Abstract
Dispersal of epithelial cells is an important aspect of tumorigenesis, and invasion. Factors such as hepatocyte growth factor induce the breakdown of cell junctions and promote cell spreading and the dispersal of colonies of epithelial cells, providing a model system to investigate the biochemical signals that regulate these events. Multiple signaling proteins are phosphorylated in epithelial cells during hepatocyte growth factor–induced cell dispersal, including c-Cbl, a protooncogene docking protein with ubiquitin ligase activity. We have examined the role of c-Cbl and a transforming variant (70z-Cbl) in epithelial cell dispersal. We show that the expression of 70z-Cbl in Madin-Darby canine kidney epithelial cells resulted in the breakdown of cell–cell contacts and alterations in cell morphology characteristic of epithelial–mesenchymal transition. Structure–function studies revealed that the amino-terminal portion of c-Cbl, which corresponds to the Cbl phosphotyrosine-binding/Src homology domain 2 , is sufficient to promote the morphological changes in cell shape. Moreover, a point mutation at Gly-306 abrogates the ability of the Cbl Src homology domain 2 to induce these morphological changes. Our results identify a role for Cbl in the regulation of epithelial–mesenchymal transition, including loss of adherens junctions, cell spreading, and the initiation of cell dispersal.
INTRODUCTION
The dissociation and migration of epithelial cell sheets are required during normal embryonic development and during pathological situations such as the dispersal of tumor cells (Gherardi, 1991). Epithelial cell dispersal is a complex process that requires the breakdown of cell–cell junctions in addition to the remodeling of the actin cytoskeleton and cell adhesion complexes. These changes contribute to a transition from an epithelial morphology toward a more mesenchymal fibroblastic phenotype, referred to as epithelial–mesenchymal transition (Boyer et al., 1996). Hepatocyte growth factor (HGF) and its receptor, the Met tyrosine kinase, is one of the predominant modulators of epithelial–mesenchymal transition described to date; it provides a model to examine the molecular signals involved in the regulation of epithelial cell dispersal (Birchmeier et al., 1996).
Little is known about the signaling pathways that regulate the dissociation and dispersal of epithelial cell sheets or colonies. To identify signal transduction pathways that are involved in the dispersal and invasion of epithelial cell sheets, we and others have performed structure-function analyses of the Met receptor tyrosine kinase with the use of an epithelial Madin-Darby canine kidney (MDCK) cell model (Komada and Kitamura, 1993; Weidner et al., 1993; Zhu et al., 1994a). Downstream from the Met receptor, the most highly tyrosine-phosphorylated proteins correspond to the Cbl and Gab1 docking proteins (Fixman et al., 1997; Nguyen et al., 1997; Maroun et al., 1999). Gab1 acts as a docking protein (Holgado-Madruga et al., 1996) that promotes a morphogenic program in response to Met activation in epithelial cells (Weidner et al., 1996; Maroun et al., 1999), whereas the function of c-Cbl in epithelial cell signaling has not been addressed.
c-Cbl becomes tyrosine phosphorylated after stimulation of multiple receptor tyrosine kinases, cytokine receptors, and integrin receptors (reviewed by Miyake et al., 1997). c-Cbl contains multiple protein interaction motifs, including tyrosine residues whose phosphorylation promotes the association of c-Cbl with numerous cytoplasmic signaling proteins, a phosphotyrosine-binding domain (PTB) that encompasses a recently identified Src homology domain 2 (SH2) (Meng et al., 1999), and a proline-rich motif. These domains allow the interaction of c-Cbl with multiple signal transducers, including the p85 subunit of phosphatidylinositol 3′ kinase, the Crk, Grb2, and Nck adaptor proteins, and Src family kinases, suggesting that c-Cbl acts as a docking protein to integrate signaling pathways (reviewed by Miyake et al., 1997). The precise biological function of c-Cbl is unclear, and both negative and positive roles for c-Cbl have been proposed.
Genetic and biochemical studies have implicated c-Cbl in the attenuation of tyrosine kinase–mediated signals. For example, Sli-1 in Caenorhabditis elegans, which shares 55% identity with the amino-terminal portion of mammalian c-Cbl, acts as a negative regulator downstream of the EGF receptor tyrosine kinase homologue Let23 (Jongeward et al., 1995; Yoon et al., 1995). Moreover, treatment of fibroblasts with Cbl antisense oligonucleotides results in enhanced EGF receptor signaling (Ueno et al., 1997), whereas overexpression of c-Cbl decreases FCγR signaling in mast cells (Ota and Samelson, 1997). This is consistent with the demonstration that c-Cbl acts as a ubiquitin ligase (Joazeiro et al., 1999; Levkowitz et al., 1999) and leads to the increased rate of ubiquitination and degradation of several receptor tyrosine kinases, including the receptors for EGF, PDGF, and colony-stimulating factor 1 (CSF-1) (Levkowitz et al., 1998; Miyake et al., 1998; Lee et al., 1999). In contrast to their negative role downstream from tyrosine kinases, treatment with antisense Cbl oligonucleotides blocks cell spreading on fibronectin surfaces (Meng and Lowell, 1998), and c-Cbl appears to play a positive role in integrin-mediated signaling downstream from Src family kinases (Tanaka et al., 1996; Manie et al., 1997; Ojaniemi et al., 1997; Zell et al., 1998).
In addition to c-Cbl, two transforming variants of this protein have been identified: v-cbl, or Cbl-N, was isolated from a Cas NS-1–transforming retrovirus and contains only the first 357 residues of c-Cbl, which encompasses the recently identified SH2/PTB domain (Langdon et al., 1989; Lupher et al., 1996; Meng et al., 1999); 70z-Cbl is a naturally occurring mutant isolated from 70z/3 preB cell lymphomas that transforms fibroblasts in culture (Andoniou et al., 1994). 70z-Cbl contains a 17-amino acid deletion in the RING domain (Andoniou et al., 1994) necessary for the ubiquitin ligase activity and exhibits an enhanced level of tyrosine phosphorylation in the absence of cell stimulation (Andoniou et al., 1994), which allows its association with SH2 domain–containing signaling proteins (van Leeuwen et al., 1999).
Because c-Cbl is implicated in Met- and integrin-dependent signaling, we have examined the role of Cbl in epithelial cell dispersal. We show that c-Cbl is phosphorylated after stimulation of the Met receptor with HGF and that this promotes the coupling of c-Cbl with signaling proteins, including the p85 subunit of phosphatidylinositol-3′-kinase (PI3′-K) and Crk. Expression of the 70z-Cbl variant in MDCK cells leads to alterations in cell morphology characteristic of epithelial–mesenchymal transition, and structure–function studies reveal that the c-Cbl SH2/PTB domain is sufficient to induce these changes in cell shape.
MATERIALS AND METHODS
Cell Culture and DNA Transfections
COS-1 and MDCK cells were maintained in DMEM containing 10% FBS (Life Technologies, Grand Island, NY). The generation of stable cell lines expressing the chimeric CSF-MET receptor has been described elsewhere (Zhu et al., 1994b). Cell lines expressing the Met receptor tyrosine kinase or hemagglutinin (HA)-tagged c-Cbl or 70z-Cbl were generated by retroviral infection of MDCK cells with the use of amphotropic retrovirus, as described previously (Rodrigues and Park, 1993). Cell lines were selected in 400 μg/ml G418. For the generation of cell populations expressing wild-type and mutant HA-tagged 70z-Cbl, the 70z-Cbl cDNA was cloned into the pRK5 expression vector at the BamHI site and cotransfected with the pLXSH vector, which confers resistance to hygromycin, by the calcium phosphate method, as described elsewhere (Wigler et al., 1979). Cell populations were selected in 300 μg/ml hygromycin.
Reagents/Constructs and Antibodies
Antibodies raised in rabbit against a carboxyl-terminal peptide of human Met (143) were used (Rodrigues et al., 1991). Anti-phosphotyrosine antibody (4G10) was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-HA antibody (HA.11) was purchased from BabCo (Richmond, CA). Anti-E-cadherin antibody was obtained from the American Type Culture Collection (Rockville, MD) hybridoma bank. Anti-vinculin antibody was purchased from Sigma Chemical (Oakville, Ontario, Canada). Anti-Cbl (C-15) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphospecific JNK and MAPK antibodies were purchased from New England Biolabs (Mississauga, Ontario, Canada). Anti-Cas antibody was kindly provided by Dr. Michel Tremblay (McGill University), and the anti-EGF receptor (EGFR) antibody was kindly provided by Drs. John Bergeron and Barry Posner (McGill University). Anti-Crk antibody was purchased from Transduction Laboratories (Mississauga, Ontario, Canada). rhCSF-1 was kindly provided by the Genetics Institute (Cambridge, MA).
Cell Stimulation, Immunoprecipitation, and Western Blotting
MDCK cells were seeded at a density of 106 cells in a 100-mm tissue culture dish, serum starved the next day in 0.02% FBS for 48 h, stimulated with 100 ng/ml CSF, 250 ng/ml (100 U) HGF, or 1 ng/ml EGF for the indicated periods, and harvested. Cells were lysed in 1 ml of lysis buffer containing 0.5% Triton X-100, 50 mM HEPES, pH 8.0, 150 mM NaCl, 10% glycerol, 2 mM EGTA, 1.5 mM MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM sodium vanadate, and 1 mM PMSF. Equal amounts of total protein were immunoprecipitated and immunoblotted as described previously (Fixman et al., 1995). Immunoreactive bands were visualized by ECL (Amersham Pharmacia Biotech, Uppsala, Sweden).
Immunofluorescence
MDCK cells expressing c-Cbl or 70z-Cbl were plated on glass coverslips (Bellco Glass, Vineland, NJ) in a 24-well dish (Nunc, Burlington, Ontario, Canada) in DMEM containing 10% FBS. After 24 h, cells were incubated for 10 min at room temperature in cytoskeletal extraction (CSK) buffer containing 10 mM 1,4-piperazindiethansulfonic acid, pH 7.0, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, and 0.5% Triton X-100 to remove proteins that are not linked to the cytoskeleton. After two washes in PBS, cells were fixed in 3.7% formaldehyde in PBS for 15 min at room temperature and washed twice in PBS. Fixed cells were treated for 10 min at room temperature with PBS containing 5% FBS and 0.1% Triton X-100 (buffer A). Anti-E-cadherin antibody (1:300 dilution in buffer A) was then added to the cells for 15 min, and after three washes in the same buffer, Cy3-conjugated goat anti-mouse immunoglobulin G (1:2000 dilution in buffer A) was added for 15 min and the cells were washed three times in buffer A. For the double staining of both vinculin and actin, cells were first incubated for 10 min with anti-vinculin antibody (1:300 dilution in buffer A), followed by a mouse anti-Alexa 488 antibody (1:1000 dilution in buffer A) for 10 min, and finally with phalloidin–TRITC for 30 min (1:2000 dilution in buffer A), with three washes in buffer A between each incubation. The glass coverslips were mounted onto slides in Immunofluore medium (ICN, Costa Mesa, CA) and visualized with a Zeiss (Thornwood, NY) Axiovert 135 incident-light fluorescence microscope.
Cell Migration Assays
Migration assays were performed with the use of a motility chamber (8 mm, 6 μm) from Neuroprobe (Gaithersburg, MD). A total of 105 cells, suspended in 25 μl of DMEM/10% FBS, were placed on the polycarbonate membrane in the upper chamber, and 29 μl of DMEM/10% FBS was placed in the lower chamber. The migration chambers were then incubated at 37οC for the indicated times and subsequently fixed in 10% formalin-buffered phosphate for 15 min at room temperature. Cells that had not migrated and remained on the upper side of the membrane were removed with a cotton applicator. Cells that had migrated through the membrane were stained with 0.1% crystal violet/20% methanol for 20 min at room temperature, and filters were washed three times before drying. The crystal violet–stained cells were solubilized in 500 μl of 10% acetic acid, and the optical densities were measured at a wavelength of 590 nm. Scatter assays were performed as described by Royal and Park (1995).
Time-lapse Video Microscopy
Cells were seeded at a low density for 24 h, and the cell medium was replaced with CO2-independent medium containing 10% FBS and 50 mM l-glutamine. The cells were subsequently placed on a heated microscope stage, and digital images of the same field were taken every 20 min for a 14-h period. The video images were processed with the use of an image-processing system (Northern Eclipse, Empix Imaging, Toronto, Ontario, Canada).
GST Association Assay
Bacteria expressing the amino-terminal SH2 domain of p85 fused to GST (GST-N-SH2 p85) were kindly provided by Dr. Tony Pawson (Samuel Lunenfield Research Institute, University of Toronto). Bacteria expressing the two Src homology domain 3 (SH3) domains of Grb2 fused to GST (GST-N+C-SH3 Grb2) were kindly provided by Dr. Michel Tremblay. The bacteria expressing the amino-terminal portion of c-Cbl (amino acids 1–356; GST-Cbl-N) fused to GST (GST-Cbl-N) as well as the mutant G306E Cbl-N (GST-G306E Cbl-N) have been described elsewhere (Lupher et al., 1996). Fusion proteins were produced by isopropyl-β-d-thiogalactopyranoside induction, and purification was carried out on glutathione–Sepharose beads (Smith and Johnson, 1988). Lysates were prepared from MDCK cell lines or populations, as described above, and incubated with ∼0.5–1.0 μg of the GST fusion proteins coupled to glutathione–Sepharose beads for 1 h at 4°C. Complexes were washed in 0.5% Triton X-100 lysis buffer and resuspended in Laemmli sample buffer. Proteins complexed with the GST fusion proteins were subject to SDS-PAGE and Western blotting, as described above.
RESULTS
Overexpression of 70z-Cbl in MDCK Cells Induces Loss of Epithelial Cell Morphology
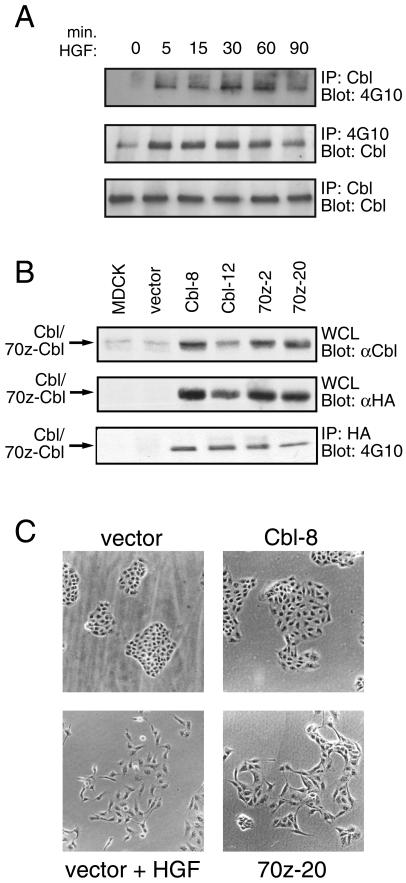
Previous studies have focused on the function of c-Cbl in hematopoietic cells and in fibroblast models; however, c-Cbl is also highly expressed in epithelial cells, including MDCK cells (Figure 1A, lower panel). To investigate the role of c-Cbl in epithelial cells and downstream from the Met receptor, colonies of MDCK cells were stimulated with HGF, endogenous c-Cbl protein was immunoprecipitated, and the immunocomplexes were analyzed by anti-phosphotyrosine immunoblotting. Phosphorylation of c-Cbl reached a peak within 5 min, was maintained for 60 min, and returned to near baseline levels after 90 min of HGF stimulation (Figure 1A, upper panel). The increase in tyrosine phosphorylation of c-Cbl was not due to changes in the level of c-Cbl protein after HGF stimulation (Figure 1A, lower panel). Moreover, proteins immunoprecipitated with anti-phosphotyrosine antibody and immunoblotted with anti-Cbl antibody revealed similar kinetics of c-Cbl phosphorylation (Figure 1A, middle panel), demonstrating HGF-dependent tyrosine phosphorylation of c-Cbl in epithelial MDCK cells.
Figure 1.
Overexpression of 70z-Cbl in MDCK cells results in alterations in cell morphology. (A) MDCK cells were serum starved for 48 h and stimulated for the indicated times with 100 U/ml HGF. Equal amounts of cell lysate were immunoprecipitated with either anti-Cbl antibody (upper and lower panels) or anti-phosphotyrosine antibody (middle panel). The proteins were resolved by SDS-PAGE and immunoblotted with anti-phosphotyrosine (upper panel) or anti-Cbl (middle and bottom panels) antibodies. (B) Proteins from whole cell lysates from stable MDCK cell lines were separated by SDS-PAGE and immunoblotted with either anti-Cbl (upper panel) or anti-HA antibody (middle panel). HA-tagged Cbl proteins were immunoprecipitated with anti-HA antibody, separated by SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (lower panel). (C) MDCK cell lines expressing vector, c-Cbl (clone 8), or 70z-Cbl (clone 20) were incubated for 24 h with or without 5 U/ml HGF, as indicated, and cell colonies were visualized by light microscopy.
To investigate the function of c-Cbl in epithelial cell dispersal, clonal lines of MDCK cells were generated expressing either HA-tagged c-Cbl or the 70z-Cbl variant. Both c-Cbl and 70z-Cbl were overexpressed compared with the endogenous levels of Cbl protein in epithelial cells, as analyzed by immunoblotting with anti-Cbl serum. When overexpressed, both c-Cbl and 70z-Cbl were constitutively phosphorylated on tyrosine to similar levels, as analyzed by immunoblotting with anti-phosphotyrosine antibody (Figure 1B). MDCK cells expressing the control vector or c-Cbl (Cbl-8) form discrete colonies when seeded at low density, although MDCK cells expressing c-Cbl are larger and flatter in morphology (Figure 1C). Strikingly, when seeded at low cell density, MDCK cell lines expressing 70z-Cbl either fail to grow in colonies or form only loose colonies (Figure 1C, showing one representative cell line 70z-20). Moreover, MDCK cells expressing 70z-Cbl lose their epithelial cobblestone morphology, adopt an elongated mesenchymal-like cell morphology, and exhibit membrane ruffling and extensions that partially resemble MDCK cells when stimulated with HGF (Figure 1C, vector + HGF).
70z-Cbl Induces Disruption of Adherens Junctions and Reorganization of Actin and Adhesion Complexes
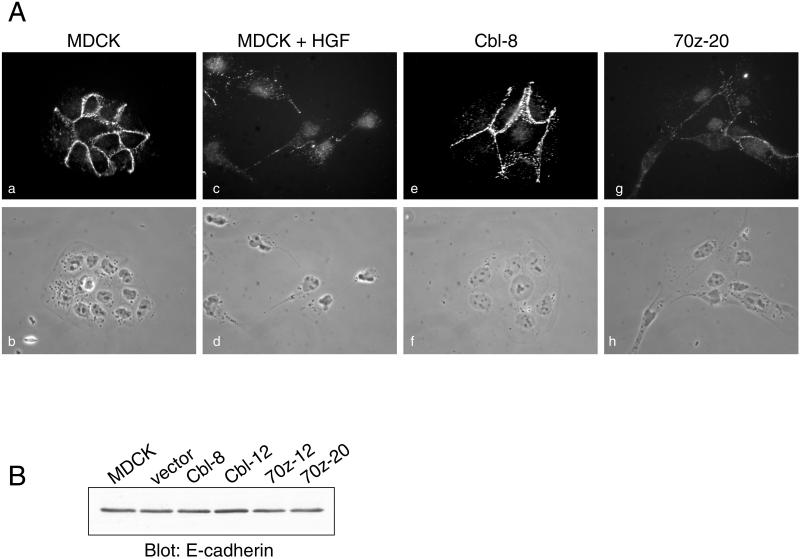
Stimulation of colonies of MDCK cells with HGF promotes the breakdown of cell–cell contacts maintained by the major epithelial adhesion molecule, E-cadherin. In response to HGF, E-cadherin becomes redistributed from insoluble complexes localized at cell–cell junctions to soluble complexes that are no longer associated with the actin cytoskeleton (Royal and Park, 1995; Potempa and Ridley, 1998) (Figure 2A, a and c). Hence, we examined whether expression of c-Cbl or 70z-Cbl affected E-cadherin localization. In MDCK cell lines expressing c-Cbl, E-cadherin is localized to cell–cell junctions and is in an insoluble compartment after treatment of cells with CSK buffer (Figure 2A, e). In contrast, MDCK cell lines expressing 70z-Cbl reveal a reduced level of E-cadherin in the insoluble fraction after CSK treatment, which corresponds to a decrease in cell–cell contacts (Figure 2A, g). However, the total level of cellular E-cadherin is unaltered in cells expressing 70z-Cbl (Figure 2B), suggesting a redistribution of E-cadherin from an insoluble to a soluble compartment in cells expressing 70z-Cbl.
Figure 2.
Overexpression of 70z-Cbl promotes the loss of cell–cell contacts. (A) Parental MDCK cells, unstimulated or stimulated with 5 U/ml HGF for 24 h, as well as stable cell lines expressing c-Cbl (clone 8) and 70z-Cbl (clone 20), were grown on glass coverslips in DMEM containing 10% FBS. Cells were treated for 10 min with CSK buffer, fixed in 3.7% formaldehyde, and then labeled with anti-E-cadherin antibody followed by Cy3-conjugated anti-mouse antiserum (a, c, e, and g). Matching phase-contrast images are shown (b, d, f, and h). Photographs were taken at a magnification of ×63. (B) Whole cell lysates were separated on an 8% SDS-PAGE gel and immunoblotted with anti-E-cadherin antibody.
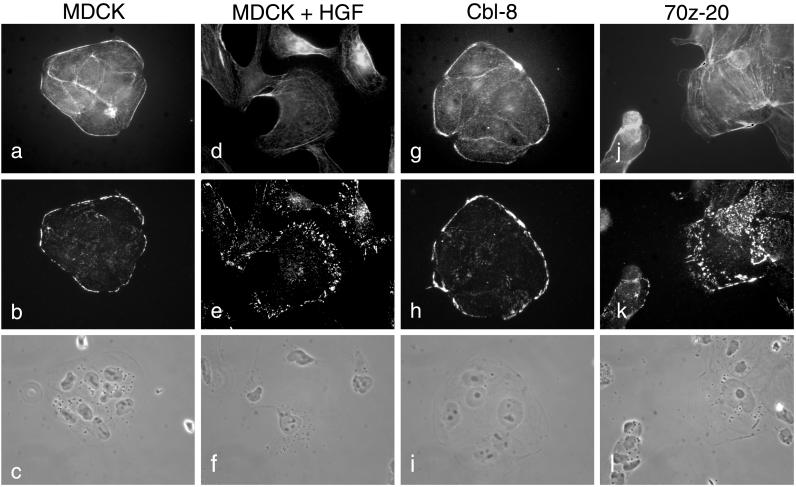
Cell dispersal and changes in cell morphology are concomitant with the reorganization of both the actin cytoskeleton and cell–extracellular matrix contacts. Therefore, we examined the impact of expression of c-Cbl or 70z-Cbl on the F-actin cytoskeleton and focal adhesion complexes by indirect immunofluorescence with the use of phalloidin and anti-vinculin antibody, respectively. In parental MDCK or c-Cbl–overexpressing cells, visualization of F-actin with phalloidin–TRITC reveals that actin is concentrated at the edge of the colonies as well as at cell–cell borders in peripheral bundles associated with the lateral membranes of the cells (Figure 3, a and g). In addition, vinculin-containing adhesion complexes are localized predominantly at the perimeter of the cell colony (Figure 3, b and h). After stimulation of MDCK cells with HGF, adhesion complexes are redistributed to the lamellipodia structures (Figure 3, d and e). In contrast, in the absence of HGF stimulation, MDCK cells expressing 70z-Cbl show a decrease in peripheral actin, and actin stress fibers appear within lamellipodia-like structures (Figure 3j). Moreover, MDCK cells expressing 70z-Cbl show a striking reorganization of vinculin-containing focal complexes from the outer edge of the colony to the large lamellipodia-like structures present in the 70z-Cbl–expressing cells, in addition to many focal contacts throughout the cells (Figure 3k). Together, these data are consistent with the overexpression of 70z-Cbl promoting a switch in equilibrium in MDCK cells from maintaining cell–cell interactions to forming cell–extracellular matrix interactions, as characterized by the breakdown of adherens junctions, and the reorganization of actin and focal complexes.
Figure 3.
Reorganization of actin and focal contacts in MDCK cells overexpressing 70z-Cbl. MDCK cells (a–c), MDCK cells stimulated with 5 U/ml HGF for 24 h (d–f), c-Cbl (clone 8; g–i), and 70z-Cbl (clone 20; j–l) were grown on glass coverslips for 24 h and treated with CSK buffer for 10 min. After fixation, the cells were double-labeled with phalloidin (a, d, g, and j) and anti-vinculin antibody (b, e, h, and k). Matching phase-contrast images are shown (c, f, i, and l). Photographs were taken at a magnification of ×63.
c-Cbl and 70z-Cbl Associate with Similar Signaling Proteins
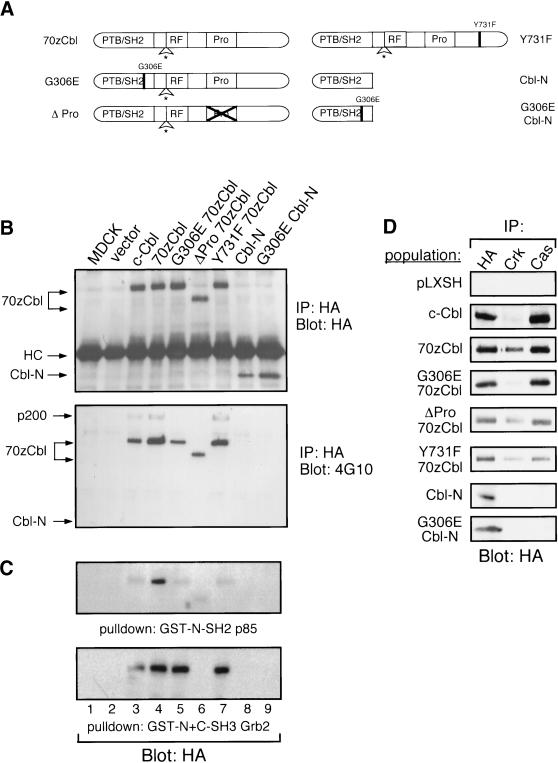
To delineate the domain(s) of 70z-Cbl that are responsible for the cell dissociation and altered cell morphology observed, a structure–function analysis was performed with the use of various mutants of 70z-Cbl, shown in Figure 4A. Association of c-Cbl with PI3′-K was suggested to be required for integrin-dependent spreading in macrophages (Meng and Lowell, 1998); thus, we examined a 70z-Cbl mutant (Y731F) that is predicted to uncouple the 70z-Cbl protein from the p85 subunit of PI3′-K (Hunter et al., 1999). The ΔPro mutant deletes the entire proline-rich region, which is predicted to bind SH3 domain–containing proteins, such as Grb2. To determine the requirement for the c-Cbl SH2/PTB domain, we assessed the activity of Cbl proteins that contained only the SH2/PTB (Cbl-N) domain (Lupher et al., 1996; Hunter et al., 1999; Meng et al., 1999) or contained a point mutation (G306E 70z-Cbl, G306E Cbl-N) corresponding to a mutation within the SH2/PTB domain that inactivates the C. elegans homologue (SLI-1) (Yoon et al., 1995).
Figure 4.
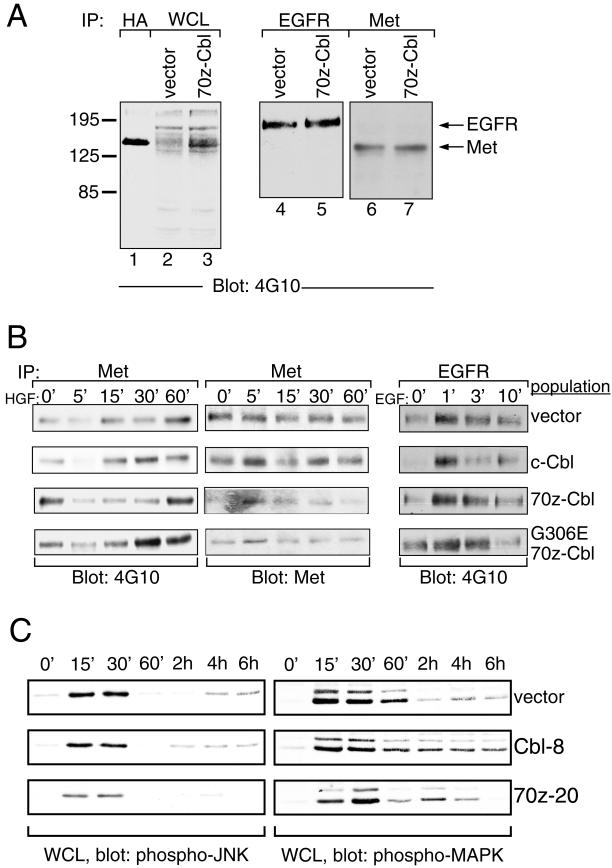
Constitutive phosphorylation of 70z-Cbl when expressed in MDCK cells. (A) Scheme of 70z-Cbl and its mutant variants. PTB/SH2, phosphotyrosine-binding SH2 domain; RF, RING finger domain; Pro, proline-rich domain. (B) 70z-Cbl and its variants were immunoprecipitated from MDCK cell populations, separated on an 8% SDS-PAGE gel, and immunoblotted with anti-HA (upper panel) or anti-phosphotyrosine (lower panel) antibodies. (C) Lysates from MDCK cell populations were incubated with the amino-terminal SH2 domain of p85 (GST-N-SH2 p85; upper panel) or the amino- and carboxyl-terminal SH3 domains of Grb2 (GST-N+C-SH3 Grb2; lower panel). Proteins were separated on an 8% SDS-PAGE gel, transferred to nitrocellulose, and immunoblotted with anti-HA antibody. (D) MDCK cell populations were immunoprecipitated with the indicated antibodies, resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-HA antibody.
Populations of MDCK cells expressing each mutant were established, and the synthesis and functional integrity of each variant protein were confirmed through immunoprecipitation and immunoblotting with an anti-HA antibody. All proteins were synthesized and expressed to similar levels in the MDCK cell populations (Figure 4B, top panel). Immunoprecipitation of c-Cbl and 70z-Cbl proteins from cell populations with anti-HA antibody and subsequent immunoblotting with anti-phosphotyrosine antibody revealed constitutive phosphorylation of c-Cbl, 70z-Cbl, G306E 70z-Cbl, ΔPro 70z-Cbl, and the Y731F 70z-Cbl proteins when overexpressed (Figure 4B, bottom panel). A decrease in phosphorylation was seen in the G306E 70z-Cbl and ΔPro 70z-Cbl mutant proteins and in c-Cbl compared with 70z-Cbl. Cbl-N and the G306E Cbl-N mutant proteins were not detectably phosphorylated.
In fibroblasts and hematopoietic cells, c-Cbl interacts with the Grb2 adaptor protein as well as with the SH2 domains of the p85 subunit of PI3′-K and the Crk adaptor protein (reviewed by Miyake et al., 1997). To establish which of these proteins interact with c-Cbl in epithelial cells, the ability of c-Cbl, 70z-Cbl, and its mutant variants to associate with these signaling proteins was examined. A GST fusion protein containing the amino- and carboxyl-terminal SH3 domains of Grb2 (GST-N+C-SH3 Grb2) associated with c-Cbl, 70z-Cbl, and the Y731F 70z-Cbl mutant but failed to associate with the ΔPro 70z-Cbl or Cbl-N proteins (Figure 4C). A GST fusion protein containing the amino-terminal SH2 domain of p85 (GST-N-SH2 p85) interacted predominantly with 70z-Cbl and to a lesser extent with c-Cbl and the G306E, ΔPro, and Y731F 70z-Cbl mutant proteins but not with Cbl-N (Figure 4C). Similarly, by coimmunoprecipitation, the Crk adaptor protein associated predominantly with 70z-Cbl and to a lesser extent with c-Cbl and the G306E, ΔPro, and Y731F 70z-Cbl mutant proteins but not with Cbl-N (Figure 4D). In addition to known associating proteins, we observed association of c-Cbl and 70z-Cbl but not Cbl-N or the G306E 70z-Cbl mutant with the multisubstrate-binding protein p130 Cas (Figure 4D).
The SH2/PTB Domain of c-Cbl (Cbl-N) Is Sufficient to Induce Epithelial-to-Mesenchymal Transition of MDCK Cells
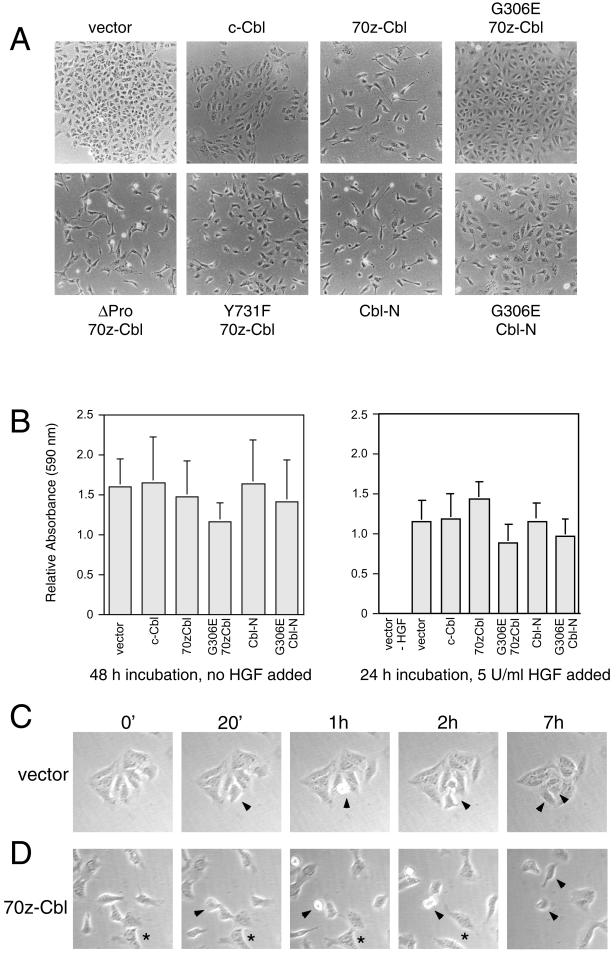
To establish if specific c-Cbl–dependent signals were required for the changes in cell shape observed in Figure 1, the morphology of MDCK cells expressing c-Cbl, 70z-Cbl, and its mutants was examined. As shown previously, MDCK cells expressing vector alone grew as colonies of tightly associated cells, whereas those expressing 70z-Cbl were dissociated and contained lamellipodia-like extensions (Figure 5A). Although the p85-binding site in c-Cbl is implicated in integrin-dependent cell spreading (Zell et al., 1998; Feshchenko et al., 1999), overexpression of the Y731F 70z-Cbl mutant, which fails to bind PI3′-K (Figure 4C), or the ΔPro 70z-Cbl mutant, which fails to bind Grb2 (Figure 4C), induced morphological changes in MDCK cells similar to 70z-Cbl (Figure 5A). Significantly, the overexpression of the SH2/PTB domain of c-Cbl (Cbl-N) induced dissociation and the acquisition of a mesenchymal phenotype in MDCK cells, similar to MDCK cells expressing 70z-Cbl. In contrast, a 70z-Cbl or Cbl-N mutant containing a single substitution that is thought to disrupt the ability of this domain to interact with phosphotyrosine residues (G306E 70z-Cbl or G306E Cbl-N) failed to induce dissociation and the acquisition of a mesenchymal phenotype when expressed in MDCK cells (Figure 5A). Thus, the SH2/PTB domain of c-Cbl is sufficient to induce dissociation and morphological changes in MDCK cells.
Figure 5.
The SH2/PTB domain of c-Cbl is sufficient to induce morphological changes. (A) MDCK cells were transfected with 70z-Cbl and its variants, as described in MATERIALS AND METHODS, and after selection in hygromycin, changes in cell morphology were visualized by light microscopy. (B) Cells expressing 70z-Cbl and its variants were placed in the upper chamber of a modified Boyden chamber (Neuroprobe; 8 mm, 6 μm) and allowed to migrate through the filter for either 48 h in the absence of HGF stimulation (left panel) or 24 h in the presence of 5 U/ml HGF (right panel). Cells that had traversed the filter were stained with crystal violet, solubilized in 10% acetic acid, and measured at an optical density of 590 nm. Each of these samples was carried out in triplicate. A value of 1 represents the relative motility rate of parental MDCK cells in this assay. MDCK cells (C) and the 70z-Cbl cell line (clone 20) (D) were seeded at low density, and after the formation of small colonies, were subject to time-lapse video microscopy. Photographs were taken of the same field every 20 min for 14 h. The arrowheads in the MDCK (C) and 70z-Cbl (D) panels indicate dividing cells, whereas the asterisks in the images of 70z-Cbl–expressing cells indicate two cells that have dissociated and dispersed.
The changes in cell morphology in MDCK cells expressing 70z-Cbl mimic the early stages of epithelial cell spreading, dissociation, and subsequent cell dispersal observed after HGF stimulation. To determine if the expression of 70z-Cbl leads to an increase in the spontaneous rate of motility of MDCK cells, as suggested by the dispersal of cell colonies, the migration rates of MDCK cell populations expressing c-Cbl, 70z-Cbl, Cbl-N, or the G306E mutant proteins were measured. As shown in Figure 5B, no difference was observed in a modified Boyden chamber assay in the overall rate of migration of individual MDCK cells expressing the control vector, c-Cbl, 70z-Cbl, or Cbl-N when seeded as single cells, either in the absence or presence of HGF. This demonstrates that expression of 70z-Cbl or Cbl-N did not enhance spontaneous or HGF-induced motility of single MDCK cells. Because these assays measure the motility of single cells before colony formation, we examined colonies of MDCK cells expressing vector alone or 70z-Cbl with the use of time-lapse photography. When observed by time-lapse photography, MDCK cells expressing vector alone maintained cell–cell contacts and formed colonies, and any dividing cells were retained within the colony (Figure 5C, arrowheads). Moreover, although several cells at the edge of the colony exhibited membrane ruffles and formed membrane extensions, these cells retained their cell–cell associations and failed to detach from the colony during a 14-h period. In contrast, MDCK cells expressing 70z-Cbl dispersed after cell division (Figure 5D, arrowheads), consistent with the observed decrease in cell–cell adhesion. Similarly, cells that appear in association are able to disperse throughout the time course (Figure 5D, asterisks). Together, these data further support the ability of 70z-Cbl to promote a decrease in cell–cell adhesion that subsequently results in cell dispersal.
Expression of 70z-Cbl or Cbl-N in MDCK Cells Does Not Detectably Enhance the Tyrosine Phosphorylation of the Met or EGF Receptors
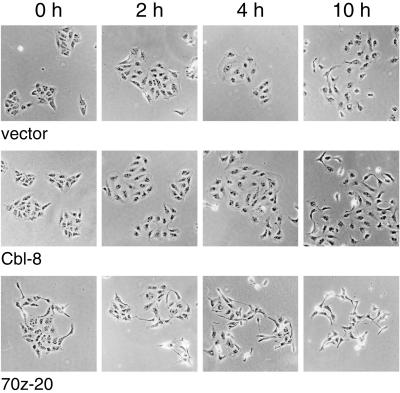
The overexpression of 70z-Cbl and Cbl-N in fibroblasts enhances the basal kinase activity and phosphotyrosine levels of the PDGF and EGF receptors in the absence of ligand and in the presence of suboptimal concentrations of ligand (Bonita et al., 1997; Thien and Langdon, 1997). This is thought to be modulated through the ability of the 70z-Cbl protein to compete with c-Cbl and release the negative regulatory ac-tivity of c-Cbl on these kinases (Joazeiro et al., 1999; Levkowitz et al., 1999). Thus, one may speculate that expression of 70z-Cbl may enhance the basal catalytic activity of the Met receptor or other receptor tyrosine kinases, which in turn promotes dissociation of MDCK cells. To establish if there was an increase in the basal phosphorylation of either the Met or EGF receptor in MDCK cell populations expressing 70z-Cbl, proteins from whole cell lysates were subjected to SDS-PAGE and immunoblotted with anti-phosphotyrosine antibodies. No significant increase was observed in total tyrosine-phosphorylated proteins in MDCK cells expressing 70z-Cbl, except for a protein of ∼120 kDa, which comigrated with 70z-Cbl (Figure 6A, lanes 1 and 3). Although both the EGF and Met receptors were phosphorylated in unstimulated MDCK cells expressing 70z-Cbl, the level of phosphorylation was not enhanced above the basal level observed in MDCK cells expressing vector alone (Figure 6A, lanes 4–7). Moreover, the phosphorylation kinetics of the Met or EGF receptor were not altered significantly in MDCK cells overexpressing c-Cbl, 70z-Cbl, and G306E 70z-Cbl compared with cells expressing vector alone (Figure 6B). Consistent with this finding, similar profiles of activity of downstream targets of the Met receptor, MAPK and JNK, were observed in MDCK cells transfected with vector alone or in cells overexpressing c-Cbl or 70z-Cbl. The baseline activities of MAPK and JNK were not increased in 70z-Cbl–expressing MDCK cells compared with MDCK or c-Cbl–expressing cells in the absence of stimulation (Figure 6C). Furthermore, after HGF stimulation, JNK and MAPK showed a similar time course of activation in all cell lines, as detected with the use of antiserum that recognizes the activated phosphorylated forms of each of these proteins (Figure 6C). In accord with the similar phosphorylation profiles of the Met receptor and downstream targets in vector and c-Cbl (Cbl-8)–expressing cells in response to HGF, these cells dispersed with similar kinetics and were fully dispersed after 10 h of HGF treatment (Figure 7). In contrast, 70z-Cbl–expressing cells, which were partially dispersed when unstimulated, showed full dispersal 4 h after HGF stimulation (Figure 7).
Figure 6.
Phosphorylation kinetics of EGF and Met receptors in cells expressing c-Cbl or 70z-Cbl. (A) Whole cell lysates (WCL) of MDCK cell populations expressing vector or 70z-Cbl were resolved on an SDS-PAGE gel and immunoblotted with anti-phosphotyrosine antibody (lanes 2 and 3). As a control for the size of 70z-Cbl, anti-HA immunoprecipitation was performed on cell lysates expressing 70z-Cbl protein (lane 1). Lysates prepared from MDCK cell populations expressing vector or 70z-Cbl were immunoprecipitated with anti-EGF receptor antibody (lanes 4 and 5) or the anti-Met receptor antibodies (lanes 6 and 7), resolved on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine antibody. (B) MDCK cell populations were serum starved for 48 h before stimulation with either 100 U/ml HGF (left and middle panels) or 1 ng/ml EGF (right panel) for the indicated times. Cell lysates were immunoprecipitated with either anti-Met (left and middle panels) or anti-EGFR (right panel) antibody. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine (left and right panels) and anti-Met (middle panel) antibodies. (C) Cell lines were stimulated with HGF for 15 min, after which cells were washed in serum-free medium and harvested at the indicated times. Whole cell lysates were subjected to immunoblotting with phosphospecific JNK or MAPK antibodies.
Figure 7.
c-Cbl–expressing MDCK cells scatter with similar kinetics as control MDCK cells. MDCK cell lines were plated in 24-well dishes and allowed to form colonies. Cells were stimulated with 5 U/ml HGF and fixed at intervals after HGF stimulation.
Cbl-N Associates with Specific Phosphotyrosine-containing Proteins in MDCK Cells
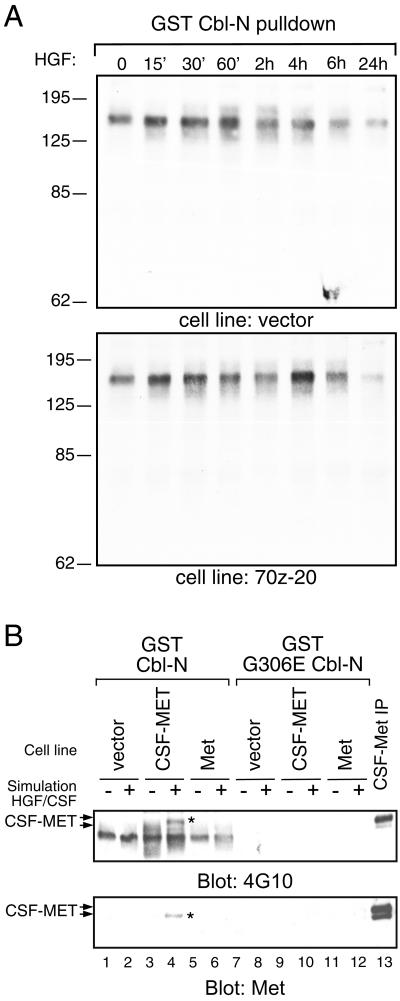
Because Cbl-N is sufficient to induce changes in cell shape and corresponds to a SH2/PTB domain that can associate with phosphotyrosine-containing proteins, we sought to establish whether a GST fusion protein expressing the amino SH2/PTB region of c-Cbl (GST-Cbl-N) associates with specific phosphoproteins in MDCK cells. Parental MDCK cells, as well as an MDCK cell line expressing 70z-Cbl, were either unstimulated or stimulated with HGF for the indicated times, and cell lysates were subject to a pulldown assay with GST-Cbl-N as well as the loss-of-function GST-G306E Cbl-N. Complexes were immunoblotted with anti-phosphotyrosine antibodies to identify tyrosine-phosphorylated proteins that associated with Cbl-N. A tyrosine-phosphorylated protein migrating at ∼130–150 kDa was detected in association with Cbl-N but not with the G306E Cbl-N mutant (Figure 8, A and B, upper panels). The presence of several phosphoproteins in this size range, including Fak, Pyk2, and Cas, as well as c-Cbl and the Met receptor was examined by Western blotting, but at the level of detection none of these correspond to any of the phosphotyrosine-associated proteins (our unpublished results and Figure 8B). However, GST-Cbl-N pulldowns in MDCK cells overexpressing a chimeric CSF-MET receptor demonstrated the ability to precipitate a phosphorylated protein of ∼150–170 kDa after CSF stimulation (Figure 8B, upper panel). Subsequent immunoblotting of the GST-Cbl-N pulldowns with anti-Met antibody revealed that the 170-kDa band was the CSF-MET receptor (Figure 8B, lower panel), demonstrating that when overexpressed CSF-MET can interact with Cbl-N in pulldown assays.
Figure 8.
Cbl-N associates with phosphoprotein(s) of ∼150 kDa. (A) Lysates prepared from MDCK cells or an MDCK cell line expressing 70z-Cbl (clone 20) were incubated with the GST fusion protein of the amino-terminal portion of c-Cbl (GST-Cbl-N). Associated proteins were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine antibody. (B) MDCK cells or MDCK cells overexpressing either the Met receptor (Met) or the chimeric CSF-MET receptor (CSF-MET) were serum starved for 48 h before stimulation with either 100 U/ml HGF (lanes 2, 6, 8, and 12) or 100 ng/ml CSF (lanes 4 and 10). Cell lysates were subject to a pulldown assay with the use of GST-Cbl-N (lanes 1–6) and GST-G306E Cbl-N (lanes 7–12). Proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine (upper panel) or anti-Met (lower panel) antibodies.
DISCUSSION
The roles of many signaling pathways involved in mitogenesis have been established, whereas only recently have studies focused on pathways leading to breakdown of cell junctions, epithelial cell dispersal, and morphogenesis in matrix culture. We have shown previously that c-Cbl is phosphorylated downstream from an oncogenic Met variant (Fixman et al., 1997). Here we show that c-Cbl is expressed in epithelial cells and that in response to HGF, c-Cbl is phosphorylated on tyrosine residues. To assist in defining the biological function of c-Cbl in epithelial dispersal and morphogenesis, we dissected the biological activities of 70z-Cbl–mediated signaling with the use of our established MDCK epithelial cell model. We show that expression of the 70z-Cbl oncogenic variant induces changes in cell shape consistent with loss of an epithelial phenotype. From structure–function studies, we demonstrate that the amino-terminal SH2/PTB domain of c-Cbl (Cbl-N) is sufficient to induce changes in cell morphology and that a point mutation at Gly-306 abrogates the ability of Cbl-N or 70z-Cbl to induce these morphological changes. In contrast, substitution of Tyr-731 for Phe or deletion of the proline-rich domain of 70z-Cbl had no effect. Our results suggest that the SH2/PTB domain of c-Cbl (Cbl-N) plays a role in the breakdown of cell–cell junctions and modulates changes in epithelial cell morphology.
Upon characterization of MDCK cells expressing 70z-Cbl, we observed remodeling of the actin cytoskeleton associated with a decrease in cortical actin and an increase in actin stress fibers present in large lamellipodia-like membrane extensions. Consistent with the reorganization of the actin cytoskeleton, vinculin-containing focal complexes were relocalized from the periphery of cells in the colony to lamellipodial structures and throughout the cell at points of contact with the extracellular matrix (Figure 3). The process of conversion of epithelial cells to a mesenchymal phenotype has been referred to as epithelial–mesenchymal transition (Hay, 1995). Hence, overexpression of 70z-Cbl promotes cell changes that resemble an epithelial–mesenchymal transition. This transition correlates with the loss of E-cadherin from an insoluble compartment at cell–cell contacts, although the total levels of E-cadherin were not altered (Figure 2). Loss of epithelial organization is a hallmark of tumor progression associated with the acquisition of increased migratory and invasive properties (Birchmeier et al., 1996). However, the quantitation of cell migration with the use of a modified Boyden chamber in the absence or presence of a migratory stimulus such as HGF revealed no increase in the rate of motility of single MDCK cells expressing 70z-Cbl relative to control cells (Figure 5), demonstrating that 70z-Cbl expression did not enhance cell motility per se. However, from time-lapse video microscopy, MDCK cells expressing 70z-Cbl were shown to be capable of breaking cell–cell contacts and cell dispersal, whereas control MDCK cells expressing vector alone were unable to break cell contacts in the absence of HGF (Figure 5, C and D). We conclude that 70z-Cbl promotes the conversion of MDCK cells from predominantly forming cell–cell interactions to favoring cell–matrix interactions that promote cell spreading. A role for c-Cbl in the formation of cell–matrix interactions is in accordance with the observation that c-Cbl is phosphorylated after integrin stimulation (Ojaniemi et al., 1997) and that antisense inhibition of c-Cbl expression is associated with reduced cell spreading in macrophages (Meng and Lowell, 1998).
Our structure–function studies have demonstrated that the amino-terminal domain of c-Cbl (Cbl-N, amino acids 1–357) is sufficient to induce loss of epithelial cell morphology. Phosphorylation and coimmunoprecipitation studies demonstrated that although 70z-Cbl is highly phosphorylated and associated with Crk, Cas, Grb2, and p85, Cbl-N is neither phosphorylated nor associated with any of these proteins. This suggests that the association of Cbl with these known downstream signaling proteins is not essential for the observed changes in epithelial morphology and enhanced spreading of Cbl-N–expressing MDCK cells. This is in contrast to the apparent requirement for c-Cbl–associated PI3′-K activity in the spreading of macrophages (Meng and Lowell, 1998; Zell et al., 1998; Feshchenko et al., 1999). However, our data support observations that the amino-terminal portion of Cbl allows a positive signal required for NFAT activation downstream from the T-cell receptor (van Leeuwen et al., 1999; Zhang et al., 1999).
The amino-terminal portion of Cbl (Cbl-N) was identified as a phosphotyrosine-binding domain with the ability to interact with phosphotyrosine residues in Zap-70 (Tyr-292) (Lupher et al., 1997), and binding was abrogated in the G306E mutant. Recent structural studies have revealed that the Cbl-N domain retains a structure common to SH2 domains, further implicating this domain as a phosphotyrosine-binding domain. This suggests that epithelial–mesenchymal transition induced by Cbl-N is possibly mediated by its ability to interact with phosphotyrosine-containing proteins. In support of this, a GST-Cbl-N fusion protein associates with phosphorylated protein(s) of ∼150 kDa in unstimulated or HGF-stimulated MDCK cells (Figure 8). As expected, a Cbl-N mutant (G306E) fails to associate with phosphotyrosine-containing proteins in MDCK cells (Figure 8), which correlates with its inability to induce an epithelial–mesenchymal transition (Figure 5).
In fibroblasts and in transient overexpression studies, the binding of 70z-Cbl or Cbl-N to the EGF and PDGF receptors enhances their tyrosine phosphorylation (Thien and Langdon, 1997). While this paper was in preparation, the c-Cbl RING finger domain was shown to interact with ubiquitin-conjugating enzymes, and this was shown to promote ligand-induced ubiquitination of the EGF receptor (Waterman et al., 1999; Yokouchi et al., 1999). Hence, a 70z-Cbl or Cbl-N protein lacking a portion or the entire RING finger domain is thought to compete with a c-Cbl SH2/PTB-domain binding site in the EGFR. As a result, endogenous c-Cbl can no longer bind, ubiquitinate, and down-regulate the receptor (Waterman et al., 1999), providing a possible mechanism for 70z-Cbl– and Cbl-N–mediated cell transformation. Consistent with this, a G306E Cbl-N mutant fails to enhance tyrosine phosphorylation of the EGF and PDGF receptors and abolishes the transforming activity of Cbl-N in NIH3T3 cells (Bonita et al., 1997; Thien and Langdon, 1997).
The Met receptor is one of the predominant regulators of epithelial–mesenchymal transition (Birchmeier et al., 1996). Met is polyubiquitinated and degraded after HGF stimulation (Jeffers et al., 1997); thus, one possible explanation for the ability of Cbl-N to promote epithelial–mesenchymal transition of colonies of epithelial cells is through the activation of the Met receptor tyrosine kinase in these cells. Although we cannot detect Met in the GST-Cbl-N pulldowns from 70z-Cbl–expressing cells, a CSF-MET fusion protein of 170 kDa, overexpressed in MDCK cells (Zhu et al., 1994b), can associate with Cbl-N in pulldown assays (Figure 8B). Hence, it is possible that the phosphoprotein(s) of ∼150 kDa that associate with the GST-Cbl-N fusion protein may also include the Met receptor tyrosine kinase β chain (145 kDa), but at levels that are undetected by immunoblotting. The positive signal induced by Cbl-N, therefore, may result in part from competition of Cbl-N with the endogenous c-Cbl protein and subsequent indirect increases of Met receptor activity. However, Met, EGFR phosphorylation, and exogenous kinase activity are not enhanced detectably in cells expressing Cbl-N or 70z-Cbl (Figure 6 and our unpublished results), nor does EGF treatment promote epithelial cell dispersal (Maroun et al., 1999; our unpublished results). Moreover, MAPK and JNK pathways activated downstream of the Met receptor after stimulation by HGF (Royal et al., 2000) are not increased in cells expressing 70z-Cbl, supporting our observations that Met phosphotyrosine levels are not increased detectably in these cells, suggesting that Cbl-N may promote epithelial–mesenchymal transition by a mechanism independent from activation of the Met receptor. In agreement with our data, a role for c-Cbl in modulating cell shape and the actin cytoskeleton in fibroblasts was recently shown to be dependent on a functional SH2 domain (Scaife and Langdon, 2000), supporting a role for the Cbl SH2 domain in the interaction with proteins that regulate the actin cytoskeleton.
Our studies suggest that an important role for c-Cbl function in epithelial cells may be to prevent inappropriate activation of signaling pathways that lead to breakdown of organized epithelia. This is consistent with the observation that c-Cbl null mice display hyperplasia of breast epithelia (Murphy et al., 1998). Additional studies are required to elucidate the molecular mechanism through which Cbl regulates epithelial morphology, and the identification of Cbl-N–associated proteins in epithelial cells is likely to provide important insights into the biology of this signaling protein.
ACKNOWLEDGMENTS
We thank members of the Park laboratory for critical reading of the manuscript and Drs. Michel Tremblay, John Bergeron, and Barry Posner for antibodies. This research was supported by operating grants from the Medical Research Council of Canada and the National Cancer Institute of Canada to M.P. Further support was given by National Institutes of Health operating grants CA76118 and CA75075 (to H.B.) and by the National Health and Medical Research Council (Canberra) (to W.L). T.M.F. is a recipient of a Cancer Research Society Studentship. L.L. is a recipient of a Studentship from the Medical Research Council of Canada. M.P. is a Scientist of the Medical Research Council of Canada.
Abbreviations used:
- EGFR
EGF receptor
- HA
hemagglutinin
- HGF
hepatocyte growth factor
- MDCK
Madin-Darby canine kidney
- PI3′-K
phosphatidylinositol-3′-kinase
- PTB
phosphotyrosine-binding domain
- SH2
Src homology domain 2
- SH3
Src homology domain 3
REFERENCES
- Andoniou CE, Thien CB, Langdon WY. Tumor induction by activated abl involves tyrosine phosphorylation of the product of the cbl oncogene. EMBO J. 1994;13:4515–4523. doi: 10.1002/j.1460-2075.1994.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 1996;156:217–226. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
- Bonita DP, Miyake S, Lupher ML, Jr, Langdon WY, Band H. Phosphotyrosine binding domain-dependent upregulation of the platelet-derived growth factor receptor alpha signaling cascade by transforming mutants of Cbl: implications for Cbl's function and oncogenicity. Mol Cell Biol. 1997;17:4597–4610. doi: 10.1128/mcb.17.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B, Valles AM, Thiery JP. Model systems of epithelium-mesenchyme transitions. Acta Anat. 1996;156:227–239. doi: 10.1159/000147849. [DOI] [PubMed] [Google Scholar]
- Feshchenko EA, Shore SK, Tsygankov AY. Tyrosine phosphorylation of C-Cbl facilitates adhesion and spreading while suppressing anchorage-independent growth of V-Abl-transformed NIH3T3 fibroblasts. Oncogene. 1999;18:3703–3715. doi: 10.1038/sj.onc.1202672. [DOI] [PubMed] [Google Scholar]
- Fixman ED, Holgado-Madruga M, Nguyen L, Kamikura DM, Fournier TM, Wong AJ, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- Fixman ED, Naujokas MA, Rodrigues GA, Moran MF, Park M. Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene. 1995;10:237–249. [PubMed] [Google Scholar]
- Gherardi E. Growth factors and cell movement. Eur J Cancer. 1991;27:403–405. doi: 10.1016/0277-5379(91)90370-s. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Hunter S, Burton EA, Wu SC, Anderson SM. Fyn associates with Cbl and phosphorylates tyrosine 731 in Cbl, a binding site for phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:2097–2106. doi: 10.1074/jbc.274.4.2097. [DOI] [PubMed] [Google Scholar]
- Jeffers M, Taylor GA, Weidner KM, Omura S, Vande Woude GF. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu Y-C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Jongeward GD, Clandinin TR, Sternberg PW. sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics. 1995;139:1553–1566. doi: 10.1093/genetics/139.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Kitamura N. The cell dissociation and motility triggered by scatter factor/hepatocyte growth factor are mediated through the cytoplasmic domain of the c-Met receptor. Oncogene. 1993;8:2381–2390. [PubMed] [Google Scholar]
- Langdon WY, Hartley JW, Klinken SP, Ruscetti SK, Morse HC., 3d v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci USA. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Wang Y, Dominguez MG, Yeung YG, Murphy MA, Bowtell DD, Stanley ER. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupher ML, Jr, Reedquist KA, Miyake S, Langdon WY, Band H. A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- Lupher ML, Jr, Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- Manie SN, Sattler M, Astier A, Phifer JS, Canty T, Morimoto C, Druker BJ, Salgia R, Griffin JD, Freedman AS. Tyrosine phosphorylation of the product of the c-cbl protooncogene is [corrected] induced after integrin stimulation [published erratum appears in Exp. Hematol. (1997). 25, 177] Exp Hematol. 1997;25:45–50. [PubMed] [Google Scholar]
- Maroun CR, Holgado-Madruga M, Royal I, Naujokas MA, Fournier TM, Wong AJ, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Lowell CA. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Sawasdikosol S, Burakoff SJ, Eck MJ. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- Miyake S, Lupher ML, Jr, Andoniou CE, Lill NL, Ota S, Douillard P, Rao N, Band H. The Cbl protooncogene product: from an enigmatic oncogene to center stage of signal transduction. Crit Rev Oncog. 1997;8:189–218. doi: 10.1615/critrevoncog.v8.i2-3.30. [DOI] [PubMed] [Google Scholar]
- Miyake S, Lupher ML, Jr, Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD. Tissue hyperplasia and enhanced T-cell signaling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Holgado-Madruga M, Maroun C, Fixman ED, Kamikura D, Fournier T, Charest A, Tremblay ML, Wong AJ, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Martin SS, Dolfi F, Olefsky JM, Vuori K. The proto-oncogene product p120(cbl) links c-Src and phosphatidylinositol 3′-kinase to the integrin signaling pathway. J Biol Chem. 1997;272:3780–3787. doi: 10.1074/jbc.272.6.3780. [DOI] [PubMed] [Google Scholar]
- Ota Y, Samelson LE. The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GA, Naujokas MA, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GA, Park M. Isoforms of the met receptor tyrosine kinase. EXS. 1993;65:167–179. [PubMed] [Google Scholar]
- Royal I, Lamarch-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of Cdc42, Rac, PAK and Rho-kinase in response to hepatocyte growth factor differentially regulate epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor induced scatter of MDCK cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Scaife RM, Langdon WY. c-Cbl localizes to actin lamellae and regulates lamellipodia formation and cell morphology. J Cell Sci. 2000;113:215–226. doi: 10.1242/jcs.113.2.215. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy JB, Baron R. c-Cbl is downstream of c-Src in a signaling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. Tyrosine kinase activity of the EGF receptor is enhanced by the expression of oncogenic 70Z-Cbl. Oncogene. 1997;15:2909–2919. doi: 10.1038/sj.onc.1201468. [DOI] [PubMed] [Google Scholar]
- Ueno H, Sasaki K, Miyagawa K, Honda H, Mitani K, Yazaki Y, Hirai H. Antisense repression of proto-oncogene c-Cbl enhances activation of the JAK-STAT pathway but not the ras pathway in epidermal growth factor receptor signaling. J Biol Chem. 1997;272:8739–8743. doi: 10.1074/jbc.272.13.8739. [DOI] [PubMed] [Google Scholar]
- van Leeuwen JE, Paik PK, Samelson LE. Activation of nuclear factor of activated T cells-(NFAT) and activating protein 1 (AP-1) by oncogenic 70Z Cbl requires an intact phosphotyrosine binding domain but not Crk(L) or p85 phosphatidylinositol 3-kinase association. J Biol Chem. 1999;274:5153–5162. doi: 10.1074/jbc.274.8.5153. [DOI] [PubMed] [Google Scholar]
- Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- Yoon CH, Lee J, Jongeward GD, Sternberg PW. Similarity of sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-cbl. Science. 1995;269:1102–1105. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]
- Zell T, Warden CS, Chan AS, Cook ME, Dell CL, Hunt SW, 3rd, Shimizu Y. Regulation of beta 1-integrin-mediated cell adhesion by the Cbl adaptor protein. Curr Biol. 1998;8:814–822. doi: 10.1016/s0960-9822(98)70323-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Elly C, Qiu L, Altman A, Liu YC. A direct interaction between the adaptor protein Cbl-b and the kinase zap-70 induces a positive signal in T cells. Curr Biol. 1999;9:203–206. doi: 10.1016/s0960-9822(99)80090-6. [DOI] [PubMed] [Google Scholar]
- Zhu H, Naujokas MA, Fixman ED, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994a;269:29943–29948. [PubMed] [Google Scholar]
- Zhu H, Naujokas MA, Park M. Receptor chimeras indicate that the met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ. 1994b;5:359–366. [PubMed] [Google Scholar]