lehmann AR, Walicka M, Griffiths DJ, Murray JM, Watts FZ, McCready S, Carr AM. The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol Cell Biol. 1995;15:7067–7080. doi: 10.1128/mcb.15.12.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FL, Sperle K, Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci USA. 1985;82:1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Maser RS, Monsen KJ, Nelms BE, Petrini JH. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol Cell Biol. 1997;17:6087–6096. doi: 10.1128/mcb.17.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K, Shore D. Yeast Ku protein plays a direct role in telomeric silencing and counteracts inhibition by rif proteins. Curr Biol. 1999;9:1123–1126. doi: 10.1016/s0960-9822(99)80483-7. [DOI] [PubMed] [Google Scholar]
- Modesti M, Hesse JE, Gellert M. DNA binding of Xrcc4 protein is associated with V(D)J recombination but not with stimulation of DNA ligase IV activity. EMBO J. 1999;18:2008–2018. doi: 10.1093/emboj/18.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny SA, Snowden CM, McCarville J, Ramsden DA. Ku recruits the XRCC4-ligase IV complex to DNA ends. Mol Cell Biol. 2000;20:2996–3003. doi: 10.1128/mcb.20.9.2996-3003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Yoo S, Dynan WS, Jung M, Dritschilo A. Ku proteins join DNA fragments as shown by atomic force microscopy. Cancer Res. 1997;57:1412–1415. [PubMed] [Google Scholar]
- Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Petrini JH. The Mre11 complex and ATM: collaborating to navigate S phase. Curr Opin Cell Biol. 2000;12:293–296. doi: 10.1016/s0955-0674(00)00091-0. [DOI] [PubMed] [Google Scholar]
- Ramsden DA, Gellert M. Ku protein stimulates DNA end joining by mammalian DNA ligases: a direct role for Ku in repair of DNA double-strand breaks. EMBO J. 1998;17:609–614. doi: 10.1093/emboj/17.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DM, Ripley S, Henderson AS, Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981;23:29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Redon C, Boon C, Johnson K, Bonner WM. Rapid histone extraction for electrophoretic analysis. Biotechniques. 2000;28:38–46. doi: 10.2144/00281bm06. [DOI] [PubMed] [Google Scholar]
- Roth DB, Wilson JH. Relative rates of homologous and nonhomologous recombination in transfected DNA. Proc Natl Acad Sci USA. 1985;82:3355–3359. doi: 10.1073/pnas.82.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiesing JA, Ball AR, Jr, Gregson HC, Alderton JM, Zhou S, Yokomori K. Identification of two distinct human SMC protein complexes involved in mitotic chromosome dynamics. Proc Natl Acad Sci USA. 1998;95:12906–12911. doi: 10.1073/pnas.95.22.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiesing JA, Gregson HC, Zhou S, Yokomori K. A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol Cell Biol. 2000;20:6996–7006. doi: 10.1128/mcb.20.18.6996-7006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harbor Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Smith GC, Cary RB, Lakin ND, Hann BC, Teo SH, Chen DJ, Jackson SP. Purification and DNA binding properties of the ataxia-telangiectasia gene product ATM. Proc Natl Acad Sci USA. 1999;96:11134–11139. doi: 10.1073/pnas.96.20.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–4. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Song K, Jung Y, Jung D, Lee I. Human Ku70 interacts with heterochromatin protein 1alpha. J Biol Chem. 2001;276:8321–8327. doi: 10.1074/jbc.M008779200. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeting of genes to specific sites in the mammalian genome. Cold Spring Harbor Symp Quant Biol. 1986;2:1101–1113. doi: 10.1101/sqb.1986.051.01.128. [DOI] [PubMed] [Google Scholar]
- van den Engh G, Trask B, Cram S, Bartholdi M. Preparation of chromosome suspensions for flow cytometry. Cytometry. 1984;5:108–117. doi: 10.1002/cyto.990050203. [DOI] [PubMed] [Google Scholar]
- Verkade HM, Bugg SJ, Lindsay HD, Carr AM, O'Connell MJ. Rad18 is required for DNA repair and checkpoint responses in fission yeast. Mol Biol Cell. 1999;10:2905–2918. doi: 10.1091/mbc.10.9.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation, and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva M, Kowalewski T, Lieber MR. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J. 1997;16:098–5112. doi: 10.1093/emboj/16.16.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannone SM, Roy S, Chan DW, Murphy MB, Huang S, Campisi J, Chen DJ. Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J Biol Chem. 2001;276:38242–38248. doi: 10.1074/jbc.M101913200. [DOI] [PubMed] [Google Scholar]